Abstract
BACKGROUND
The forkhead transcription factor Foxo3 is a master regulator and potent suppressor of primordial follicle activation. Loss of Foxo3 function in the mouse leads to premature ovarian failure due to global follicle activation. Here we show that the mouse Foxo3 locus is haploinsufficient, and that Foxo3−/+ females undergo early reproductive senescence consistent with an increased rate of primordial follicle utilization. Then, to determine if heterozygous or homozygous polymorphisms or mutations of the human orthologue FOXO3 contribute to premature ovarian failure (POF) or idiopathic primary amenorrhea (PA), we sequenced the exons and flanking splice sequences of the gene in a large number of women with idiopathic POF (N=273) or PA (N=29). A total of 8 single nucleotide polymorphisms (SNPs) were identified, revealing a substantial amount of genetic variation at this locus. Allelic frequencies in control samples excluded several of these variants as causal. For the remaining variants, site-directed mutagenesis was performed to assess their functional impact. These rare sequence variants were not associated with significant decreases in FOXO3 activity.
CONCLUSIONS
Taken together, our findings suggest that, despite the potential for FOXO3 haploinsufficiency to cause ovarian failure, FOXO3 mutations or common SNPs are not a common cause of either POF or PA.
Keywords: FOXO3, female infertility, premature ovarian failure, primary amenorrhea, menopause
Introduction
The median age at menopause is 51 years. However, approximately 1% of women develop ovarian insufficiency prior to the age of 40, and 0.1% prior to the age of 30 (Coulam et al., 1986). Based on these epidemiologic findings, premature ovarian failure (POF) is usually defined as the development of hypergonadotropic hypergonadism prior to the age of 40. POF results in amenorrhea/infertility, and has systemic consequences (such as reduced bone density) due to sex steroid deficiency (Kalantaridou and Nelson, 2000). POF has distinct etiologies, including chemo/radiotherapy, galactosemia, X-chromosome abnormalities, and autoimmune oophoritis (Aittomaki et al., 1995; Bione et al., 1998; Bione and Toniolo, 2000; Prueitt et al., 2002; Rossetti et al., 2004; Zinn and Ross, 2001). However, approximately 90% of POF cases are idiopathic. Case-control studies have shown that affected women have a much higher positive family history of POF than controls, suggesting the existence of as yet unknown hereditary factors influencing POF (Cramer et al., 1995; Pal and Santoro, 2002). Non-genetic factors (most significantly tobacco smoke) also influence menopausal age, demonstrating that POF may be influenced by a combination of hereditary and environmental influences (Cramer and Xu, 1996).
Biopsy studies have uniformly found that most POF cases are due to accelerated depletion of primordial follicles (Board et al., 1979; Russell et al., 1982; Starup and Sele, 1973; Zarate et al., 1970), indicating that POF, like physiologic menopause, in many cases is due to gradual loss and eventual depletion of primordial follicles. This suggests that intrinsic abnormalities of (or damage to) primordial follicles affecting their initial endowment or survival are the unifying pathophysiologic bases for POF. What mechanism(s) could account for accelerated depletion of primordial follicles in idiopathic POF? Defects in the normal physiologic processes that deplete primordial follicles likely play a major role. Two such mechanisms include: 1) oocyte apoptosis (Tilly, 2001), and 2) primordial follicle activation (PFA), also known as follicle initiation (McGee and Hsueh, 2000). Because both oocyte apoptosis and PFA occur continually prior to menopause, even subtle defects in either process could, over a woman’s lifetime, have a significant cumulative impact on primordial follicle numbers and therefore influence the age at menopause.
