Abstract
Studies were conducted to determine the possibility that voluntary exercise could enhance regenerative effects of gene therapy via Schwann cells (SC) over-expressing FGF-2. Sedentary or exercise rehabilitation conditions were therefore provided shortly after reconstructing 10 mm sciatic nerve gaps in rats with silicone grafts. Exercise for 7 days elevated mRNA levels of regeneration associated proteins (GAP-43 and synapsin I) in lumbar spinal cord and dorsal root ganglia of SC transplanted, in contrast to non-cellular reconstructed rats. FGF-2 gene therapy followed by 25–27 days of exercise did enhance regeneration of myelinated axons in comparison to sedentary animals. Four weeks after surgery mRNA levels of regeneration associated proteins were significantly higher in lumbar spinal cord of running compared to sedentary SC transplanted animals. Our results suggest that voluntary exercise could reinforce the beneficial effects of SC transplantation and FGF-2 gene therapy in peripheral nerve reconstruction approaches.
Keywords: Ex vivo gene therapy, Schwann cells, peripheral nerve regeneration, transplantation
Introduction
Peripheral nerve injury is a common burden associated with many types of domestic and work accidents. The peripheral nervous system (PNS), displaying an intrinsic regenerative capacity, provides the opportunity for implementation of therapeutics to enhance healing. Schwann cells (SC), the myelinating glia cells of the PNS, are crucially involved in the recovery of injured peripheral nerves. However, regeneration often fails where long gaps have to be bridged. A promising therapeutic approach to promote nerve regeneration is interposition of artificial nerve grafts, bio-engineered to combine synthetic guidance channels and regeneration-promoting cells [10].
Fibroblast growth factor-2 (FGF-2) shows regeneration-promoting properties when released from synthetic nerve grafts [4]. Studies on manipulations of the FGF-2 gene system in mice support a physiological role of FGF-2 in PNS regeneration and suggest putative therapeutic applications [7].
FGF-2 is expressed in 18kD, 21kD, and 23kD isoforms in the rat [3]. In previous studies we found transplanted genetically modified SC over-expressing high molecular weight-FGF-2 (FGF-221/23kD) sufficient to promote regeneration of myelinated axons across long distances [9, 15]. FGF-221/23kD gene therapy has been shown to support early recovery of sensory functions whereas transplantation of SC over-expressing FGF-218kD did influence motor recovery [9]. Although gene therapy is an innovative approach in peripheral nerve repair [8], transplanting genetically modified SC alone has so far not promoted sufficient functional motor recovery. Accordingly, we have sought the use of combined approaches to enhance the outcome of gene therapy.
There is evidence that exercise has an impact on peripheral axon regeneration after crush injury [12]. We hypothesized that axonal regeneration across nerve gaps and mechanisms related to reestablishment of functional innervation could be enhanced by a combination of SC transplantation with running exercise in a nerve gap repair approach. To test this hypothesis, we bridged 10 mm gaps in transected adult rat sciatic nerves with silicone nerve guides. Silicone grafts were either filled with extracellular matrix (non-cellular transplant), non-transfected SC (SC transplant), or genetically modified SC over-expressing FGF-2 isoforms (SC transplant for gene therapy). During rehabilitation, animals were randomly exposed to sedentary or voluntary exercise housing conditions.
The main objective of this study was to determine whether the combination of FGF-2 gene therapy via transplanted SC and voluntary exercise during rehabilitation could promote axonal regeneration across peripheral nerve gaps. We evaluated mRNA levels of regeneration-associated proteins in lumbar spinal cord and dorsal root ganglia. In particular, we measured levels of the growth cone associated protein GAP-43 to have an idea of axonal growth and the synaptic vesicle protein synapsin I (SYN-I) to estimate of changes in synaptic plasticity.
Materials and methods
Preparation of transfected cells and synthetic nerve grafts
Primary SC were prepared and genetically modified as described above [9, 15]. The sciatic nerves of neonatal Sprague-Dawley rats were dissected. After enzymatic dissociation and primary seeding, treatment with arabinoside C (1mM) for 2 days and incubation with α-Thy1 antibody-coupled magnetic beads (Dynabeads, Dynal, Denmark) removed contaminating fibroblast, resulting in about 99% pure SC cultures.
