Abstract
Isothiocyanates are found in cruciferous vegetables such as broccoli, Brussels sprouts, cauliflower, and cabbage. Epidemiologic studies suggest that cruciferous vegetable intake may lower overall cancer risk, including colon and prostate cancer. Sulforaphane (SFN) is an isothiocyanate found in cruciferous vegetables and is especially high in broccoli and broccoli sprouts. SFN has proved to be an effective chemoprotective agent in cell culture, carcinogen-induced and genetic animal cancer models, as well as in xenograft models of cancer. Early research focused on the “blocking activity” of SFN via Phase 2 enzyme induction, as well as inhibition of enzymes involved in carcinogen activation, but there has been growing interest in other mechanisms of chemoprotection by SFN. Recent studies suggest that SFN offers protection against tumor development during the “post-initiation” phase and mechanisms for suppression effects of SFN, including cell cycle arrest and apoptosis induction are of particular interest. In humans, a key factor in determining the efficacy of SFN as a chemoprevention agent is gaining an understanding of the metabolism, distribution and bioavailability of SFN and the factors that alter these parameters. This review discusses the established anti-cancer properties of SFN, with an emphasis on the possible chemoprevention mechanisms. The current status of SFN in human clinical trials also is included, with consideration of the chemistry, metabolism, absorption and factors influencing SFN bioavailability.
Keywords: sulforaphane, prostate cancer, colorectal cancer, chemoprevention
1 Introduction
Cancer is the second leading cause of death in the United States. With over 1.4 million people estimated to be diagnosed with cancer in 2007, preventive measures that target the various steps involved in cancer initiation and progression could significantly decrease the incidence and mortality of cancer. In particular, the use of dietary chemoprevention strategies has gained significant interest. Research investigating the use of diet-derived chemoprevention compounds may have significant impact on qualifying or changing recommendations for high-risk cancer patients and thereby increase their survival through simple dietary choices with easily accessible foods. Epidemiologic studies suggest that cruciferous vegetable intake may lower overall cancer risk, including colon and prostate cancer, particularly during the early stages [1; 2]. However, in vitro and in vivo data provide evidence that increasing cruciferous vegetable intake provides protection at every stage of cancer progression. Thus, there is growing interest in identifying the specific chemoprotective constituents in cruciferous vegetables and their mechanisms of action at all stages of cancer.
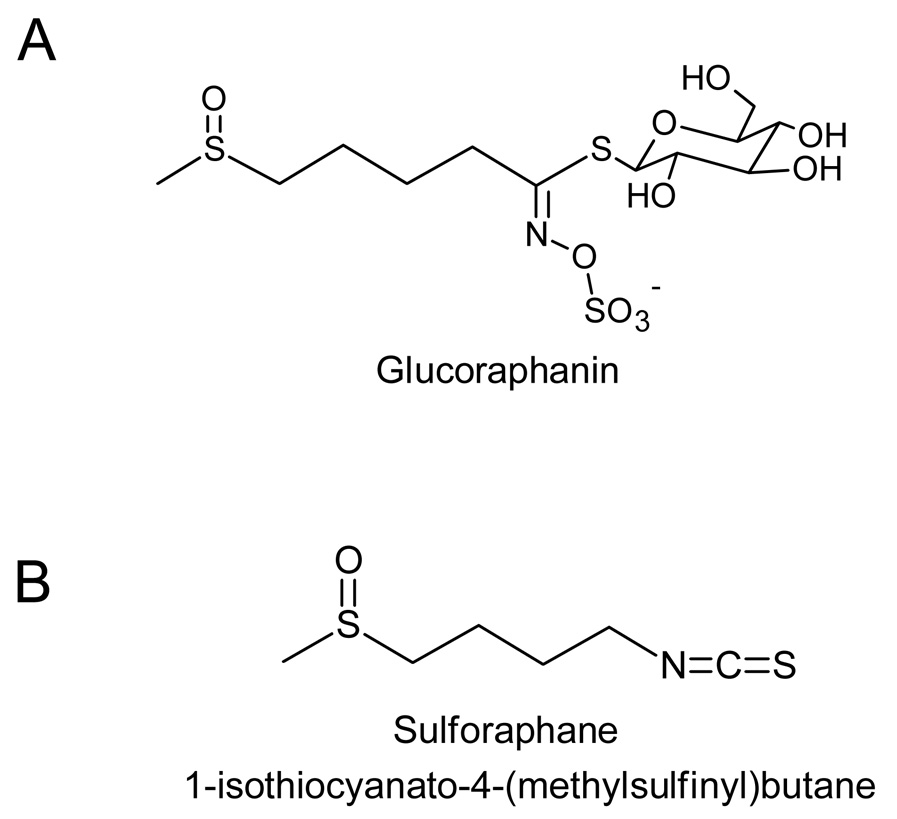
One such family of chemoprotective constituents are isothiocyanates (ITC) which are formed by hydrolysis of their precursor parent compounds glucosinolates. Within the plant, glucosinolate content can vary greatly between and within members of the Cruciferae family depending on cultivation environment and genotype [3] and there are over 120 glucosinolates in the various varieties of cruciferous vegetables, each yielding different aglycone metabolic products including isothiocyanates [4]. The general structure of glucosinolate consists of a β-D-thioglucose group, a sulfonated oxime group, and a variable side chain. Many of the anticancer effects observed from cruciferous vegetables have been attributed to the ITCs rather than their parent glucosinolates. Two important and well studied isothiocyanates derived from cruciferous vegetables are sulforaphane (SFN) and indole-3-carbinol (I3C). The glucosinolate precursor to SFN, glucoraphanin, is abundant in broccoli, cauliflower, cabbage, and kale with the highest concentration found in broccoli and broccoli sprouts [5]. Hydrolysis of glucoraphanin to its aglycone product SFN requires the activity of myrosinase enzymes released from the plant during consumption and other myrosinase enzymes present in our gut. The structures of glucoraphanin and SFN are shown in Figure 1. This review will focus on SFN in cancer development. A more in-depth review of I3C is presented elsewhere [6].
Figure 1.
Structures of glucosinolate precursor (A) glucoraphanin and its isothiocyanate hydrolysis product (B) sulforaphane.
The mechanisms of SFN chemoprevention have been well studied and reveal diverse responses depending upon the stage of carcinogenesis. For another comprehensive review of the possible molecular mechanisms of chemoprevention by SFN, refer to a recent review by Juge et al [7]. SFN can function by blocking initiation via inhibiting phase 1 enzymes that convert procarcinogens to proximate or ultimate carcinogens, and by inducing phase 2 enzymes that detoxify carcinogens and facilitate their excretion from the body. Once cancer is initiated, SFN can act via several mechanisms that modulate cell growth and cell death signals to suppress cancer progression. Prostate and colon cancer are the 1st and 3rd most prevalent cancers in men in the United States, respectively. This review will discuss overall function and metabolism of SFN, with a focus on the mechanisms for chemoprevention of prostate and colorectal cancer development and discuss its capacity to act during both initiation and post-initiation stages. In particular, this review will discuss novel targets of SFN for chemoprevention, including mechanisms of cell cycle arrest, epigenetic regulation and modulation cell signals.
