Abstract
This investigation examined the visuomotor tracking abilities of persons with apraxia of speech (AOS) or conduction aphasia (CA). In addition, tracking performance was correlated with perceptual judgments of speech accuracy. Five individuals with AOS and four with CA served as participants, as well as an equal number of healthy controls matched by age and gender. Participants tracked predictable (sinusoidal) and unpredictable signals using jaw and lip movements transduced with strain gauges. Tracking performance in participants with AOS was poorest for predictable signals, with decreased kinematic measures of cross-correlation and gain ratio and increased target-tracker difference. In contrast, tracking of the unpredictable signal by participants with AOS was performed as well as for other groups (e.g. participants with CA, healthy controls). Performance of the subjects with AOS on the predictable tracking task was found to strongly correlate with perceptual judgments of speech. These findings suggest that motor control capabilities are impaired in AOS, but not in CA. Results suggest that AOS has its basis in motor programming deficits, not impaired motor execution.
In their taxonomy of motor speech disorders, Darley, Aronson, and Brown (1975) claimed that apraxia of speech (AOS) has its basis in the impaired planning and programming of speech motor patterns. Controversy about AOS in the ensuing decades focused on whether the disorder was best defined from a motoric or a linguistic perspective (for review see Ballard, Granier, & Robin, 2000; McNeil, Robin, & Schmidt, 2008). Data suggest that AOS is a disorder of motor control of the speech production system (e.g. Ballard & Robin, 2007; Itoh & Sasanuma, 1984; Kelso & Tuller, 1981; Kent & McNeil, 1987; Maas et al., 2006; McNeil, Caligiuri, & Rosenbek, 1989; McNeil, Weismer, Adams, & Mulligan, 1990). However, the database on movement control in AOS is remarkably limited. Consequently, an understanding and description of the movement patterns of speakers with apraxia remains elusive.
A problem with much of the literature on AOS is that most studies have used individuals with multiple problems (e.g., apraxia and aphasia) or provided incomplete subject descriptions (McNeil et al., 2008). Relatively few studies have examined the speech or motor capabilities of participants with AOS with no concomitant problems such as aphasia or dysarthria (see McNeil et al., 1990; Robin, Bean, & Folkins, 1989 for exceptions).
Kinematic studies using speech stimuli in AOS are few and those available have produced contradictory results. For example, while Forrest and colleagues found impaired inter-articulatory coordination in speakers with apraxia (Forrest, Adams, McNeil, & Southwood, 1991), Robin et al. (1989) found normal upper and lower lip onset asynchronies in speakers with apraxia. The lack of consistent kinematic findings in individuals with AOS suggests further study is needed.
Assessment of non-speech movements of the speech production mechanism appears to be a fruitful avenue of research in participants with motor speech disorders (McClean, Beukelman, & Yorkston, 1987), allowing the analysis of motor control deficits without the confound implicit in tasks requiring linguistic processing (see Ballard, Robin, & Folkins, 2003; Ballard, Solomon, Robin, Moon, & Folkins, 2008; Folkins et al., 1995; for a review of nonspeech assessment of motor speech disorders, although cf. Ziegler, 2003). In one example of such work, McNeil et al. (1990) reported abnormal non-speech movement control in subjects with AOS using fine force and position control tasks. These researchers noted that speakers with AOS or ataxic dysarthria had greater instability of the articulators during non-speech fine force and position control tasks than did healthy speakers or those with conduction aphasia (CA). However, they did not attempt to relate the abnormal non-speech control measures to speech.
A non-speech task that appears to be useful in the study of coordination of the speech production system is visuomotor oral tracking of a moving target (McClean et al., 1987). Tracking tasks have a number of important advantages over fine force or position control tasks. Most importantly, tracking tasks are dynamic rather than static in nature (Moon, Zebrowski, Robin, & Folkins, 1993). In addition, tracking sinusoidal signals results in movement gestures where the peak velocity occurs approximately in the middle of the movement, not unlike the pattern observed for speech movements (Gracco & Abbs, 1986).
Critical to the current study, tracking a moving target is an appropriate task to test the motor planning/programming hypothesis of AOS. The visuomotor tracking paradigm examines aspects of motor control related to the planning of movement patterns by requiring participants to follow either predictable (sinusoidal) or unpredictable (random) signals. In theory, when tracking a predictable target it is possible to develop a model of the target motion. Data supporting this hypothesis have shown that normal performance during the tracking of predictable targets is phase synchronous or phase ahead of the target signal (Flowers, 1978; Moon et al., 1993). By contrast, subjects tend to phase lag when tracking unpredictable signals, which lends further support for the model-driven hypothesis of predictable tracking. In addition, participants continue to accurately produce predictable movements after the target has been removed, suggesting that predictable tracking is model-driven (Ballard & Robin, 2007).
Thus, it appears that tracking predictable targets is based on an internal representation of the target motion. Since predictable tracking uses an internal model, based on perceptual representations, to guide movement accuracy, it may be well-described by current models of speech production, including the DIVA model (Directions into Velocities of Articulators Guenther, Ghosh, & Tourville, 2006; Guenther, Hampson, & Johnson, 1998).
In the DIVA model, feedforward control processes are used to derive the appropriate articulatory parameters for a known, predictable movement. These processes may be conceived of as stored internal models or plans of target motions, similar to generalized motor programs (GMPs) in schema theory (Schmidt & Lee, 2005). Feedback processes, in contrast, provide sensory input to the feedforward system to monitor the accuracy of movements and update feedforward processes based on altered conditions.
In predictable tracking, once a stable internal model (feedforward command) is established, feedback control signals are monitored only periodically to ensure movement accuracy and to enable smooth movement transitions. In contrast, tracking unpredictable targets does not allow for development of or following an internal plan of movement patterns. Rather, tracking an unpredictable signal requires participants to constantly attend to the target and make changes in their movement pattern based on the on-line feedback from the external signal. As a result, participants show significant phase lag during unpredictable tracking and the movements are “jerky” as a result of the constant adjustment to error. The result is that overall tracking accuracy for unpredictable targets is poorer than that found for predictable tracking (Moon et al., 1993).
An important issue in understanding AOS is to distinguish performance of speakers with AOS from those who have aphasia. Recent studies in AOS have included participants with CA as a control group (e.g. Clark & Robin, 1998; Seddoh, Robin, Sim, & Hageman, 1996). The rationale behind inclusion of a group with CA is to compare errors made by patients with aphasia to those made by patients with AOS. If the two groups of patients differ on a task, it is presumed that the mechanisms underlying each disorder are different. Study of participants with CA in comparison to AOS is logical since the predominant production error in CA is the phonemic paraphasia (Damasio & Damasio, 1980). CA is also similar to AOS in that both groups of patients have difficulty with repetition of speech (see Damasio & Damasio, 1980; Goodglass & Kaplan 1983 for CA and Darley et al., 1975; Duffy, 2005 for AOS). While the speech errors in AOS are thought to be motoric, those in CA are considered linguistically-based. Thus, even though both groups produce somewhat similar surface behaviors, the pathogenesis of these errors is thought to be different. Yet kinematic data on speech or non-speech movements are extremely scarce in CA.
