Abstract
Objective
Ischemia reperfusion (IR) injury, occurring during heart attacks, hemorrhagic shock, and bypass and transplant surgeries, impairs microcirculatory function and nitric oxide (NO) synthesis. We report the regulation of endothelial and inducible NO synthase (eNOS and iNOS) proteins as a consequence of the application of continuous mode diagnostic frequency ultrasound application following IR injury.
Methods
Animals were assigned to one of 5 groups for microcirculatory assessment or western blot analysis (WB) as follows: 1) IR+iNOS inhibition (1400W); and 2) IR+1400W+ultrasound for microcirculatory assessment, 3) Control; 4) IR; and 5) IR+ultrasound for WB. Functional capillary density and microvascular diameter, flow velocity, and flow were monitored for microcirculatory assessment. Skin tissue samples were harvested for WB. 2.49 MHz continuous ultrasound was used for irradiation.
Results
Both the inhibition of iNOS alone and iNOS inhibition with ultrasound irradiation positively influenced the microcirculation of observed animals relative to baseline values. Ultrasound exposure resulted in a significant production of eNOS protein in skin tissue harvested 24 h into reperfusion (p < 0.01). iNOS levels from the same tissue of irradiated animals were found to be significantly decrease 0.5 h into reperfusion (p < 0.05).
Conclusion
Protection from lasting IR injury effects in the microcirculation, with continuous mode diagnostic frequency ultrasound, results from augmented eNOS levels during late reperfusion. Ultrasound inhibited iNOS production during early reperfusion may also confer protection from IR injury.
PACS codes
87.51.+e; 87.59.Mt
Keywords: ischemia reperfusion, ultrasound, microcirculation, eNOS, iNOS
INTRODUCTION
The beneficial effects of nitric oxide (NO) in the circulation and microcirculation have been widely documented [3, 24, 26, 32, 37]. Reports demonstrate its vasodilatory properties and its role in maintaining and regulating blood flow in healthy tissues [18, 19]. Increase of tissue damage following initial injury, through microvascular impairment and leukocyte endothelial cell interaction, has been attributed to the decreased production of NO [6, 11, 26, 33]. Injury in diseased or abnormal tissue leading to microcirculatory dysfunction has also been associated with inhibited NO synthesis [27, 40]. Analysis of the increased impairment of microcirculatory function and tissue injury following NO inhibitory treatment [31, 32] led to the identification of the NO synthase (NOS) enzyme and its endothelial and inducible isoforms, eNOS and iNOS respectively.
Studies on the prevention and treatment of ischemia reperfusion (IR) injury [5, 6, 33, 34, 36] have shown that eNOS and iNOS activity leads to differences in NO production encompassing both negative and positive effects. eNOS activity is most commonly reported as beneficial [11, 17], contrastingly, a majority of findings showing that iNOS derived NO has a detrimental influence [15, 34] on damaged tissue.
The usual protocol for analysis of the influence of NO on IR involves the use of exposed tissue from anesthetized animals, and the local manipulation of NO production thus avoiding responses due to systemic effects [25]. These investigations are therefore clinically relevant in the context of cardiac angioplasty or bypass and transplantations, involving the ligation of vasculature or the removal of a vascular block.
IR injury seen clinically in victims of heart attack, stroke, hemorrhagic shock, and transplant recipients, does not always afford the possibility of exposing the damaged tissue for treatment. Furthermore, such invasive actions may increase tissue damage through the procedure itself or possible infection. The noninvasive use of ultrasound irradiation, during circulatory occlusion and IR injury, should be advantageous in mitigating tissue damage in exposed [7, 39] and unexposed [8, 16] tissue.
The objective of this present study was to differentiate between eNOS and iNOS activity during IR injury with ultrasound exposure. Specifically this study examines the production and regulation of eNOS and iNOS protein due to the application of continuous mode diagnostic frequency ultrasound during IR injury following reperfusion. Our previous work reported significant improvement with ultrasound application after an extended period of reperfusion, which was partially negated with Nω-nitro-l-arginine methyl ester (l-NAME) treatment [16] therefore we will also investigate the hypothesis that effects of ultrasound in IR injury are due to the specific reversal of iNOS NO production impairment in the endothelium.
MATERIALS AND METHODS
Animal preparation
Investigations were carried out using the hamster window chamber model (chamber), implemented in Golden Syrian male hamsters (Charles River, Boston, MA), weight range of 50 to 75g, allowing the elimination of anesthetic influence and tissue exposure during the investigations. The complete technique has been previously described [10, 12]. Animal surgeries, chamber and carotid artery catheterization, were performed under general anesthesia, 50 mg/kg ip injection of pentobarbital sodium [13]. TPX used in place of the glass cover slip incorporates the protective properties of glass with greater acoustical coupling [9]. The Guide for the Care and Use of Laboratory Animals (National Research Council, 1996) was followed for animal handling and provided care, with all experiments approved by the local Animal Subjects Committee.
Inclusion criteria
Animals used were free of edema, visually evident injury in the window area, and infection in the surgical sites. Initial observations were carried out at least 3 days after chamber implantation, in order to allow for tissue recovery and stabilization.