2 Molecular targets/Anticancer properties of Sulforaphane
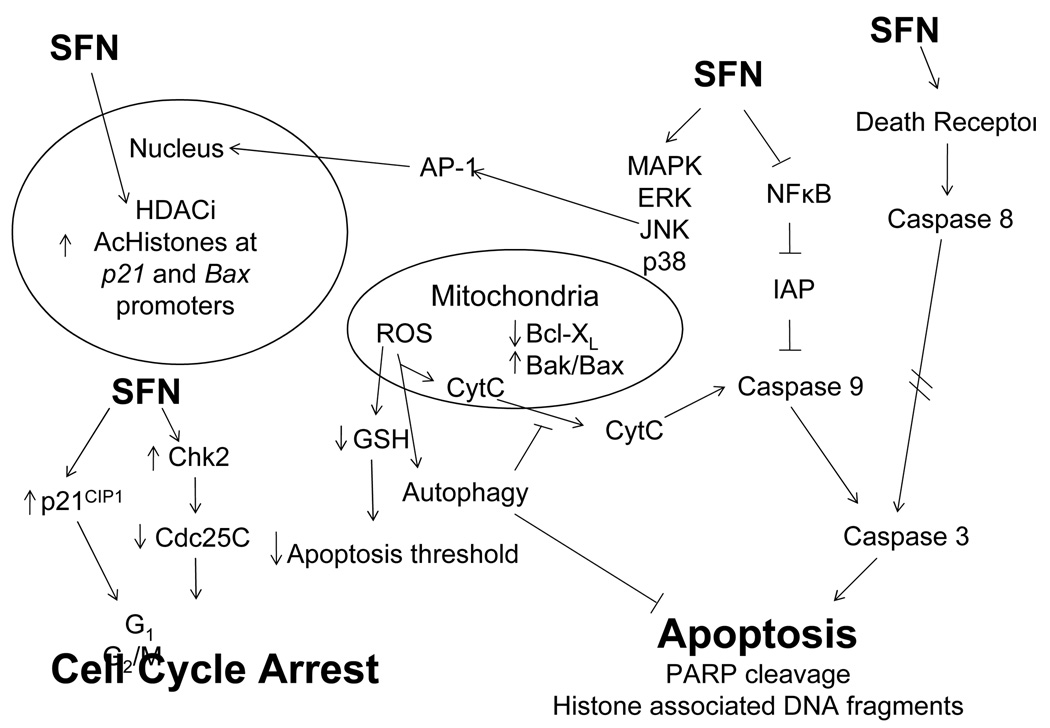
The molecular targets of SFN vary depending upon cancer stage and target tissue. Recent work has clearly implicated multiple targets of SFN action. Since SFN was first identified in 1992 as a potential chemopreventive agent [8], there has been a sharp increase in PubMed citations mentioning SFN. Early research focused on Phase 2 enzyme induction by SFN, as well as inhibition of enzymes involved in carcinogen activation, but there has been growing interest in other mechanisms of chemoprotection by SFN. Recent studies also suggest that SFN offers protection against tumor development during the “post-initiation” phase and mechanisms for “suppression” effects of SFN are of particular interest. At the initiation stage of cancer, SFN blocks carcinogenesis through inhibition of Phase 1 enzymes and induction of Phase 2 enzymes. A summary of the “blocking” targets are shown in Table 1. These detoxification enzymes play an integral role in cellular resistance to carcinogenic insults. Post initiation, SFN can act to suppress cancer development through various molecular targets that are involved in controlling cell proliferation, differentiation, apoptosis, or cell cycle. A summary of “suppression” targets is presented diagrammatically in Figure 2. These targets may be aberrantly activated or silenced, depending on each specific case, and thus allow initiated cells to survive and proliferate. SFN can modulate these targets and redirect their activities towards apoptosis or cell cycle arrest, thereby eliminating initiated cells from the general population.
Table 1.
Effects of sulforaphane on “blocking” mechanisms.
| Category | Gene | Change in activity |
|---|---|---|
| Phase 1 enzyme | CYP1A1 | Decrease |
| CYP2B1/2 | Decrease | |
| CYP3A4 | Decrease | |
| CYP4A10 | Increase | |
| CYP4A14 | Increase | |
| CYP39A1 | Increase | |
| Phase 2 enzyme | NQO-1 | Increase |
| GST | Increase | |
| Γ-GCS | Increase | |
| UGT | Increase | |
| Antioxidants | Thioredoxin reductase 1 and 3 | Increase |
| Glutathione peroxidase 3 | Increase | |
| Proteasome subunits | Alpha 1 and 3, beta 5, and 26S | Increase |
| Cell cycle and cell growth | Cyclins D1, E2 and T2 | Increase |
| CDK 4, 7 and 9 | Increase | |
| Stress response | Heat shock proteins 1, 1 alpha, 1 beta, 8 and 105 | Increase |
| Transcription factors | CREB binding protein | Increase |
| Aryl hydrocarbon receptor nuclear translocator-like | Increase |
Figure 2.
Proposed “suppression” mechanisms of chemoprevention by SFN leading to alteration in cell cycle arrest, apoptosis and/or growth inhibition.
2.1 Blocking mechanisms: phase I and phase II enzymes
SFN can modulate phase 1 metabolism through direct interactions with cytochrome P450 enzymes (CYP) or regulating their transcript levels within the cell. Phase 1 enzymes usually involve oxidation, reduction, or hydrolysis and generally lead to detoxification, but are also involved in converting procarcinogens to carcinogens. Inhibition of phase 1 enzymes is thought to be an important step in blocking chemically-induced carcinogenesis. A dose dependent inhibition of CYP1A1 and CYP2B1/2 by SFN was seen in rat hepatocytes. Similarly in human hepatocytes, SFN decreased the activity of CYP3A4 by decreasing its mRNA levels [9]. Thus, SFN can modulate the levels and activities of various phase 1 enzymes leading to reduced activation of procarcinogens. There is additional indirect evidence that SFN can modulate the activity of different CYP enzymes. For a more thorough review, refer to Myzak et al [10].
The “blocking activity” of SFN has received significant attention and, although CYP inhibition is of interest, most work has focused on Phase 2 enzyme induction via antioxidant response element (ARE)-driven gene expression. ARE-driven targets include NAD(P)H:quinone reductase (NQO1), heme oxygenase 1 (HO1) and gamma-glutamylcysteine synthetase (γ-GCS), a rate-limiting enzyme in glutathione (GSH) synthesis. Regulation of these genes, involved in detoxification of carcinogens and oxidants, is mediated by nuclear factor E2-factor related factor (Nrf2), a member of the basic leucine-zipper NF-E2 family, which binds to ARE sites as a cis-acting element in the 5’-flanking region of the genes for many of these Phase 2 enzymes.
Kelch-like ECH-associated protein 1 (Keap1) is a cysteine rich protein which, in its dimeric form, interacts with Nrf2 sequestering it the cytosol, thereby inhibiting its transcriptional activity. Several models have been suggested to explain how Keap1 regulates Nrf2. In the most widely accepted model, two cysteine residues, C273 and C288, are important for the dissociation of Keap1 from Nrf2. SFN is able to react with the thiol groups of Keap1 and form thionacyl adducts promoting Nrf2 dissociation from Keap1 and subsequent activation of ARE-driven gene expression.
The ability of SFN to activate these Nrf2 driven detoxifying genes is well documented both in vitro and in vivo. Potent induction of phase 2 enzymes has been found in prostate cell lines treated with 0.1–15 µM SFN. Treatment with SFN or broccoli sprout extracts strongly induced NQO1 activity due to increased transcription of NQO-1 [11]. GSH is a crucial substrate in the metabolism of SFN. In human prostate cells, GSH synthesis was induced by SFN treatment due to increased levels of γ-GCS light chain mRNA but not γ-GCS heavy chain, although high levels of γ-GCS heavy chain message were present. This induction occurred concomitant with an increase in intracellular GSH levels and induction of glutathione S-transferase alpha (GST-α) and microsomal GST [11]. More recent in vivo work in the F-344 rat model confirmed phase 2 enzyme induction in the prostate and colon after dietary supplementation of a broccoli or SFN diet. In one study the rats were fed a broccoli diet and a 4.5 fold induction of NQO1 activity was observed in the rat colon [12]. In another study, rats gavaged with 50 mg/kg/day SFN for five days had increased NQO-1, total GST, and GST-µ activities in the prostate. In other tissues such as liver, kidney, and bladder, SFN induced NQO-1 and total GST activity, with the highest induction in the bladder [13]. Finally, the induction of phase 2 enzymes by SFN is also apparent in humans. In vivo jejunal perfusion of broccoli extracts (equivalent to ~1.2 g dry weight broccoli) resulted in an induction of GSTA1 and UGT1A1 in exfoliated enterocytes [14].
Importantly, the induction of these blocking genes after SFN treatment is dependent on Nrf2 as revealed from work done in Nrf2 knock out mice. Transciptional profiling of the small intestine from wild-type Nrf2 mice (nrf2+/+) and Nrf2 knock out mice (nrf2−/−) fed 9 µmol/day SFN revealed upregulation of the detoxification genes NQO-1, GST, γ-GCS, and UDP-glucuronosyltransferases (UGT). In addition, antioxidant genes glutathione peroxidase, glutathione reductase, ferritin, and haptaglobin were also affected. The upregulation of these genes by SFN was significantly blunted in the Nrf2 knockout mice [15]. Similar responses have been found in other tissues. In the liver, γ-GCS was induced as well as two GST-α subunits and three GST-µ subunits following 90 mg SFN/kg body weight. Many other gene groups were identified including antioxidants, ubiquitin/proteasome systems, cell cycle and cell growth, transcription factors, and several others [16]. Another group reported an increase in NQO1 and GST activities in the stomach, small intestine, and liver of wild-type but not Nrf2 knockout mice after being fed a diet containing broccoli seed extracts. This was accompanied by increased protein levels of GSTA1/2, GSTA3, and GSTM1/2 in the wild-type mice [17]. These studies defined the Nrf2 dependent SFN targets that are altered in the stomach, small intestine, and liver, tissues that play a critical role in absorption and detoxification, respectively. Collectively, these results highlight the importance of SFN in blocking the initiation stage of cancer by inhibiting phase 1 enzymes and stimulating phase 2 enzymes.
