Abstract
We have developed a noninvasive detection method for expression of viral-mediated gene transfer. A recombinant adenovirus was constructed by using the gene for arginine kinase (AK), which is the invertebrate correlate to the vertebrate ATP-buffering enzyme, creatine kinase. Gene expression was noninvasively monitored using 31P-magnetic resonance spectroscopy (31P-MRS). The product of the AK enzyme, phosphoarginine (PArg), served as an MRS-visible reporter of AK expression. The recombinant adenovirus coding for arginine kinase (rAdCMVAK) was injected into the right hindlimbs of neonatal mice. Two weeks after injection of rAdCMVAK, a unique 31P-MRS resonance was observed. It was observable in all rAdCMVAK injected hindlimbs and was not present in the contralateral control or the vehicle injected limb. PArg and phosphocreatine (PCr) concentrations were calculated to be 11.6 ± 0.90 and 13.6 ± 1.1 mM respectively in rAdCMVAK injected limbs. AK activity was demonstrated in vivo by monitoring the decreases in PArg and ATP resonances during prolonged ischemia. After 1 h of ischemia intracellular pH was 6.73 ± 0.06, PCr/ATP was decreased by 77 ± 8%, whereas PArg/ATP was decreased by 50 ± 15% of basal levels. PArg and PCr returned to basal levels within 5 min of the restoration of blood flow. AK activity persisted for at least 8 mo after injection, indicating that adenoviral-mediated gene transfer can produce stable expression for long periods of time. Therefore, the cDNA encoding AK provides a useful reporter gene that allows noninvasive and repeated monitoring of gene expression after viral mediated gene transfer to muscle.
Keywords: nuclear magnetic resonance, gene therapy, adenovirus, creatine kinase, arginine kinase
Recombinant viruses provide highly efficient vehicles for the delivery of transgenes to both skeletal and cardiac muscle (1, 2). Recombinant adenoviruses have been used to replace missing proteins in mouse models of muscular dystrophies (3, 4), as well as to create muscles that secrete therapeutic proteins (2, 5, 6). Previous studies have shown that direct injection of recombinant adenovirus into neonatal murine muscle can result in long-term, efficient expression in >60% of myofibers (7). However, there is tremendous variability in the extent and length of expression. If gene therapy in humans becomes a reality, then monitoring of gene transfer efficiency and expression in clinical settings would currently require invasive techniques (8). Therefore, an obvious need exists for noninvasive tools to measure the efficacy of gene transfer. Despite the recent advent of new methods to image gene expression in vivo in transparent tissues (9, 10) and using positron emitting radioisotopes (11), MRI, and magnetic resonance spectroscopy (MRS) have the greatest potential for use in noninvasive gene transfer assessment. MRS is a classic tool for performing noninvasive measurements of metabolic status of tissues without the use of ionizing or nephrotic agents. MRS has the added advantage that spectral information can be obtained in seconds and can be used to determine enzymatic rates in vivo quantitatively.
An ideal reporter gene must satisfy a number of criteria. First, it must provide a unique signal so that transgene expression can be monitored against a background of natively expressed genes. Second, it must be small enough so that it can be expressed with therapeutic genes in bicistronic constructs so that it serves as a direct marker for the entire virus. Third, it must not interfere with normal tissue function. In our approach, we have constructed an adenovirus expressing arginine kinase (AK) that satisfies the criteria listed above.
Creatine kinase (CK) catalyzes a near-equilibrium, dead end reaction in a variety of vertebrate tissues (primarily nerve and muscle). An analogous reaction that provides buffering in invertebrate tissue is catalyzed by AK:
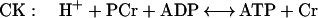 |
1 |
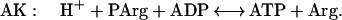 |
2 |
The equilibrium constant for the CK (Eq. 1) reaction is approximately 166, whereas under the same conditions it is approximately 10-fold less for AK (Eq. 2) (12, 13). Measurements of the levels of free Arg in the cytoplasm of mammalian muscle range from 0.1 to 1.0 mM (14). Thus, we anticipated that if AK was introduced into mammalian muscle, the muscle would produce phosphoarginine (PArg) at equilibrium concentrations in the range of 3–30 mM. Based on previous studies on invertebrate muscle (12, 15, 16), PArg would provide a unique phosphorus NMR signal of magnitude and position that could be easily recognized in vertebrate muscle. In addition, we hypothesized that the combined expression of AK and CK could be beneficial to the cell under conditions of low ATP/adenosine 5′-diphosphate and intracellular pH (12). In this study, AK expression was achieved in the hindlimb muscles of mice by the direct injection of a recombinant adenovirus encoding for an AK cDNA (rAdCMVAK). AK expression was monitored noninvasively by using 31P-magnetic resonance spectroscopy (31P-MRS.). Herein, we demonstrate that both the expression and the activity of the AK transgene can be measured noninvasively and followed as a function of time with 31P-MRS.
