Abstract
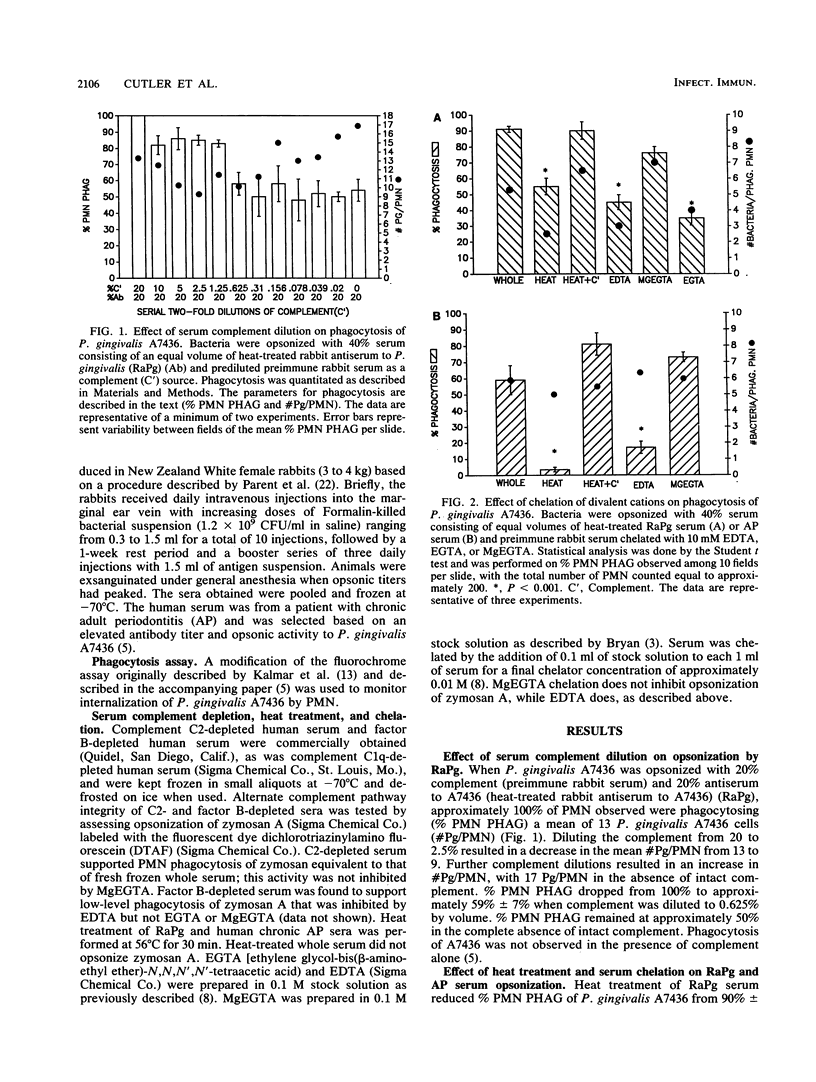
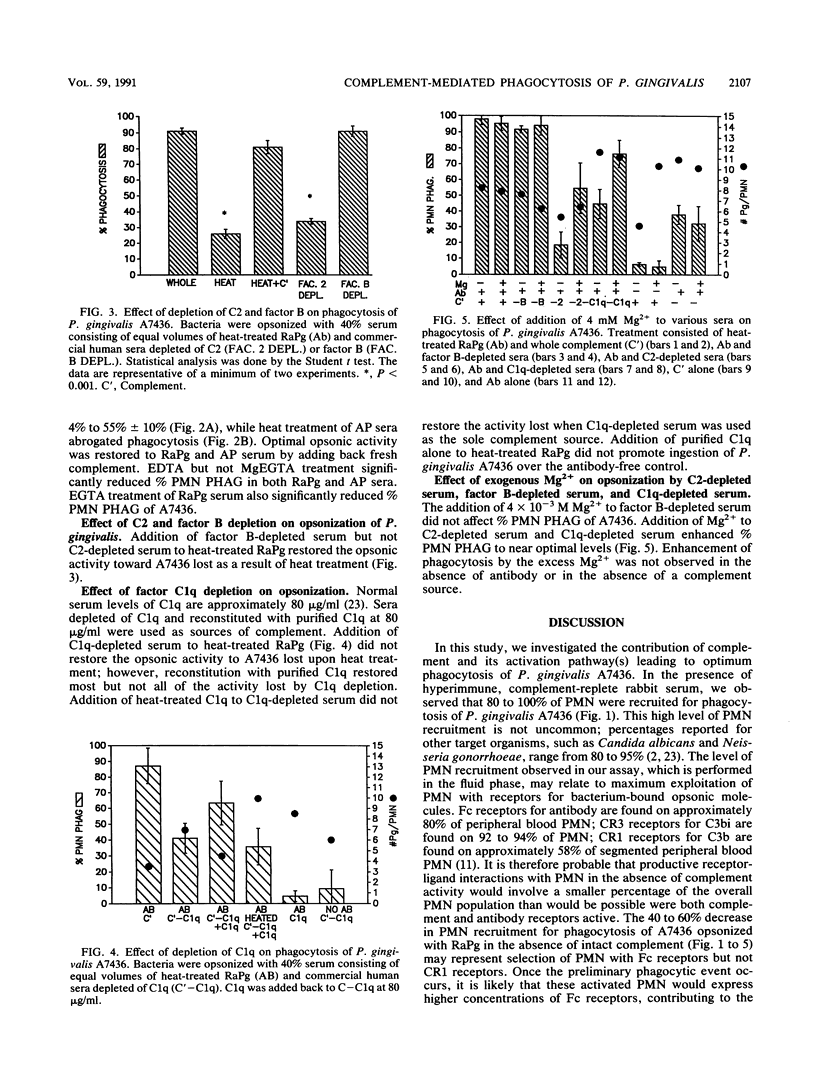
It has been suggested that the ability of Porphyromonas gingivalis to proteolyse complement, as well as its production of a capsule, contributes to resistance to phagocytosis by polymorphonuclear leukocytes. In this report, the opsonic role of serum complement and its activation pathways were investigated, using individual sera heat treated or depleted of factors B, C2, and C1q and the divalent cations Mg2+ and Ca2+. A fluorochrome microassay was used to quantitate phagocytosis of P. gingivalis A7436 by human polymorphonuclear leukocytes. Heat treatment of rabbit antiserum to P. gingivalis (RaPg) (56 degrees C, 30 min) resulted in a reduction in phagocytosis from 100% to 55% +/- 5%, while heat treatment of chronic adult periodontal disease serum abrogated phagocytosis. The heat-labile activity of RaPg was fully restored with MgEGTA-chelated rabbit serum but not EDTA- or EGTA-chelated rabbit serum. The addition of serum depleted of factor B but not C2 or C1q restored most of the heat-labile activity; however, the factor B-depleted serum was suspect, due to low-level opsonization of zymosan (inhibitable by EDTA but not MgEGTA). Adding C1q at 80 micrograms/ml to serum depleted of C1q restored much but not all of the activity lost through heat treatment or through depletion of C1q. A large part of opsonic activity with C2- and C1q-depleted sera was enhanced by the addition of 4 x 10(-3) M Mg2+. The data indicate that although opsonophagocytosis of P. gingivalis A7436 is dependent on the classical complement pathway, a significant contribution is made by an antibody-dependent alternate pathway.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bindon C. I., Hale G., Brüggemann M., Waldmann H. Human monoclonal IgG isotypes differ in complement activating function at the level of C4 as well as C1q. J Exp Med. 1988 Jul 1;168(1):127–142. doi: 10.1084/jem.168.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerknes R. Flow cytometric assay for combined measurement of phagocytosis and intracellular killing of Candida albicans. J Immunol Methods. 1984 Aug 3;72(1):229–241. doi: 10.1016/0022-1759(84)90451-4. [DOI] [PubMed] [Google Scholar]
- Bryan C. S. Sensitization of E. coli to the serum bactericidal system and to lysozyme by ethyleneglycoltetraacetic acid. Proc Soc Exp Biol Med. 1974 Apr;145(4):1431–1433. doi: 10.3181/00379727-145-38028. [DOI] [PubMed] [Google Scholar]
- Bryant R. E., Jenkins D. E., Jr Calcium requirements for complement dependent hemolytic reactions. J Immunol. 1968 Oct;101(4):664–668. [PubMed] [Google Scholar]