Over-expression of FGF-2-isoforms was induced by SC transfection using MetafecteneTM (Biontex, Germany) and plasmid constructs with FGF-218kD or FGF-221/23kD in pCI-neo. Transfected SC were positively selected for their co-expressed resistance to Geneticin (G418). Successful transfection/FGF-2 over-expression was monitored in Western blot analysis [9].
For transplantation, transfected cells were withdrawn from serum and appropriate cell numbers (end concentration: 114 × 105 cells/µl) were prepared and stored on ice. Directly prior to implantation, SC were re-suspended in growth factor reduced Matrigel (Becton Dickinson, Germany) and injected in sterile silicone nerve grafts (13 mm length, 1.5 mm diameter, 0.4 mm wall thickness; VWR International, Germany). Each animal received approximately 1.7 × 106 cells. For non-cellular conditions, grafts were solely filled with Matrigel.
Nerve graft transplantation
72 adult female Sprague-Dawley rats (180 g; Charles River, USA) were individually housed in a 12/12h light/dark vivarium with food and water ad lib. Animals were anaesthetized with Isoflurane (2–2.5%, Mobile Laboratory Animal Anesthesia System, USA). Body temperature during anesthesia was stabilized by placing the rats on an electric warming pad. Under aseptic conditions, the animals` left sciatic nerves were exposed by skin incision along the femur and separation of biceps femoris and superficial gluteal muscles. Nerves were transected at mid-thigh. The proximal nerve end was sutured (9/0 Ethilon®II, Ethicon, USA) 2 mm and the distal nerve end 1 mm inside the lumen of the silicone tube so to leave a 10 mm gap between the stumps. Muscle layers (4/0 resorbable suture, Ethicon) and skin (3/0 non-resorbable suture, Ethicon) were sutured. Transplantation experiments were performed on consecutive days in order to pool sufficient animal numbers/group.
Exercise paradigm and animal groups
Three days after surgery, rats were randomly assigned to single housing in either sedentary control groups (n=6/nerve graft type, Tab.1) without access to a running wheel or voluntary exercise groups (n=6/nerve graft type, Tab.1). The exercise apparatus consisted of a common polyethylene rat cage (30×40×20cm) equipped with a running wheel (diameter 31.8 cm, width 10 cm) that rotated on the shaft against a 100g-resistance in either direction. Wheel revolutions were recorded hourly (Vitalviewer Data Acquisition System Software, Minimitter Company Inc., USA). Sciatic nerve injured animals are capable of running [12]. Animals did voluntary exercise until the morning of sacrifice; there was no significant difference in running activity between groups. Running distances [km] during initial 24h after onset of exercise are exemplarily listed in Tab.1.
Table 1.
Overview of animal groups and periods and intensity of exercise.
| Experimental paradigm | Type of nerve transplant | Number of animals | Running distance [km] during initial 24 h [Mean ± SEM] |
|---|---|---|---|
| A | Non-cellular: | ||
| Matrigel w/o cells | n = 12 | ||
| Cell transplantation | (Sedentary:6, Exercise:6) | 1.94 ± 0.38 km | |
| + | SC transplant: | ||
| 7 days of exercise | SCphysiol | n = 12 | 2.26 ± 0.35 km |
| (Sedentary:6, Exercise:6) | |||
| B | SC transplant: | ||
| SCphysiol | n = 12 | ||
| Cell transplantation | (Sedentary:6, Exercise:6) | 1.92 ± 0.42 km | |
| + | SCvector alone | n = 12 | |
| FGF-2 gene therapy | (Sedentary:6, Exercise:6) | 1.15 ± 0.14 km | |
| + | SC-FGF-218kD | n = 12 | |
| 25–27days of | (Sedentary:6, Exercise:6) | 1.56 ± 0.35 km | |
| exercise | SC-FGF-221/23kD | n = 12 | |
| (Sedentary:6, Exercise:6) | 2.04 ± 0.18 km | ||
Experiments were carried out in accordance to NIH Guide for Care and Use of Laboratory Animals. Table 1 summarizes how two experimental paradigms were combined with two rehabilitation periods: (A) a short term of voluntary exercise (7d) was used to evaluate the effects of SC transplantation combined with exercise on regulation of regeneration-associated proteins (Tab.1A). (B) mid term of 25–27d exercise was used to test the impact of FGF-2 gene therapy followed by exercise on regulation of regeneration-associated proteins as well as axonal regeneration (Tab.1B).