Studies of CA have suggested that, in part, the speech sound errors may be motoric in origin. For instance, Kent and McNeil (1987) noted that the interpretation of substitution errors found in CA is problematic since these errors are assigned to a single level of the speech production system. They interpreted data from acoustic and perceptual analyses to support a possible motor component in CA. Moreover, McNeil and colleagues (1990) reported that non-speech force and position in CA were significantly better than that of patients with AOS or dysarthria, but poorer than normal, perhaps supporting a motoric component to the disorder. By contrast, Clark and Robin (1998) reported that subjects with CA had normal motor control using a non-speech task in which novel movement patterns were taught. Consequently, an unambiguous interpretation of the mechanisms underlying CA does not exist. The study of non-speech tracking performance in CA should provide further insight into the motor control abilities of these individuals.
As part of a larger investigation aimed at understanding speech motor control and its impairments, the visuomotor tracking paradigm was utilized to examine oral motor control in AOS and CA. We tested the hypothesis that persons with AOS would have difficulty with programming lip and jaw movements as evidenced by difficulty tracking predictable signals, but perform normally on motor tasks that do not require planning or prediction, as evidenced by normal performance while tracking unpredictable signals. If data support this hypothesis, it would point to motor programming difficulty in AOS. We further hypothesized that participants with CA would show normal motor ability as shown by unimpaired tracking of predictable and unpredictable signals. Preliminary data from four speakers with apraxia (Hageman, Robin, Moon, & Folkins, 1994) showed that participants had difficulty tracking predictable targets (lower than normal cross-correlations), but performed at normal levels when tracking unpredictable targets. In that study, cross-correlation and phase difference were the only variables studied and no attempt was made to relate the results to speech performance.
The current study was conducted to explore the tracking abilities of speakers with apraxia in greater detail than our earlier study (Hageman et al., 1994). In this study, the number of participants in the AOS group was increased and a CA group was included in order to examine the motor capabilities related to that type of aphasic disturbance. The study was expanded in two additional ways from the preliminary report. First, as reported in Moon et al. (1993), we examined a number of different dependent variables in addition to cross-correlation and phase (see methods below). Second, since our long-term interest is in the relation between speech and non-speech performance, we examined the correlation between perceptual ratings of speech (i.e., overall speech defectiveness, overall articulatory imprecision, and intelligibility) and tracking performance. If tracking shares some of the motoric demands of speech production, then tracking performance should strongly relate to perceptual judgments.
Method
Participants
The experimental groups were comprised of six participants with apraxia of speech (AOS group), four of whom participated in Hageman et al. (Hageman et al., 1994) and four participants with conduction aphasia (CA group), for whom acoustic data have been previously reported (Seddoh et al., 1996). Participants with AOS had a “pure” form of the disorder (no aphasia or apparent dysarthria on clinical testing (see below). The AOS group ranged in age from 35 to 80 years (mean= 66.83 years). The CA group ranged in age from 21 to 66 years (mean= 40.50 years). Normal speakers also participated in the experiment and were matched for gender and age (within six months) to each member of the AOS and CA groups. Since there were significant differences in the average ages of the two brain-injured groups (p < .05), two groups of control participants, one for the apraxia group (AOS-C) and one for the aphasic group (CA-C), were used in all analyses. Demographic information on individuals with brain injury is shown in table 1. All healthy controls reported normal speech, language and hearing and had no history of neurological or uncorrected vision disorders.
Table 1.
Brain-damaged participants’ demographic information
| Subject | Age | Gender | Handedness* | Education | Job History |
|---|---|---|---|---|---|
| AOS1 | 35 | Female | +100 | 12 | Homemaker |
| AOS2 | 55 | Male | +100 | 12 | Farmer |
| AOS3 | 66 | Male | +100 | 16 | Elec. Engineer |
| AOS4 | 72 | Female | +100 | 12 | Homemaker |
| AOS5 | 68 | Male | +100 | 16 | Retired |
| AOS6 | 80 | Male | +100 | 16 | Retired |
| CA1 | 49 | Male | +100 | 12 | Factory Foreman |
| CA2 | 29 | Male | +100 | 13 | College Student |
| CA3 | 21 | Male | +100 | 12 | Blue Collar Job |
| CA4 | 66 | Male | +100 | 12 | Retired Postal |
Based on modified Oldfield Geschwind Questionnaire +100 = full right handedness
All participants with brain injury underwent extensive speech, language, neuropsychological, and neurological evaluation based on a standard protocol of a large program project grant. The neuropsychological test battery examined verbal and performance IQ using the Wechsler Adult Intelligence Scale, Revised (Wechsler, 1987). Memory function in verbal and nonverbal domains was assessed using the Wechsler Memory Scale (Russell, 1975), the Rey Auditory-Verbal Learning Test (Rey, 1941) for verbal memory, and the Benton Visual Retention Test (Benton, 1974 (Benton, 1974)) and the Complex Figure Delayed Recall Test (Osterrieth, 1944) for visuospatial memory. Visual perception was tested using the Judgement of Line Orientation Test (Benton, 1974). Constructional abilities were tested with the Complex Figure Test-Copy (Osterrieth, 1944), drawing to dictation, and a three-dimensional block design test. Executive control was assessed using information from the personal interview, the MMPI (Graham, 1987), and the Wisconsin Card Sorting Test (Grant & Berg, 1948). Language was assessed using The Multilingual Aphasia Examination (Benton & Hamsher, 1978), portions of the Boston Diagnostic Aphasia Examination (Goodglass & Kaplan, 1983), the Token Test (De Renzi & Vignolo, 1962). All nonverbal testing was within normal limits. Language data are shown in Table 2. Information on overall severity was based on the aphasia tests, reviews of the chart, patient interview, and the judgments of a speech-language pathologist (DAR) and a neuropsychologist.
Table 2.
Brain-damaged participants: Language and neuropsychological information1 (1 = Normal : 2 = Mild Impairment ; 3 = Moderate Impairment ; 4 = Severe Impairment)
| LANGUAGE2 | ||||||
|---|---|---|---|---|---|---|
| Subject | Spontaneous Speech | Auditory Comp. | Repetition | Naming | Reading Comp. | |
| AOS1 | Nonfluent | 1 | 3 | 1 | 1 | |
| AOS2 | Nonfluent | 1 | 3 | 1 | 1 | |
| AOS3 | Nonfluent | 1 | 4 | 1 | 1 | |
| AOS4 | Nonfluent | 2 | 3 | 2 | 1 | |
| AOS5 | Nonfluent | 1 | 4 | NA3 | 1 | |
| AOS6 | Nonfluent | 1 | 4 | NA3 | 1 | |
| CA1 | Fluent | 3 | 4 | 3 | 3 | |
| CA2 | Fluent | 3 | 4 | 3 | 3 | |
| CA3 | Fluent | 2 | 3 | 3 | 2 | |
| CA4 | Fluent | 2 | 3 | 3 | 2 | |
|
| ||||||
| VISUAL MEMORY
| ||||||
| Subject | Performance IQ4 | Perception5 | Construction6 | Executive Control7 | Verbal | Nonverbal |
|
| ||||||
| AOS1 | 1 | 1 | 1 | 1 | 1 | 1 |
| AOS2 | 1 | 1 | 1 | 1 | 1 | 1 |
| AOS3 | 1 | 1 | 1 | 1 | 1 | 1 |
| AOS4 | 1 | 1 | 1 | 1 | NA | 1 |
| AOS5 | 1 | 1 | 1 | 1 | NA | 1 |
| CA1 | 1 | 1 | 1 | 1 | NA | 1 |
| CA2 | 1 | 1 | 1 | 1 | NA | 1 |
| CA3 | 1 | 1 | 1 | 1 | NA | 1 |
| CA4 | 1 | 1 | 1 | 1 | 1 | 1 |
Note: Neuropsychological data from Dr. Daniel Tranel, Chief of Neuropsychology, UIHC
NA- could not test- speech or language too impaired,
See Lezak (1983) for reference to tests,
Multilingual Aphasia Examination, Boston Diagnostic Aphasia Examination, Token Test
Weschler Adult Intelligence Scale Revised,
Facial Recognition Test, Line Orientation Test
Complex Figure Test- Copy, Drawing to dictation, Three-Dimensional Block Design Test
Clinic Interview, MMPI, Wisconsin Card Sorting Test,
Wechsler Memory Scale, Key-Auditory-Verbal Learning Test, Benton Visual Retention Test, Complex Figure Delay Recall Test
Neuroimaging data for lesion localization were obtained from MRI examinations, taken at least 3 months post-stroke, using a standardized lesion plotting method (Damasio & Damasio, 1989). Lesion information is provided in Table 3.