2.2 Suppression via anti-proliferative mechanisms
2.2.1 Cell cycle arrest
One hallmark of cancer is hyperproliferation due to loss of cell cycle regulatory mechanisms. The key regulators of cell cycle progression are the cyclin-dependent kinases (CDKs), cyclins, and CDK inhibitors. The regulation of CDK complexes is dependent upon the phosphorylation status of the various components of the complex and whether or not CDK inhibitors are bound. The cyclin/CDK complexes promote cell cycle progression while the CDK inhibitors promote cell cycle arrest. Research suggests that the action of SFN on various CDKs, cyclins, and CDK inhibitors is complex and the regulation is likely affected by cell type, dose of treatment, and time of exposure.
In vitro experiments with SFN indicate a pronounced role for cell cycle arrest in its anticancer properties. In both prostate and colon cancer cells a predominant G2/M cell cycle arrest is observed. The G2/M transition in the cell cycle requires an active cyclinB/CDK1 complex. Phosphorylation of CDK1 in its ATP binding loop by Wee1 and MYT1 (membrane associated tyrosine/threonine 1) kinases inactivates the complex while an opposing phosphatase, cell division cycle 25 (Cdc25) removes these inhibitory phosphorylations and promotes the progression into M-phase. Thus far, three mammalian Cdc25 isoforms have been identified; Cdc25A, Cdc25B, and Cdc25C. Chk1 and Chk2 kinases can phosphorylate and inactivate the various Cdc25 isoforms. Therefore Chk2 inactivates Cdc25 and the cyclinB/CDK complex arresting the cells in G2/M phase. In PC3 cells treated with 20 µM SFN a G2/M cell cycle arrest occurred with concomitant decreases in levels of cyclin B1, Cdc25B, and Cdc25C. Cdc25C was phosphorylated at Ser-216 by Chk2 and sequestered to the cytoplasm by 14-3-3β. This same Chk2-dependent G2/M arrest was seen in the HCT116 human colon cancer cell line [18]. Likewise in LnCap prostate cancer cells, a dramatic increase in G2/M phase arrest occurred in a concentration- and time-dependent manner concomitant with induction of cyclin B1, Chk2 kinase, and down-regulation of Cdk1 and Cdc25C protein levels [19]. In addition, 10 µM SFN treatment in DU145 prostate cancer cells reduced cell viability and induced G2/M cell cycle arrest [20].
Although G2/M arrest is the predominant stage of cell cycle arrest induced by SFN [21; 22], arrest at other phases of the cell cycle occurs in both prostate and colon cancer cells. In HT-29 cells, G1 cell cycle arrest occurred concomitant with an increase in p21CIP1, and a decrease in cyclin D1, cyclin A, and c-myc [23]. A G1/S block in LnCap and DU145 prostate cells has also been reported at concentrations at or below 10 µM [24; 25].
Evidence indicates that dose and duration of SFN exposure may be responsible for these divergent cell fates. In human colon adenocarcinoma Caco-2 cells, a G2/M arrest was observed at a dose of 20 µM SFN, whereas concentrations >20 µM induced accumulation of sub-G1 cells and loss of mitochondrial membrane potential [26]. Length of exposure to SFN also appears to play an important role. In p53 wild-type 40-16 colon cancer cells, transient SFN treatment for up to 6 h resulted in a reversible G2/M arrest and cytostatic growth effects. In contrast, exposures >12h resulted in irreversible G2/M arrest and subsequent apoptosis. Interestingly, the cytostatic effects seen with 12 h exposure was sustained up to 72 h after SFN removal and IC50s were indistinguishable from 72 h exposures [27].
The tumor suppressor and cell cycle inhibitor protein p21 appears to play an important role in SFN-induced cell cycle arrest. An induction of p21 is consistently observed regardless of cell type and p53 status. In vitro, SFN treatment induced p21 expression in both p53 negative colon cell lines HT-29 and Caco-2 [21]. In vivo, surgically resected colon tissue from three human volunteers treated with SFN for 2 h exhibited a strong induction of p21 in cancer tissue but not in normal tissue in 2 out of 3 volunteers [28]. In LnCap prostate cancer cells, p53 and p21 were induced by 20 µM SFN. However, induction of p21 by SFN is also apparent in p53-null PC3 prostate cancer cells, suggesting p53-independent regulation of p21 [29]. Supporting this p53-independent mechanism in LnCap cells SFN-induced cell cycle arrest occurred after induction of p21 but not p53. Interestingly, in these experiments using LnCap cells, p21 siRNA knockdown potentiated cell cycle arrest with no affect on apoptosis as detected by histone fragmentation indicating a role for p21 in protecting against SFN-mediated arrest [19]. This role of p21 merits further investigation in other cell lines. Taken together these data indicate a consistent induction of p21 following SFN administration in both p53 dependent and independent contexts.
2.2.2 Apoptosis
Apoptosis, or programmed cell death, can be accomplished either through the death-receptor caspase cascades or the mitochondria caspase cascades. Caspases are the effectors of apoptosis and some of the hallmarks of apoptosis are cytoplasmic histone associated DNA fragments, poly (ADP-ribose) polymerase (PARP) cleavage, changes in Bcl-2 protein family ratios (increased proapoptotic proteins and decreased antiapoptotic proteins), and cytochrome C release from the mitochondrial membrane. There is substantial and compelling evidence for SFN mediated apoptosis in both prostate and colon cancer cells, but this may depend on SFN dose.
Administration of 10 µM SFN reduced cell viability and induced apoptosis as indicated by PARP cleavage and increased release of histone associated DNA fragments in DU145 prostate cancer cells [20]. In HCT116 colon cancer cell lines (both wild-type for p53 (40-16) or p53 knockout (379.2)), 15 µM SFN induced activation of caspase 7 and caspase 9, and apoptosis independent of p53. This was accompanied by a consistent decrease in Bcl-XL protein levels. Although the levels of Bax and Bak protein were not consistently decreased a time-dependent change in Bax and Bak to Bcl-XL protein ratios in favor of the pro-apoptotic factors Bax and Bak was observed concomitant with increased PARP cleavage [30]. In HT-29 colon cancer cells, treatment with 15 µM SFN increased Bax expression, release of cytochrome C from mitochondria, and PARP cleavage [22]. In PC3 prostate cancer cell line apoptosis induction was apparent as indicated by an increase in sub-G0/G1 DNA content of treated cells, cytoplasmic histone associated DNA fragments, PARP cleavage, and an increased Bax:Bcl-2 ratio. Induction of apoptosis was associated with activation of caspases 3, 8, and 9 [31]. This same group performed a PC3 xenograft experiment to determine the effects of SFN administration in vivo. In this experiment tumor growth was significantly inhibited as indicated by reduction in tumor volume and weight. Increased apoptosis and expression levels of Bax were also reported [31]. From these data it is clear that apoptosis is induced in both prostate and colon cancer cells through the death receptor and mitochondrial pathways.
2.2.3 Histone deacetylase (HDAC) inhibition
HDAC inhibition is emerging as a fascinating and promising field in cancer chemoprevention and therapy. Increased HDAC activity and expression is common in many cancer malignancies, and can result in repression of transcription that results in a de-regulation of differentiation, cell cycle and apoptotic mechanisms. Moreover, tumor suppressor genes, such as p21 appear to be targets of HDACs and are “turned off”, or transcriptional silenced, by deacetylation. HDAC1, a class I HDAC, is over-expressed and localized in the nucleus in hormone refractory prostate cancer [32]. It has also been shown that Sirt1, the predominant class III NAD+ dependent HDAC, is over-expressed in both human and mouse prostate cancers [33]. In human patient samples, global decreases in histone acetylation state corresponded with increased grade of cancer and risk of prostate cancer recurrence [34]. In the Apcmin mouse model for colon cancer, the loss of Apc results in an over-expression of HDAC2. Moreover, HDAC2 knockdown promotes cell death in HT-29 colon cancer cells [35]. Taken together these findings support the hypothesis that overactive HDAC activity and hypoacetylation may contribute to prostate and colon cancer progression.