Nerve grafts were differentially filled in order to build experimental subgroups: (1) Matrigel w/o cells (non-cellular control, n=12 animals, experimental paradigm A, Tab.1A) or SC resuspended in Matrigel as follows: (2) non-transfected, SCphysiol (n=12, each for experimental paradigm A, Tab.1A, and B, Tab.1B), (3) empty-vector transfected, SCvector alone (n=12, experimental paradigm B, Tab.1B) or SC genetically modified to over-express FGF-2 isoforms (4) SC-FGF-218kD (n=12, experimental paradigm B, Tab.1B) or (5) SC-FGF-221/23kD (n=12, experimental paradigm B, Tab.1B).
Evaluation of mRNA levels of regeneration associated proteins – Taqman RT-PCR
All rats were sacrificed by decapitation around 8 AM. The entire spinal cord and L4–L6 dorsal root ganglia (DRG) were rapidly dissected. Subsequently, the lumbar spinal enlargement was identified, separated, frozen on dry ice and stored at −70°C. The side ipisilateral to surgery was used for biochemical analyses. The RNA STAT-60 kit (TEL-TEST, USA) was used for total RNA extraction. The mRNAs for GAP-43 and SYN-I were measured by TaqMan real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, USA). The technique is based on the ability to directly detect the RT-PCR product with no downstream processing. This is accomplished with monitoring the increase in fluorescence of a dye-labeled DNA probe specific for each factor under study plus a probe specific for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene used as endogenous control. Total RNA (100ng) was converted into cDNA using TaqMan EZ RT-PCR Core reagents (Applied Biosystems). The sequences of probes, forward and reverse primers (Integrated DNA Technologies, USA) were GAP-43: probe 5′-CTCATAAGGCTGCAACCAAAATTCAGGCT-3′, forward primer 5′-GATGGTGTCAAACCGGAGGAT-3′, reverse primer 5′-CTTGTTATGTGTCCACGGAAGC-3′ and SYN-I: probe 5′-CATGGCACGTAATGGAGACTACCGCA-3′, forward primer 5′-CCGCCAGCTGCCTTC-3′, reverse primer 5′-TGCAGCCCAATGACCAAA-3′. The RT reaction conditions were: initial step to activate uracil glycosylase (UNG, 2min at 50°C), reverse transcription (30min at 60°C) and completion by UNG deactivation (95°C, 5min). The 40 cycles of the two-step PCR reaction conditions were 20s at 94°C and 1min at 62°C.
Processing of regenerated tissue for morphometrical analysis
Silicone grafts together with eventually incorporated regenerated tissue were explanted after sacrifice of animals by end of experimental paradigm B (cell transplantation +/− gene therapy + sedentary or exercise, Tab.1B). Nerve grafts were fixed in fixative according to Karnovsky (2% paraformaldehyde, 2.5% glutaraldehyde in 0.2 M sodium cacodylat buffer, pH 7.3) for 24h. Afterwards, tubes were longitudinally opened, and the tissue cables bridging the nerve gap were removed and rinsed three times with 0.1 M sodium cacodylat buffer containing 7.5% saccharose. Postfixation was performed in 1% OsO4 for 1.5h. Staining of myelin sheaths was done as described above [9, 15] in 1% potassium dichromate (24h) followed by 25% ethanol (24h) and incubation in hematoxylin (0.5% in 70% Ethanol, 24h). After dehydration, tissue was epon embedded. Semi-thin (1µm) transverse sections were cut at mid-tube level and additionally stained with toluidine blue. Sections were observed under light microscopy (BX60, Leica, Germany) at 80x magnification. The total number of regenerated myelinated axons on the entire cross sectional area was determined. Additionally, ultra-structures were analyzed using an EM 9 2S electron microscope (Zeiss, Germany) with regard to unmyelinated axons.
Statistical analysis
GraphPad InStat Version 3.0 software (GraphPad Software Inc., USA) was used to perform specific statistical tests for specific experiments as mentioned in particular in the Results section.