Table 3.
Lesion Information (All Brain-damaged participants had a single left hemispheric stroke)
| Subject | Time Post Onset (Yrs.) | Lesion* |
|---|---|---|
| AOS1 | 8 | Left 44, 45, 6 |
| AOS2 | 7 | Left 44, 4, 6 |
| AOS3 | 7 | Left 44, 45, 6 |
| AOS4 | 6 | Left 44, 37, 40, 8 |
| AOS5 | 13 | Left 44, 45, 6, 1, 2, 3, 37 |
| CA1 | 5 | Left 77, 42, 39, 22 |
| CA2 | 5 | Left 22, 41, 42, 37 |
| CA3 | 3 | Left 37, 39, 42, 22 |
| CA4 | 2 | Left 37, 39, 40, 22 |
Lesion data reported in Brodmann’s system by Dr. Hanna Damasio using a standard using a standard plotting analysis system.
The participants with AOS fit selection criteria provided by McNeil et al. (2008) and used in our earlier studies (Hageman et al., 1994; Robin et al., 1989). Each of the individuals with AOS demonstrated a slow speech rate (characterized by extended segment and intersegment durations), a predominance of distortions of sounds as the primary error type, consistency in regard to type of error and place of error during repeated trials of the same sounds in the same context, prosodic anomalies, and sequential motion rates that were markedly poorer (slower and more distorted) than alternating motion rates. The diagnosis of CA was based on results of the aphasia examinations and by the criteria described by Goodglass and Kaplan (1983) and Damasio (1981). The hallmark of CA is the predominance of phonemic paraphasias and inordinate difficulty with repetition of speech. Thus, inclusion criteria for both groups are identical to those used by McNeil and colleagues (Kent & McNeil, 1987; McNeil et al., 1990; Odell, McNeil, Rosenbek, & Hunter, 1991). Severity of AOS was determined by clinician judgments and by results of the perceptual analysis (see below). All participants were assessed by two speech-language pathologists (authors DAR and CH) and found to not demonstrate any characteristics of dysarthria on clinical examination. In particular, there were no voice or resonatory anomalies, nor were any of the articulators weak as indicated by resistance testing with a tongue blade.
Procedures
A detailed presentation of visuomotor tracking with the lips, jaw, and larynx is described by Moon et al. (1993). In brief, a horizontal bar (1.5 inches wide), which moved vertically, was displayed on an oscilloscope screen as a target for tracking. In this study we report only on lip and jaw tracking because all participants with apraxia were unable to complete the Fo tracking task, presumably because the task demand (sustaining phonation and tracking with the voice) was too high. All others were able to complete the Fo tracking task. Transduced signals from the articulators (lower lip, jaw) were displayed as a dot centered horizontally on the bar. Participants were instructed to keep the dot on the bar throughout the extent of the bar’s vertical movement.
Transduction of articulator movements was accomplished using a standard strain gauge cantilever system (Barlow, Cole, & Abbs, 1983; Muller & Abbs, 1979). The strain gauges were affixed to the lower lip and the jaw with pieces of double-sided tape and a bite block was used to stabilize the jaw during lip tracking. Participants were seated in a dental chair and their heads immobilized using a wall-mounted cephalostat. Four tracking conditions were employed for each articulator. These included sine waves of 0.3, 0.6, and 0.9 Hz (i.e., predictable target frequencies) and an unpredictable signal that was comprised of ten equal amplitude frequencies ranging from 0.1 to 1.0 Hz in 0.1 Hz steps. Presentation order of tracking conditions was randomized across and within subject. Jaw and lip tracking tasks were completed in random order. All participants received a 60 second practice trial of tracking at each of the four conditions prior to collection of data used in the analyses to ensure they understood the task (Seddoh et al., 1996).
Each participant completed several speech tasks, including oral reading, picture description, and informal talking tasks. The speech samples were video recorded and 30-second samples of the picture description task were dubbed onto audiotapes for perceptual analyses. These analyses involved six speech-language pathologists with at least 5 years experience who rated the speech samples in three domains, including overall speech defectiveness (OSD), overall articulatory imprecision (OAI) and intelligibility (Seddoh et al., 1996). OSD was defined as any deficit in speech production in any dimension whereas OAI was considered only a deficit in articulation. Intelligibility was defined as how much speech was understood by the listener. A ten-point equal-appearing interval scale was used with 1 being normal and 10 being severely defective. Intra-judge reliability was based on a repeat presentation of 10% of the samples from all participants and was 99%. Inter-judge reliability was 94% for OSD, 96% for OAI, and 89% for intelligibility. Measures of OSD, OAI, and intelligibility made by the six judges were averaged and used for further analysis including group comparisons and correlational analyses with kinematic measures.
Data Analysis
As detailed in Moon et al. (1993), lip and jaw movement and target signal were then digitized at 50 Hz and low-pass filtered using a 25 Hz cut-off frequency. A total of sixty seconds of tracking was obtained. An automatic in-house analysis routine (see Moon et al., 1993) parceled the data into six 10-second samples of the tracking signal that were then extracted and used in the analyses. Measures obtained by comparing the target and transduced movement signal included cross-correlation (the highest correlation obtained across the entire range of possible phase shifts), gain ratio (the amplitude of the participant’s predominant tracking frequency divided by the amplitude of the target signal as determined by FFT analysis), phase shift, and the average target-tracker difference (TTD). All four tracking measures were obtained for predictable conditions, as these analyses were conducted relative to a specific target frequency. Cross-correlation and average target-tracker difference were analyzed further to contrast tracking performance with predictable signals and performance with an unpredictable signal and to determine whether this contrast was different for AOS and CA subjects.
