Abstract
Endothelin (ET) receptor blockade delays the progression of diabetic nephropathy; however, the mechanism of this protection is unknown. Therefore, the aim of this study was to test the hypothesis that ETA receptor blockade attenuates superoxide production and inflammation in the kidney of diabetic rats. Diabetes was induced by streptozotocin (HG), and Sham rats received vehicle treatments. Some rats also received the ETA antagonist ABT-627 (Sham+ABT and HG+ABT; 5 mg·kg−1·day−1, n=8–10/group). During the 10 week study, urinary microalbumin was increased in HG rats and this effect was prevented by ETA receptor blockade. Indices of oxidative stress, urinary excretion of thiobarbituric acid reactive substances (TBARS), 8-hydroxy2-deoxyguanosine (8-OHdG), and H2O2 and plasma TBARS, were significantly greater in HG rats than sham rats. These effects were not prevented by ABT-627. Additionally, renal cortical expression of 8-OHdG and NADPH oxidase subunits was not different between HG and HG+ABT rats. ETA receptor blockade attenuated increases in macrophage infiltration and urinary excretion of TGF-β and PGE2 metabolites in HG rats. Thus, while ABT-627 did not alleviate oxidative stress in HG rats, inflammation and production of inflammatory mediators were reduced in association with prevention of microalbuminuria. These observations indicate that ETA receptor activation mediates renal inflammation TGF-β production in diabetes, and are consistent with the postulate that ETA blockade slows progression of diabetic nephropathy via an anti-inflammatory mechanism.
Keywords: Diabetes, Endothelin, Kidney, Reactive Oxygen Species, Inflammation
Selective endothelin type A (ETA) receptor antagonists as well as combined ETA/ endothelin type B (ETB) receptor antagonists prevent the development of hypertension, renal vasoconstriction, proteinuria, increased renal matrix protein expression, and renal structural injury in streptozotocin (STZ)-induced diabetes (1,2,3). Endothelin-1 (ET-1) stimulates hypertrophy, proliferation, and extracellular matrix accumulation in the kidney, primarily via ETA receptor stimulation (4,5). Therefore, it is reasonable to hypothesize that renal ET-1 contributes to the fibrosis associated with diabetic nephropathy.
Experimental and clinical diabetes have been linked to enhanced reactive oxygen species production (6,7,8). The effects of increased oxidative stress include vasoconstriction, vascular smooth muscle cell growth and migration, endothelial dysfunction, modification of extracellular matrix proteins, and increased renal sodium reabsorption. Several models of experimental diabetes exhibit increased activity of NADPH oxidase, the predominant source of superoxide in the kidney (9), and NADPH oxidase inhibition reduces proteinuria and renal injury in diabetes (8). Accumulating evidence suggests that ETA receptor activation increases the production of reactive oxygen species in the vasculature and in the kidney. In endothelin-dependent models of hypertension, ET-1 stimulated NADPH oxidase production of superoxide in carotid arteries (10), and ETA receptor blockade reduced vascular superoxide generation (11, 12). In ET-1 infused animals, vascular and renal oxidative stress were increased (13,14).
Inflammation is also believed to play a key role in the pathogenesis of diabetic nephropathy. Macrophage accumulation correlates with renal injury in STZ-diabetic nephropathy (15,16); however, the mechanisms of macrophage recruitment in the kidney are not completely understood (17). Cytokines and growth factors are thought to mediate the effects of high glucose on the kidney (18). Transforming growth factor-beta (TGF-β), a hypertrophic and fibrogenic cytokine, has received attention as one of the major factors involved in the development of diabetic kidney disease (19,20). TGF-β1 mRNA and protein levels are increased in both the glomerular and tubular compartments of various models of experimental diabetes in rats and mice (21,22).
ET-1 may be involved in the induction of inflammatory mechanisms in the kidney of diabetic rats including the production of cytokines and growth factors and chemoattraction of macrophages (23,24). ET-1 promotes macrophage infiltration in the rat kidney (25), and ETA receptor blockade suppresses macrophage infiltration in the renal cortex in non-diabetic nephropathies (26) and attenuates the increase in TGF-β mRNA in glomeruli from diabetic rats (27).
Because ET-1 stimulates the production of reactive oxygen species and inflammatory mediators, we proposed that enhanced activation of the ETA receptor promotes renal injury in type 1 diabetes mellitus via increased production of reactive oxygen species and inflammatory mediators. Therefore, the aim of this study was to test the hypothesis that ETA receptor blockade attenuates oxidative stress and inflammation in the kidney of diabetic rats.
METHODS
Animal Model
All experiments were performed using male Sprague-Dawley rats (200–250 g, Harlan Laboratories, Indianapolis, IN) in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved and monitored by the Medical College of Georgia IACUC. Blood glucose levels were measured (Accu-Check III model 766; Boehringer Mannheim, Indianapolis, IN), and each rat was weighed and assigned randomly to one of four groups: normal rats receiving vehicle treatments (Sham), diabetic rats with partial insulin replacement to maintain modest hyperglycemia (HG), Sham rats receiving the ETA receptor antagonist ABT-627 (Sham+ ABT), and HG rats receiving ABT-627 (HG+ABT). ABT-627 (5 mg·kg−1·day−1) was administered via the drinking water to facilitate continuous administration (28). The concentration of ABT-627 in the water was adjusted weekly to maintain appropriate dosing and to account for the increased water intake in STZ-treated rats. ABT-627 has 1000-fold selectivity for the ETA receptor over ETB receptors (28).
Rats were anesthetized on Day 0 with isoflurane (IsoFlo, Abbott Laboratories, North Chicago, IL) to facilitate intravenous injection of either 65 mg/kg STZ (Sigma Chemical, St. Louis, MO; HG and HG+ABT rats) or vehicle (Sham and Sham+ABT rats). The next day, blood glucose levels were measured and the rats were anesthetized to facilitate subcutaneous insertion of a 2.3×2.0 mm sustained-release insulin implant (HG and HG+ABT rats, Linshin Canada, Scarborough, Ontario, Canada) or a 2.3×2.0 mm palmitic acid implant (vehicle; Sham and Sham+ ABT rats). Blood glucose and body weight were measured at 3- to 4-day intervals for 10 weeks. Periodically, rats were placed in metabolic cages for 24-h urine collection and food and water intake measurements. After 10 weeks, rats were anesthetized with pentobarbital sodium (Nembutal, 65 mg/kg ip; Abbott Laboratories, North Chicago, IL), and blood and kidneys were harvested.