Ischemia reperfusion injury
A pressure tourniquet, described previously [13, 30], was used to induce complete ischemia in the chamber. Flow of the area studied was interrupted by compressing a rubber ring against the chamber window tissue. The degree of compression was controlled by tightening a screw connected to the chamber, and the tissue within the ring was monitored until obtaining complete occlusion (blood flow cessation) of all vessels [30]. Continuous ischemia was ensured through periodic inspection. The release of the tourniquet marked the end of ischemia and initiation of reperfusion.
iNOS inhibition
N-(3-aminomethyl) benzylacetamidine dihydrochloride (1400W, Sigma) treatment was administered to appropriate animal groups as a bolus injection 1 min before the release of the tourniquet. The volume for infusion of 1400W solution was less than 5% of systemic blood volume, an estimate of 7% of hamster total body weight on day one of the experiment. Injections where dosed at 5 mg/kg in each group, a dose previously shown to inhibit iNOS in mice IR models [36]. The sample solution of 2.5 ml/g (0.1 to 0.15 ml) was infused through the carotid artery by hand and the catheter flushed with heparinized saline.
Ultrasound exposure
All animals exposed to ultrasound were suspended over the transducer using a three-pronged clamp and support ring stand. Exposure to ultrasound, for a 20 min period, began 5 min following the onset of reperfusion. The transducer (Valpey Fisher Corporation immersion transducer, part # IL0208HP, nominal frequency 2.25 MHz, element diameter 1 in.) was secured in a water bath, acting as a heat sink for the transducer head.
Animal exposure was accomplished using an open ended plastic cone affixed to the face of the transducer, filled with degassed water. Ultrasound was generated using a function generator (30 MHz Synthesized Function Generator, model DS345; Stanford Research Systems, Sunnyvale, CA) with a continuous sine wave output, 2.49 MHz frequency, and 10 Vp-p amplitude settings. The frequency and amplitude settings, currently used in clinical applications, were chosen to in order to facilitate adaptation of the experimental setup and effects to potential future clinical applications. Resultant pressure and power density produced is about 0.5 MPa and 8.33 W/cm2, respectively. (The transducer was calibrated using a hydrophone in the Ferrara Laboratory, Department of Biomedical Engineering, University of California, Davis).
Experimental groups
Animals were randomly assigned into one of 5 groups for microcirculatory assessment or Western Blot analysis (WB) as follows: IR+1400W group (n=5), IR+1400W+ultrasound group (n=5), control group (n=3), IR group (n=3), or IR+ultrasound group (n=3).
Microvascular experimental setup
Conscious animals were placed in plexi-glass restraining tubes, allowing for protrusion of chamber and minimized movement. Tubes containing hamsters were then fixed to the stage of an upright transillumination intravital microscopic (BX51WI; Olympus, New Hyde Park, NY). Projection onto a charge-coupled device camera (COHU 4815) connected to a videocassette recorder (AG-7355; JVC, Tokyo, Japan) enabled viewing of microscopic images on a monitor. Baseline vessel site selection used a 40x (LUMPFL-WIR, numerical aperture 0.8; Olympus) water immersion objective. The same measurement sites were followed throughout each experiment to allow direct comparison with baseline levels (Figure 1).
Figure 1.
Schematic outline of the microvascular and chamber tissue harvest experimental protocol for each group, not including control group. US, ultrasound.
Functional capillary density
Contrast enhancement between flowing red blood cells (RBCs) and tissue was accomplished using a BG12 (420 nm) band pass filter. Functional capillary density (FCD), functioning capillaries presented the flow of at least one RBC during the observation period of 30 sec, was determined in 13 to 25 stepwise vertically successive microscopic fields, (a region of about 1.7 to 1.9 mm2). Location of the initial field was identified by an anatomical feature in order to allow recognition in repeated measurements.
Fluorescent microscopy
Vessel diameter and FCD were assessed using epiillumination and fluorescein isothiocyanate, bound to dextran (MW 150,000; FITC-Dextran 150 Sigma Chemical, St. Louis, MO; 0.1 ml of a 12.5 mg/ml saline), during repeated measurements. FITC-Dextran was injection 5 to 10 min before individual repeated assessment periods. Intravital fluorescent microscopy used an additional mercury 100 W lamp and appropriate filters.
Microhemodynamics
Vessel diameter (D) was obtained online using a video image-shearing technique [21]. Real-time arteriolar and venular blood flow velocity measurements were achieved using the photodiode cross-correlation method [20] (Photo Diode/Velocity Tracker model 102B; Vista Electronics, San Diego, CA). Measured microvascular centerline velocity (V) was corrected using the corresponding vessel size correction factor to obtain the mean RBC velocity (Va) [29]. Blood flow (Q) was calculated using the measured and calculated parameters as Q = π Va*(D/2)2. This calculation has been found appropriate for tubes of 15 to 80 μm internal diameters [29] and assumes a parabolic velocity profile.
Western blot analysis experimental setup
Skin samples from animals in IR and IR+ultrasound groups were harvested in place of microcirculatory assessment at the proper assessment periods; 0.5, 2, or 24 h after the on set of reperfusion, following a 4 h period of ischemia. Samples from control group animals were harvested prior to IR. All tissue samples were harvested under anesthesia, quickly weighed, placed into individual tubes and snap frozen.