The statistical design (conducted by a biostatistician, author GW on this paper) utilized separate mixed model analyses of variance (ANOVA) for each dependent variable (cross correlation, target tracker difference, phase shift, and gain). Since the AOS and CA groups had different controls, ANOVAs were performed separately for each articulator in the two groups. Note that severity of speech impairment spanned a wide range in the group with AOS (see Table 4). Due to the variance resulting from this range and the limited number of participants, it was also important to examine the descriptive trends in the data. These descriptive trends show important differences between groups. Cross-correlations were converted into Fisher’s z values before analysis but the other measures were not transformed. The within subject factors were structure (lip, jaw) and tracking condition (predictable targets at 0.3, 0.6, and 0.9 Hz). For cross-correlation and TTD, contrast t-tests for the predictable targets vs the unpredictable target were run in a separate analysis for each structure. For gain ratio and phase shift, only the predictable targets were analyzed. Alpha level was set at 0.05 for the ANOVAs.
Table 4.
Perceptual ratings for participants with apraxia of speech (AOS), apraxia of speech controls (AOS-C), participants with conduction aphasic (CA) and conduction aphasic controls (CA-C)
| ARTICULATORY PRECISION
| ||||
|---|---|---|---|---|
| S# | AOS | AOS-C | CA | CA-C |
| 1 | 3.17 | 9.33 | 8.5 | 9 |
| 2 | 8.16 | 9.33 | 8.8 | 8.83 |
| 3 | 9.17 | 8.83 | 8.6 | 9.17 |
| 4 | 8.33 | 9.67 | 8.6 | 8.83 |
| 5 | 7.83 | 9.33 | ||
| 6 | 7.33 | 8.17 | ||
|
| ||||
| ARTICULATORY DEFECTIVENESS
| ||||
| S# | AOS | AOS-C | CA | CA-C |
|
| ||||
| 1 | 2.17 | 9.17 | 8.8 | 8 |
| 2 | 8.5 | 9.67 | 9.33 | 9.3 |
| 3 | 8.83 | 9.83 | 9.12 | 9.67 |
| 4 | 8.33 | 9.67 | 9 | 8.83 |
| 5 | 7 | 9.67 | ||
| 6 | 8.17 | 8.83 | ||
|
| ||||
| INTELLIGIBILITY
| ||||
| S# | AOS | AOS-C | CA | CA-C |
|
| ||||
| 1 | 3.5 | 9.75 | 9.5 | 8.75 |
| 2 | 9 | 10 | 9.5 | 9.5 |
| 3 | 9.5 | 10 | 9.25 | 9.75 |
| 4 | 8.75 | 10 | 9.75 | 10 |
| 5 | 7.5 | 10 | ||
| 6 | 9.25 | 9.75 | ||
Results
Visuomotor Tracking
Cross-correlation
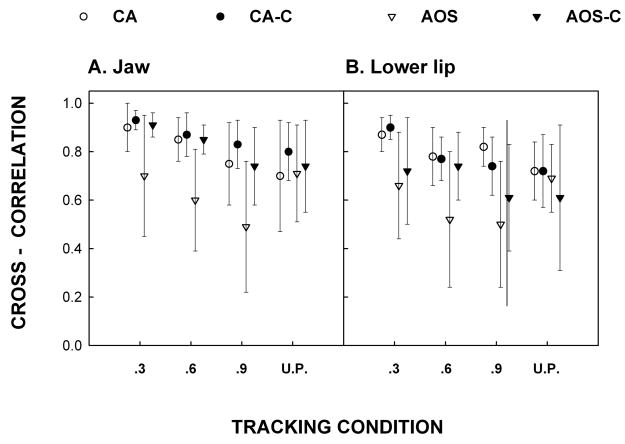
Means and standard errors for cross-correlations are shown for each group for each tracking condition in figures 1a (jaw) and 1b (lower lip). The mixed model ANOVA for the aphasia group for the lower lip revealed no significant group effect [F(1, 3) = 0.14, p = .7327] or group by tracking condition interaction [F(2, 6) = 1.50, p = .2966]. Inspection of the descriptive trends in the figures also suggests no differences between participants with CA and their controls for any of the tracking conditions. For the jaw, the aphasia group analysis also revealed no significant differences for group [F(1, 3) = 0.82, p = .4325] or interaction [F(2, 6) = .09, p= .9179]. T-test comparisons revealed no significant differences for either structure in the unpredictable tracking condition. Thus, for both structures the participants with CA did not differ from their controls for either of the tracking conditions (predictable or unpredictable).
Figure 1.
Cross-correlation between the target signal and participants’ movement for the Jaw (1a) and Lower Lip (1b) for the three predictable tracking conditions and the unpredictable condition (U.P.). Open circles refer to participants with Conduction Aphasia (CA), closed circles to the age-matched controls for CA (CA-C), open triangles to participants with Apraxia of Speech (AOS), and closed triangles for their age-matched controls (AOS-C).
The mixed model ANOVA for the AOS group for the lower lip revealed no significant group effect [F(1, 5) =3.34, p= .1274] or group by tracking condition interactions [F(2, 10) = 0.77, p = .4901]. However, inspection of Figure 1 shows that AOS participants performed more poorly than their controls for each of the predictable tracking conditions (especially at higher frequencies of .6 and .9 Hz). It is interesting to note that the controls for the AOS group performed more poorly than the controls for the CA group, an observation that may reflect the older age of these individuals (see (Ballard, Robin, Woodworth, & Zimba, 2001). For the jaw, there was a significant group effect [F(1,5) = 9.68, p = .0265] but no significant interaction [F(2, 10) = 1.72, p = .2277]. As is obvious from inspection of the data in Figure 1, mean tracking for all three frequencies of predictable signals was lower in the AOS group than in controls. There were no significant differences between the AOS group and their controls for unpredictable tracking for either structure. Thus, for the AOS group, tracking performance was poorer than controls when tracking predictable targets but unimpaired when tracking an unpredictable signal.
For the AOS group, the contrast between predictable and unpredictable targets was significant for the jaw [t(24) = −2.50, p = 0.019] and approached significance for the lip [t(24) = −1.92, p = 0.0670]. For the CA group the contrast did not approach significance for the jaw [t(24) = 0.34, p = 0.7393] or lip [t(24) = 0.34, p = 0.7382]. The interaction between predictability and diagnostic group approached significance for the jaw [t(24) = −1.84, p = 0.0774] and was insignificant for the lip [t(24) = −1.28, p = 0.2124]. However, particularly for the jaw, it is important to understand that an interaction can be estimated with only one quarter the precision of a main effect, and consequently the lack of significance should not be over-interpreted.
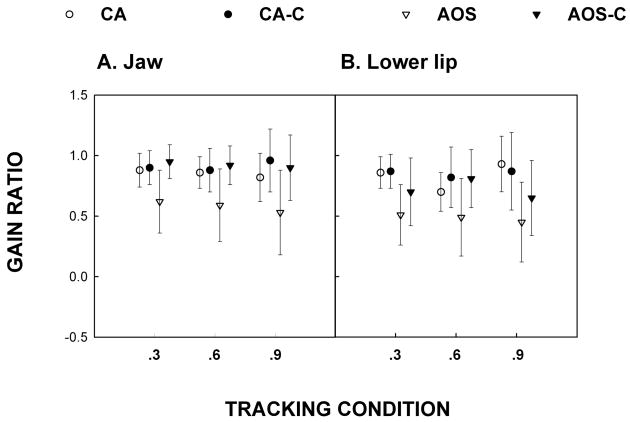
Gain ratio
Means and standard errors of gain ratios for each of the three predictable tracking conditions are shown in figure 2a (jaw), 2b (lower lip). Results of the mixed model ANOVA for the CA group for the lower lip revealed no significant group effect [F(1, 3) = 0.32, p = .6114] or group by tracking condition interaction [F(2, 6) = 1.39, p = .3189]. Similarly, for the jaw there was no significant group effect [F(1,3) = 0.63, p = .4856] or interaction [F(2,6) = 0.88, p = .4605]. Visual inspection of Figure 2 provides data supporting the finding that on average participants with aphasia were not different from controls in terms of the amplitude of their lower lip movements during predictable tracking.