Results
Experimental paradigm A - SC transplantation plus exercise (Tab.1A)
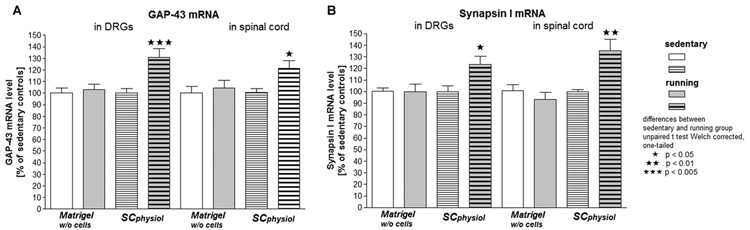
We examined the effects of exercise after nerve gap repair on molecular markers of regeneration. In the Matrigel w/o cells group, 7d of exercise did not affect GAP-43 (Fig.1A) and SYN-I (Fig.1B) mRNA levels, in neither ipsilateral L4–L6 DRGs nor ipsilateral lumbar spinal cord region. In contrast, after SCphysiol transplantation, GAP-43 and SYN-I mRNA levels were significantly increased in animals after 7d of exercise compared to sedentary animals (DRG: p<0.005 for GAP-43, p<0.05 for SYN-I; lumbar spinal cord: p<0.05 for Gap-43, p<0.01 for SYN-I; statistical test: unpaired t-test Welch corrected).
Figure 1.
mRNA levels (mean±SEM ) of GAP-43 (A) and synapsin I (B) in ipsilateral L4–L6 dorsal root ganglia (DRG) and lumbar spinal cord segments of rats with Matrigel w/o cells or SCphysiol transplants and housed for 7d sedentary or running.
Experimental paradigm B - SC transplantation and FGF-2 gene therapy plus exercise (Tab.1B)
Experimental paradigm A showed an enhancing effect of short-term exercise on mRNA levels of regeneration-associated proteins after SC transplantation. In experimental paradigm B, the effects of FGF-2 gene therapy plus 25–27d exercise on mRNA levels of GAP43 and SYN-I, macroscopic tissue regeneration and axonal regeneration (number of myelinated axons) were evaluated. Silicone nerve grafts were filled with non-transfected SC (SCphysiol), SC transfected with empty vector (SCvector alone), or SC over-expressing FGF-2 isoforms, SC-FGF-218kD or SC-FGF-221/23kD (Tab.1B).
mRNA levels of regeneration associated proteins
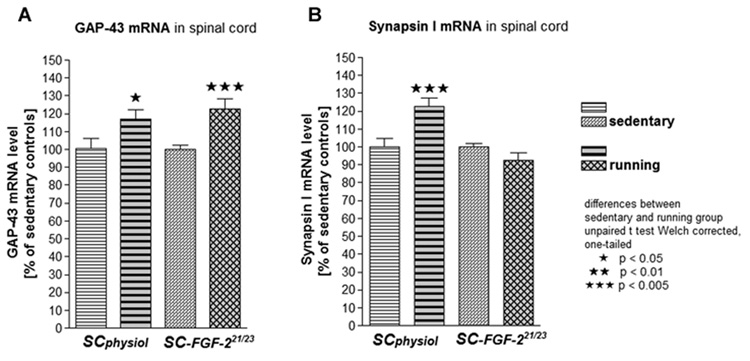
Ipsilateral lumbar spinal cord enlargement was analyzed in SCphysiol and SC-FGF-221/23kD groups, which demonstrated the most interesting results in axonal regeneration (see below). GAP-43 mRNA levels were significantly higher in excercising animals than sedentary animals in both conditions (Fig.2A). Exercise did increase mRNA levels of GAP43 by 17% in SCphysiol animals (p<0.05) and by 22.8% in SC-FGF-221/23kD animals (p<0.005). In SCphysiol animals, exercise significantly increased SYN-I mRNA levels as compared to sedentary conditions (p<0.005, unpaired t-test Welch corrected; Fig.2B).
Figure 2.
mRNA levels (mean±SEM) of GAP-43 (A) and synapsin I (B) in ipsilateral lumbar spinal cord after SC transplantation and FGF-221/23kD gene therapy in sedentary or running rehabilitation for approx. 4 weeks after surgery.
Macroscopic tissue regeneration
In the SCphysiol group, 3 of 6 animals in both housing conditions showed regenerated tissue bridging the 10 mm nerve gaps. Exercise resulted in a marginal difference in gap bridging tissue regeneration in 3 of 6 running animals as compared to 2 of 6 in sedentary SCvector alone animals. Running SC-FGF-218kD animals displayed gap-bridging tissue in 4 of 6 animals, while no tissue regeneration was found in sedentary animals. Transplantation of SC-FGF-221/23kD resulted in the best overall tissue regeneration rate, with gap-bridging tissue in 5 of 6 sedentary and 4 of 6 running animals.