Preparation of lysates from hamster skin tissue
Frozen samples were cut into smaller pieces, placed in homogenization buffer, 10ml/g tissue (50 mM Tris, pH 7.5, 150 mM NaCl and complete protease inhibitor cocktail (Roche)) and homogenized with a Dounce homogenizer. Samples were solubilized by addition of 1/10th volume of 1% TritonX-100 and 600 mM octylglucoside. After incubating on ice for 20 min, the samples were centrifuged at 14,000 g for 20 minutes. Supernatant was collected and protein amounts were determined by BCA protein assay (Pierce) using bovine serum albumin as a standard. Equal amount of proteins were separated on NuPAGE® Bis-Tris Gels (Invitrogen) using MOPS running buffer and transferred to polyvinylidene fluoride (PVDF) membranes.
Immunoblot
After blocking with 5% BSA in Tris-buffered saline (TBS), membranes were incubated with primary antibodies against eNOS (monoclonal, BD Biosciences) or iNOS (monoclonal, BD Biosciences) and β-Tubulin (polyclonal, Santa Cruz Biotechnology, Inc.) at 4°C overnight in TBS 0.1% Tween 20 (TBST). Bound primary antibodies were detected with horseradish peroxidase–conjugated secondary antibodies: goat–anti-mouse IgG (Pierce, 1/5000) and goat–anti-rabbit IgG (Pierce, 1/5000), respectively, followed by chemiluminescent SuperSignal substrate (Pierce). Band intensity was quantified on unsaturated X-ray film by a digital image analyzer (Quantity-One; BioRad). All comparisons were made relative to individual band intensities of the quantified β-Tubulin of each sample.
Data analysis
Results are presented as means ± standard deviation. Data comparisons made within groups, measurement time points, and between group controls and treatments were analyzed using the unpaired student’s t-test or one sample t-test, with a hypothetical mean of 1.00. Microhemodynamic measurements were compared to baseline levels obtained before the experimental procedure and data are presented as ratios relative to baseline values. As stated above the same baseline vessels and capillary fields were assessed through repeated measurements for direct comparisons to be completed, allowing for more robust statistics in small sample populations. Differences were considered significant for p < 0.05. All statistics were calculated using GraphPad Prism 4.01 (GraphPad Software, Inc.; San Diego, CA).
RESULTS
Microcirculation: IR+1400W and IR+ultrasound+1400W groups
Both the inhibition of iNOS alone and ultrasound exposure with iNOS inhibition positively influenced the microcirculation of animals under study. Animals exposed to ultrasound had decreased FCD at the 0.5 h observation, below that of unexposed animals after IR and 1400W treatment. However, at both the 2 and 24 h assessments FCD of ultrasound exposed animals exceeded baseline and unexposed animal values. Differences in FCD did not reach significance at anytime during reperfusion (Table 1).
Table 1.
Changes in functional capillary density due to ischemia reperfusion injury and 1400W treatment, with and without 20 min of ultrasound exposure. Data are presented as mean ± standard deviation. IR14, IR+1400W group; US14, IR+1400W+ultrasound group.
| Time Point | Functional Capillary Density | |
|---|---|---|
| IR14 | US14 | |
| 0.5 h | 0.79 ± 0.13 | 0.77 ± 0.29 |
| 2 h | 0.88 ± 0.19 | 1.08 ± 0.28 |
| 24 h | 0.92 ± 0.13 | 1.01 ± 0.39 |
Vessel diameters shown in Table 2, where measured and classified as above or below baseline values for all repeated observations during reperfusion in both microcirculatory assessment groups. Arteriolar diameters of unexposed animal, at 24 h of reperfusion, were significantly increased compared to baseline values. Venules of both exposed and unexposed animals showed significantly increased diameters relative to baseline at each assessment period (Table 2).
Table 2.
Changes in arteriolar and venular diameters due to ischemia reperfusion injury and 1400W treatment, with and without 20 min of ultrasound exposure. 1400W alone provoked a significant long term increase in arteriolar diameter, relative to baseline levels, at 24 h of reperfusion. Both 1400W treatment and ultrasound exposure and 1400W treatment alone resulted in significantly increased diameters throughout the reperfusion period, 5, 2, and 24 h. Data are presented as mean ± standard deviation. Values excluded for p>0.05. IR14, IR+1400W group; US14, IR+1400W+ultrasound group.
| Time Point | Arteriolar Diameter | Venular Diameter | |||||
|---|---|---|---|---|---|---|---|
| IR14 | p | US14 | IR14 | p | US14 | p | |
| 0.5 h | 1.05 ± 0.31 | 0.99 ± 0.20 | 1.16 ± 0.19** | 0.001 | 1.31 ± 0.26*** | 0.0002 | |
| 2 h | 1.14 ± 0.20 | 1.10 ± 0.20 | 1.25 ± 0.17*** | <0.0001 | 1.25 ± 0.19*** | <0.0001 | |
| 24 h | 1.18 ± 0.21* | 0.03 | 1.07 ± 0.13 | 1.13 ± 0.17** | 0.0022 | 1.16 ± 0.21** | 0.0064 |
A similar trend was seen in arteriolar and venular flow velocity for all animals treated with 1400W. Flow velocity levels at 0.5 h of reperfusion where increased at 2 h but returned to decreased levels at 24 h into reperfusion. Decreased levels of flow velocity in exposed animals were found throughout reperfusion, relative to the unexposed group. The measured arteriolar flow velocities of ultrasound exposed animals were elevated above baseline values, compared to those of unexposed animals. The reverse response was true for venular flow velocities. Animals without ultrasound exposure had raised values compared to exposed animals; with both groups resulting in values above baseline. Increased flow velocities of unexposed animals researched significance, relative to baseline values, at 2 h of reperfusion (Table 3).