Figure 2.
Gain ratio for Jaw (2a) and Lower Lip (2b). This measure refers to the amplitude of the predominant frequency divided by the target amplitude as determined by FFT analysis.
Analyses of the AOS group for the lower lip revealed no significant differences for the group effect [F(1, 5) = 4.82, p = .0795] or the interaction [F(2, 10) = 0.50, p = .6202], although the group effect approached significance. The AOS group on average produced half the required amplitude of movement. For the jaw, there was a significant group effect [F (1, 5) = 13.48, p = .0144] but no significant interaction [F(2, 10) = 0.11, p = .8998]. Inspection of figure 2 shows that on average the AOS group produced lower movement amplitudes than the control group and moved approximately half the requisite distance during predictable tracking. In sum, participants with CA had normal gain ratios while those with AOS had reduced movement amplitudes during predictable jaw tracking.
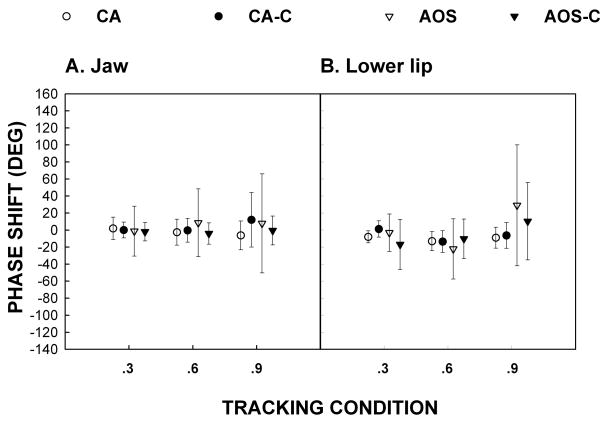
Phase Shift
Means and standard deviations of the degree of phase shift in tracking predictable signals for all groups are shown in figure 3a (jaw) and 3b (lower lip). In this figure, a negative shift implies a phase lead (i.e. tracking ahead of the target) and a positive shift a phase lag (i.e. tracking behind the target). A mixed model ANOVA on the lower lip for the CA group revealed no significant group effect [F(1, 3) = 0.90, p = .4129] or interaction [F (2, 6) = 1.48, p = .3004]. No significant group effect [F(1, 3) = 0.82, p = .4308] or interaction [F(2, 6) = 2.51, p = .1612] was found for the jaw. Similarly, the AOS group analysis for the lower lip yielded no significant group effect [F(1, 5) = 0.43, p = .5415] or interaction [F(2, 10) = 2.22, p = .1593]. For the jaw no significant group effect [F(1, 5) = 1.04, p = 0.3541] or interaction [F(2, 10) = 0.92, p = .4312] was found. Inspection of Figure 3 supports the statistical analyses in that generally phase differences were equivalent, with no difference between experimental participants and their controls.
Figure 3.
Phase shift for Jaw (3a) and Lower Lip (3b).
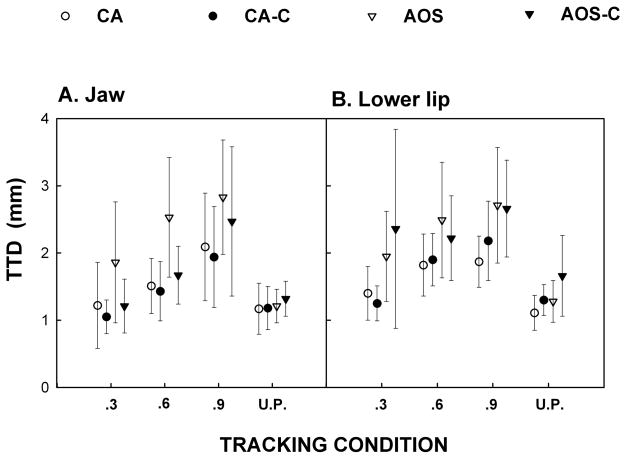
Average target-tracker difference (TTD)
The final dependent variable was the TTD for the jaw (Figure 4a) and the lip (Figure 4b). Mixed model ANOVA of predictable lower lip tracking for the CA group revealed no significant group effect [F(1, 3) = 0.17, p = .7080] and no significant interaction [F(2, 6) = 1.11, p = .3891]. Similarly, for the jaw, no significant group effects were found [F(1, 3) = 0.39, p = .5757] or interaction [F(2, 6) = 0.02, p = .9840]. T-test analysis revealed no significant group difference for the unpredictable tracking condition for either the lower lip or the jaw. Inspection of the trends observed in Figure 4 support the conclusion that on average the CA group performed similarly to controls for all tracking conditions.
Figure 4.
Target-tracker difference (TTD) for Jaw (4a) and Lower Lip (4b). This measure represents the average difference in millimeters between the target and the participant’s movements.
Mixed model ANOVA of the AOS group for the lower lip revealed no significant group effect [F(1, 5) = 0.05, p = .8253] or interaction [F(2, 10) = 1.86, p = .2054]. For the jaw, analysis revealed no significant differences for the group effect [F(1, 5) = 3.66, p = .1138] or for the interaction [F(2, 10) = 0.74, p = .5031]. T-test analyses revealed no significant group differences for the lip or the jaw. However, inspection of Figure 4 suggests that for the jaw, the AOS group had higher average TTD (i.e., performed more poorly) than did the controls for all predictable tracking conditions, but not for the unpredictable tracking condition.
For the AOS group, the contrast between predictable and unpredictable targets was significant for the jaw [t(24) = 2.09, p=0.0475] but insignificant for the lip [t(24) = 1.37, p=0.1844]. For the CS group the contrast did not approach significance for jaw [t(24) = 0.34, p=0.7393] or lip [t(24) = 0.35, p=0.7264]. The interaction between predictability and diagnostic group was not significant for jaw [t(24) = 1.06, p = 0.2996] or for lip [t(24) = 0.59, p = 0.5607]. However, particularly in the case of the jaw, it must be understood that an interaction can be estimated with only one quarter the precision of a main effect, and emphasize that the lack of significance should not be over-interpreted.
Perceptual Ratings of Speech and Tracking Performance
In order to examine possible relations between tracking performance and speech ability Pearson Product Moment correlations were performed on the dependent measures of the tracking performance and perceptual ratings of speech along the dimensions of OSD, OAI, and intelligibility. Perceptual ratings for the group of apraxic speakers were significantly poorer than those for the other three participant groups, who did not differ significantly from one another. Thus, correlational analyses were performed only for the participants with AOS.