Axonal regeneration
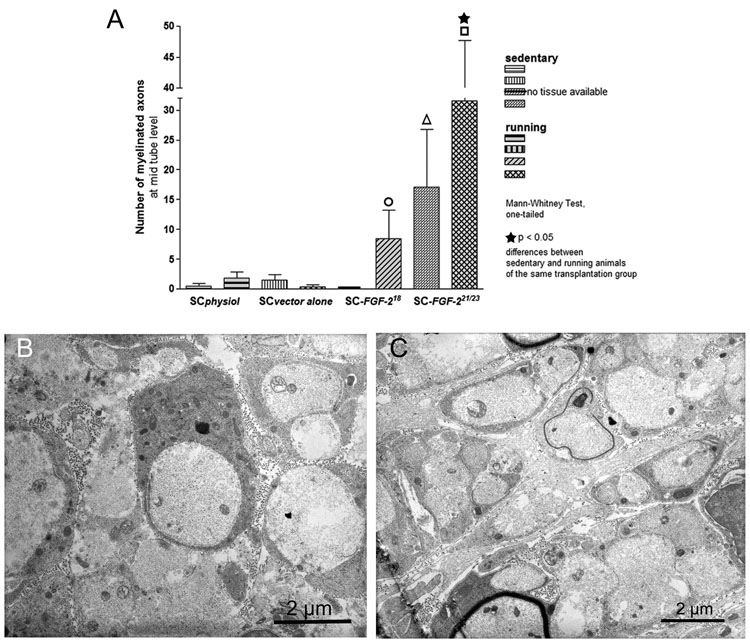
Morphometrical analysis of semi-thin cross sections through gap bridging tissue at mid-tube level (5 mm distal of the proximal nerve end) revealed the number of regenerated myelinated axons. Statistical analysis consisting of a one-tailed P value, was performed by Mann-Whitney Test for all transplants comparing sedentary or running animals group by group. Additionally, for each transplantation group, sedentary and running animals were compared. Sedentary SC-FGF-221/23kD animals displayed a significant higher number of myelinated axons compared to sedentary SCphysiol animals (Fig.3A, p<0.05 indicated by Δ). Exercised SC-FGF-221/23kD animals showed significantly more myelinated axons in comparison to sedentary SC-FGF-221/23kD animals (Fig.3A, p<0.05, indicated by ★). Furthermore, exercise in SC-FGF-221/23kD animals resulted in significant higher numbers of myelinated axons compared to exercise in all other transplantation conditions (Fig.3A, p<0.001–0.005 indicated by □). No tissue was available for myelinated axon counts in sedentary SC-FGF-218kD animals. Exercising SC-FGF-218kD animals displayed significantly more myelinated axons compared to running SCvector alone animals (Fig.3A, p<0.05, indicated by ○).
Figure 3.
(A) Number counts of myelinated axons in cross sections of gap bridging tissue cable at mid-tube level (mean±SEM). SC-FGF-221/23kD group: significant difference between sedentary and running animals marked by ★ (p<0.05); differences to all other running animals indicated by □ (p<0.005); difference of sedentary animals to sedentary SCphysiol animals indicated by Δ (p<0.05). Difference between running SC-FGF-218kD and running SCvector alone animals marked by ○ (p<0.05).
Transmission electron microscopy revealed presence of regenerated unmyelinated axons (B) in running SC-FGF-218kD and (C) sedentary SC-FGF-221/23kD animals.
Suggesting that a 4 weeks regeneration period might be rather short for remyelination of axons regenerating across a nerve gap, transmission electron microscopy demonstrated that unmyelinated axons did regenerate in the chosen observation period in running SC-FGF-218kD animals (Fig.3B) as well as sedentary SC-FGF-221/23kD animals (Fig.3C).
Summary & Discussion
We found elevation of mRNA levels of regeneration associated GAP-43 and SYN-I in the lumbar spinal cord and dorsal root ganglia ipsilateral to a reconstructed peripheral nerve gap. This occurred when running was performed during the early rehabilitation phase after using SC filled silicone grafts for reconstruction. Under non-cellular transplantation conditions no effect of exercise occured. Growth cone activation (GAP-43) and increased synaptic plasticity (SYN-I) of spinal motoneurons and sensory neurons projecting into the proximal nerve stump might be induced by changes in the target tissue. However, our result suggests that the stimulus for growth cone activation and neuronal plasticity could only be transmitted to the growth cones across a peripheral nerve gap in presence of transplanted SC.