Several clinical trials are currently ongoing aimed at establishing the chemotherapeutic efficacy of HDAC inhibitors, based on evidence that cancer cells undergo cell cycle arrest, differentiation and apoptosis in vitro, and that tumor volume and/or tumor number may be reduced in animal models. Strikingly, the effects of HDAC inhibition occur preferentially in cancer cells and not normal cells [36]. Recent research has shown that SFN and its metabolites act as HDAC inhibitors.
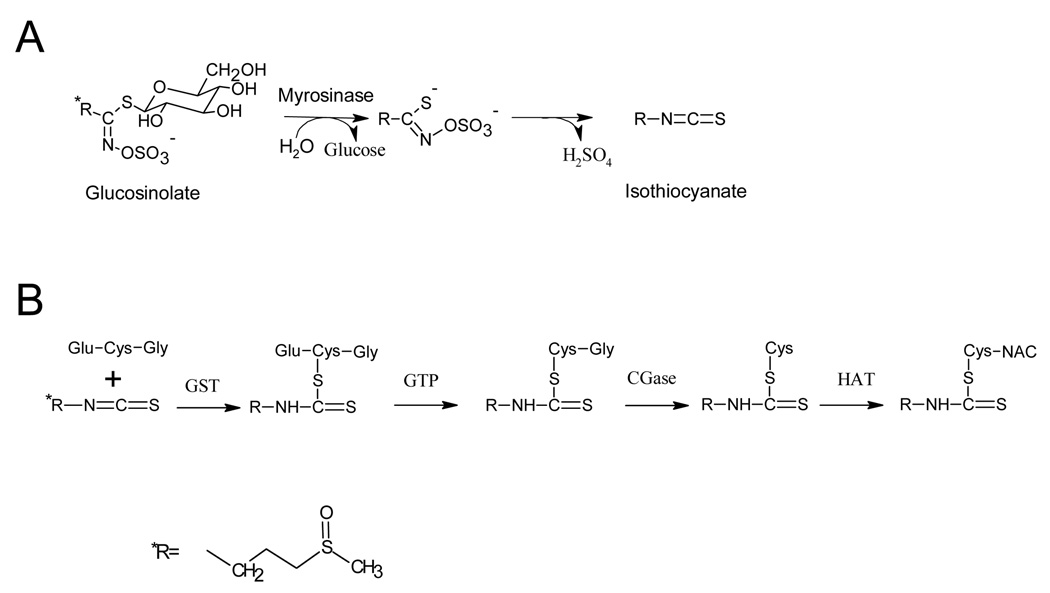
SFN is metabolized via the mercapturic acid pathway, starting with GSH conjugation by GST and subsequent steps generate SFN-cysteine (SFN-Cys) followed by SFN-N-acetylcysteine (SFN-NAC) (Figure 3). Biochemical assays found that SFN metabolites did indeed inhibit HDAC activity in vitro, the greatest inhibition involving SFN-NAC and SFN-Cys. Molecular modeling in the active site of an HDAC enzyme provided evidence that SFN-Cys is acting as a competitive inhibitor [37]. In BPH1, PC3, and LnCap prostate cancer cells, SFN inhibited HDAC activity with a concomitant increase in global histone acetylation, increased acetylated histone H4 interactions with the P21 and Bax promoter, and induction of p21 and Bax mRNA and protein levels [29]. The same effects were seen in HCT116 human colorectal cancer cells treated with SFN; namely HDAC inhibition, increase global histone acetylation, and selective increase in histone acetylation at the p21 promoter [37]. HDAC inhibition coincided with the induction of G2/M phase cell cycle arrest and apoptosis as indicated by multi-caspase activation [29]. HT-29 colon cancer cells, which lack endogenous Nrf2 protein, and Nrf2−/− mouse embryonic fibroblasts both exhibited an HDAC inhibitory response to SFN treatment. These results indicated the possibility of a separate SFN chemoprevention pathway distinct from the classic Nrf2 pathway [38].
Figure 3.
Metabolism of sulforaphane involving (A) hydrolysis of glucosinolate to its isothiocyanate via myrosinase enzyme activity and (B) metabolism of SFN via the mercapturic acid pathway. GST=Glutathione-S-Transferase; GTP= γ-Glutamyltranspeptidase; CGase=Cysteinylglycinase; HAT=; Histone acetyltransferase
In vivo, mice were given a single oral gavage dose of 10 µM SFN or SFN-NAC and HDAC inhibition was observed with a concomitant increase in acetylated histones and induction of p21 in the colonic mucosa. In dietary studies, Apcmin mice were fed ~6 µmol SFN/day for 10 weeks. In these experiments a significant decrease in intestinal polyps and an increase in global acetylated histones H3 and H4 were observed, with specific increases at the Bax and p21 promoters [39]. In PC3 xenograft studies, dietary SFN supplementation resulted in slower tumor growth and significant HDAC inhibition in the xenografts, as well as in the prostate and circulating peripheral blood mononuclear cells [38]. From these studies it can be concluded that HDAC inhibition represents a novel chemoprevention mechanism by which SFN might promote cell cycle arrest and apoptosis.
2.2.4 MAPK
Mitogen-activated protein kinases (MAPKs) belong to the superfamily of serine/threonine kinases including the extracellular signal-regulated kinases (ERK), c-Jun NH2-termial kinases (JNK), and p38. Each is believed to play a role in carcinogenesis and cancer development. An important downstream effector protein of MAPKs is Activator protein-1 (AP-1), a dimeric basic region-leucine zipper (bZIP) protein that is activated by different MAPKs. The AP-1 family is comprised of heterogeneous and complex dimeric interacting partners, with divergent downstream targets depending on tissue context and cellular stimuli. Modulation of AP-1 members can have effects on both promoting and inhibiting carcinogenesis. These divergent responses observed are likely dependent on genetic background, cell type, tumor state, and signaling networks that are affected in response to specific agents.
In prostate and colon cancer, the activation of AP-1 by SFN appears to be a factor that plays an important role in the regulation of cell death. In HT-29 colon cancer cells activation of AP-1 luciferase activity occurred at low concentrations of SFN treatment (≤ 35 µM) while it was inhibited at high concentration (≥ 50 µM). In corroboration with AP-1 activation, cyclin D1 levels increased at low concentrations of SFN and decreased at high concentrations. Interestingly, while AP-1 activity and cyclin D1 protein levels increased at lower concentrations and then decreased at higher concentrations, cell viability decreased in a direct dose dependent manner. Activation of the p46-JNK isoforms and not the p54-JNK isoforms was reported indicating that the p54-JNK isoform may be responsible for activation of AP-1 [40]. Further work in HT-29 cells revealed that SFN was able to decreased cell viability and activate the MAPK pathways ERK, JNK, and p38. Furthermore, consistent with the previous report, activated JNK was able to decrease cyclin D1 levels at high concentrations of SFN [23]. SFN treatment in human colon adenocarcinoma cells Caco-2 induced ERK activation but, in contrast to the above studies, not JNK or p38 [26]. In prostate cancer cells it was reported that the ERK and JNK pathways are necessary to induce cell death in PC3 cells but only the JNK pathway in DU14 cells [20; 41]. Another report by Kong et al reveals that SFN can inhibit p38 activity. This is significant because active p38 can phosphorylate Nrf2 and promotes its association with Keap1 thereby suppressing Nrf2 translocation to the nucleus, thus inhibiting the activation of phase 2 enzymes [42]. Taken together these data indicate a diverse range of MAPK responses and possible cellular outcomes after SFN treatment in both prostate and colon cancer cells and highlights the importance of evaluating MAPK pathways in light of tissue context.
2.2.5 NFκB
The nuclear factor kappa-B (NFκB) is a heterodimeric transcription factor that consists of a p50 and p65 subunit and, when active, promotes inflammatory gene expression, cell proliferation and cell survival. Inactive NFκB is normally found in the cytosol, bound to its inhibitory subunits (IκBs). Upon activation, IκBs are rapidly phosphorylated by IκB kinases (IKK), and ubiquitinated leading to IκB degradation and subsequent release of NFκB and translocation to the nucleus. Constitutive activation of NFκB is common in various human malignancies, including colon and prostate cancer, and leads to up-regulation of genes encoding adhesion molecules, inflammatory cytokines, growth factors, and anti-apoptotic genes [43; 44]. Thus, inhibition of NFκB activation has been postulated as a key target for cancer chemoprevention.