Telemetric measurements
Rats were anesthetized with pentobarbital sodium anesthesia, and telemetry transmitters (Data Sciences, St. Paul, MN) were implanted in the abdominal aorta according to manufacturer’s specifications. Rats were allowed to recover from surgery and returned to individual housing for 1 wk prior to initiation of data acquisition. Arterial pressure and heart rate waveforms were continuously recorded for ten seconds every ten minutes.
Immunoblotting
The renal cortex was pulverized while frozen and placed in ice-cold homogenization buffer (volume/weight ratio of 10; 50 mmol/L Tris·HCl - pH 7.4, 0.1 mmol/L EDTA, 0.1 mmol/L EGTA, 250 mmol/L sucrose, 0.1% BME, 10% glycerol) in the presence of protease inhibitors (1 mmol/L PMSF, 2 μmol/L leupeptin, 1 μmol/L pepstatin A, and 0.1% aprotinin) and homogenized on ice with a glass-teflon homogenizer. Protein concentrations were determined by standard Bradford assay, and Western blotting was performed as previously described (7). Bands were detected and densitometric analysis was performed with the Odyssey Imaging System (LI-COR Biosciences, Lincoln, NE). Primary antibodies included rabbit polyclonal antibodies for COX2 (Cayman Chemical, Ann Arbor, MI) and the NADPH oxidase subunits p22 phox, p47 phox, and p67 phox (Santa Cruz Biotechnology, Santa Cruz, CA). Equal protein loading was verified by probing for β-actin (Sigma Chemicals, St. Louis, MO).
Immunohistochemical analysis
Kidneys were perfused with 4% paraformaldehyde in 100 mmol/L dibasic sodium phosphate buffer and immersed in 4% paraformaldehyde solution overnight at room temperature, transferred to 70% ethanol for 24 hours and paraffin-embedded. The kidneys were sectioned at a thickness of 4 μm onto Superfrost plus slides and processed as previously described (7). Slides were incubated in the absence or presence of primary antibodies to COX2 (Cayman Chemical, Ann Arbor, MI), 8-hydroxy-2’deoxyguanosine (8-OHdG, Oxis International, Portland, OR), or ED-1 (CD58, Serotec, Kidlington, Oxford, UK) in humidity chambers overnight at 4°C, followed by incubation with peroxidase-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) or goat anti-mouse IgG (Serotec, Kidlington, Oxford, UK) for 30 minutes at room temperature. To assess renal injury, kidney sections were stained using a Periodic Acid-Schiff stain kit, Gomori’s Tricrome Stain Kit, blue collagen (Richard Allen Scientific, Kalamazoo, MI), or Picro-Sirius Red according to standard methods (29). The stained sections were viewed with an Olympus BX40 microscope (Olympus America, Melville, NY) on brightfield setting fitted with a digital camera (Olympus DP12, Olympus America, Melville, NY). For semiquantitative evaluation, Dr. Yamamoto examined histologic sections in a blind fashion for mesangial proliferation, mesangial expansion, interstitial mononuclear cell infiltration, and arteriolar thickening. For quantification of ED-1, positive cells were counted in 425 mm by 320 mm sections of kidney cortex (magnification 40X).
Urine and plasma assays
Urinary concentrations of electrolytes were determined by ion-selective electrodes (Synchron EL-ISE, Beckman Instruments, Brea, CA). Plasma and urine creatinine concentrations were measured by the picric acid method adapted for microtiter plates (30). Creatinine and picric acid were obtained from Sigma Chemical Company (St. Louis, MO). Urinary and plasma immunoreactive ET concentrations were measured by radioimmunoassay (Amersham Pharmacia Biotech, Arlington Heights, IL) and chemiluminescent immunoassay (R and D Systems, Minneapolis, MN), respectively. Plasma and urinary measures of oxidative stress were measured using an OXItek thiobarbituric acid reactive substances (TBARS) assay kit (ZeptoMetrix, Buffalo, NY), an Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Molecular Probes, Eugene, OR), and a BIOXYTECH 8-hydroxy-2’deoxyguanosine (8-OHdG)-EIA Kit (Oxis International, Portland, OR). Urinary concentrations of transforming growth factor-beta1 (TGF-β1) were determined by a TGFβ1 Emax Immunoassay System (Promega Corp., Madison, WI). Urinary concentrations of the metabolites of prostaglandin E2 (PGEM), prostacyclin (6-keto-prostaglandin F1α), and thromboxane A2 (TXB2) were measured using enzyme immunoassay kits from Cayman Chemical (Ann Arbor, MI).
Statistical analysis
Values are expressed as mean ± SEM. A probability value < 0.05 was considered significant. The significance of differences between groups for blood pressure data were evaluated with an ANOVA for repeated measures, followed by Duncan’s multiple range post hoc tests. Between group comparisons for other measurements were performed using an ANOVA combined with post hoc contrasts (Statistica, StatSoft, Inc, Tulsa, OK).
RESULTS
Characteristics of experimental animals
On Day 0, the rats weighed 302±2 g and had blood glucose levels averaging 92±2 mg/dl (n=40). Twenty-four hours after STZ injection, blood glucose levels were elevated in HG and HG+ABT rats (465±14 mg/dl; n=24), confirming the onset of diabetes. As shown in Table 1, blood glucose levels were elevated in HG and HG+ABT rats to a similar degree, and blood glucose levels of Sham rats were not changed by treatment with ABT-627. Relative to Sham and Sham+ABT rats, animals in the HG and HG+ABT groups gained less weight over the study period and displayed renal hypertrophy, hyperphagia, polydipsia, polyuria, and glomerular hyperfiltration (as estimated by creatinine clearance). None of these parameters differed significantly between HG and HG+ABT rats. Plasma ET-1 concentration was increased by ABT-627 treatment only in HG rats. Hyperglycemia increased urinary ET-1 excretion, and this was unaffected by ABT-627 treatment (Figure 1).
Table 1.