Methods
Viral Construct.
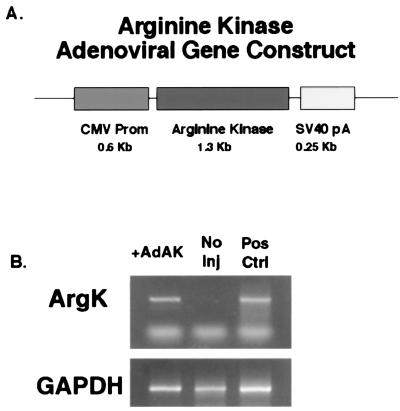
The cDNA for Drosophila melanogaster AK was amplified from an adult Drosophila cDNA library (5′-Stretch, CLONTECH) using the PCR (sense strand, CGCCCTTTTACAATGGTCAAT; antisense strand, GATTGTTGCGTATGCCCCGAA). The cDNA fragment was then ligated into the BamHI–HinDIII sites of the pAdLox vector (17). Expression was driven by CMV promoter and was stabilized by a simian virus 40 polyadenylation sequence (SV40pA). Fig. 1 shows a schematic for this gene construct. An ΔE1–ΔE3 adenovirus was prepared from the final construct (rAdCMVAK) by using published methods (17) by the University of North Carolina Vector Core.
Figure 1.
(A) Schematic diagram for rAdCMVAK gene construct. Expression was driven by nonspecific CMV promoter and was stabilized by an simian virus 40 polyadenylation sequence (SV40pA). From this construct, a ΔE1–ΔE3 adenovirus was prepared by using published methods (17). (B) RT-PCR was used to detect the presence of AK transcripts in rAdCMVAK-injected muscles. Total RNA isolated from frozen tissue was subjected to RT-PCR by using oligonucleotides specific for Drosophila AK and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). AK transcripts were detected in rAdCMVAK-injected muscles (+AdAK) but not in the contralateral control (No Inj). AK and GAPDH primers served as positive controls for the procedure (Pos Ctrl).
Injections.
All experiments involving animals were approved by the University of Pennsylvania's Animal Care and Use Committee. Forty microliters of 10% glycerol/PBS containing approximately 1010 adenoviral particles were injected into the interstitial space of the anterior and posterior muscle compartment of the right hindlimb of anesthetized C57BL/6 neonatal mice between 1 and 3 days of age. Once the mice regained consciousness, they were returned to the animal facility until further study.
Detection of Transgene Expression.
The reverse transcriptase–PCR (RT-PCR) was used to detect the presence of AK transcripts in rAdCMVAK-injected muscles. Total RNA was isolated from frozen tissue using a commercial kit (RNAqueous, Ambion, Austin, TX) and was subjected to reverse transcription and PCR (Perkin–Elmer) using oligonucleotides specific for Drosophila AK (sense primer, TGCCGAGGCTTACACAG; antisense primer, AAGTGGTCGTCGATCAG). Primers that amplified glyceraldehyde 3-phosphate dehydrogenase (sense primer, TGAAGGTCGGAGTCAACGGATTTGGT; antisense primer, CATGTGGGCCATGAGGTCCACCAC) served as a positive control for the procedure.
NMR.
High resolution NMR spectra were recorded from mouse hindlimbs using a 5-mm diameter surface coil double tuned to 1H (300 MHz) and 31P (121 MHz) on a Bruker 300 MHz AMX spectrometer. Mice were anesthetized and secured to a home built vertical probe such that the surface coil could be positioned over the anterior portion of the lower hindlimb. Magnetic field homogeneity was adjusted using the free proton signal, resulting in a typical full width half maximum of 0.2 parts per million (ppm). 31P-MRS spectra were obtained with a pulse repetition time of 5.4 s, a pulse width of 20 micro seconds, a spectral width of 12,000 Hz, and 4,096 complex data points. Peak areas, chemical shifts, and line widths were measured by using time domain analysis. All chemical shifts were determined relative to the phosphocreatine (PCr) resonance. Intracellular pH was determined based on the Pi chemical shift.