Several labs have shown down-regulation of NFκB activity with SFN administration in prostate and colon cancer cells. This was observed in PC3 cells treated with 20 µM SFN in which reduced nuclear localization of p65-NFκB occurred after 1 h [45]. Additionally in PC3 cells inhibition of NFκB activity coincided with expression of downstream targets, VEGF, cyclin D1, and Bcl-XL. Decreased nuclear translocation and activation of p65-NFκB was attributed to the inhibition of IKK phosphorylation, thereby attenuating IκBα phosphorylation and degradation [46]. Similar effects were seen in HT-29 colon cancer cells, depending on SFN dose. Specifically, SFN strongly inhibited LPS induced NFκB activity at doses as low as 10 µM, but a dose of 50 µM SFN was required for significantly decreased LPS induced IκBα phosphorylation. Decreased cell viability was observed at 25 µM SFN which correlated to an induction of apoptosis via caspase 3 [47]. Interestingly, a unique biphasic response was observed in LnCap cells, with an initial increase in NFκB activity at 6–12 h, followed by inhibition 24 h after treatment. The inhibitor of apoptosis (IAP) family is one family of the downstream factors that are up-regulated by NFκB activation. In LnCap and PC3 cells, the modulation of IAP levels was proportional to the level of NFκB activity [45]. Collectively, these results indicate that SFN can affect proliferation signals and apoptotic signals via modulation of NFκB activity.
2.2.6 ROS
The production of reactive oxygen species (ROS) has been postulated to be a key mechanism by which SFN induces apoptosis. SFN administration to PC3 prostate cancer cells resulted in ROS generation, which was accompanied by disruption of mitochondrial membrane potential, cytosolic release of cytochrome C, and apoptosis. All of these effects were reversed with administration of the antioxidant N-acetylcysteine and overexpression of catalase [48]. The authors indicated that conjugation of SFN with GSH, a necessary step in SFN metabolism, depletes the intracellular concentration of GSH and potentially lowers the oxidative stress threshold of the cell. In their experiments SFN treatment increases mitochondrial ROS production and induces apoptosis as indicated by release of cytochrome C via both death-receptor and mitochondrial caspase cascades [48]. In HT-29 colon cancer cells treated with 50 µM SFN, the cell cycle arrest response were blocked by addition of antioxidants NAC or GSH, indicating that generation of ROS was indispensable for growth arrest under the assay conditions used [23]. Generally, high doses of SFN are needed in order to induce ROS production, but one group reported a transient rise in ROS in DU145 after treatment with only 10 µM SFN [20]. Mitochondrial ROS generation and disruption of the mitochondrial membrane potential have both been shown to induce the formation of acidic vesicular organelles and autophagy in PC3 and LnCap human prostate cells at a dose of 40 µM SFN. This response has unique morphological effects and, interestingly, has the ability to inhibit mitochondrial cytochrome C release and apoptosis [49]. Therefore, ROS production after SFN exposure has the ability to influence cell death in prostate and colon cancer cells.
3 Metabolism and bioavailability
The metabolism of SFN is summarized in Figure 3. The initial reaction involves enzymatic hydrolysis of glucoraphanin, the glucosinolate precursor of SFN, found in the plant. This reaction is catalyzed by myrosinase, a β-thioglucosidase, which cleaves the glycone from the glucosinolate forming glucose, hydrogen sulfate and one of many different aglycones (e.g. thiocyanate, ITC, or a nitrile) depending on the glucosinolate, reaction pH, and availability of ions [50]. At neutral pH, the major glucosinolate hydrolysis products are stable isothiocyanates. After absorption, SFN is predominantly metabolized via the mercapturic acid pathway. In these reactions, the electrophilic central carbon of the -N=C=S group in SFN reacts with the sulfhydryl group of GSH to form a dithiocarbamate GSH conjugate. The enzymes that catalyze GSH conjugation to SFN are the family of GST enzymes, and polymorphisms in these enzymes have a significant impact on overall ITC metabolism (discussed below). Interestingly, SFN is also able to induce its own metabolism via induction of GSTs. The final steps in SFN metabolism is formation of SFN-Cys and ultimately SFN-NAC [51].
The absorption and bioavailability of SFN is affected by several factors. The first factor involves the hydrolysis of SFN from glucoraphanin via myrosinase activity. This initial step is critical because only the ITC form is thought to be biologically active and exhibits the desired anticancer properties. Importantly, mammalian cells do not have endogenous myrosinase activity. Instead myrosinases are found in the plant or the gut microbial flora. In the cruciferous plant, the myrosinase enzyme is physically separated from the glucosinolate by the plant cell wall, but upon physical disruption through chopping, cutting, and/or chewing, the enzyme is released and the ITC is released. However, myrosinase is heat labile and thus cooking procedures can inactivate the enzyme and significantly reduce the bioavailability of SFN up to 3-fold [52]. Another source of myrosinase activity is the intestinal microbial flora. Evidence from experiments done with isolated human fecal bacteria [53] and others using F344 rats dosed ip with glucoraphanin, indicates that glucoraphanin can be converted to SFN by colonic microbial flora and that enterohepatic circulation is requisite for efficient metabolism [54]. However, the bioavailability of SFN is six times less when metabolism of the glucosinolate to the ITC had not occurred prior to ingestion [55], revealing a strong reliance on plant myrosinase activity, as opposed to the intestinal gut flora. Nonetheless, an important factor determining inter-individual SFN bioavailability is variability in the gut microbial flora when the non-hydrolyzed glucosinolate is consumed.
The last factor that affects SFN bioavailability is related to polymorphisms in phase 2 SFN metabolizing genes, such as GSTs, which play a significant role in determining the detoxifying ability of an organism. In general, GST enzymes catalyze the conjugation of GSH to electrophiles such as SFN. There are six different classes of GST isoenzymes; α, µ, π, θ, σ, and κ, and each functional unit is composed of two subunits. Each is designated by the abbreviated Roman capital of each Greek letter followed by a number indicating subunit composition (ex. GSTµ1=GSTM1). In general, the substrate specificity for the different isoenzymes overlaps but, specifically, each class has varying degrees of reactivity for different substrates. GST null genotypes are quite prevalent in the population, with up to 50% of people being GSTM1 null and 47% GSTT1 null.
The effect that GST genotype has on cancer development and chemoprevention is complex. In the context of high cruciferous vegetable intake, evidence is mounting in favor of a GST null genotype providing a protective effect against lung, colon, and breast cancers [56; 57]. Since GST activity plays a critical role in SFN metabolism and subsequent excretion, lower GST activity in individuals with GST polymorphisms could result in slower elimination and longer exposure to isothiocyanates after cruciferous vegetable consumption. For example, one study investigating a population in Singapore reported higher ITC excretion among GSTT1 positive individuals in comparison to GSTT1 null [58], implying shorter exposure times to the potential beneficial metabolites of SFN for GSTT1 positive individuals. This finding corroborates with a later study in Singapore, indicating that GST null genotype coupled with high cruciferous vegetable intake reduced the risk of colorectal cancer [59]. However, other studies have shown that the GSTM1 null genotype produced a slight increase in the area under time-concentration curve (AUC) for metabolite concentrations in plasma, a significantly higher rate of SFN metabolite excretion, and a higher percentage of SFN excreted 24 h after ingestion, indicating shorter retention times of SFN and its metabolites [60]. In addition, in other studies, a GSTM1 null genotype had a non-significant increase in prostate cancer risk, while men in the high vegetable consuming group that have GSTM1 present exhibited the greatest reduction in cancer risk [1]. These inconsistencies may be explained by differences in the predominant ITC consumed (3-butenyl-ITC and4-pentenyl-ITC versus SFN), the class of GST null genotype present (GSTT1 versus GSTM1), or the cancer of interest (colorectal versus prostate). Despite these conflicting results, the impact of polymorphism on nutrient bioavailability is an important area of research that will aid in our understanding of response variability to SFN and other phytochemicals in human populations.