Table 3.
Changes in arteriolar and venular flow velocity due to ischemia reperfusion injury and 1400W treatment, with and without 20 min of ultrasound exposure. Venular flow velocities at 2 h of reperfusion were significantly increased, relative to baseline values, in unexposed animals. Data are presented as mean ± standard deviation. Values excluded for p>0.05. IR14, IR+1400W group; US14, IR+1400W+ultrasound group.
| Time Point | Arteriolar Flow Velocity | Venular Flow Velocity | |||
|---|---|---|---|---|---|
| IR14 | US14 | IR14 | p | US14 | |
| 0.5 h | 0.94 ± 0.57 | 1.15 ± 0.48 | 0.99 ± 0.65 | 0.91 ± 0.49 | |
| 2 h | 1.29 ± 0.66 | 1.41 ± 1.41 | 1.33 ± 0.38*** | 0.0001 | 1.15 ± 0.47 |
| 24 h | 1.09 ± 0.49 | 1.29 ± 0.54 | 1.02 ± 0.52 | 0.97 ± 0.43 | |
Calculated microvascular flow (Table 4), for arterioles and venules, resulted in increased levels throughout reperfusion compared to baseline levels for both animal groups. As with flow velocity there was a trend of increased microvascular flow levels at 2 h, compared to 0.5 h, returning to decreased levels at 24 h in both animals groups. Significantly increased venular flow was obtained, compared to baseline values, at 0.5 h of reperfusion in unexposed animals and 2 h of reperfusion in both exposed and unexposed animals.
Table 4.
Calculated changes in arteriolar and venular flow due to ischemia reperfusion injury and 1400W treatment, with and without 20 min of ultrasound exposure. Venular flows, compared to baseline, significantly increased at both 2 and 24 h of reperfusion in animals exposed to ultrasound and 2 h of reperfusion in unexposed animals. Data are presented as mean ± standard deviation. Values excluded for p>0.05. IR+1400W, IR+1400W group; US14, IR+1400W+ultrasound group.
| Time Point | Arteriolar Flow | Venular Flow | ||||
|---|---|---|---|---|---|---|
| IR14 | US14 | IR14 | p | US14 | p | |
| 0.5 h | 1.19 ± 0.99 | 1.13 ± 0.60 | 1.38 ± 1.02 | 1.59 ± 1.12*** | 0.0002 | |
| 2 h | 1.86 ± 1.43 | 1.74 ± 1.09 | 2.08 ± 0.71*** | <0.0001 | 1.75 ± 0.86** | 0.0024 |
| 24 h | 1.48 ± 0.70 | 1.51 ± 0.70 | 1.32 ± 0.87 | 1.36 ± 0.78 | ||
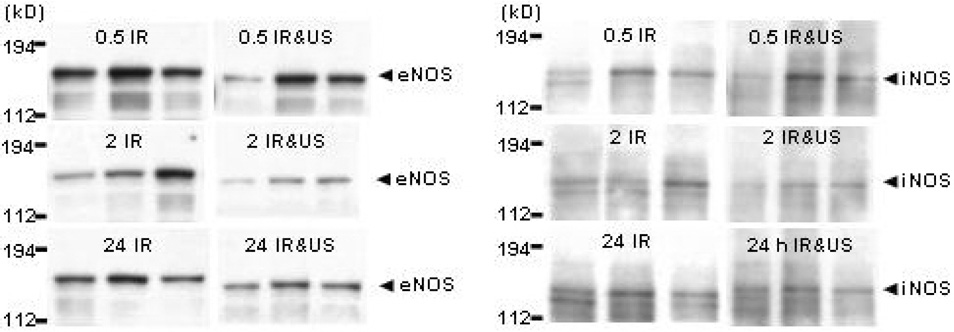
Presence of eNOS and iNOS
Figure 2 shows WB of eNOS and iNOS protein, in three individual hamster chamber skin samples; in controls and in animals subjected to IR injury with or without ultrasound exposure. Ultrasound exposure resulted in a significant production of eNOS protein in the chamber skin samples. Skin samples harvested from ultrasound exposed animals 24 h into reperfusion showed a significant production of eNOS protein (p < 0.01) compared to unexposed animals. eNOS protein levels in exposed animals decreased slightly from 0.5 to 2 h of reperfusion. Increased eNOS presence during 24 h of reperfusion after ultrasound exposure was significant compared to amounts produced during 0.5 and 2 h of reperfusion (p < 0.05). Skin samples harvested from unexposed animals showed a continuous decrease in eNOS production from 0.5 through 24 h of reperfusion without reaching significance (Table 5).
Figure 2.
Western blot analysis of NOS protein content in 3 individual hamster chamber tissue samples of animals subjected to ischemia reperfusion injury with or without ultrasound exposure. Tissue was harvested at 0.5, 2, or 24 h of reperfusion. β-Tubulin band intensities of samples are not shown. Left Panel: eNOS protein content. Right Panel: iNOS protein content. IR, IR group; IR&US, IR+ultrasound group.