Characteristics of Sham, Sham rats treated with ABT-627 (Sham+ ABT), HG, and HG rats treated with ABT-627 (HG+ABT) after 10 weeks of treatment. Excretory data were derived from 24-h urine collections in metabolic cages within 48 h of sacrifice. Plasma samples were obtained under anesthesia immediately prior to sacrifice.
| Sham | Sham+ ABT | HG | HG+ ABT | |
|---|---|---|---|---|
| Body Weight (g) | 415±12 | 434±6 | 331±7* | 331±7* |
| Kidney Weight (g) | 1.4±0.1 | 1.4±0.1 | 1.9±0.1* | 1.6±0.1 |
| Kidney:Body Weight (mg/g) | 3.3±0.2 | 3.2±0.4 | 5.6±0.3* | 4.9±0.2* |
| Blood [Glucose] (mg/dl) | 82±1 | 93±2 | 461±16* | 454±11* |
| Food Intake (g/day) | 14±2 | 18±1 | 38±1* | 38±1* |
| Water Intake (ml/day) | 25±2 | 27±3 | 180±12* | 167±16* |
| Urine Flow (ml/day) | 14±1 | 18±2 | 158±10* | 131±17* |
| Creatinine Clearance (ml/min) | 1.0±0.1 | 1.2±0.1 | 1.5±0.1* | 1.3±0.1* |
| Plasma [ET-1] (pg/ml) | 0.7±0.1 | 0.9±0.1 | 0.6±0.1 | 1.7±0.2*# |
Values are means ± SEM, n=6–11,
p < 0.05 vs Sham,
p < 0.05 vs HG.
Figure 1.
Urinary ET-1 (A) and microalbumin (B) excretion in Sham, Sham rats treated with ABT-627 (Sham+ABT), HG, and HG rats treated with ABT-627 (HG+ ABT). Values are means ± SE. * p< 0.05 vs sham, † p<0.05 vs HG+ABT, n=4–6.
Blood pressure and heart rate measurements
Figure 2 illustrates mean arterial pressure (MAP) and heart rate (HR) in Sham, Sham+ABT, HG, and HG+ABT rats over a 5 day baseline period and the 10 week treatment period. ABT-627 significantly reduced MAP throughout the treatment period in Sham and HG rats (p<0.05) with no effect on HR. Hyperglycemia had no effect on MAP or HR for the first 7 weeks of treatment, but MAP and HR were decreased in the HG group during the last 2–3 weeks of treatment (p<0.05). The Sham group maintained a constant MAP throughout the 10 week period.
Figure 2.
Mean arterial pressure and heart rate in conscious Sham, Sham+ABT, HG, and HG+ABT rats during the 10-wk course of study. Values are means for 24 hour periods, reported every 5 days. * p< 0.05 vs sham, † p<0.05 ABT vs untreated, n=4–6.
Measures of renal injury
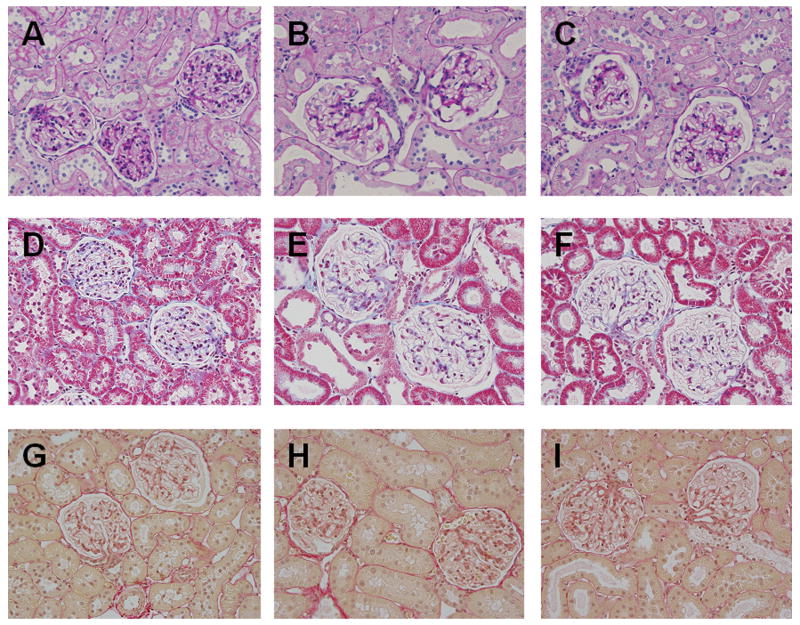
HG rats exhibit increased microalbumin excretion, compared to Sham rats, as early as 2 wks after STZ injection and this effect was sustained over the entire 10 wks of the study. ABT-627 treatment prevented microalbuminuria in diabetic rats (Figure 1). As shown in Figure 3, histological examination using Periodic Acid-Schiff staining (basement membranes, glycoproteins), Gomori’s trichrome staining (collagen), and picrosirius red staining (connective tissue and fibers) revealed no remarkable changes in kidney structure of HG rats compared to Sham rats or ABT-627 treated HG rats after 10 weeks of diabetes.
Figure 3.
Representative images of renal histology of Sham (A, D, and G), HG (B, E, and H) and HG+ABT rats (C, F, and I) rats after 10 weeks of treatment (original magnification = 40X). Periodic Acid-Schiff stain (A, B, and C), Gomori’s trichrome stain (D, E, and F), and picro Sirius red staining (G, H, and I).
Measures of oxidative stress
Urinary excretion of hydrogen peroxide and TBARS were significantly elevated after only 2 wks of hyperglycemia, and remained at these levels for the duration of the study (Figure 4). Moreover, plasma TBARS and urinary excretion of 8-OHdG (measured only at the end of the 10-wk study) were also significantly increased in HG rats compared to Sham rats. ETA receptor blockade did not prevent the hyperglycemia-induced increase in any of these indices of oxidative stress. Additionally, neither immunohistochemical staining of 8-OHdG nor expression of the NADPH oxidase subunits p22phox, p47phox, or p67phox was altered by ABT-627 treatment in the renal cortex of HG rats (Figure 4).
Figure 4.
Indices of oxidative stress in Sham, Sham+ABT, HG and HG+ABT rats (n=5–11). Urinary excretion of (A) hydrogen peroxide and (B) TBARS (MDA equivalents) were measured at 2, 6, and 10 weeks after injection of STZ or vehicle. (C) Plasma TBARS and 8-hydroxy-2’-deoxyguanosine (8-OHdG) excretion measured at the 10 wk time point. Typical immunohistochemical localization of 8-OHdG is provided for the renal cortex of (D) HG and (E) HG+ABT. (F) Western blot analysis of renal cortical expression of the NADPH oxidase subunits p22phox, p47phox, and p67phox in each group, normalized to β–actin. * p< 0.05 vs sham
Analysis of inflammatory and pro-fibrotic markers
In the cortex of HG rats, the number of cells that stained positive for ED-1, a macrophage marker, was significantly greater than in Sham rats (Figure 5). ABT-627 treatment reduced the hyperglycemia-induced increase in the number of ED-1 positive cells. Urinary excretion of TGF-β was increased in HG rats and ABT-627 treated HG rats compared to Sham rats (Figure 5) during the early part of the study (2–6 weeks). After 10 weeks, there was a further increase in TGF-β excretion in the HG group, and ABT-627 attenuated this increase.