4 Pharmacokinetics
The ability of SFN to be distributed throughout the body and reach target tissues has been investigated in vitro, in mouse models and in human subjects. In the human small intestine, SFN can be efficiently absorbed and conjugated to GSH. Human perfusion experiment showed that 74 ±29% of SFN from broccoli extracts can be absorbed in the jejunum and that a portion of that returns to the lumen of the jejunum as SFN-GSH [14]. Pharmacokinetic studies in both rats and humans also support that SFN can be distributed in the body and reach µM concentrations in the blood. In rats, following a 50 µmol gavage of SFN, detectable SFN was evident after 1 h and peaked at ~20 µM at 4 h, with a half life of approximately 2.2 h [61]. In human subjects given single doses of 200 µmol broccoli sprouts isothiocyanate preparation, ITC plasma concentrations peaked between 0.943–2.27 µmol/L 1 h after feeding, with half life times of 1.77 ±0.13 h [62]. Once SFN is distributed there is evidence that it can accumulate in tissues and produce anticancer blocking and suppressing effects. In a recent pilot study in human mammary tissue, an oral dose of broccoli sprout preparation containing 200 µmol SFN 1 h prior to tissue removal showed mean accumulation of 1.45±1.12 pmol/mg in the right breast and 2.00±1.95 pmol/mg in the left breast. In these tissues the detoxification genes NQO1 and heme oxygenase-1 (HO-1) were measured providing proof of principle that these measures can be made in a clinical trial [63]. In another study mice supplemented with 300 or 600 ppm SFN, accumulated SFN and SFN-GSH plasma concentrations of 124 to 254 nM and 579 to 770 nM, respectively. Also SFN and SFN-GSH concentrations in the small intestine were between 3 and 13 nmol/g of tissue and 14–32 nmol/g of tissue, respectively, which is equivalent to roughly 3–30 µM of total SFN. Notably, the accumulation of SFN in colonic tissue corresponded with decreased adenoma formation in these animals [64]. The last aspect of SFN pharmacokinetics concerns its excretion. When efficient metabolism of glucoraphanin occurs, SFN-NAC is the primary SFN metabolite excreted in the urine [12; 51; 52; 54]. In humans, SFN and its metabolites were excreted with first-order kinetics [53; 62] and most data indicate that SFN and its metabolites are cleared from the body within 72 h of dosing. From these data it can be extrapolated that maintenance of SFN concentrations in the body can be achieved by consuming recommended servings of cruciferous vegetables once a day. Collectively, the published data indicate that SFN can be absorbed, reach µM concentrations in the blood, accumulate in tissues, and be maintained to achieve the anticancer effects.
5 Preclinical and clinical studies
In preclinical rodent models, there is significant data supporting the chemopreventive effects of SFN at several stages of carcinogenesis. SFN supplementation administered both pre- and post–initiation decreased colonic aberrant crypt foci in azoxymethane (AOM)-induced rats. In contrast, supplementation with SFN-NAC was only effective post-initiation, suggesting different mechanisms of action between parent SFN and its metabolites [65]. SFN supplementation also decreased polyp formation in Apcmin mice [39; 64]. In other suppression studies, supplementation with SFN also decreased tumor growth in prostate xenograft studies [31; 66].
To date very few human clinical trials have evaluated the effects of SFN on cancer outcome, however, several pilot and phase 1 human SFN trials have been conducted utilizing different sources of SFN. The first study was a randomized, placebo-controlled, double-blind phase 1 clinical trial of healthy volunteers that used glucoraphanin or isothiocyanate as the SFN source [67]. The phase 1 trial consisted of three study groups; 25 µmol of glucosinolate, 100 µmol of glucosinolate, or 25 µmol ITC for 7 days and examined parameters of safety, tolerance, and pharmacokinetics. Importantly, there were no significant toxicities associated with taking the extracts at the doses employed. A second study was a randomized placebo-controlled chemoprevention trial performed in Qidong, People’s Republic of China which used a hot drinking water infused with 3-day old broccoli sprouts [68]. Residents of Qidong are at high risk for development of hepatocellular carcinoma, in part due to consumption of aflatoxin-contaminated foods, and are exposed to high levels of the airborne toxin phenanthrene. There was an inverse association for the excretion of dithiocarbamates and urinary aflatoxin-DNA adducts and trans, anti-phenanthrene tetraol, a metabolite of the combustion product phenanthrene in the intervention arm. Thus, an inverse correlation between SFN treatment and excretion of carcinogens was detected, suggesting induction of one or more phase 2 enzymes. Interestingly, in the Qidong trial, although there was consistency within an individual between doses, there was significant inter-individual variability in bioavailability of the dithiocarbamates. A third small preliminary human study interested in determining if the HDAC inhibition effects observed in cell culture and mice could be translated into humans was performed. After ingestion of 68g of broccoli sprouts, a significant decrease in HDAC activity was evident in peripheral blood mononuclear cells with a concomitant increase in acetylated histones H3 and H4 [66]. The most recent pilot human study was performed in 8 healthy women who were undergoing elective reduction mammoplasty. Here the women were given an oral dose of broccoli sprout preparation containing 200 µmol SFN 1 h prior to breast surgery. Mean epithelial-/stromal-enriched breast tissue dithiocarbamate concentration was 1.45±1.12 and 2.00±1.95 pmol/mg tissue for the right and left breast, respectively. NQO1 and HO-1 transcript levels were measured in both breasts of all subjects [63]. These clinical trials provide the important link to human relevance for SFN as a promising anticancer agent.
6 Conclusions
Cancer is a dynamic and multifaceted disease that impacts morbidity and mortality in every country in the world. Prostate and colon cancers are highly prevalent in the U.S., collectively representing 18% of all cancer deaths in men. Lifestyle changes can potentially eliminate one third of these cancers, and epidemiological data indicates an inverse relationship between cruciferous vegetable intake and cancer risk. SFN has been postulated to be one of the principle ITCs found in cruciferous vegetables that possesses cancer chemopreventive properties. One of the challenges in cancer treatment is that each stage of cancer development presents unique obstacles to efficacious targeting and treatment of cancer. Since cancer is a multi-factorial process, with several molecular alterations, targeting more than one pathway by a chemopreventive agent is highly desirable. The ability of SFN to target both blocking mechanisms, via alteration in Phase 1 and Phase 2 enzymes, and suppressing mechanisms post-initiation via multiple cell proliferation targets (see Figure 2), make SFN a highly promising dietary chemoprevention and therapeutic agent.
An important key area of research that needs to be further addressed is the metabolism, bioavailability, and efficacy of SFN treatment in human populations. In clinical trials, SFN treatment appears to be safe and well tolerated. The blocking action of SFN is evident in the induction of phase 2 enzymes and inverse relationship between dithiocarbamate excretion and carcinogen excretion after ingestion of SFN. The suppression action of SFN is verified in humans by ex vivo induction of p21 in resected colon and HDAC inhibition response in peripheral blood cells. However, other cancer suppressive mechanisms identified in cell culture and mouse models have not been studied in humans. Additionally, more extensive clinical studies are required to elucidate the changes in key cancer suppression biomarkers, such as apoptosis and cell cycle arrest, as well as tumor outcomes. Although preliminary clinical studies have shown promise, larger human studies are necessary in order to understand the full potential of SFN for cancer chemoprevention. In particular, a greater understanding of the mechanisms leading to inter-individual variation in responses to SFN supplementation is needed to delineate efficacious dose response, source of SFN (whole food or supplement), and effects of genotype in the population at large.
In closing, it is important to mention that there are other glucosinolates and metabolites found in cruciferous vegetables which possess similar chemistry, metabolism, and anticancer effects as SFN (eg. glucobrassicin- I3C, gluconasturtiin- phenethylisothiocyanate, glucoerucin-erucin (sulfide analog of SFN), glucoiberin-iberin). Also, there is a large body of research that has examined SFN effects on many other cancers such as breast, hepatic, bladder, osteosarcoma, glioblastoma, leukemia, pancreatic, and melanoma. Although larger scale clinical trials are necessary, dietary SFN shows promise as a safe and effective anti-cancer strategy that includes incorporating easily accessible foods into an individual’s regular diet.