Table 5.
Identification of individual nitric oxide synthase (eNOS and iNOS) protein expression levels during reperfusion assessment periods, relative to individual band intensities of quantified β-Tubulin of each sample. Data are presented as mean ± standard deviation. Values excluded for p>0.05.
| Time Point | eNOS Protein Level | iNOS Protein Level | ||||
|---|---|---|---|---|---|---|
| IR | IR+US | p | IR | IR+US | p | |
| 0.5 h | 0.71 ± 0.15 | 0.87 ± 0.44 | 0.43 ± 0.14 | 0.15 ± 0.10 | ||
| 2 h | 0.70 ± 0.27 | 0.63 ± 0.23 | 0.43 ± 0.28 | 0.17 ± 0.05* | 0.046 | |
| 24 h | 0.52 ± 0.21 | 1.83 ± 0.31**, +, ^^ | 0.004, 0.037, 0.006 | 0.32 ± 0.09 | 0.20 ± 0.09 | |
p < 0.05 vs. IR.
p < 0.01 vs. IR.
p < 0.05 vs. 0.5 h IR+ultrasound.
p < 0.01 vs. 2 h IR+ultrasound.
Levels of iNOS protein analyzed from the same harvested skin samples of animals exposed to ultrasound were lower at all time points, compared to unexposed animals. A significant decrease between chamber tissue iNOS levels was seen at 0.5 h into reperfusion in animals exposed to ultrasound, compared to those that were not. iNOS levels in unexposed animal skin increased slightly from 0.5 to 2 h of reperfusion and then decreased below 0.5 h levels in the 24 h sample. Ultrasound exposed animals had a slight steady increase in iNOS level through 24 h of reperfusion (Table 5).
DISCUSSION
The principle finding of this study is that exposure to diagnostic frequency continuous ultrasound after 4 h of complete ischemia decreased the production of iNOS at 0.5 h while increasing the production of eNOS at 24 h of reperfusion. Also the beneficial effect of iNOS inhibition, during ischemia reperfusion was confirmed. These outcomes were obtained by exposing the tissue for a 20 min period to continuous wave ultrasound, starting 5 min after the onset of reperfusion. Inhibition of iNOS resulted in a lasting early recovery of microvascular function of both animals groups, often significantly surpassing baseline values from 0.5 through 24 h of reperfusion.
The significant mitigation of IR injury following ultrasound exposure in a variety of tissues compared to unexposed tissues was previously reported [7, 16, 39]. We hypothesize that our results do not show a corresponding difference in microcirculatory function, between ultrasound exposed and unexposed tissues, as a consequence of the beneficial effects due to iNOS inhibition. It is likely that a maximal decrease in microcirculatory injury was the result of iNOS inhibition alone, therefore the added beneficial effects due to ultrasound exposure would not provide any further improvement of microvascular function. The prevention of iNOS production through genetic manipulation [34] and treatment with iNOS specific inhibitors [22, 43] during IR results in decreased injury and improved recovery. We also show that IR injury in the chamber model [16] is eliminated by treatment with the iNOS specific inhibitor 1400W. Although there is evidence supporting the benefit of iNOS [2, 28, 41], our results specifically show that inhibition of iNOS expression attenuates microvascular injury during IR.
WB of chamber tissue samples using an anti-iNOS antibody showed increased, above baseline, iNOS concentration during early reperfusion in both animal groups. We hypothesize that attenuated long-term IR damage is partially attributed to the decreased presence of iNOS in animals exposed to ultrasound, compared to unexposed animals. The upregulation of iNOS and or NO synthesis through the iNOS pathway induced apoptosis during in vitro [23] and in vivo ischemia reperfusion studies [42]. The build up of toxic metabolites during ischemia in addition to the influx of oxygen and subsequent reactive oxygen species (ROS) formation upon reperfusion leads to microvascular dysfunction in IR studies [14, 35]. The increased production and presence of iNOS resulting from both ischemia and reperfusion enhances the early injury of vessel endothelial and surrounding tissue cells of animals not exposed to ultrasound. Microvascular iNOS levels increased from baseline intensify all IR injurious effects. Whether damage is temporary or permanent, however, depends on the severity of the injury as well as the effect of any protective and or preventative mechanisms.
The microvascular protective influences related to eNOS activity in the production of NO have been well documented. Inhibition of eNOS and or the production of NO through the eNOS pathway while subjecting tissue to ischemia and subsequent reperfusion [4, 11, 33], results in increased circulatory dysfunction and tissue injury, compared to uninhibited groups. Ultrasound exposed chamber samples, compared to unexposed samples, demonstrated increased eNOS production with WB utilizing an anti-eNOS antibody. Increased eNOS production currently seen during late reperfusion (24 h) confirms our previous work showing improved microcirculatory function of ultrasound exposed animals 24 h into reperfusion [16]. The enhanced production of eNOS with ultrasound exposure extends our hypothesis for the attenuation of long-term IR damage. Twenty-four hours after the onset of reperfusion is sufficient time for the subsistence of IR effects and the establishment of a new level of homeostasis. A surplus in NO production at this later time point would greatly benefit microvessel function.