Figure 5.
Pro-fibrotic and inflammatory markers in Sham, Sham+ABT, HG, and HG+ABT rats. (A) Urinary excrretion of TGF-β (n=4–5; * p<0.05 vs Sham, † p<0.05 vs HG+ABT). (B) Quantification of ED-1+ cells in the renal cortex.
Urinary analysis of prostanoid production
Urinary excretion rates were determined for PGE metabolite (PGEM), 6-keto PGF1α (6 keto), and TxB2. PGEM is a measure of PGE2 production. 6-keto PGF1α is formed by the hydration of prostacyclin and is an estimate of prostacyclin production. TxA2 is rapidly hydrolyzed to TxB2. Because of its transient nature, TxB2 measurements reflect renal TxA2 production (31). Urinary excretion of all three prostanoid metabolites was greater in HG rats compared to Sham rats (p< 0.01, Figure 6), with the increase in PGEM excretion evident beginning at the 2-wk time point. There were no differences in urinary excretion of 6-keto PGF1α or TxB2 between HG rats and HG rats treated with the ETA receptor antagonist; however, ABT-627 prevented the further rise in PGEM excretion observed in HG rats at the end of the 10 week study (p=0.001). ABT-627 had no effect on prostanoid excretion in Sham rats.
Figure 6.
Prostaglandin excretion by Sham, Sham+ABT, HG, and HG+ABT rats. (A) Time course of PGEM excretion. (B) 6-keto PGF1α and TxB2 excretion at the 10 week time point. (n=4–5; * p<0.05 vs Sham, † p<0.05 vs HG)
Expression of cortical cyclooxygenase-2
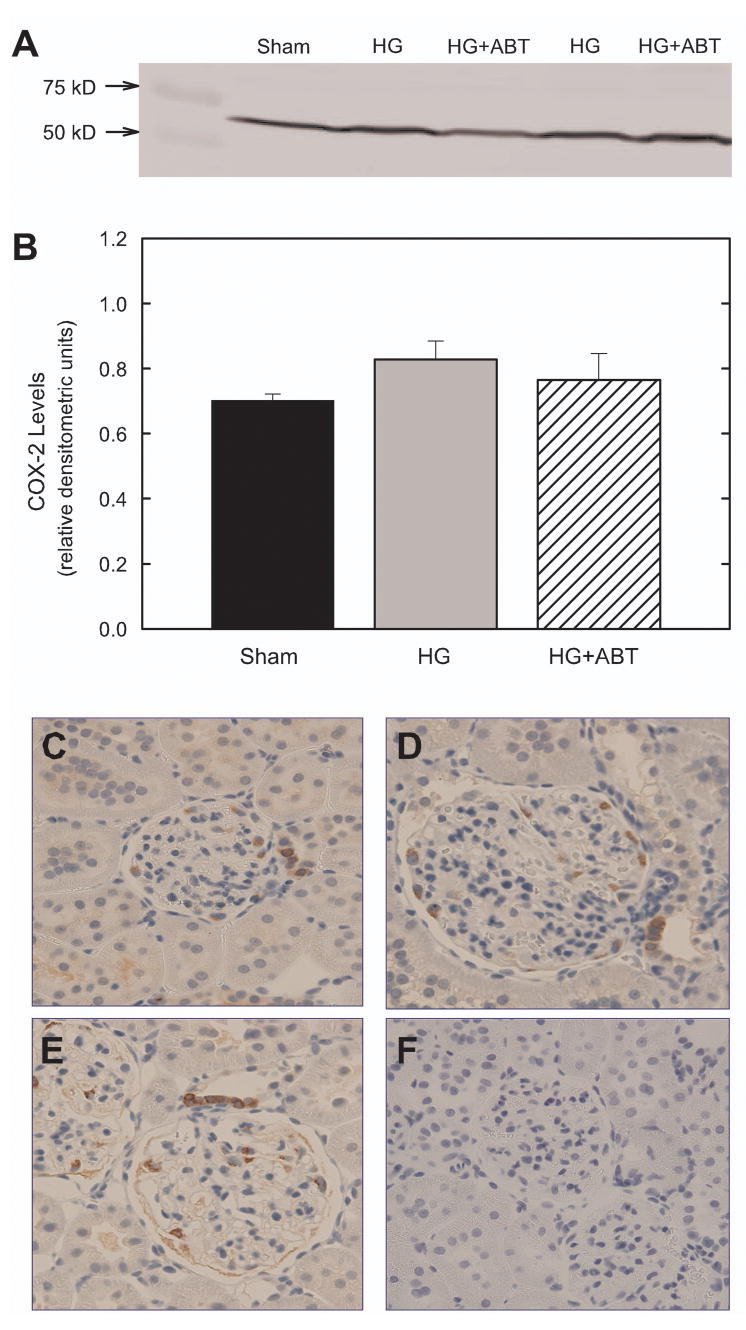
Although there was a trend for higher expression of COX-2 in the cortex of HG rats, ABT-627 had no effect on cortical COX-2 expression in hyperglycemic animals (Figure 7). Furthermore, immunohistochemical analysis revealed no difference in the localization or expression of COX-2 in the renal cortex of any group (Figure 7, panels C-F).
Figure 7.
Renal cortical expression of cyclooxygenase-2.after 10 weeks of treatment. (A) Representative Western Blot. (B) Densitometric analysis (normalized to β-actin). n=5–6. Immunohistochemical localization of cyclooxygenase-2 in the renal cortex of Sham (C), HG (D) and HG+ABT rats (E) after 10 weeks of treatment. Panel F is a negative control (no primary antibody).
DISCUSSION
In experimental models of diabetes, ETA receptor antagonists as well as combined ETA / ETB receptor antagonists have been shown to delay the progression of diabetic nephropathy (1,2,3). Although the protective effects of ET receptor blockade have been previously demonstrated, the mechanism of this protection is unknown. Therefore, the purpose of this study was to determine if enhanced activation of the ETA receptor promotes renal injury in STZ-induced diabetes mellitus via increased production of reactive oxygen species and inflammatory mediators. Ten weeks after the induction of diabetes, HG rats exhibited glomerular hyperfiltration, as estimated by creatinine clearance, and had decreased blood pressure compared to Sham rats. These animals also exhibited increased urinary excretion of ET-1, but no change in plasma levels, observations that together indicate increased renal ET-1 production in diabetes. Blockade of the ETA receptor prevented the development of albuminuria in this model of type 1 diabetes, although no major structural alterations were evident in the kidneys of the HG rats at this relatively early stage of diabetes. Oxidative stress was increased in the diabetic rats; however, the production of reactive oxygen species was not attenuated by treatment with the ETA receptor antagonist, ABT-627. In contrast, ETA receptor blockade attenuated the macrophage infiltration and blunted the increase in urinary excretion of TGF-β and PGE2 metabolites in diabetic rats. These data implicate ETA receptor activation in the renal inflammatory and pro-fibrotic, but not pro-oxidant, events that arise during type 1 diabetes.