- Cutler C. W., Kalmar J. R., Arnold R. R. Phagocytosis of virulent Porphyromonas gingivalis by human polymorphonuclear leukocytes requires specific immunoglobulin G. Infect Immun. 1991 Jun;59(6):2097–2104. doi: 10.1128/iai.59.6.2097-2104.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Prez R. M., Bryan C. S., Hawiger J., Colley D. G. Function of the classical and alternate pathways of human complement in serum treated with ethylene glycol tetraacetic acid and MgCl2-ethylene glycol tetraacetic acid. Infect Immun. 1975 Jun;11(6):1235–1243. doi: 10.1128/iai.11.6.1235-1243.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P. Comparison of ethyleneglycoltetraacetic acid and its magnesium salt as reagents for studying alternative complement pathway function. Infect Immun. 1977 Apr;16(1):124–128. doi: 10.1128/iai.16.1.124-128.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Fries L. F., Siwik S. A., Malbran A., Frank M. M. Phagocytosis of target particles bearing C3b-IgG covalent complexes by human monocytes and polymorphonuclear leucocytes. Immunology. 1987 Sep;62(1):45–51. [PMC free article] [PubMed] [Google Scholar]
- Genco C. A., Cutler C. W., Kapczynski D., Maloney K., Arnold R. R. A novel mouse model to study the virulence of and host response to Porphyromonas (Bacteroides) gingivalis. Infect Immun. 1991 Apr;59(4):1255–1263. doi: 10.1128/iai.59.4.1255-1263.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin F. M., Jr Roles of macrophage Fc and C3b receptors in phagocytosis of immunologically coated Cryptococcus neoformans. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3853–3857. doi: 10.1073/pnas.78.6.3853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar J. R., Arnold R. R., Warbington M. L., Gardner M. K. Superior leukocyte separation with a discontinuous one-step Ficoll-Hypaque gradient for the isolation of human neutrophils. J Immunol Methods. 1988 Jun 13;110(2):275–281. doi: 10.1016/0022-1759(88)90115-9. [DOI] [PubMed] [Google Scholar]
- Kalmar J. R., Arnold R. R., van Dyke T. E. Direct interaction of Actinobacillus actinomycetemcomitans with normal and defective (LJP) neutrophils. J Periodontal Res. 1987 May;22(3):179–181. doi: 10.1111/j.1600-0765.1987.tb01561.x. [DOI] [PubMed] [Google Scholar]
- Michl J., Pieczonka M. M., Unkeless J. C., Silverstein S. C. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J Exp Med. 1979 Sep 19;150(3):607–621. doi: 10.1084/jem.150.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E. Microbiology of periodontal disease. J Periodontal Res. 1987 Sep;22(5):335–341. doi: 10.1111/j.1600-0765.1987.tb01595.x. [DOI] [PubMed] [Google Scholar]