Acknowledgements
Work presented here was from studies supported in part by NIH grants CA65525 (RHD), CA80176 (RHD), CA90890 (RHD), CA122959 (RHD), CA122906 (EH), CA107693 (EH), the Oregon Agricultural Experiment Station, as well as NIEHS center grant P30 ES00210.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Joseph MA, Moysich KB, Freudenheim JL, Shields PG, Bowman ED, Zhang Y, Marshall JR, Ambrosone CB. Cruciferous vegetables, genetic polymorphisms in glutathione s-transferases m1 and t1, and prostate cancer risk. Nutrition and Cancer. 2004;50:206–213. doi: 10.1207/s15327914nc5002_11. [DOI] [PubMed] [Google Scholar]
- 2.Lin HJ, Probst-Hensch NM, Louie AD, Kau IH, Witte JS, Ingles SA, Frankl HD, Lee ER, Haile RW. Glutathione transferase null genotype, broccoli, and lower prevalence of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 1998;7:647–652. [PubMed] [Google Scholar]
- 3.Mosbah AFB, Kushad M, Kurilich Anne C, Juvik John A, Klein Barbara P, Wallig Mathew A, Jeffrey Elizabeth H. Variation of glucosinolates in vegetable crops of brassica oleracea. J. Agric. Food Chem. 1999;47:1541–1548. doi: 10.1021/jf980985s. [DOI] [PubMed] [Google Scholar]
- 4.Mithen R. Glucosinolates - biochemistry, genetics and biological activity. Plant Growth Regulation. 2001;34:91–103. [Google Scholar]
- 5.TP Zhang Y, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacological Research. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maheo K, Morel F, Langouet S, Kramer H, Le Ferrec E, Ketterer B, Guillouzo A. Inhibition of cytochromes p-450 and induction of glutathione s-transferases by sulforaphane in primary human and rat hepatocytes. Cancer Res. 1997;57:3649–3652. [PubMed] [Google Scholar]
- 10.Myzak MC, Dashwood RH. Chemoprotection by sulforaphane: Keep one eye beyond keap1. Cancer Letters. 2006;233:208–218. doi: 10.1016/j.canlet.2005.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks JD, Paton VG, Vidanes G. Potent induction of phase 2 enzymes in human prostate cells by sulforaphane. Cancer Epidemiol Biomarkers Prev. 2001;10:949–954. [PubMed] [Google Scholar]
- 12.Keck AS, Qiao Q, Jeffery EH. Food matrix effects on bioactivity of broccoli-derived sulforaphane in liver and colon of f344 rats. J. Agric. Food Chem. 2003;51:3320–3327. doi: 10.1021/jf026189a. [DOI] [PubMed] [Google Scholar]
- 13.Jones SB, Brooks JD. Modest induction of phase 2 enzyme activity in the f-344 rat prostate. BMC Cancer. 2006;6:62. doi: 10.1186/1471-2407-6-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petri N, Tannergren C, Holst B, Mellon FA, Bao Y, Plumb GW, Bacon J, O'Leary KA, Kroon PA, Knutson L, Forsell P, Eriksson T, Lennernas H, Williamson G. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase ii enzymes, in human jejunum in vivo. Drug Metab Dispos. 2003;31:805–813. doi: 10.1124/dmd.31.6.805. [DOI] [PubMed] [Google Scholar]
- 15.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 16.Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, Lin W, Reddy B, Chan JY, Kong AN. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of c57bl/6j mice and c57bl/6j/nrf2 (−/−) mice. Cancer Lett. 2006;243:170–192. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 17.McWalter GK, Higgins LG, McLellan LI, Henderson CJ, Song L, Thornalley PJ, Itoh K, Yamamoto M, Hayes JD. Transcription factor nrf2 is essential for induction of nad(p)h:Quinone oxidoreductase 1, glutathione s-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates. J. Nutr. 2004;134:3499S–3506S. doi: 10.1093/jn/134.12.3499S. [DOI] [PubMed] [Google Scholar]
- 18.Singh SV, Herman-Antosiewicz A, Singh AV, Lew KL, Srivastava SK, Kamath R, Brown KD, Zhang L, Baskaran R. Sulforaphane-induced g2/m phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of cell division cycle 25c. J. Biol. Chem. 2004;279:25813–25822. doi: 10.1074/jbc.M313538200. [DOI] [PubMed] [Google Scholar]
- 19.Herman-Antosiewicz A, Xiao H, Lew KL, Singh SV. Induction of p21 protein protects against sulforaphane-induced mitotic arrest in lncap human prostate cancer cell line. Mol Cancer Ther. 2007;6:1673–1681. doi: 10.1158/1535-7163.MCT-06-0807. [DOI] [PubMed] [Google Scholar]
- 20.Cho SD, Li G, Hu H, Jiang C, Kang KS, Lee YS, Kim SH, Lu J. Involvement of c-jun n-terminal kinase in g2/m arrest and caspase-mediated apoptosis induced by sulforaphane in du145 prostate cancer cells. Nutr Cancer. 2005;52:213–224. doi: 10.1207/s15327914nc5202_11. [DOI] [PubMed] [Google Scholar]
- 21.Parnaud G, Li P, Cassar G, Rouimi P, Tulliez J, Combaret L, Gamet-Payrastre L. Mechanism of sulforaphane-induced cell cycle arrest and apoptosis in human colon cancer cells. Nutr Cancer. 2004;48:198–206. doi: 10.1207/s15327914nc4802_10. [DOI] [PubMed] [Google Scholar]
- 22.Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont M-A, Chevolleau S, Gasc N, Tulliez J, Terce F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in ht29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- 23.Shen G, Xu C, Chen C, Hebbar V, Kong A-N. P53-independent g1 cell cycle arrest of human colon carcinoma cells ht-29 by sulforaphane is associated with induction of p21cip1 and inhibition of expression of cyclin d1. Cancer Chemotherapy and Pharmacology. 2006;57:317–327. doi: 10.1007/s00280-005-0050-3. [DOI] [PubMed] [Google Scholar]
- 24.Chiao JW, Chung FL, Kancherla R, Ahmed T, Mittelman A, Conaway CC. Sulforaphane and its metabolite mediate growth arrest and apoptosis in human prostate cancer cells. Int J Oncol. 2002;20:631–636. doi: 10.3892/ijo.20.3.631. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Liu D, Ahmed T, Chung FL, Conaway C, Chiao JW. Targeting cell cycle machinery as a molecular mechanism of sulforaphane in prostate cancer prevention. Int J Oncol. 2004;24:187–192. [PubMed] [Google Scholar]
- 26.Jakubikova J, Sedlak J, Mithen R, Bao Y. Role of pi3k/akt and mek/erk signaling pathways in sulforaphane- and erucin-induced phase ii enzymes and mrp2 transcription, g2/m arrest and cell death in caco-2 cells. Biochem Pharmacol. 2005;69:1543–1552. doi: 10.1016/j.bcp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 27.Gerlinde Pappa HBCG. Biphasic modulation of cell proliferation by sulforaphane at physiologically relevant exposure times in a human colon cancer cell line. Molecular Nutrition & Food Research. 2007;51:977–984. doi: 10.1002/mnfr.200700115. [DOI] [PubMed] [Google Scholar]
- 28.Traka M, Gasper AV, Smith JA, Hawkey CJ, Bao Y, Mithen RF. Transcriptome analysis of human colon caco-2 cells exposed to sulforaphane. J Nutr. 2005;135:1865–1872. doi: 10.1093/jn/135.8.1865. [DOI] [PubMed] [Google Scholar]
- 29.Myzak MC, Hardin K, Wang R, Dashwood RH, Ho E. Sulforaphane inhibits histone deacetylase activity in bph-1, lncap and pc-3 prostate epithelial cells. Carcinogenesis. 2006;27:811–819. doi: 10.1093/carcin/bgi265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pappa G, Lichtenberg M, Iori R, Barillari J, Bartsch H, Gerhauser C. Comparison of growth inhibition profiles and mechanisms of apoptosis induction in human colon cancer cell lines by isothiocyanates and indoles from brassicaceae. Mutat Res. 2006;599:76–87. doi: 10.1016/j.mrfmmm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured pc-3 human prostate cancer cells and retards growth of pc-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 32.Kalipso Halkidou LGSCHYLDENCNR. Upregulation and nuclear recruitment of hdac1 in hormone refractory prostate cancer. The Prostate. 2004;59:177–189. doi: 10.1002/pros.20022. [DOI] [PubMed] [Google Scholar]
- 33.Huffman DM, Grizzle WE, Bamman MM, Kim J-s, Eltoum IA, Elgavish A, Nagy TR. Sirt1 is significantly elevated in mouse and human prostate cancer. Cancer Res. 2007;67:6612–6618. doi: 10.1158/0008-5472.CAN-07-0085. [DOI] [PubMed] [Google Scholar]
- 34.Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, Kurdistani SK. Global histone modification patterns predict risk of prostate cancer recurrence. Nature. 2005;435:1262–1266. doi: 10.1038/nature03672. [DOI] [PubMed] [Google Scholar]