Correlations between perceptual ratings for speakers with apraxia and their tracking performance are shown in table 5. In no instances did tracking performance on the unpredictable signal for cross correlation and gain significantly correlate with the perceptual ratings. However, inspection of the table reveals that strong correlations were found between predictable target tracking and perceptual ratings of speech, particularly for the dependent variables cross-correlation and gain. The dependent variables phase shift and target-tracker difference did not correlate strongly with the perceptual data. The correlations with perceptual data for the cross-correlation and gain were particularly robust for the tracking frequency of 0.9 Hz. In fact, for the lower lip, a clear trend is seen in which correlations were lowest for the 0.3 Hz signal and highest for the 0.9 Hz signal. Although less clear, the jaw results show a similar trend.
Table 5.
Pearson Product-moment Correlations between tracking performance (lip and jaw) and perceptual measures of intelligibility, articulatory precision and articulatory defectiveness
| Intelligibility | Precision | Defectiveness | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tracking condition | 0.3 | 0.6 | 0.9 | NP | 0.3 | 0.6 | 0.9 | NP | 0.3 | 0.6 | 0.9 | NP |
|
| ||||||||||||
| Best Correlation
| ||||||||||||
| Lip | 0.40 | 0.80 | 0.80 | 0.10 | 0.30 | 0.75 | 0.90 | 0.08 | 0.42 | 0.75 | 0.89 | 0.15 |
| Jaw | 0.80 | 0.60 | 0.70 | 0.40 | 0.67 | 0.41 | 0.69 | 0.44 | 0.78 | 0.41 | 0.74 | 0.49 |
|
| ||||||||||||
| Target Tracker Difference
| ||||||||||||
| Lip | 0.30 | 0.40 | 0.20 | 0.60 | −0.20 | −0.20 | −0.20 | −0.60 | −0.30 | −0.40 | −0.30 | −0.70 |
| Jaw | 0.60 | 0.30 | 0.20 | 0.50 | −0.50 | −0.10 | −0.20 | −0.60 | −0.60 | −0.30 | −0.20 | −0.60 |
|
| ||||||||||||
| Gain
| ||||||||||||
| Lip | 0.40 | 0.80 | 0.80 | 0.39 | 0.75 | 0.90 | 0.43 | 0.80 | 0.86 | |||
| Jaw | 0.70 | 0.80 | 0.80 | 0.65 | 0.66 | 0.73 | 0.72 | 0.75 | 0.82 | |||
|
| ||||||||||||
| Phase
| ||||||||||||
| Lip | 0.50 | 0.40 | 0.40 | 0.62 | −0.40 | −0.50 | 0.57 | −0.40 | −0.40 | |||
| Jaw | 0.10 | 0.70 | 0.00 | −0.30 | −0.60 | 0.00 | −0.10 | −0.70 | 0.01 | |||
Note: Significant correlations (|r| > 0.60 at α = 0.05) are underlined.
Discussion
Results of the current investigation support the hypothesis that AOS has its basis in impaired motor control. Data from this study and previous investigations of non-speech motor tasks (Ballard & Robin, 2007; Clark & Robin, 1998; McNeil et al., 1990) are strong indicators that the motoric involvement in people with AOS is not restricted to speech. Therefore, it appears that information about non-speech motor programming that presumably taps into similar motor processes and demands as those found in speech can assist in understanding AOS. By contrast, these data did not support a motor contribution to conduction aphasia, or at least the tracking tasks did not reveal a similar type of impairment as that found in AOS.
Participants with CA performed comparably to their age-matched controls on all tracking conditions for both the lip and the jaw. Similarly, a previous acoustic study on the speech of these CA subjects did not support a motoric view of CA, but did support motoric contributions to AOS (Seddoh et al., 1996). Specifically, acoustic changes in AOS all followed linguistic lengthening rules whereas changes in duration in CA were not consistent with vowel lengthening rules. Clark and Robin (1998) reported no motor involvement in CA using a non-speech motor learning task while finding that motor programming is impaired in AOS. In particular, they found that matching a new oral movement pattern with the jaw resulted in abnormal error of relative timing as well as absolute time and space in AOS but not CA. These data are at slight variance with McNeil et al. (1990), who reported that speakers with CA might have motor involvement based on fine force control data.
In contrast, participants with AOS showed impairment tracking predictable targets of varying frequencies, as was evident in the qualitative graphical analyses of the data. In particular, as the predictable target speed increased, AOS performance decreased disproportionately to the decrease observed in the other groups (figures 1–4). Thus, as the task demand increased during predictable tracking, participants with AOS were more adversely affected than controls or individuals with CA. In contrast, tracking performance on the unpredictable targets was no different in speakers with AOS than in other groups, suggesting that not all aspects of motor control are impacted in AOS. These findings are clear evidence that individuals with AOS do not have impairment of motor execution abilities, i.e. dysarthria. More importantly, these results suggest that motor control processes used by healthy speakers when the task prohibits the use of an internal model (e.g. feedback control) may be intact in speakers with AOS.
Interpretation of the current study suggests that AOS results from a deficit of feedforward motor control processes, as conceptualized in the DIVA model of speech processing (Guenther et al., 2006). Specifically, the direct neural mappings between predictable targets and spatio-temporal movement parameters, which operate with minimal feedback from sensory input in healthy adults, may not function properly in AOS. Impairment at this level manifests as inefficiency in movements which would normally benefit from prior experience or learning, or movements which are highly predictable, such that they might be encoded into an internal model vis-à-vis feedforward mappings. While healthy controls and participants with CA in this study benefited from tracking a predictable target, as observed in high cross correlations and gain ratios and low target-tracker differences, participants with AOS demonstrated relative impairment on these measures.
However, the ability of participants with AOS to normally track unpredictable signals, a task that does not permit the development of an internal model, suggests a sparing of feedback control processes in these individuals. Note that unpredictable tracking in normal participants is poorer than predictable tracking, suggesting that this is a highly demanding task. Specifically, while tracking of predictable targets requires only periodic reference to sensory input to ensure that accurate performance is maintained, unpredictable tracking necessitates more frequent monitoring, as participants must rely on the visual target to successfully complete the task. Thus, movement trajectories are planned in very short segments, with the external signal used to guide movements.
Data from this study suggest that participants with AOS are better able to evaluate the external signal than one that is driven by internal models or programs. The performance for unpredictable signals suggests that monitoring of movements and comparing them to an external target is difficult for both healthy controls and those with AOS. Since unpredictable tracking cannot rely on internal models of structures, both groups (unimpaired and AOS) must follow the target and make adjustments after visual feedback thereby reducing performance.
This account of relatively intact feedback motor control processes in people with AOS is supported by data from a recent study of visuomotor tracking with and without feedback (Ballard & Robin, 2007). Using a slightly different paradigm than that used here, Ballard and Robin examined tracking performance of predictable targets in one condition with visual target feedback provided and another in which the target was initially present (10 seconds) and then removed (i.e. “pseudo”-tracking). Participants with AOS demonstrated significantly better kinematic measures of tracking (e.g. increased cross-correlations and gain ratios; reduced target-tracker differences) with visual feedback relative to the no-feedback condition, which requires reference to an accurate internal model of the target.
A recent study of bite block vowel production in AOS also suggests that feedback control is relatively spared in persons with the disorder (Jacks, In Press). Speakers with AOS as well as healthy controls showed similar patterns of increased acoustic vowel targeting error with large and small bite blocks placed between the teeth than in unconstrained vowel production. Although error was increased in the bite block conditions, suggesting increased variability characteristic of feedback control mechanisms, it was similarly increased for both groups. These findings suggest that the ability to utilize ongoing sensory feedback, in this case proprioceptive information, is relatively unimpaired in persons with AOS. Together, these studies suggest that while high level motor planning or programming is impaired in AOS, not all aspects of motor control are deficient.