Basal high energy phosphate levels were measured in the mouse hindlimbs starting at 2 wk after injection of rAdCMVAK and continued until 8 mo after injection. AK activity was detected in vivo by monitoring the degradation of PArg levels during circulatory occlusion. Prolonged ischemia was produced by the application of a tourniquet around the upper thigh muscles.
In Vitro Force Measurement.
The extensor digitorum longus (EDL) was removed from the hind limb, retaining the proximal and distal tendon. The intact muscle was immersed in a Ringer's solution buffered to pH 7.4 with 25 mM Hepes, which was continuously oxygenated and maintained at 25 ± 0.5° C. Muscles were mounted horizontally in the muscle bath, attached by tendinuous insertions to a post at one end and to the lever of a dual mode servomotor system at the other. Muscle length was adjusted to the length (L0) at which maximal twitch force is reached. Stimulation was delivered via two platinum plate electrodes, which were positioned along the length of the muscle. The maximal tetanic force was determined by using 120 Hz, 500-ms supramaximal pulses.
Results
MRS Measurement of AK Expression in Skeletal Muscle.
Solution experiments.
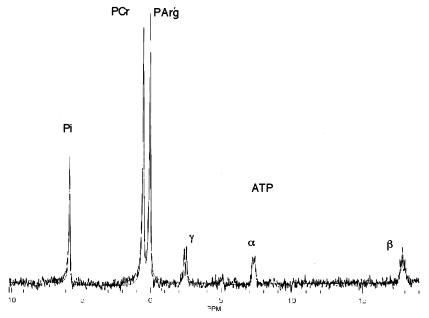
To determine the feasibility of measuring AK activity in vertebrate muscle a solution of the expected physiological concentrations of ATP, Pi, PCr, and PArg was constructed. As seen in Fig. 2, PArg was well resolved from PCr and γATP with a chemical shift of 0.47 ppm relative to PCr. In addition, the solution was used to determine the appropriate spectral analysis package to analyze both phantom and mouse skeletal muscle data with the minimal amount of user bias. Based on these preliminary studies a time domain Hankel single value decomposition algorithm (18) was used (Fig. 2) and user peak selection was avoided.
Figure 2.
31P-MRS spectra of a model solution containing expected physiological concentrations of ATP, Pi, PCr, and PArg. The model solution consisted of 30 mM PCr, 10 mM Pi, 30 mM PArg, and 8 mM ATP in 0.4 ml of distilled water. In solution, PArg was well resolved from PCr and γATP with a chemical shift of 0.47 ppm relative to PCr. The model solution was used to determine the appropriate spectral analysis package for the analysis of both phantom and mouse skeletal muscle data with the minimal amount of user bias. The solid line is the best fit to the free induction decay using a Hankel single value decomposition algorithm (18), which avoided peak selection and the use of previous knowledge.
Detection of AK Expression in Skeletal Muscle by NMR.
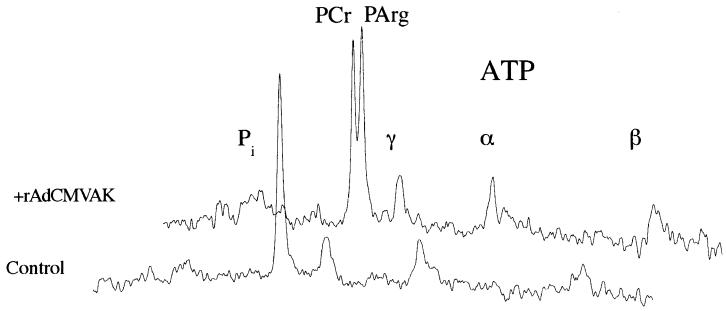
Due to the small volume of muscle in the neonatal mouse hindlimb (1- to 5-day-old), the earliest possible 31P-MRS measurements were made 12–13 days after gene delivery. At this time point, a unique resonance was observed at 0.49 ppm relative to the PCr resonance. This resonance at 0.49 ± 0.01 (n = 24) ppm away from the PCr resonance was observable in all of the injected limbs and was not present in the contralateral control leg or vehicle-injected limbs (Fig. 3). The presence of AK transcripts in rAdCMVAK injected muscles was confirmed by RT-PCR (Fig. 1B). The PArg resonance was observed in rAdCMVAK muscles up to 8 mo after gene delivery, indicating persistent expression of AK. The chemical shift of PCr relative to γATP was 2.46 ± 0.02 ppm, was not different between experimental groups, and was the same as previously reported in murine muscle (19). Both PCr and PArg existed as single, well-defined Lorentzian resonances with line widths of 44 ± 2.7 Hz and 32 ± 2.0 Hz, respectively.