Relative to our previous studies where sciatic nerves in sedentary animals were allowed to regenerate for 12 weeks [9], the outcome of gap bridging tissue regeneration was poor in the current SCphysiol, SCvector only and SC-FGF-218kD groups. This apparent discrepancy may be related to the shorter regeneration period currently analyzed. However, results from the SC-FGF-221/23kD group are in accordance to our previous work, demonstrating best macroscopic tissue regeneration after FGF-221/23kD gene therapy.
Focusing on axonal regeneration across the nerve gaps, comparably low numbers of myelinated axons have currently been found after 4 weeks of regeneration as compared to 12 weeks in previous studies. However, an increased regeneration of myelinated axons due to FGF-221/23kD gene therapy is seen in the current as well as previous studies [9, 15]. Furthermore, the current study clearly demonstrates that exercise significantly increased the number of early regenerating myelinated axons after FGF-221/23kD gene therapy (Fig.3A).
The results suggest that exercise is supporting regenerative capacities of the FGF-2 system. It has been shown that physical exercise positively regulates hippocampal FGF-2 expression [5]. Consequently, exercise could support functions of the FGF-2 system, e.g., by activating the FGF-receptor system helping FGF-2 introduced by gene therapy to better interact with regenerating axons crossing the nerve gap. Furthermore, neurotrophic molecules induced by exercise are known to exert positive effects on synaptic plasticity [18]. Exercise is also known to increase neurotrophin expression in the stimulated muscles [6]. FGF-2 could develop symbiotic effects with these molecular changes triggering re-establishment of motor functions in long-term experiments.
Regenerating peripheral axons grow faster when electrically activated [1] or exposed to exercise [13]. Accordingly, exercise has been suggested to display enhanced action on regeneration when axons undergo outgrowth [2]. Our current findings underscore this idea showing that SC transplantation and FGF-2 gene transfer likely increase the susceptibility of axons to exercise. Increase of GAP-43 mRNA levels in spinal cord after 4 weeks of exercise in SC transplanted animals further supports this view. Additionally, FGF-212/23kD gene therapy plus prolonged voluntary exercise resulted in a dramatic increase of spinal GAP-43 mRNA levels relative to gene therapy in sedentary animals. Growth cones of regenerating axons could have been stimulated by the enriched environment produced by exercise stimulated growth assisting SC, high amounts of regeneration promoting FGF-221/23kD delivered by gene therapy and exercise-induced neurotrophic factors/signals [18].
It has been described that 21–28 days of exercise helped functional recovery after peripheral nerve crush injuries [14, 16]. In the current study, exercise induced increase of SYN-I mRNA levels suggest an influence on vesicular trafficking/synaptic vesicle release in regenerating spinal neurons. According to results from gene microarray investigations on exercise related events in rat hippocampus [11], exercise is likely to result in an increased release of signaling and neurotrophic factors in the spinal cord, DRGs, and axonal sprouts. Gene array analyses were not included to the current non-exploratory study, but the demonstrated changes in SYN-1 mRNA levels indicate that rehabilitation exercise could result in changes in synaptic plasticity as needed for consolidation of recovered peripheral nerve function. For transection and peripheral nerve gap reconstruction no satisfactory functional motor recovery is reported so far after a period of 4 weeks independently of any surgical or rehabilitation treatment. In the current study, evaluation of the sciatic function index did also not reveal functional motor recovery (data not shown).
Longer observation periods as well as different rehabilitation exercise regimes still need to be tested. It is important to determine protocols to implement voluntary exercise in conjunction with bio-engineered peripheral nerve reconstruction. The exercise regimes might be important for proper maintenance of the inserted graft. Maintained denervated muscle activity is reported not only to increase and modify trophic factor release from muscle but also potassium channel distribution on regenerating nerve fibers [13]. Therefore it is conceivable that extended intensities or training periods could have negative effects on muscles, as well as on regenerating axons or on maintenance of freshly formed tissue.
Conclusion
We have demonstrated in a proof-of-principle-way that voluntary exercise during early rehabilitation sufficiently supports axonal regeneration across a bio-engineered peripheral nerve gap. This is of high interest given the restricted time frame for muscle reinnervation before atrophy. SC transplantation and FGF-221/23kD gene therapy pave the way for exercise-induced changes. Additionally, growth cone activation and elevated synaptic plasticity due to exercise shown by increased GAP-43 and SYN-I mRNA levels show the promise of reestablishment and consolidation of functional muscle reinnervation. However, the optimal combinations of nerve grafts, neurotrophic factors for gene therapy and rehabilitation training program must be further elucidated.