Inflammation plays a key role in the pathogenesis of diabetic nephropathy. Our data indicate that macrophage infiltration is increased in the renal cortex of diabetic rats and attenuated by treatment with ABT-627. This is consistent with reports that ETA receptor blockade suppresses macrophage infiltration in the renal cortex of rats with aldosterone-induced hypertension (25) and hypokalemia-induced renal tubulointerstitial injury (26). Previous studies have shown that macrophage infiltration precedes development of structural changes in the diabetic kidney (15,16). In a non-diabetic model of endothelin-dependent kidney fibrosis, an increase in the density of CD4-positive lymphocytes and macrophages was observed in the kidney well before the onset of fibrosis (32). These data and our own support the theory that chronic renal inflammation is one of the first detectable changes that can be observed during the progression of renal injury. Although renal hypertrophy was evident in HG rats, we did not observe significant structural changes in the kidney during this study although some previous reports have shown glomerular enlargement, matrix expansion, interstitial fibrosis and arteriolopathy during early diabetes in the STZ model (33–36). These differences may be due to the strain or gender of rats used or the use of insulin to maintain moderate hyperglycemia.
Additionally, we found that urinary excretion of TGF-β, a pro-fibrotic cytokine implicated in the development of diabetic nephropathy, is attenuated by blockade of the ETA receptor. This is consistent with the work of Nakamura, et al (21), which demonstrated that treatment with FR139317, another selective ETA receptor antagonist, reduced TGF-β mRNA in the glomeruli of diabetic rats and supports a role of ETA receptor activation in the progression of renal injury associated with hyperglycemia. The source of TGF-β in our experiments is currently unknown. However, it is known that the activation of macrophages stimulates TGF-β secretion (37). It is possible that ET-1 stimulates the renal recruitment of macrophages that, in turn, promote the release of TGF-β. Such a mechanism may account for the attenuation of TGF-β excretion by ABT-627 in our studies.
PGE2 is an important regulator of vascular tone, glomerular filtration, renin release, and sodium transport in the kidney (38). Inhibition of COX-2 (39) or the PGE receptor EP-1 (40) prevents proteinuria and reduces renal injury in diabetes. Furthermore, in genetically hypertensive rats, the PGE2/EP-1 signaling pathway has been shown to play an important role in the development of renal injury (41). Therefore, the present study tested the hypothesis that the beneficial effects of ETA receptor blockade are in part due to a reduction in prostanoid production. We found that excretion of PGE metabolites was reduced in diabetic rats treated with ABT-627, although excretion of prostacyclin and thromboxane metabolites was unchanged. Our data suggest that ETA receptor activation contributes to PGE2 production in diabetic rats. ET-1 can regulate the renal production of PGE2 by influencing the expression of the enzymes responsible for the synthesis of PGE2 in the kidney, namely COX-2 and PGE synthase. Although activation of ETA receptors results in stimulation of COX-2 to produce PGE2 in renal mesangial cells (42), our results suggest that the decrease in PGE2 excretion during ETA receptor blockade in diabetes is not due to a reduction of COX-2 expression in the renal cortex. Alternatively, macrophages can release PGE2 in response to inflammatory signals (43–44) and can also stimulate PGE2 release from neighboring fibroblasts (45). Therefore, it is possible that blockade of ETA receptors could reduce PGE2 production in diabetic rats by reducing inflammation and macrophage recruitment in the kidney.
Our finding that blood pressure and heart rate are decreased in HG rats compared to Sham rats is consistent with previous studies in STZ-induced diabetic rats (46–48). These changes may reflect the decline in cardiac function that are arises during this stage of the disease (49). In contrast, some groups have reported no change (50–51) or an increase in blood pressure in diabetic rats (52). It is possible that the discrepancies in these studies may be due to differences in the severity or duration of diabetes, use of anesthetics, or method of blood pressure measurement. In any case, the diabetes-induced alterations in these cardiovascular parameters in the present study were unaffected by ABT-627 and, thus, apparently arise independent of ETA receptor activation.
Based on findings that ETA receptor activation stimulates NADPH oxidase to produce superoxide (10,13,53) and that the NADPH oxidase inhibitor apocynin prevents proteinuria and reduces renal injury in diabetes (8), we proposed that blockade of ETA receptors would reduce oxidative stress in the kidney of diabetic rats and therefore reduce renal injury. To address this postulate, we measured lipid peroxidation, hydrogen peroxide excretion, DNA damage, and expression of NADPH oxidase subunits as indices of oxidative stress. Although widely used and accepted, these are indirect measures of reactive oxygen species production that do not reveal the sites of reactive oxygen species production or action and have significant limitations. The TBARS assay may overestimate MDA levels in the urine due to cross-reactivity with other aldehydes, sugars, and amino acids (54). It is not clear whether urinary excretion of MDA, hydrogen peroxide, and 8-OHdG reflects renal or systemic production of reactive oxygen species. Thus, oxidative stress in the renal cortex was targeted by immunostaining for 8-OHdG (an indicator of oxidative DNA damage) and Western blot analysis of NADPH oxidase subunits in renal cortical homogenates. While it is possible that ETA receptor blockade had localized effects that were not detected by these methods, our data revealed no effect of ABT-627 on these systemic or renal measures of oxidative stress in STZ-induced diabetic rats, even though it attenuated the development of albuminuria. These observations refute the postulate that oxidative stress results from ETA receptor activation in diabetes. Although ETA receptor blockade reduces oxidative stress in ET-1-dependent models of hypertension, it may not be effective in diabetes because hyperglycemia can generate reactive oxygen species through multiple pathways (55). Alternatively, it is possible that ET-1 enhances oxidative stress via activation of ETB receptors in the diabetic rat. ET-1 stimulates the production of reactive oxygen species in endothelial cells (56) and in the rat aorta (53) via an ETB receptor dependent mechanism. We also cannot exclude the possibility that hyperglycemia-induced renal oxidative stress in diabetes provides the stimulus for increased ET-1 production. Indeed, studies have shown that superoxide and hydrogen peroxide can increase ET-1 production in human mesangial cells and diabetic rat glomeruli (57–58).