Figure 3.
In vivo basal 31P spectra from the hindlimbs of a 6-mo-old mouse. 31P-MRS spectra from the rAdCMVAK-injected limb (Upper spectrum) reveal a 31P resonance at the chemical shift for PArg that is not present in the contralateral control limb (Lower spectrum).
The PArg/γATP ratio in the experimental hindlimb was 1.25 ± 0.12 whereas the PCr/γATP was 1.31 ± 0.12. The total PCr + PArg/γATP was 2.56 ± 0.16 in the rAdCMVAK-injected leg and PCr/γATP was 2.65 ± 0.58 in control limbs. To calculate absolute PCr and PArg concentration, perchloric acid extracts of injected and uninjected EDLs were used to determine ATP levels by high performance liquid chromatagraphy (20). Based on the measured ATP value of 8.7 mM in the injected limb and following correction for saturation, PArg and PCr were calculated to be 11.6 ± 0.85 and 13.6 ± 1.1 mM in the rAdCMVAK-injected muscles. Using the combined equilibrium of CK and AK, free Arg levels were calculated to range from 0.3 to 0.6 mM in the rAdCMVAK-injected muscles.
Detection of AK Activity in Skeletal Muscle.
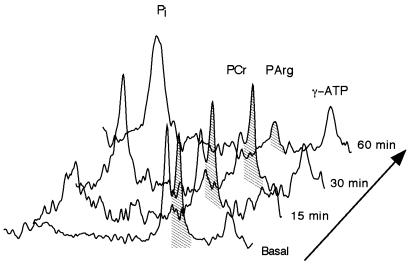
AK activity was determined in vivo by monitoring the decreases in PArg, PCr, and ATP resonances during prolonged ischemia (Fig. 4). After 1 h of ischemia intracellular pH was 6.73 ± 0.06, PCr/ATP decreased by 77 ± 8%, whereas PArg/ATP decreased by 50 ± 15% of basal levels (n = 6). PArg was completely resynthesized within 5 min of the restoration of blood flow. PArg depletion was demonstrated up to 8 mo in rAdCMVAK muscle. These results demonstrate AK activity and the equlibration of the PArg pool with cytoplasmic ATP.
Figure 4.
Stack plot of changes in high energy phosphates during prolonged ischemia in the rAdCMVAK-injected limb. During circulatory occlusion, PCr levels are depleted and inorganic Pi levels rise within 15 min of ischemia followed by the depletion of PArg.
In Vitro Mechanics.
The expression of AK in the EDL did not have a detrimental effect on maximal force production. The EDLs of 6-mo-old mice (n = 3) were removed for the determination of in vitro maximal tetanic muscle force. There was no significant difference in maximal tetanic force between rAdCMVAK and the contralateral control EDL muscles. Furthermore, the specific forces were not different from uninjected control EDLs.
Discussion
We have shown that AK can serve as a noninvasive monitoring system for viral-mediated gene delivery. This transgene upholds a number of criteria necessary for a gene marker in that it is small, nontoxic, and unique against the mammalian background. In addition, the expression of this particular marker in striated muscle introduces an additional thermodynamic buffer into a highly energetic tissue. This can provide insight into the role of phosphagen kinases in cellular function.
The general implication of these results are twofold. First, AK can be used as a noninvasive marker for gene transfer in vertebrate skeletal muscles. Secondly, skeletal muscles that express a combination of CK and AK should have an extended thermodynamic buffering range (12, 21). The CK equilibrium is poised to buffer ATP levels during the initial burst of ATPase activity, whereas AK buffering is greater under low ATP and intracellular pH levels.
AK as a Noninvasive Marker Gene.