WB of both NOS isoforms eNOS and iNOS supports our hypothesis relating to the efficacy of ultrasound exposure in the reduction of IR injury and the existence of both fast and slow acting responses to stimulation [16]. Ultrasound exposure during IR downregulates the quantity of iNOS produced at the 0.5 h assessment point constitutes a rapid response to stimulation during early reperfusion, while upregulating eNOS production 24 h into reperfusion is a delayed stimulus response. However, contrasting our previous conclusion on the use of l-NAME treatment with ultrasound exposure, we now propose that l-NAME treatment negatively influences the delayed, slow acting stimulation effect. Negatively affecting the rapid response to ultrasound stimulation should produce results similarly to those obtained with iNOS inhibition (Table 1–Table 4).
From a clinical viewpoint the application of ultrasound would appear to be particularly useful in situations involving ischemia reperfusion syndromes associated with the re-establishment of flow in ischemic tissue. In these conditions the ultrasound treatment would specifically diminish tissue injury, with the additional feature that it has no known side effects, and it is applied externally, via an essentially non invasive method. Furthermore, ultrasound exposure used in combination with an eNOS derived NO releasing compound could result in improved beneficial outcomes, compared to ultrasound alone, during medical procedures requiring the initiation of ischemia and reperfusion, organ transplantations, or reperfusion due to an ischemic event, cardiac angioplasty and bypass.
In summary attenuation of IR induced tissue damage by ultrasound exposure and NO production has previously been reported following acute ischemic stroke [1], femoral thrombosis [38], muscular ischemia [39], and complete vascular occlusion [7, 16], mitigating injury and preventing further tissue damage. The present study extends our understanding of the underlying pathophysiological basis of the damage produced in the microcirculation by IR injury, and how this is mitigated by the application of ultrasound irradiation. We show that continuous wave diagnostic frequency ultrasound exposure affects the levels of iNOS and eNOS leading, respectively, to rapid and protracted effects. Our findings provide further evidence that the beneficial effects of ultrasound exposure are related to NO. They also suggest that the effects of ultrasound exposure are advantageous in the treatment of IR injured tissue and that long term benefits appear to be related to early therapeutic intervention.
ACKNOWLEDGEMENTS
The authors thank Froilan P. Barra and Cynthia Walser for the surgical preparation of the animals. The authors gratefully acknowledge Dr. Jiunn-chern (Gene) Yeh (La Jolla Bioengineering Institute) for his training in, assistance with, and discussion of the Western blot analysis and results. The authors also acknowledge Dr. Dustin E. Kruse (University of California, Davis) for his discussion on and assistance with ultrasonics and the calibration of our ultrasound transducer. This work has been supported by the NIH grant HL 40696.
Supported by NIH grant HL 04069
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, Montaner J, Saqqur M, Demchuk AM, Moye LA, Hill MD, Wojner AW. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351(21):2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 2.Arnaud C, Godin-Ribuot D, Bottari S, Peinnequin A, Joyeux M, Demenge P, Ribuot C. Inos is a mediator of the heat stress-induced preconditioning against myocardial infarction in vivo in the rat. Cardiovasc Res. 2003;58(1):118–125. doi: 10.1016/s0008-6363(02)00812-x. [DOI] [PubMed] [Google Scholar]
- 3.Becker BF, Kupatt C, Massoudy P, Zahler S. Reactive oxygen species and nitric oxide in myocardial ischemia and reperfusion. Z Kardiol. 2000;89 Suppl 9:IX/88–IX/91. doi: 10.1007/s003920070037. [DOI] [PubMed] [Google Scholar]
- 4.Bertuglia S, Giusti A. Role of nitric oxide in capillary perfusion and oxygen delivery regulation during systemic hypoxia. Am J Physiol Heart Circ Physiol. 2005;288(2):H525–H531. doi: 10.1152/ajpheart.00426.2004. [DOI] [PubMed] [Google Scholar]
- 5.Bertuglia S, Giusti A. Microvascular oxygenation and oxidative stress during postischemic reperfusion. Po2, ros, and no during reperfusion. Adv Exp Med Biol. 2005;566:23–29. doi: 10.1007/0-387-26206-7_4. [DOI] [PubMed] [Google Scholar]
- 6.Bertuglia S, Colantuoni A, Intaglietta M. Capillary reperfusion after l-arginine, l-nmma, and l-nna treatment in cheek pouch microvasculature. Microvasc Res. 1995;50:162–174. doi: 10.1006/mvre.1995.1050. [DOI] [PubMed] [Google Scholar]