Acknowledgements
We thank Dr. Ghiani and Dr. de Vellis for permission to work at their cell culture lab facilities, Dr. Vaynmann for discussion and help in animal handling. Thanks to Dr. Claus for providing plasmids used in this study, to Dr. Jungnickel for advices in morphometrical analysis. For excellent technical assistance, we thank N. Heidrich, K. Kuhlemann and M. Wesemann. Financial support: German Research Foundation (research stipend to KH), Kogge-Stiftung (to KH), NIH NS50465 (to FGP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J Neurosci. 2000;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doyle LM, Roberts BL. Exercise enhances axonal growth and functional recovery in the regenerating spinal cord. Neuroscience. 2006;141:321–327. doi: 10.1016/j.neuroscience.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 3.Florkiewicz RZ, Sommer A. Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proc Natl Acad Sci U S A. 1989;86:3978–3981. doi: 10.1073/pnas.86.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujimoto E, Mizoguchi A, Hanada K, Yajima M, Ide C. Basic fibroblast growth factor promotes extension of regenerating axons of peripheral nerve. In vivo experiments using a Schwann cell basal lamina tube model. J Neurocytol. 1997;26:511–528. doi: 10.1023/a:1015410023132. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Pinilla F, Dao L, So V. Physical exercise induces FGF-2 and its mRNA in the hippocampus. Brain Res. 1997;764:1–8. doi: 10.1016/s0006-8993(97)00375-2. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 7.Grothe C, Haastert K, Jungnickel J. Physiological function and putative therapeutic impact of the FGF-2 system in peripheral nerve regeneration--lessons from in vivo studies in mice and rats. Brain Res Brain Res Rev. 2006;51:293–299. doi: 10.1016/j.brainresrev.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Haastert K, Grothe C. Gene therapy in peripheral nerve reconstruction approaches. Curr Gene Ther. 2007;7:221–228. doi: 10.2174/156652307780859035. [DOI] [PubMed] [Google Scholar]
- 9.Haastert K, Lipokatic E, Fischer M, Timmer M, Grothe C. Differentially promoted peripheral nerve regeneration by grafted Schwann cells over-expressing different FGF-2 isoforms. Neurobiol Dis. 2006;21:138–153. doi: 10.1016/j.nbd.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Lundborg G. Alternatives to autologous nerve grafts. Handchir Mikrochir Plast Chir. 2004;36:1–7. doi: 10.1055/s-2004-820870. [DOI] [PubMed] [Google Scholar]
- 11.Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;16:1107–1116. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 12.Molteni R, Zheng JQ, Ying Z, Gomez-Pinilla F, Twiss JL. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci U S A. 2004;101:8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rasband MN, Trimmer JS, Schwarz TL, Levinson SR, Ellisman MH, Schachner M, Shrager P. Potassium channel distribution, clustering, and function in remyelinating rat axons. J Neurosci. 1998;18:36–47. doi: 10.1523/JNEUROSCI.18-01-00036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarikcioglu L, Oguz N. Exercise training and axonal regeneration after sciatic nerve injury. Int J Neurosci. 2001;109:173–177. doi: 10.3109/00207450108986533. [DOI] [PubMed] [Google Scholar]
- 15.Timmer M, Robben S, Muller-Ostermeyer F, Nikkhah G, Grothe C. Axonal regeneration across long gaps in silicone chambers filled with Schwann cells overexpressing high molecular weight FGF-2. Cell Transplant. 2003;12:265–277. doi: 10.3727/000000003108746821. [DOI] [PubMed] [Google Scholar]
- 16.van Meeteren NL, Brakkee JH, Hamers FP, Helders PJ, Gispen WH. Exercise training improves functional recovery and motor nerve conduction velocity after sciatic nerve crush lesion in the rat. Arch Phys Med Rehabil. 1997;78:70–77. doi: 10.1016/s0003-9993(97)90013-7. [DOI] [PubMed] [Google Scholar]
- 17.Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19:283–295. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- 18.Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Voluntary exercise increases neurotrophin-3 and its receptor TrkC in the spinal cord. Brain Res. 2003;987:93–99. doi: 10.1016/s0006-8993(03)03258-x. [DOI] [PubMed] [Google Scholar]