The use of a MRS-visible marker has the obvious clinical benefit of monitoring transgene expression without removing the tissue from the subject. Adenoviruses are known to infect nonreplicating cell types such as terminal-differentiated myocytes. Muscle has been shown to be an easy target for adenoviral gene transfer in vivo, rendering muscle a viable tissue for gene therapy and the endogenous production of therapeutic secreted proteins (2, 5, 6). In this study, an adenovirus was injected into neonates to avoid an immune response that normally occurs in adult muscle (7). With the advent of high titer recombinant adeno-associated viruses, which lack immunogenic response, the prospect of human gene therapy using adeno-associated viruses is becoming a reality (2, 3, 6). Because of the large volume of tissues necessary to target in humans, methods need to be developed to assess the degree of gene transfer achieved. Moreover, because of the possibility that only transient expression (22) of the therapeutic gene is achieved, this method should be nondamaging, nontoxic, and capable of sequential measures. Whereas MR strategies to monitor gene expression have relied on receptor-mediated or probe activation strategies (23–26), xenografts (27), and the expression of an endogenous gene (28), here, we have presented a noninvasive MRS method capable of directly monitoring the expression of an unique, nontoxic gene marker in vivo. This method has the advantage that when tissue specificity or when inducible expression is desired, the reporter and therapeutic gene are under control of the same promoter. Furthermore, this method is not hampered by indicator delivery limitations. We demonstrated that AK expression in murine muscle resulted in the production of a unique resonance noninvasively observable with 31P-MRS. This resonance cannot be explained as a shift in the PCr peak or by changes in line width because it remained a well-defined, narrow resonance 2.46 ppm upfield from the γ-ATP in all limbs as previously reported (19). Due to the high concentration (≈12 mM) and the large chemical shift of PArg compared to other 31P-MRS visible metabolites, it was possible to repeatedly monitor the transgene product (PArg) and AK enzyme activity in vivo. Future experiments will be aimed at using saturation transfer studies to determine the simultaneous flux through CK and AK at rest and during exercise and its relationship to AK content (29, 30).
Energetic Consequences of AK Expression.
We have focused on AK as a noninvasive marker for gene transfer, but AK may prove to have an additional benefit to transiently ischemic muscle. We have added an additional cytoplasmic ATP buffer to vertebrate skeletal muscle by using an invertebrate transgene. The CK system is poised to keep the ATP/adenosine 5′-diphosphate at very high levels, enabling high ATPase fluxes at the onset of burst activity at the cost of PCr. Unfortunately, PCr cannot continue to sustain its thermodynamic buffer capacity at low ATP/adenosine 5′-diphosphate ratios that exist during sustained activity or prolonged ischemia. Ultimately, ATP levels are depleted, rigor ensues, and Ca2+ accumulation occurs in the sarcoplasm resulting in the loss of membrane integrity and cell death. On the other hand, invertebrate phosphagens exist that could also serve to buffer ATP utilization at low PCr values (12). Based on the difference in the CK and AK equilibrium constants, if the two enzymes exist in the same cell, then the initial flux through the AK reaction would be small compared with that through CK (12). As PCr is depleted, the ATPase flux will be primarily supported by AK. Thus the coexpression of AK and CK should prove beneficial to skeletal cells under conditions of prolonged ischemia or fatiguing conditions. Following the introduction of AK into the mammalian muscle cytoplasm a large PArg pool is expected to be formed without disturbing the normal levels of ATP or PCr (13). However, upon depletion of PCr which would occur in ischemia, CK can no longer buffer changes in ATP. At this point, the PArg pool will continue to buffer changes in ATP levels. In addition, the AK reaction will tend to slow the fall of pH. Direct support for this hypothesis is the observation that vertebrate muscle is known to take up the synthetic creatine analog cyclocreatine at high concentrations. When cyclocreatine is the substrate the CK equilibrium is reduced to 3% of that when native Cr is the substrate. Cyclocreatine also has been shown to delay ischemia-induced damage in both skeletal and cardiac muscle (21, 31). AK also buffers the free arginine pool and unlike creatine can be used by a number of different pathways. Of primary interest is whether transient increases in free arginine and/or PArg effect NO production and muscle function.
Implications for Gene Therapy.