- Mouton C., Hammond P. G., Slots J., Genco R. J. Serum antibodies to oral Bacteroides asaccharolyticus (Bacteroides gingivalis): relationship to age and periondontal disease. Infect Immun. 1981 Jan;31(1):182–192. doi: 10.1128/iai.31.1.182-192.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Eberhard H. J., Polley M. J., Calcott M. A. Formation and functional significance of a molecular complex derived from the second and the fourth component of human complement. J Exp Med. 1967 Feb 1;125(2):359–380. doi: 10.1084/jem.125.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Okuda K., Takazoe I. Detection of specific antibody in adult human periodontitis sera to surface antigens of Bacteroides gingivalis. Infect Immun. 1987 Mar;55(3):832–834. doi: 10.1128/iai.55.3.832-834.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niekrash C. E., Patters M. R. Simultaneous assessment of complement components C3, C4, and B and their cleavage products in human gingival fluid. II. Longitudinal changes during periodontal therapy. J Periodontal Res. 1985 May;20(3):268–275. doi: 10.1111/j.1600-0765.1985.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K., Schreiber R. D., Müller-Eberhard H. J. Formation of the initial C3 convertase of the alternative complement pathway. Acquisition of C3b-like activities by spontaneous hydrolysis of the putative thioester in native C3. J Exp Med. 1981 Sep 1;154(3):856–867. doi: 10.1084/jem.154.3.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent R., Mouton C., Lamonde L., Bouchard D. Human and animal serotypes of Bacteroides gingivalis defined by crossed immunoelectrophoresis. Infect Immun. 1986 Mar;51(3):909–918. doi: 10.1128/iai.51.3.909-918.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S. C., Densen P. Opsonophagocytosis of Neisseria gonorrhoeae: interaction of local and disseminated isolates with complement and neutrophils. J Infect Dis. 1985 Jan;151(1):33–41. doi: 10.1093/infdis/151.1.33. [DOI] [PubMed] [Google Scholar]
- Schenkein H. A. Failure of Bacteroides gingivalis W83 to accumulate bound C3 following opsonization with serum. J Periodontal Res. 1989 Jan;24(1):20–27. doi: 10.1111/j.1600-0765.1989.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Schenkein H. A., Genco R. J. Gingival fluid and serum in periodontal diseases. II. Evidence for cleavage of complement components C3, C3 proactivator (factor B) and C4 in gingival fluid. J Periodontol. 1977 Dec;48(12):778–784. doi: 10.1902/jop.1977.48.12.778. [DOI] [PubMed] [Google Scholar]
- Schenkein H. A. The effect of periodontal proteolytic Bacteroides species on proteins of the human complement system. J Periodontal Res. 1988 May;23(3):187–192. doi: 10.1111/j.1600-0765.1988.tb01356.x. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff A. J., van Steenbergen T. J., de Graaff J. The role of black-pigmented Bacteroides in human oral infections. J Clin Periodontol. 1988 Mar;15(3):145–155. doi: 10.1111/j.1600-051x.1988.tb01561.x. [DOI] [PubMed] [Google Scholar]