- 7.Bertuglia S, Giusti A, Picano E. Effects of diagnostic cardiac ultrasound on oxygen free radical production and microvascular perfusion during ischemia reperfusion. Ultrasound Med Biol. 2004;30(4):549–557. doi: 10.1016/j.ultrasmedbio.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Birnbaum Y, Luo H, Atar S, Fishbein MC, Brasch AV, Nagai T, Pal D, Nishioka T, Chae JS, Zanelli C, Peterson TM, Siegel RJ. Noninvasive transthoracic low frequency ultrasound augments thrombolysis in a canine model of acute myocardial infarction--evaluation of the extent of st-segment resolution. J Thromb Thrombolysis. 2001;11(3):229–234. doi: 10.1023/a:1011964904848. [DOI] [PubMed] [Google Scholar]
- 9.Booi RC, Krucker JF, Goodsitt MM, O'Donnell MO, LeCarpentier GL, Roubidoux MA, Fowlkes JB, Carson PL. Evaluation of thin compression paddles for mammographically compatible ultrasound. Ultrasonics Symposium, 2004 IEEE; 2004. pp. 2129–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabrales P, Tsai AG, Intaglietta M. Perfluorocarbon in microcirculation during ischemia reperfusion. J Am Coll Surg. 2007;204(2):225–235. doi: 10.1016/j.jamcollsurg.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Chang WL, Chung CH, Wu YC, Su MJ. The vascular and cardioprotective effects of liriodenine in ischemia-reperfusion injury via no-dependent pathway. Nitric Oxide. 2004;11(4):307–315. doi: 10.1016/j.niox.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Endrich B, Asaishi K, Gotz A, Messmer K. Technical report--a new chamber technique for microvascular studies in unanesthetized hamsters. Res Exp Med (Berl) 1980;177(2):125–134. doi: 10.1007/BF01851841. [DOI] [PubMed] [Google Scholar]
- 13.Friesenecker B, Tsai AG, Instaglietta M. Capillary perfusion during ischemia-reperfusion in subcutaneous connective tissue and skin muscle. Am J Physiol. 1994;267(6 Pt 2):H2204–H2212. doi: 10.1152/ajpheart.1994.267.6.H2204. [DOI] [PubMed] [Google Scholar]
- 14.Grace PA. Ischaemia-reperfusion injury. Br J Surg. 1994;81(5):637–647. doi: 10.1002/bjs.1800810504. [DOI] [PubMed] [Google Scholar]
- 15.Gunnett CA, Lund DD, McDowell AK, Faraci FM, Heistad DD. Mechanisms of inducible nitric oxide synthase-mediated vascular dysfunction. Arterioscler Thromb Vasc Biol. 2005;25(8):1617–1622. doi: 10.1161/01.ATV.0000172626.00296.ba. [DOI] [PubMed] [Google Scholar]
- 16.Hightower CM, Intaglietta M. The use of diagnostic frequency continuous ultrasound to improve microcirculatory function after ischemia-reperfusion injury. Microcirculation. 2007;14(6):571–582. doi: 10.1080/10739680701410280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hines IN, Harada H, Flores S, Gao B, McCord JM, Grisham MB. Endothelial nitric oxide synthase protects the post-ischemic liver: Potential interactions with superoxide. Biomed Pharmacother. 2005;59(4):183–189. doi: 10.1016/j.biopha.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, Snyder SH, Burnett AL. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci U S A. 2002;99(6):4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Intaglietta M, Tompkins WR. System for the measurement of velocity of microscopic particles in liquids. IEEE Trans Biomed Eng. 1971;18(5):376–379. doi: 10.1109/tbme.1971.4502869. [DOI] [PubMed] [Google Scholar]
- 21.Intaglietta M, Tompkins WR. On-line measurement of microvascular dimensions by television microscopy. J Appl Physiol. 1972;32(4):546–551. doi: 10.1152/jappl.1972.32.4.546. [DOI] [PubMed] [Google Scholar]
- 22.Ito Y, Abril ER, Bethea NW, McCuskey RS. Role of nitric oxide in hepatic microvascular injury elicited by acetaminophen in mice. Am J Physiol Gastrointest Liver Physiol. 2004;286(1):G60–G67. doi: 10.1152/ajpgi.00217.2003. [DOI] [PubMed] [Google Scholar]
- 23.Jiang H, Koubi D, Zhang L, Kuo J, Rodriguez AI, Hunter TJ, Gautam SC, Levine RA. Inhibitors of inos protects pc12 cells against the apoptosis induced by oxygen and glucose deprivation. Neurosci Lett. 2005;375(1):59–63. doi: 10.1016/j.neulet.2004.10.067. [DOI] [PubMed] [Google Scholar]
- 24.Koeppel TA, Thies JC, Schemmer P, Trauner M, Gebhard MM, Otto G, Post S. Inhibition of nitric oxide synthesis in ischemia/reperfusion of the rat liver is followed by impairment of hepatic microvascular blood flow. J Hepatol. 1997;27(1):163–169. doi: 10.1016/s0168-8278(97)80297-8. [DOI] [PubMed] [Google Scholar]
- 25.Kozlov AV, Sobhian B, Duvigneau JC, Costantino G, Gemeiner M, Nohl H, Redl H, Bahrami S. Nitric oxide synthase inhibitors affect nitric oxide synthesis in normoxic but not in ischemic organs during intestinal ischemia and early reperfusion. J Lab Clin Med. 2002;140(5):303–311. doi: 10.1067/mlc.2002.128180. [DOI] [PubMed] [Google Scholar]