We have previously shown that the forkhead transcription factor Foxo3 is a master regulator and potent suppressor of PFA. Loss of Foxo3 function in the mouse leads to premature ovarian failure due to global PFA occurring almost immediately after the completion of primordial follicle assembly within a few days of birth (Castrillon et al., 2003). These findings raised the possibility that mutations or naturally occurring polymorphisms of the human orthologue FOXO3 (95% sequence identity at the amino acid level) might contribute to POF or idiopathic primary amenorrhea (PA), a related condition resulting from primordial follicle depletion prior to menarche (Nakano et al., 1982; Timmreck and Reindollar, 2003). Idiopathic POF and PA may thus be manifestations of fundamentally similar disease processes, with PA representing a more severe phenotype. Lastly, heterozygosity for FOXO3 mutations may be biologically significant given that a number of hereditary disorders are due to loss of function mutation of a single forkhead transcription factor allele (haploinsufficiency) (Crisponi et al., 2001; Erickson, 2001).
Here we address these questions in both the mouse and human. Lifetime serial breeding assays measuring fecundity and age of menopause were used to test whether Foxo3 is haploinsufficient (i.e. mutation of single allele has phenotypic consequences). This was followed by a comprehensive sequence analysis of the human FOXO3 coding sequence in over 300 women with POF or PA and an evaluation of mutations at this locus to determine whether they contribute to the etiology of these diseases.
Materials and methods
Serial breeding assay
Single pair matings were established between 3 week-old Foxo3+/− females and Foxo3+/+ siblings and six week-old FVB males to assess fertility. The generation and validation of this Foxo3 null allele (FVB background) has been previously described (Castrillon et al., 2003). A total of nine matings per genotype were initially established. Breedings were checked daily for births and all offspring were removed by postnatal day 3 to minimize stress on mothers. A priori, it was established that 1) matings were to be stopped after twelve weeks with no live births, 2) matings in which the female developed any serious disease or morbidity were to be censored from the analysis (this was the case for one Foxo3+/+ female that became lethargic at six months of age; necropsy revealed a large lung adenocarcinoma, a common spontaneous tumor in this genetic background) (Mahler et al., 1996).
Collection of premature ovarian failure DNA samples
Genomic DNA was isolated from blood leukocytes using standard methods. For the POF samples, ages ranged from 19 to 41. POF and PA cases were obtained in clinics from diverse geographical locations as follows: Italy (n=93), North America (n=164), France (n=25), Germany (n=10), and South Korea (n=10). The normal control samples (n=22) used to identify common sequence variants were of North American origin. All genomic DNA samples were unamplified with the exception of the Italian cohort, which was subjected to one round of whole genome amplification prior to PCR. These studies were performed following approval by review boards at each participating institution, and informed consent was obtained from all patients.
Human FOXO3 sequencing
The two coding exons were amplified from genomic DNA using two sets of primers: Exon 1 with FOXO3-E1F: GAGAGGAGAGCGCGAGAG and FOXO3-E1R: ACTCCGACGAATCCGAGAC, Exon 2 with FOXO3-E2F: TATATCATCTGGGTGCTCGGTTTT and FOXO3-E2R: CCCCTCATCCCCATATTGTTATT. Following a 10 min hot start, DNA was amplified for thirty five cycles (95° 30sec, 55.4° 30sec, 72° 2min) in 1× Buffer with 1.5mM MgCl2, 0.25mM dNTPs, 0.5μM each primer, 5% DMSO, ~50ng genomic DNA, and 5U Taq Gold, (Applied Biosystems) in a total volume of 50μl. The amplified samples were subjected to agarose gel electrophoresis to ensure single bands and then purified using the High Pure PCR Product Purification Kit (Roche). Samples were sequenced at the UTSW McDermott Sequencing Center using Big Dye Terminator 3.1 chemistry (Applied Biosystems Inc.) with the above primers and run on a 3730xl DNA analyzer (ABI). A third primer was also used to completely sequence exon 2 (FOXO3-E2M: AGTCCAGGACGATGATGCGC). Sequence analysis and heterozygous base calling was aided by Sequencher 4.6 (Genecodes) and Phred/Phrap/Consed (Ewing et al., 1998). All tracings were also visually inspected for quality assurance.