In conclusion, most studies agree that the effects of ET-1 include remodeling, vasoconstriction, vascular smooth muscle cell proliferation, glomerulosclerosis, and induction of cytokine expression. Excessive ET-1 production or ETA receptor activation in the kidney of diabetic rats contributes to the development of diabetic nephropathy; however, the specific sites of ET-1 action in the diabetic kidney and the pathways by which ET-1 induces renal injury have not been fully elucidated. The current study suggests that ET-1 via the ETA receptor may induce renal injury, at least in part, by promoting macrophage infiltration and stimulating production of TGF-β and PGE2. The roles of inflammatory mediators and PGE2 in the regulation of ET-1-induced diabetic renal injury remain to be investigated. By identifying signaling pathways necessary for the pathological effects of ET-1, we can gain a better understanding of the development of diabetic nephropathy.
Acknowledgments
The authors thank Hiram Ocasio, Jacqueline Musall, and Heather Walker for expert technical assistance. This work was supported by a Predoctoral Fellowship from the PhRMA Foundation (to J.M.S.) and grants from the National Institutes of Health (DK 063416 to P.K.C. and J.S.P., HL 60653 to J.SP. and HL 74167 to D.M.P.). J.S. Pollock and D.M. Pollock are Established Investigators of the American Heart Association.
References
- 1.Ding SS, Qiu C, Hess P, Xi JF, Zheng N, Clozel M. Chronic endothelin receptor blockade prevents both early hyperfiltration and late overt diabetic nephropathy in the rat. J Cardiovasc Pharmacol. 2003;42:48–54. doi: 10.1097/00005344-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Cosenzi A, Bernobich E, Trevisan R, Milutinovic N, Borri A, Bellini G. Nephroprotective effect of bosentan in diabetic rats. J Cardiovasc Pharmacol. 2003;42:752–756. doi: 10.1097/00005344-200312000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Hocher B, Schwarz A, Reinbacher D, Jacobi J, Lun A, Priem F, Bauer C, Neumayer HH, Raschack M. Effects of endothelin receptor antagonists on the progression of diabetic nephropathy. Nephron. 2001;87:161–169. doi: 10.1159/000045906. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Garre D, Ruiz-Ortega M, Ortego M, Largo R, Lopez-Armada MJ, Plaza JJ, Gonzalez E, Egido J. Effects and interactions of endothelin-1 and angiotensin II on matrix protein expression and synthesis and mesangial cell growth. Hypertension. 1996;27:885–892. doi: 10.1161/01.hyp.27.4.885. [DOI] [PubMed] [Google Scholar]
- 5.Sorokin A, Kohan DE. Physiology and pathology of endothelin-1 in renal mesangium. Am J Physiol Renal Physiol. 2003;285:F579–F589. doi: 10.1152/ajprenal.00019.2003. [DOI] [PubMed] [Google Scholar]
- 6.Son SM, Whalin MK, Harrison DG, Taylor WR, Griendling KK. Oxidative stress and diabetic vascular complications. Curr Diab Rep. 2004;4:247–252. doi: 10.1007/s11892-004-0075-8. [DOI] [PubMed] [Google Scholar]
- 7.Ishii N, Patel KP, Lane PH, Taylor T, Bian K, Murad F, Pollock JS, Carmines PK. Nitric oxide synthesis and oxidative stress in the renal cortex of rats with diabetes mellitus. J Am Soc Nephrol. 2001;12:1630–1639. doi: 10.1681/ASN.V1281630. [DOI] [PubMed] [Google Scholar]
- 8.Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int. 2005;67:1890–1898. doi: 10.1111/j.1523-1755.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 9.Modlinger PS, Wilcox CS, Aslam S. Nitric oxide, oxidative stress, and progression of chronic renal failure. Semin Nephrol. 2004;24:354–365. doi: 10.1016/j.semnephrol.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Fink GD, Watts SW, Northcott CA, Galligan JJ, Pagano PJ, Chen AF. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation. 2003;107:1053–105. doi: 10.1161/01.cir.0000051459.74466.46. [DOI] [PubMed] [Google Scholar]
- 11.Callera GE, Touyz RM, Teixeira SA, Muscara MN, Carvalho MH, Fortes ZB, Nigro D, Schiffrin EL, Tostes RC. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension. 2003;42:811–817. doi: 10.1161/01.HYP.0000088363.65943.6C. [DOI] [PubMed] [Google Scholar]
- 12.Elmarakby AA, Loomis ED, Pollock JS, Pollock DM. ETA Receptor Blockade Attenuates Hypertension and Decreases Reactive Oxygen Species in ETB Receptor-Deficient Rats. J Cardiovasc Pharmacol. 2004;44:S7–S10. doi: 10.1097/01.fjc.0000166205.66555.40. [DOI] [PubMed] [Google Scholar]
- 13.Sedeek MH, Llinas MT, Drummond H, Fortepiani L, Abram SR, Alexander BT, Reckelhoff JF, Granger JP. Role of reactive oxygen species in endothelin-induced hypertension. Hypertension. 2003;42:806–810. doi: 10.1161/01.HYP.0000084372.91932.BA. [DOI] [PubMed] [Google Scholar]
- 14.Elmarakby AA, Loomis ED, Pollock JS, Pollock DM. NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1. Hypertension. 2005;45:283–287. doi: 10.1161/01.HYP.0000153051.56460.6a. [DOI] [PubMed] [Google Scholar]
- 15.Chow FY, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in streptozotocin-induced diabetic nephropathy: potential role in renal fibrosis. Nephrol Dial Transplant. 2004;19:2987–2996. doi: 10.1093/ndt/gfh441. [DOI] [PubMed] [Google Scholar]
- 16.Sassy-Prigent C, Heudes D, Mandet C, Belair MF, Michel O, Perdereau B, Bariety J, Bruneval P. Early glomerular macrophage recruitment in streptozotocin-induced diabetic rats. Diabetes. 2000;49:466–475. doi: 10.2337/diabetes.49.3.466. [DOI] [PubMed] [Google Scholar]