- 26.Kurose I, Wolf R, Grisham MB, Aw TY, Specian RD, Granger DN. Microvascular responses to inhibition of nitric oxide production. Role of active oxidants. Circ Res. 1995;76(1):30–39. doi: 10.1161/01.res.76.1.30. [DOI] [PubMed] [Google Scholar]
- 27.Leonard AM, Chafe LL, Montani JP, Van Vliet BN. Increased salt-sensitivity in endothelial nitric oxide synthase-knockout mice. Am J Hypertens. 2006;19(12):1264–1269. doi: 10.1016/j.amjhyper.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Guo Y, Tan W, Stein AB, Dawn B, Wu WJ, Zhu X, Lu X, Xu X, Siddiqui T, Tiwari S, Bolli R. Gene therapy with inos provides long-term protection against myocardial infarction without adverse functional consequences. Am J Physiol Heart Circ Physiol. 2006;290(2):H584–H589. doi: 10.1152/ajpheart.00855.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipowsky HH, Zweifach BW. Application of the "two-slit" photometric technique to the measurement of microvascular volumetric flow rates. Microvasc Res. 1978;15(1):93–101. doi: 10.1016/0026-2862(78)90009-2. [DOI] [PubMed] [Google Scholar]
- 30.Menger MD, Steiner D, Messmer K. Microvascular ischemia-reperfusion injury in striated muscle: Significance of "no reflow". Am J Physiol. 1992;263(6 Pt 2):H1892–H1900. doi: 10.1152/ajpheart.1992.263.6.H1892. [DOI] [PubMed] [Google Scholar]
- 31.Nagase S, Isobe H, Ayukawa K, Sakai H, Nawata H. Inhibition of nitric oxide production increases dimethylnitrosamine-induced liver injury in rats. J Hepatol. 1995;23(5):601–604. doi: 10.1016/0168-8278(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 32.Nishida J, McCuskey RS, McDonnell D, Fox ES. Protective role of no in hepatic microcirculatory dysfunction during endotoxemia. Am J Physiol. 1994;267(6 Pt 1):G1135–G1141. doi: 10.1152/ajpgi.1994.267.6.G1135. [DOI] [PubMed] [Google Scholar]
- 33.Pudupakkam S, Harris KA, Jamieson WG, DeRose G, Scott JA, Carson MW, Schlag MG, Kvietys PR, Potter RF. Ischemic tolerance in skeletal muscle: Role of nitric oxide. Am J Physiol. 1998;275(1 Pt 2):H94–H99. doi: 10.1152/ajpheart.1998.275.1.H94. [DOI] [PubMed] [Google Scholar]
- 34.Qi WN, Chen LE, Zhang L, Eu JP, Seaber AV, Urbaniak JR. Reperfusion injury in skeletal muscle is reduced in inducible nitric oxide synthase knockout mice. J Appl Physiol. 2004;97(4):1323–1328. doi: 10.1152/japplphysiol.00380.2004. [DOI] [PubMed] [Google Scholar]
- 35.Seal JB, Gewertz BL. Vascular dysfunction in ischemia-reperfusion injury. Ann Vasc Surg. 2005;19(4):572–584. doi: 10.1007/s10016-005-4616-7. [DOI] [PubMed] [Google Scholar]
- 36.Sharp BR, Jones SP, Rimmer DM, Lefer DJ. Differential response to myocardial reperfusion injury in enos-deficient mice. Am J Physiol Heart Circ Physiol. 2002;282(6):H2422–H2426. doi: 10.1152/ajpheart.00855.2001. [DOI] [PubMed] [Google Scholar]
- 37.Steiner DR, Gonzalez NC, Wood JC. Interaction between reactive oxygen species and nitric oxide in the microvascular response to systemic hypoxia. J Appl Physiol. 2002;93(4):1411–1418. doi: 10.1152/japplphysiol.00251.2002. [DOI] [PubMed] [Google Scholar]
- 38.Suchkova VN, Baggs RB, Francis CW. Effect of 40-khz ultrasound on acute thrombotic ischemia in a rabbit femoral artery thrombosis model: Enhancement of thrombolysis and improvement in capillary muscle perfusion. Circulation. 2000;101(19):2296–2301. doi: 10.1161/01.cir.101.19.2296. [DOI] [PubMed] [Google Scholar]
- 39.Suchkova VN, Baggs RB, Sahni SK, Francis CW. Ultrasound improves tissue perfusion in ischemic tissue through a nitric oxide dependent mechanism. Thromb Haemost. 2002;88(5):865–870. [PubMed] [Google Scholar]
- 40.Toda N, Nakanishi-Toda M. Nitric oxide: Ocular blood flow, glaucoma, and diabetic retinopathy. Prog Retin Eye Res. 2007;26(3):205–238. doi: 10.1016/j.preteyeres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 41.Tribl B, Bateman RM, Milkovich S, Sibbald WJ, Ellis CG. Effect of nitric oxide on capillary hemodynamics and cell injury in the pancreas during pseudomonas pneumonia-induced sepsis. Am J Physiol Heart Circ Physiol. 2004;286(1):H340–H345. doi: 10.1152/ajpheart.00234.2003. [DOI] [PubMed] [Google Scholar]
- 42.Vinas JL, Sola A, Genesca M, Alfaro V, Pi F, Hotter G. No and nos isoforms in the development of apoptosis in renal ischemia/reperfusion. Free Radic Biol Med. 2006;40(6):992–1003. doi: 10.1016/j.freeradbiomed.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, Looney CG, Qi WN, Chen LE, Seaber AV, Stamler JS, Urbaniak JR. Reperfusion injury is reduced in skeletal muscle by inhibition of inducible nitric oxide synthase. J Appl Physiol. 2003;94(4):1473–1478. doi: 10.1152/japplphysiol.00789.2002. [DOI] [PubMed] [Google Scholar]