Site-directed mutagenesis and luciferase assays
Mutations in a wild-type FOXO3 cDNA were introduced using the Quick Change Site-Directed Mutagenesis Kit (Stratagene) per the manufacturer’s instructions. The resulting mutants were sequenced to confirm the introduction of the desired mutation. NIH3T3 cells (105/well) were seeded in 24 well plates and cultured for 24 hrs. The cells were then co-transfected with Firefly luciferase reporter construct (0.5 μg), tk-Rennila luciferase reporter construct (0.25 μg) and FOXO3 control and mutant constructs (0.25 μg). Following 36 hrs of transfection the cells were lysed in passive cell lysis buffer (100 μl), and luciferase activity was assayed using the Dual-Luciferase Reporter assay system (Promega) per the manufacturer’s instructions.
Results
Foxo3 haploinsufficiency in the mouse
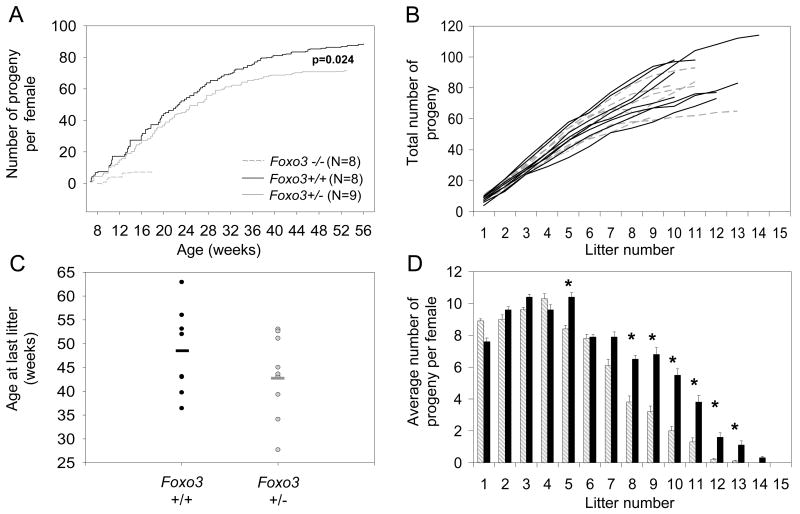
To explore the possibility of Foxo3 haploinsufficiency in mice, we performed lifetime serial breeding assays, a powerful tool for identifying subtle reproductive phenotypes, particularly those that relate to the utilization and survival of primordial follicles. Single pair matings were established prior to the onset of sexual maturity, and the total lifetime number of progeny for heterozygous and control sibling females was documented over time (see Methods). This analysis revealed a small, but statistically significant decrease in fecundity (p = 0.024, T-test) in Foxo3−/+ females relative to Foxo3+/+ siblings (Figure 1A). Importantly, plotting the data for individual females showed that this is an overall class effect, and not an incidental finding due to e.g. a single subfertile female (Figure 1B). Foxo3−/+ females also showed a trend towards earlier reproductive senescence (5 weeks earlier on average) as determined by comparing maternal ages at the time of their final live births (Figure 1C). This is preceded by a gradual decrease in fecundity/litter size (Figure 1D). Notably, however, this is associated with an inverse increase in the number of progeny initially (litter #1); although this effect is small in magnitude, it verges on statistical significance (p=0.059, one-sided T-test). This observation is consistent with the notion that Foxo3 heterozygosity results in a slight increase in the rate of PFA, particularly as PFA begins at the time of birth and thus has been in effect for ~9 weeks by the time of the first litter. Thus in summary, serial breeding assays identified a Foxo3 haploinsufficiency effect not detected in previous morphologic analyses (Castrillon et al., 2003), demonstrating that mutation of a single Foxo3 allele in mice results in a small but significant decrease in fecundity and acceleration of ovarian failure, consistent with an increased rate of PFA and utilization of primordial follicles.
Figure 1.
Foxo3 haploinsufficiency in the mouse. For all panels, gray = Foxo3 heterozygotes, black = sibling wild-type controls. (A) Serial breeding assays showing average number of progeny per female as a function of maternal age. (B) Individual line plots of same data in panel A showing that differences are a genotypic class effect. (C) Onset of reproductive senescence as determined by maternal age at the time of last live birth. Solid bars represent the median. (D) Average number of progeny per genotypic class showing age-dependent decrease in fecundity in Foxo3−/+ female mice. Timepoints for which p<0.05 threshold (T-test) are marked with an asterisk.