We have verified that adenovirus gene delivery in newborn mice can result in persistent expression of the foreign genetic material for at least 8 mo. 31P-MRS offers the ability to rapidly and repeatedly monitor AK expression in the same muscle permitting the study of the influence of growth, aging, and disease status on gene expression. The coding sequence for AK is small enough (1.3 kb) that it can be coexpressed with growth and angiogenic factors and other small proteins in adeno-associated virus expression systems (32), which are limited in packing size (4.4 kb). These results demonstrate the presence of PArg in the unlocalized 31P-MRS spectra of small muscles (<30 mg); however, in larger muscles heterogeneity in gene expression throughout the muscle is expected due to a lack of global delivery. Therefore, future experiments will be geared toward providing localized information by the direct imaging of PArg and PCr levels spatially. Recent advances in MRI technology have permitted the imaging of PCr, ATP, and Pi in the human forearm muscles by using a clinical MRI scanner (33). Our preliminary measurements have demonstrated the feasibility of such a technique to image regional (4-ml volumes of interest) PCr content in the rabbit hindlimb in <8 min. Current experiments are aimed at producing the spectral selectivity necessary to image both PCr and PArg in the rabbit hindlimb expressing AK. AK as a gene marker is not limited to skeletal muscle, it could be applied in any tissues in which the free arginine levels are above 0.2 mM such that the resulting PArg resonance is above the noise limit. This approach has previously been shown to be successful by the expression of CK in the transgenic mouse liver (28).
Acknowledgments
We would like to acknowledge the help of Rudy Stuppard for the HPLC measurements, Daria Shoturma for her expert technical assistance, and the University of North Carolina Vector Core for the preparation of rAdCMVAK virus. This work was supported by grants from the National Institutes of Health (AR/NS43648) and the Muscular Dystrophy Association.
Abbreviations
- RT-PCR
reverse transcriptase-PCR
- PCr
phosphocreatine
- PArg
phosphoarginine
- rAdCMVAK
adenovirus coding for arginine kinase
- ppm
parts per million
- MRS
magnetic resonance spectroscopy
- EDL
extensor digitorum longus
- CMV
cytomegalovirus
- AK
arginine kinase
- CK
creatine kinase
References
- 1.Woo Y J, Zhang J C, Vijayasarathy C, Zwacka R M, Englehardt J F, Gardner T J, Sweeney H L. Circulation. 1998;98:II255–II261. [PubMed] [Google Scholar]
- 2.Barton-Davis E R, Shoturma D I, Musaro A, Rosenthal N, Sweeney H L. Proc Natl Acad Sci USA. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haecker S E, Stedman H H, Balice-Gordon R J, Smith D B, Greelish J P, Mitchell M A, Wells A, Sweeney H L, Wilson J M. Hum Gene Ther. 1996;7:1907–1914. doi: 10.1089/hum.1996.7.15-1907. [DOI] [PubMed] [Google Scholar]
- 4.Ragot T, Vincent N, Chafey P, Vigne E, Gilgenkrantz H, Couton D, Cartaud J, Briand P, Kaplan J C, Perricaudet M, et al. Nature (London) 1993;361:647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- 5.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner J M. Am J Pathol. 1999;154:355–363. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao G P, Qu G, Faust L Z, Engdahl R K, Xiao W, Hughes J V, Zoltick P W, Wilson J M. Hum Gene Ther. 1998;9:2353–2362. doi: 10.1089/hum.1998.9.16-2353. [DOI] [PubMed] [Google Scholar]
- 7.Stratford-Perricaudet L D, Makeh I, Perricaudet M, Briand P. J Clin Invest. 1992;90:626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gussoni E, Blau H M, Kunkel L M. Nat Med. 1997;3:970–977. doi: 10.1038/nm0997-970. [DOI] [PubMed] [Google Scholar]