Although individuals with AOS in the present study showed clearly reduced performance on tasks that would benefit from the development of an internal model of a target movement, they appeared to compensate for their reduced motor control capabilities during the tracking task, in that they were able to approximate the target, albeit not perfectly. Consideration of the gain ratio findings may provide insight into how people with AOS compensate for motor deficits. Specifically, it was found that individuals with AOS had lower gain ratios (i.e. a measure of the amplitude of action compared to the amplitude of the target) compared to all other groups. While one interpretation is that AOS results in difficulty with amplitude parameter assignment during the activation of motor programming units, another way to view this finding is that the systematic reduction of movement amplitude in AOS is the result of compensation for an inefficient motor control system. That is, even though the speakers with AOS had the capability to move greater distances than required by the task, they may undershoot the targets when tracking predictable signals to reduce the complexity of the task, since moving greater distances places greater demand on the movement system. Using a different task, Clark and Robin (1998) have shown that some speakers with apraxia have difficulty assigning parameters to movement patterns. As such, it is unclear if the motor system strategically plans movements of smaller amplitude to reduce the control demands, or if the control of amplitude is disrupted as control resources are allocated to potentially more critical aspects of the movement. As suggested by Ballard and Robin (2007), this would occur during the activation and implementation of the speech motor program and the integration of feedback into the action to refine the movement pattern.
The perceptual ratings for participants with AOS also bear comment in that they were significantly poorer for overall speech defectiveness (OSD), overall articulatory imprecision (OAI), and intelligibility compared to all other groups, who did not differ from each other. Correlation between tracking performance for predictable signals and perceptual ratings of speech was completed for the subjects with AOS and strong correlations were obtained for the variables of cross correlation and gain ratio, especially at the highest tracking speed. These data provide further support for the importance of tracking data with regard to speech ability. The participants with AOS who scored lowest on OSD, OAI and intelligibility also had the lowest tracking scores (i.e. low cross correlations and low gain ratios). Thus it appears that the non-speech tracking task may be useful in understanding the motor control impairment in AOS, a disorder primarily characterized by impaired speech ability.
Clinical Implications
If tracking predictable targets is difficult for all persons with AOS, then it may be that differential performance between predictable and unpredictable tracking will serve as a diagnostic marker for AOS. While more data are needed, this is an intriguing hypothesis. To this end, we know that speakers with ataxic dysarthria, in contrast to those with AOS, have difficulty tracking both predictable and unpredictable targets (Hageman et al., 1994). Replication of this finding has been obtained for individuals with AOS and aphasia (Ballard & Robin, 2007). Future studies with a larger number of participants and various types of motor speech disorders are needed to support this hypothesis.
There is also at least one treatment implication of the current study. That motor control is disrupted in AOS suggests that treatments for AOS should be driven by knowledge of how the motor system learns. Thus, these data support our earlier contention (Ballard et al., 2000; McNeil et al., 2008) that principles of motor control will promote recovery of speech skills in persons with AOS. In fact, one study (Knock, Ballard, Robin, & Schmidt, 2000) has shown that random practice, compared to blocked practice, facilitates the learning of speech sounds in severe AOS.
Summary
Speakers with AOS have difficulty tracking predictable targets and their difficulty with unpredictable targets is no different than unimpaired speakers. By contrast, participants with CA performed similarly to healthy control speakers on all tasks. These results point to an impairment in feedforward control processes (i.e. generalized motor programs) as the primary deficit in AOS, with relatively spared feedback control systems. Furthermore, the tracking data in participants with AOS were highly correlated with perceptual ratings of their speech, suggesting that certain non-speech tasks may prove useful in understanding the mechanism underlying speech motor control disorders.
Acknowledgments
This research was supported in part by grants from the National Institute on Deafness and Other Communication Disorders (P60-DC00976) and the National Institute of Neurological Disease and Stroke (PO NO19632). We thank Kirrie Ballard, Edwin Maas, and Roger Ingham for their helpful comments on an earlier draft of this paper. We also thank the patients who served as participants in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Donald A. Robin, Research Imaging Center; Departments of Physical Therapy, Neurosurgery, and Otolaryngology, The University of Texas Health Science Center at San Antonio, San Antonio, TX Honors College, The University of Texas at San Antonio, San Antonio, TX.
Adam Jacks, Research Imaging Center, The University of Texas Health Science Center at San Antonio.
Carlin Hageman, Department of Communication Disorders, The University of Northern Iowa, Cedar Falls, Iowa.
Heather C. Clark, Department of Language, Reading, Exceptionalities, Appalachian State University, Boone, North Carolina
George Woodworth, Department of Statistics and Actuarial Science, The University of Iowa, Iowa City, Iowa.
References
- Ballard KJ, Granier JP, Robin DA. Understanding the nature of apraxia of speech: Theory, analysis, and treatment. Aphasiology. 2000;14(10):969–995. [Google Scholar]
- Ballard KJ, Robin DA. Influence of continual biofeedback on jaw pursuit-tracking in healthy adults and in adults with apraxia plus aphasia. Journal of Motor Behavior. 2007;39(1):19–28. doi: 10.3200/JMBR.39.1.19-28. [DOI] [PubMed] [Google Scholar]
- Ballard KJ, Robin DA, Folkins JW. An integrative model of speech motor control: A response to Ziegler. Aphasiology. 2003;17(1):37–48. [Google Scholar]
- Ballard KJ, Robin DA, Woodworth G, Zimba LD. Age-Related Changes in Motor Control During Articulator Visuomotor Tracking. Journal of Speech, Language and Hearing Research. 2001;44(4):763–777. doi: 10.1044/1092-4388(2001/060). [DOI] [PubMed] [Google Scholar]
- Ballard KJ, Solomon NP, Robin DA, Moon JB, Folkins JW. Nonspeech Assessment of the Speech Production Mechanism. In: McNeil MR, editor. Clinical Management of Sensorimotor Speech Disorders. 2. New York: Thieme Medical Publishers; 2008. [Google Scholar]
- Barlow SM, Cole KJ, Abbs JH. A new head-mounted lip-jaw movement transduction system for the study of motor speech disorders. Journal of Speech and Hearing Research. 1983;26(2):283–288. doi: 10.1044/jshr.2602.283. [DOI] [PubMed] [Google Scholar]