- 17.Atkins RC. Macrophages in renal injury. Am J Kidney Dis. 1998;31:xiv–xiv11. doi: 10.1016/s0272-6386(14)70003-4. [DOI] [PubMed] [Google Scholar]
- 18.Ziyadeh FN. Mediators of Diabetic Renal Disease: The Case for TGF-β as the Major Mediator. J Am Soc Nephrol. 2004;15:S55–S57. doi: 10.1097/01.asn.0000093460.24823.5b. [DOI] [PubMed] [Google Scholar]
- 19.Ziyadeh FN. Evidence for the involvement of transforming growth factor-β in thepathogenesis of diabetic kidney disease: Are Koch’s postulates fulfilled? Curr Pract Med. 1998;1:87–89. [Google Scholar]
- 20.Reeves WB, Andreoli TE. Transforming growth factor beta contributes to progressive diabetic nephropathy. Proc Natl Acad Sci U S A. 2000;97:7667–7669. doi: 10.1073/pnas.97.14.7667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura T, Fukui M, Ebihara I, Osada S, Nagaoka I, Tomino Y, Koide H. mRNA expression of growth factors in glomeruli from diabetic rats. Diabetes. 1993;42:450–456. doi: 10.2337/diab.42.3.450. [DOI] [PubMed] [Google Scholar]
- 22.Hill C, Flyvbjerg A, Gronbaek H, Petrik J, Hill DJ, Thomas CR, Sheppard MC, Logan A. The renal expression of transforming growth factor-beta isoforms and their receptors in acute and chronic experimental diabetes in rats. Endocrinology. 2000;141:1196–1208. doi: 10.1210/endo.141.3.7359. [DOI] [PubMed] [Google Scholar]
- 23.Johnston CI, Risvanis J, Naitoh M, Tikkanen I. Mechanism of progression of renal disease. J Hypertens. 1998;16:S3–S7. [PubMed] [Google Scholar]
- 24.Touyz RM, Turgeon A, Schiffrin EL. Endothelin A receptor blockade improves renal function and doubles the life span of stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2000;35:S300–S304. doi: 10.1097/00005344-200036051-00088. [DOI] [PubMed] [Google Scholar]
- 25.Tostes RC, Touyz RM, He G, Chen X, Schiffrin EL. Contribution of endothelin-1 to renal activator protein-1 activation and macrophage infiltration in aldosterone-induced hypertension. Clin Sci. 2002;103:25S–30S. doi: 10.1042/CS103S025S. [DOI] [PubMed] [Google Scholar]
- 26.Suga S, Yasui N, Yoshihara F, Horio T, Kawano Y, Kangawa K, Johnson RJ. Endothelin A receptor blockade and endothelin B receptor blockade improve hypokalemic nephropathy by different mechanisms. J Am Soc Nephrol. 2003;14:397–406. doi: 10.1097/01.asn.0000046062.85721.ac. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura T, Ebihara I, Fukui M, Tomino Y, Koide H. Effect of a specific endothelin receptor A antagonist on mRNA levels for extracellular matrix components and growth factors in diabetic glomeruli. Diabetes. 1995;44:895–899. doi: 10.2337/diab.44.8.895. [DOI] [PubMed] [Google Scholar]
- 28.Winn M, Von Geldern TW, Opgenorth TJ, Jae HS, Tasker AS, Boyd SA, Kester JA, Mantei RA, Bal R, Sorensen BK, Wu-Wong JR, Chiou WJ, Dixon DB, Novosad EI, Hernandez L, Marsh KC. 2,4-Diarylpyrrolidine-3-carboxylic acids: potent ETA selective endothelin receptor antagonists. 1. Discovery of A-127722. J Med Chem. 1996;39:1039–1048. doi: 10.1021/jm9505369. [DOI] [PubMed] [Google Scholar]
- 29.Sweat F, Puchtler H, Rosenthal SI. Sirius red F3BA as a stain for connective tissue. Arch Pathol. 1964;78:69–72. [PubMed] [Google Scholar]
- 30.Allcock GH, Venema RC, Pollock DM. ETA receptor blockade attenuates the hypertension but not renal dysfunction in DOCA-salt rats. Am J Physiol Regulatory Integrative Comp Physiol. 1998;275:R245–252. doi: 10.1152/ajpregu.1998.275.1.R245. [DOI] [PubMed] [Google Scholar]
- 31.Patrono C, Ciabattoni G, Pugliese F, Pierucci A, Blair IA, FitzGerald GA. Estimated rate of thromboxane secretion into the circulation of normal humans. J Clin Invest. 1986;77:590–594. doi: 10.1172/JCI112341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hocher B, Schwarz A, Slowinski T, Bachmann S, Pfeilschifter J, Neumayer HH, Bauer C. In-vivo interaction of nitric oxide and endothelin. J Hypertens. 2004;22:111–119. doi: 10.1097/00004872-200401000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Fabris B, Candido R, Carraro M, Fior F, Artero M, Zennaro C, Cattin MR, Fiorotto A, Bortoletto M, Millevoi C, Bardelli M, Faccini L, Carretta R. Modulation of incipient glomerular lesions in experimental diabetic nephropathy by hypotensive and subhypotensive dosages of an ACE inhibitor. Diabetes. 2001;50:2619–2624. doi: 10.2337/diabetes.50.11.2619. [DOI] [PubMed] [Google Scholar]
- 34.Tone A, Shikata K, Sasaki M, Ohga S, Yozai K, Nishishita S, Usui H, Nagase R, Ogawa D, Okada S, Shikata Y, Wada J, Makino H. Erythromycin ameliorates renal injury via anti-inflammatory effects in experimental diabetic rats. Diabetologia. 2005;48:2402–2411. doi: 10.1007/s00125-005-1945-6. [DOI] [PubMed] [Google Scholar]
- 35.Kalender B, Ozturk M, Tuncdemir M, Uysal O, Dagistanli FK, Yegenaga I, Erek E. Renoprotective effects of valsartan and enalapril in STZ-induced diabetes in rats. Acta Histochem. 2002;104:123–130. doi: 10.1078/0065-1281-00643. [DOI] [PubMed] [Google Scholar]
- 36.Anjaneyulu M, Chopra K. Diltiazem attenuates oxidative stress in diabetic rats. Ren Fail. 2005;27:335–344. [PubMed] [Google Scholar]
- 37.Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol. 2006;17:368–377. doi: 10.1681/ASN.2005080859. [DOI] [PubMed] [Google Scholar]
- 38.Breyer MD, Jacobson HR, Breyer RM. Functional and molecular aspects of renal prostaglandin receptors. J Am Soc Nephrol. 1996;7:8–17. doi: 10.1681/ASN.V718. [DOI] [PubMed] [Google Scholar]