- 9.Misteli T, Spector D L. Nat Biotechnol. 1997;15:961–964. doi: 10.1038/nbt1097-961. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs W R, Jr, Barletta R G, Udani R, Chan J, Kalkut G, Sosne G, Kieser T, Sarkis G J, Hatfull G F, Bloom B R. Science. 1993;260:819–822. doi: 10.1126/science.8484123. [DOI] [PubMed] [Google Scholar]
- 11.Gambhir S S, Barrio J R, Phelps M E, Iyer M, Namavari M, Satyamurthy N, Wu L, Green L A, Bauer E, MacLaren D C, et al. Proc Natl Acad Sci USA. 1999;96:2333–2338. doi: 10.1073/pnas.96.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellington W R. J Exp Biol. 1989;143:177–194. doi: 10.1242/jeb.143.1.177. [DOI] [PubMed] [Google Scholar]
- 13.Sweeney H L. Med Sci Sports Exer. 1994;26:30–36. [PubMed] [Google Scholar]
- 14.Bergström J, Alvestrand A, Fürst P, Hultman E, Sahlin K, Vinnars E, Widström A. Clin Sci Mol Med. 1976;51:589–599. doi: 10.1042/cs0510589. [DOI] [PubMed] [Google Scholar]
- 15.Rao B D, Buttlaire D H, Cohn M. J Biol Chem. 1976;251:6981–6986. [PubMed] [Google Scholar]
- 16.Nageswara Rao B D, Cohn M. J Biol Chem. 1981;256:1716–1721. [PubMed] [Google Scholar]
- 17.Hardy S, Kitamura M, Harris-Stansil T, Dai Y, Phipps M L. J Virol. 1997;71:1842–1849. doi: 10.1128/jvi.71.3.1842-1849.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Boogaart A, Howe F A, Rodrigues L M, Stubbs M, Griffiths J R. NMR Biomed. 1995;8:87–93. doi: 10.1002/nbm.1940080207. [DOI] [PubMed] [Google Scholar]
- 19.Steeghs K, Benders A, Oerlemans F, de Haan A, Heerschap A, Ruitenbeek W, Jost C, van Deursen J, Perryman B, Pette D, et al. Cell. 1997;89:93–103. doi: 10.1016/s0092-8674(00)80186-5. [DOI] [PubMed] [Google Scholar]
- 20.Wiseman R W, Moerland T S, Chase P B, Stuppard R, Kushmerick M J. Anal Biochem. 1992;204:383–389. doi: 10.1016/0003-2697(92)90255-6. [DOI] [PubMed] [Google Scholar]
- 21.Roberts J J, Walker J B. Am J Physiol. 1982;243:H911–H916. doi: 10.1152/ajpheart.1982.243.6.H911. [DOI] [PubMed] [Google Scholar]
- 22.Ye X, Rivera V M, Zoltick P, Cerasoli F, Jr, Schnell M A, Gao G, Hughes J V, Gilman M, Wilson J M. Science. 1999;283:88–91. doi: 10.1126/science.283.5398.88. [DOI] [PubMed] [Google Scholar]
- 23.Bogdanov A, Jr, Weissleder R. Trends Biotechnol. 1998;16:5–10. doi: 10.1016/S0167-7799(97)01150-5. [DOI] [PubMed] [Google Scholar]
- 24.Moore A, Basilion J P, Chiocca E A, Weissleder R. Biochim Biophys Acta. 1998;1402:239–249. doi: 10.1016/s0167-4889(98)00002-0. [DOI] [PubMed] [Google Scholar]
- 25.de Marco G, Bogdanov A, Marecos E, Moore A, Simonova M, Weissleder R. Radiology. 1998;208:65–71. doi: 10.1148/radiology.208.1.9646794. [DOI] [PubMed] [Google Scholar]
- 26.Moats R F, Fraser S E, Mead T J. Angew Chem Int Ed Engl. 1997;36:726–727. [Google Scholar]
- 27.Stegman L D, Themetulla A, Beattie B, Kievit E, Lawrence T S, Blasberg R G, Tjuvajev J G, Ross B D. Proc Natl Acad Sci USA. 1999;96:9821–9826. doi: 10.1073/pnas.96.17.9821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koretsky A P, Bronsan M J, Chen L H, Chem J D, Van Dyke T. Proc Natl Acad Sci USA. 1990;87:3112–3116. doi: 10.1073/pnas.87.8.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer R A, Kuchmerick M J, Brown T R. Am J Physiol. 1982;242:C1–C11. doi: 10.1152/ajpcell.1982.242.1.C1. [DOI] [PubMed] [Google Scholar]
- 30.Bittl J A, DeLayre J, Ingwall J S. Biochemistry. 1987;26:6083–6090. doi: 10.1021/bi00393a021. [DOI] [PubMed] [Google Scholar]
- 31.Osbakken M, Ito K, Zhang D, Ponomarenko I, Ivanics T, Jahngen E G, Cohn M. Cardiology. 1992;80:184–195. doi: 10.1159/000175002. [DOI] [PubMed] [Google Scholar]
- 32.Rabinowitz J E, Salmulski J. Curr Opin Biotech. 1998;9:470–475. doi: 10.1016/s0958-1669(98)80031-1. [DOI] [PubMed] [Google Scholar]
- 33.Greenman R L, Elliott M A, Vandenborne K, Schnall M D, Lenkinski R E. Magn Reson Med. 1998;39:851–854. doi: 10.1002/mrm.1910390523. [DOI] [PubMed] [Google Scholar]