- Benton AL. The Revised Visual Retention Test. 4. New York: Psychological Corporation; 1974. [Google Scholar]
- Benton AL, Hamsher K. Multilingual Aphasia Examination. Iowa City: University of Iowa; 1978. [Google Scholar]
- Clark HM, Robin DA. Generalized motor programme and parameterization in apraxia of speech and conduction aphasia. Aphasiology. 1998;12(78):699–713. [Google Scholar]
- Damasio AR. The nature of aphasia: Signs and syndromes. Acquired aphasia. 1981:51–65. [Google Scholar]
- Damasio H, Damasio AR. The anatomical basis of conduction aphasia. Brain. 1980;103(2):337–350. doi: 10.1093/brain/103.2.337. [DOI] [PubMed] [Google Scholar]
- Damasio H, Damasio AR. Lesion analysis in neuropsychology. New York: Oxford University Press; 1989. [Google Scholar]
- Darley F, Aronson A, Brown J. Motor Speech Disorders. Philadelphia: W.B. Saunders Inc; 1975. [Google Scholar]
- De Renzi E, Vignolo LA. The token test: A sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- Duffy JR. Motor speech disorders: Substrates, differential diagnosis, and management. St. Louis, MO: Elsevier Mosby; 2005. [Google Scholar]
- Flowers KA. Some frequency response characteristics of Parkinson’s disease. Brain. 1978;101:19–34. doi: 10.1093/brain/101.1.19. [DOI] [PubMed] [Google Scholar]
- Folkins JW, Moon JB, Luschei ES, Robin DA, Tye-Murray N, Moll KL. What can nonspeech tasks tell us about speech motor disabilities. Journal of Phonetics. 1995;23:139–147. [Google Scholar]
- Forrest K, Adams S, McNeil MR, Southwood H. Kinematic, electromyographic, and perceptual evaluation of speech apraxia, conduction aphasia, ataxic dysarthria, and normal speech production. In: Moore CA, Yorkston KM, Beukelman DR, editors. Dysarthria and apraxia of speech: Perspectives on management. Baltimore: Paul H. Brookes Publishing Co; 1991. pp. 147–171. [Google Scholar]
- Goodglass H, Kaplan E. Assessment of Aphasia and Related Disorders. 2. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Gracco VL, Abbs JH. Variant and invariant characteristics of speech movements. Experimental Brain Research. 1986;65(1):156–166. doi: 10.1007/BF00243838. [DOI] [PubMed] [Google Scholar]
- Graham JR. The MMPI: a practical guide. Oxford University Press; 1987. [Google Scholar]
- Grant DA, Berg EA. Wisconsin Card Sort Test. Odessa, FL: Psychological Assessment Resources; 1948. [Google Scholar]
- Guenther FH, Ghosh SS, Tourville JA. Neural modeling and imaging of the cortical interactions underlying syllable production. Brain and Language. 2006;96:280–301. doi: 10.1016/j.bandl.2005.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH, Hampson M, Johnson D. A theoretical investigation of reference frames for the planning of speech movements. Psychological review. 1998;105(4):611–633. doi: 10.1037/0033-295x.105.4.611-633. [DOI] [PubMed] [Google Scholar]
- Hageman CF, Robin DA, Moon JB, Folkins JW. Oral motor tracking in normal and apraxic speakers. Clinical Aphasiology. 1994;22:219–229. [Google Scholar]
- Itoh M, Sasanuma S. Articulatory movements in apraxia of speech. In: Rosenbek JC, McNeil MR, Aronson AE, editors. Apraxia of Speech: Physiology, acoustics, linguistics, management. San Diego, CA: College-Hill Press; 1984. pp. 135–165. [Google Scholar]
- Jacks A. Bite block vowel production in apraxia of speech. Journal of Speech, Language and Hearing Research. doi: 10.1044/1092-4388(2008/066). In Press. [DOI] [PubMed] [Google Scholar]
- Kelso JA, Tuller B. Toward a theory of apractic syndromes. Brain and Language. 1981;12(2):224–245. doi: 10.1016/0093-934x(81)90016-x. [DOI] [PubMed] [Google Scholar]
- Kent RD, McNeil MR. Relative timing of sentence repetition in apraxia of speech and conduction aphasia. In: Ryalls JH, editor. Phonetic approaches to speech production in aphasia and related disorders. San Diego, CA: College-Hill Press; 1987. pp. 181–200. [Google Scholar]
- Knock TR, Ballard KJ, Robin DA, Schmidt RA. Influence of order of stimulus presentation on speech motor learning: A principled approach to treatment for apraxia of speech. Aphasiology. 2000;14(56):653–668. [Google Scholar]
- Maas E, Robin DA, Steinhauer V, Ballard KJ, Magnuson C, Wright D. Motor programming in apraxia of speech: A reaction time approach; Paper presented at the Paper presented to the International Conference on Motor Speech.2006. [Google Scholar]
- McClean MD, Beukelman DR, Yorkston KM. Speech-Muscle Visuomotor Tracking in Dysarthric and Nonimpaired Speakers. Journal of Speech and Hearing Research. 1987;30(2):276–282. doi: 10.1044/jshr.3002.276. [DOI] [PubMed] [Google Scholar]
- McNeil MR, Caligiuri M, Rosenbek JC. Clinical Aphasiology Conference. San Diego: College-Hill Press; 1989. A comparison of labiomandibular kinematic durations, displacements, velocities, and dysmetrias in apraxic and normal adults; pp. 173–193. [Google Scholar]
- McNeil MR, Robin DA, Schmidt RA. Apraxia of Speech: Definition, Differentiation, and Treatment. In: McNeil MR, editor. Clinical Management of Sensorimotor Speech Disorders. 2. New York, NY: Thieme; 2008. pp. 311–344. [Google Scholar]
- McNeil MR, Weismer G, Adams S, Mulligan M. Oral structure nonspeech motor control in normal, dysarthric, aphasic and apraxic speakers: isometric force and static position control. Journal of Speech and Hearing Research. 1990;33(2):255–268. doi: 10.1044/jshr.3302.255. [DOI] [PubMed] [Google Scholar]
- Moon JB, Zebrowski P, Robin DA, Folkins JW. Visuomotor tracking ability of young adult speakers. Journal of Speech and Hearing Research. 1993;36(4):672–682. doi: 10.1044/jshr.3604.672. [DOI] [PubMed] [Google Scholar]
- Muller EM, Abbs JH. Strain gauge transduction of lip and jaw motion in the midsagittal plane: Refinement of a prototype system. Journal of the Acoustical Society of America. 1979;65:481. doi: 10.1121/1.382348. [DOI] [PubMed] [Google Scholar]
- Odell K, McNeil MR, Rosenbek JC, Hunter L. Perceptual characteristics of vowel and prosody production in apraxic, aphasic, and dysarthric speakers. Journal of Speech and Hearing Research. 1991;34(1):67–80. doi: 10.1044/jshr.3401.67. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Filetest de copie d’une figure complexe. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encéphalopathie traumatique: les problèmes. Archives de Psychologie. 1941;28(112):286–340. [Google Scholar]
- Robin DA, Bean C, Folkins JW. Lip movement in apraxia of speech. Journal of Speech and Hearing Research. 1989;32(3):512–523. doi: 10.1044/jshr.3203.512. [DOI] [PubMed] [Google Scholar]
- Russell EW. A Multiple Scoring Method for the Assessment of Complex Memory Functions. Journal of Consulting and Clinical Psychology. 1975;43:6. [Google Scholar]
- Schmidt RA, Lee T. Motor Control and Learning: A Behavior Emphasis. Champaign, Ill: Human Kinetics; 2005. [Google Scholar]
- Seddoh SAK, Robin DA, Sim HS, Hageman C. Speech timing in apraxia of speech versus conduction aphasia. Journal of Speech and Hearing Research. 1996;39(3):590–603. doi: 10.1044/jshr.3903.590. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- Ziegler W. Speech motor control is task-specific: Evidence from dysarthria and apraxia of speech. Aphasiology. 2003;17(1):3–36. [Google Scholar]