- 35.Zhu P, Martin E, Mengwasser J, Schlag P, Janssen K-P, Gottlicher M. Induction of hdac2 expression upon loss of apc in colorectal tumorigenesis. Cancer Cell. 2004;5:455–463. doi: 10.1016/s1535-6108(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone RW. Histone-deacetylase inhibitors: Novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 37.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: Inhibition of histone deacetylase. Cancer Res. 2004;64:5767–5774. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 38.Dashwood RH, Ho E. Dietary histone deacetylase inhibitors: From cells to mice to man. Semin Cancer Biol. 2007;17:363–369. doi: 10.1016/j.semcancer.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in apc-minus mice. Faseb J. 2006;20:506–508. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeong WS, Kim IW, Hu R, Kong AN. Modulation of ap-1 by natural chemopreventive compounds in human colon ht-29 cancer cell line. Pharm Res. 2004;21:649–660. doi: 10.1023/b:pham.0000022412.69380.d7. [DOI] [PubMed] [Google Scholar]
- 41.Xu C, Shen G, Yuan X, Kim JH, Gopalkrishnan A, Keum YS, Nair S, Kong AN. Erk and jnk signaling pathways are involved in the regulation of activator protein 1 and cell death elicited by three isothiocyanates in human prostate cancer pc-3 cells. Carcinogenesis. 2006;27:437–445. doi: 10.1093/carcin/bgi251. [DOI] [PubMed] [Google Scholar]
- 42.Keum Y-S, Yu S, Chang PP-J, Yuan X, Kim J-H, Xu C, Han J, Agarwal A, Kong A-NT. Mechanism of action of sulforaphane: Inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma hepg2 cells. Cancer Res. 2006;66:8804–8813. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- 43.Rayet B, Gelinas C. Aberrant rel/nfkb genes and activity in human cancer. Oncogene. 1999;18:6938–6947. doi: 10.1038/sj.onc.1203221. [DOI] [PubMed] [Google Scholar]
- 44.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor nf-{{kappa}}b. J. Clin. Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Choi S, Lew KL, Xiao H, Herman-Antosiewicz A, Xiao D, Brown CK, Singh SV. D,l-sulforaphane-induced cell death in human prostate cancer cells is regulated by inhibitor of apoptosis family proteins and apaf-1. Carcinogenesis. 2007;28:151–162. doi: 10.1093/carcin/bgl144. [DOI] [PubMed] [Google Scholar]
- 46.Xu C, Shen G, Chen C, Gelinas C, Kong AN. Suppression of nf-kappab and nf-kappab-regulated gene expression by sulforaphane and peitc through ikappabalpha, ikk pathway in human prostate cancer pc-3 cells. Oncogene. 2005;24:4486–4495. doi: 10.1038/sj.onc.1208656. [DOI] [PubMed] [Google Scholar]
- 47.Jeong WS, Kim IW, Hu R, Kong AN. Modulatory properties of various natural chemopreventive agents on the activation of nf-kappab signaling pathway. Pharm Res. 2004;21:661–670. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 48.Singh SV, Srivastava SK, Choi S, Lew KL, Antosiewicz J, Xiao D, Zeng Y, Watkins SC, Johnson CS, Trump DL, Lee YJ, Xiao H, Herman-Antosiewicz A. Sulforaphane-induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J. Biol. Chem. 2005;280:19911–19924. doi: 10.1074/jbc.M412443200. [DOI] [PubMed] [Google Scholar]
- 49.Herman-Antosiewicz A, Johnson DE, Singh SV. Sulforaphane causes autophagy to inhibit release of cytochrome c and apoptosis in human prostate cancer cells. Cancer Res. 2006;66:5828–5835. doi: 10.1158/0008-5472.CAN-06-0139. [DOI] [PubMed] [Google Scholar]
- 50.Bones AM, Rossiter JT. The myrosinase-glucosinolate system, its organisation and biochemistry. Physiologia Plantarum. 1996;97:194–208. [Google Scholar]
- 51.Kassahun K, Davis M, Hu P, Martin B, Baillie T. Biotransformation of the naturally occurring isothiocyanate sulforaphane in the rat: Identification of phase i metabolites and glutathione conjugates. Chem. Res. Toxicol. 1997;10:1228–1233. doi: 10.1021/tx970080t. [DOI] [PubMed] [Google Scholar]
- 52.Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DKW, Botero-Omary M, Chung F-L. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutrition & Cancer. 2000;38:168–178. doi: 10.1207/S15327914NC382_5. [DOI] [PubMed] [Google Scholar]
- 53.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 54.Bheemreddy RM, Jeffery EH. The metabolic fate of purified glucoraphanin in f344 rats. J. Agric. Food Chem. 2007;55:2861–2866. doi: 10.1021/jf0633544. [DOI] [PubMed] [Google Scholar]
- 55.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts: Metabolism and excretion in humans. Cancer Epidemiol Biomarkers Prev. 2001;10:501–508. [PubMed] [Google Scholar]
- 56.Seow A, Vainio H, Yu MC. Effect of glutathione-s-transferase polymorphisms on the cancer preventive potential of isothiocyanates: An epidemiological perspective. Mutat Res. 2005;592:58–67. doi: 10.1016/j.mrfmmm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Lampe JW, Peterson S. Brassica, biotransformation and cancer risk: Genetic polymorphisms alter the preventive effects of cruciferous vegetables. J Nutr. 2002;132:2991–2994. doi: 10.1093/jn/131.10.2991. [DOI] [PubMed] [Google Scholar]
- 58.Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, Coetzee GA, Yu MC. Urinary total isthiocyanate (itc) in a population-based sample of middle-aged and older chinese in singapore: Relationship with dietary total itc and glutathione s-transferase m1/t1/p1 genotypes. Cancer Epidemiol Biomarkers Prev. 1998;7:775–781. [PubMed] [Google Scholar]
- 59.Seow A, Yuan JM, Sun CL, Van Den Berg D, Lee HP, Yu MC. Dietary isothiocyanates, glutathione s-transferase polymorphisms and colorectal cancer risk in the singapore chinese health study. Carcinogenesis. 2002;23:2055–2061. doi: 10.1093/carcin/23.12.2055. [DOI] [PubMed] [Google Scholar]
- 60.Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione s-transferase m1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82:1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 61.Hu R, Hebbar V, Kim BR, Chen C, Winnik B, Buckley B, Soteropoulos P, Tolias P, Hart RP, Kong AN. In vivo pharmacokinetics and regulation of gene expression profiles by isothiocyanate sulforaphane in the rat. J Pharmacol Exp Ther. 2004;310:263–271. doi: 10.1124/jpet.103.064261. [DOI] [PubMed] [Google Scholar]
- 62.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: Pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 63.Cornblatt BS, Ye L, Dinkova-Kostova AT, Erb M, Fahey JW, Singh NK, Chen M-SA, Stierer T, Garrett-Mayer E, Argani P, Davidson NE, Talalay P, Kensler TW, Visvanathan K. Preclinical and clinical evaluation of sulforaphane for chemoprevention in the breast. Carcinogenesis. 2007;28:1485–1490. doi: 10.1093/carcin/bgm049. [DOI] [PubMed] [Google Scholar]
- 64.Hu R, Khor TO, Shen G, Jeong WS, Hebbar V, Chen C, Xu C, Reddy B, Chada K, Kong AN. Cancer chemoprevention of intestinal polyposis in apcmin/+ mice by sulforaphane, a natural product derived from cruciferous vegetable. Carcinogenesis. 2006;27:2038–2046. doi: 10.1093/carcin/bgl049. [DOI] [PubMed] [Google Scholar]
- 65.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 66.Myzak MC, Tong P, Dashwood WM, Dashwood RH, Ho E. Sulforaphane retards the growth of human pc-3 xenografts and inhibits hdac activity in human subjects. Exp Biol Med (Maywood) 2007;232:227–234. [PMC free article] [PubMed] [Google Scholar]
- 67.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, Stephenson KK, Wade KL, Ye L, Talalay P. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: A clinical phase i study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 68.Kensler TW, Chen J-G, Egner PA, Fahey JW, Jacobson LP, Stephenson KK, Ye L, Coady JL, Wang J-B, Wu Y, Sun Y, Zhang Q-N, Zhang B-C, Zhu Y-R, Qian G-S, Carmella SG, Hecht SS, Benning L, Gange SJ, Groopman JD, Talalay P. Effects of glucosinolate-rich broccoli sprouts on urinary levels of aflatoxin-DNA adducts and phenanthrene tetraols in a randomized clinical trial in he zuo township, qidong, people's republic of china. Cancer Epidemiol Biomarkers Prev. 2005;14:2605–2613. doi: 10.1158/1055-9965.EPI-05-0368. [DOI] [PubMed] [Google Scholar]