- 39.Cheng HF, Wang CJ, Moeckel GW, Zhang MZ, McKanna JA, Harris RC. Cyclooxygenase-2 inhibitor blocks expression of mediators of renal injury in a model of diabetes and hypertension. Kidney Int. 2002;62(3):929–939. doi: 10.1046/j.1523-1755.2002.00520.x. [DOI] [PubMed] [Google Scholar]
- 40.Makino H, Tanaka I, Mukoyama M, Sugawara A, Mori K, Muro S, Suganami T, Yahata K, Ishibashi R, Ohuchida S, Maruyama T, Narumiya S, Nakao K. Prevention of diabetic nephropathy in rats by prostaglandin E receptor EP1-selective antagonist. J Am Soc Nephrol. 2002;13:1757–1765. doi: 10.1097/01.asn.0000019782.37851.bf. [DOI] [PubMed] [Google Scholar]
- 41.Suganami T, Mori K, Tanaka I, Mukoyama M, Sugawara A, Makino H, Muro S, Yahata K, Ohuchida S, Maruyama T, Narumiya S, Nakao K. Role of prostaglandin E receptor EP1 subtype in the development of renal injury in genetically hypertensive rats. Hypertension. 2003;42:1183–1190. doi: 10.1161/01.HYP.0000101689.64849.97. [DOI] [PubMed] [Google Scholar]
- 42.Hughes AK, Padilla E, Kutchera WA, Michael JR, Kohan DE. Endothelin-1 induction of cyclooxygenase-2 expression in rat mesangial cells. Kidney Int. 1995;47:53–61. doi: 10.1038/ki.1995.6. [DOI] [PubMed] [Google Scholar]
- 43.Lazarus M, Kubata BK, Eguchi N, Fujitani Y, Urade Y, Hayaishi O. Biochemical characterization of mouse microsomal prostaglandin E synthase-1 and its colocalization with cyclooxygenase-2 in peritoneal macrophages. Arch Biochem Biophys. 2002;397:336–341. doi: 10.1006/abbi.2001.2614. [DOI] [PubMed] [Google Scholar]
- 44.Naraba H, Murakami M, Matsumoto H, Shimbara S, Ueno A, Kudo I, Ohishi S. Segregated Coupling of Phospholipases A2, Cyclooxygenases, and Terminal Prostanoid Synthases in Different Phases of Prostanoid Biosynthesis in Rat Peritoneal Macrophages. Journal of Immunol. 1998;160:2974–2982. [PubMed] [Google Scholar]
- 45.Lefkowith JB, Needleman P. Arachidonate metabolism in renal injury. Adv Prostaglandin Thromboxane Leukot Res. 1985;13:121–130. [PubMed] [Google Scholar]
- 46.Nagareddy PR, Xia Z, McNeill JH, MacLeod KM. Increased expression of iNOS is associated with endothelial dysfunction and impaired pressor responsiveness in streptozotocin-induced diabetes. Am J Physiol Heart Circ Physiol. 2005;289:H2144–H2152. doi: 10.1152/ajpheart.00591.2005. [DOI] [PubMed] [Google Scholar]
- 47.Katovich MJ, Hanley K, Strubbe G, Wright BE. Effects of streptozotocin-induced diabetes and insulin treatment on blood pressure in the male rat. Proc Soc Exp Biol Med. 1995;208:300–306. doi: 10.3181/00379727-208-43860. [DOI] [PubMed] [Google Scholar]
- 48.Yu Z, McNeill JH. Blood pressure and heart rate response to vasoactive agents in conscious diabetic rats. Can J Physiol Pharmacol. 1992;70:1542–1548. doi: 10.1139/y92-221. [DOI] [PubMed] [Google Scholar]
- 49.Mihm MJ, Seifert JL, Coyle CM, Bauer JA. Diabetes related cardiomyopathy Time dependent echocardiographic evaluation in an experimental rat model. Life Sciences. 2001;69:527–542. doi: 10.1016/s0024-3205(01)01141-9. [DOI] [PubMed] [Google Scholar]
- 50.Chang KS, Lund DD. Alterations in the baroreceptor reflex control of heart rate in streptozotocin diabetic rats. J Mol Cell Cardiol. 1986;18:617–624. doi: 10.1016/s0022-2828(86)80969-5. [DOI] [PubMed] [Google Scholar]
- 51.Kiff RJ, Gardiner SM, Compton AM, Bennett T. The effects of endothelin-1 and NG-nitro-L-arginine methyl ester on regional haemodynamics in conscious rats with streptozotocin-induced diabetes mellitus. Br J Pharmacol. 1991;103:1321–1326. doi: 10.1111/j.1476-5381.1991.tb09787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katayama S, Lee JB. Hypertension in experimental diabetes mellitus. Renin-prostaglandin interaction. Hypertension. 1985;7:554–561. doi: 10.1161/01.hyp.7.4.554. [DOI] [PubMed] [Google Scholar]
- 53.Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS. Endothelin Mediates Superoxide Production and Vasoconstriction through Activation of NADPH Oxidase and Uncoupled Nitric-Oxide Synthase in the Rat Aorta. J Pharmacol Exp Ther. 2005;315:1058–1064. doi: 10.1124/jpet.105.091728. [DOI] [PubMed] [Google Scholar]
- 54.Meagher EA, FitzGerald GA. Indices of lipid peroxidation in vivo: strengths and limitations. Free Radical Biology and Medicine. 2000;28:1745–1750. doi: 10.1016/s0891-5849(00)00232-x. [DOI] [PubMed] [Google Scholar]
- 55.Brownlee M. The Pathobiology of Diabetic ComplicationsL A Unifylin Mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 56.Dong F, Zhang X, Wold LE, Ren Q, Zhang Z, Ren J. Endothelin-1 enhances oxidative stress, cell proliferation and reduces apoptosis in human umbilical vein endothelial cells: role of ETB receptor, NADPH oxidase and caveolin-1. British J Pharmacol. 2005;145:323–333. doi: 10.1038/sj.bjp.0706193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen HC, Guh JY, Shin SJ, Tsai JH, Lai YH. Reactive oxygen species enhances endothelin-1 production of diabetic rat glomeruli in vitro and in vivo. J Lab Clin Med. 2000;135:309–315. doi: 10.1067/mlc.2000.105616. [DOI] [PubMed] [Google Scholar]
- 58.Hughes AK, Stricklett PK, Padilla E, Kohan DE. Effect of reactive oxygen species on endothelin-1 production by human mesangial cells. Kidney Int. 1996;49:181–189. doi: 10.1038/ki.1996.25. [DOI] [PubMed] [Google Scholar]