Case selection and direct PCR sequencing strategy
Cases spanned a wide range of ethnicities and geographical regions, including North America, Europe (France, Italy, and Germany), and South Korea (see Methods for details). Idiopathic POF cases (n=273 total) were defined by standard diagnostic criteria (amenorrhea =4 months prior to the age of 40, persistently elevated FSH in the menopausal range for the local laboratory), and no known cytogenetic abnormalities or history of chemotherapy/radiotherapy. PA cases (N=29) were similarly defined by standard clinical criteria including absence of known cytogenetic abnormalities. To identify single nucleotide polymorphisms (SNPs) common in the general population, 22 normal control samples were also sequenced.
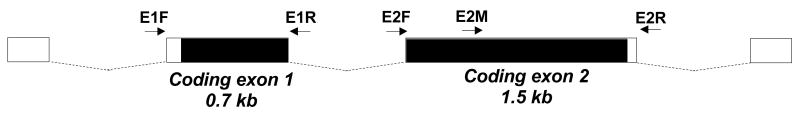
The human FOXO3 locus consists of 4 exons, only two of which are coding. Both coding exons were amplified from genomic peripheral lymphocyte DNA for direct PCR sequencing. To sequence the coding exon 1 PCR product (0.7 kb), the PCR primers were reused for sequencing reactions, whereas the coding exon 2 PCR product (1.5 kb) required sequencing with the PCR primers and an additional internal primer (Figure 2). All single nucleotide polymorphisms (SNPs) were confirmed on repeat PCR reactions.
Figure 2.
Schematic of the human FOXO3 gene and sequencing strategy. The FOXO3 gene (Chromosome 10 at location 42,304,235–42,365,032 [Ensembl v36]) consists of 2 noncoding exons (white boxes) and 2 coding exons (coding regions in black), referred to as coding exons 1 and 2 in this manuscript. The positions of the primers used for PCR amplification and sequencing are shown (arrows).
Identification of SNPs in the FOXO3 locus
Among the total of 273 and 29 POF + PA samples, 8 SNPs were identified in the coding portions of the 2 exons (Table 1). All 8 polymorphisms were heterozygous in the majority of cases where they were present although some of the more common SNPs were homozygous in a small number of patients. Of the 8 SNPs, 4 are predicted to alter a single amino acid in the FOXO3 protein, whereas the other 4 are silent and thus not likely to be causally related to POF/PA, although we do note that recent studies have demonstrated that, at least in rare cases, such noncoding changes can have substantial functional impact (Kimchi-Sarfaty et al., 2007). Of the 4 coding changes, one variant (p.Ala140Val) was also common in controls, and hence clearly not disease-causing. Only 1 of the 8 SNPs was identified in the Ensembl GeneSNPView database (Table 1).
Table 1.
FOXO3 sequence variants in POF/PA cases and controls. The incidence of homozygotes in disease cases is listed in parentheses if non-zero. Nucleotide positions correspond to reference cDNA sequence NM_001455 (NCBI); amino acid positions correspond to reference predicted protein NP_001446. SNP database searches were carried out using the Ensembl GeneSNPView search tool that compiles a number of SNP databases; the reference number in parentheses corresponds to the corresponding refSNP ID.
| Exon 1 | Exon 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Nucleotide position | c.159C>T | c.280C>T | c.281T>C | c.419C>T | c.504C>T | c.1021G>A | c.1156C>T | c.1857C>T |
| Reference sequence | GCC.GCC.GAC | CTC.CTT.GAG | CTC.CTT.GAG | GGG.GCG.GCT | ACC.CGC.GCC | GAT.GCG.CCT | AAC.CTC.ATG | GGG.AGC.TTG |
| Observed variation | GCC.GCT.GAC | CTC.TTT.GAG | CTC.CCT.GAG | GGG.GTG.GCT | ACC.CGT.GCC | GAT.ACG.CCT | AAC.TTC.ATG | GGG.AGT.TTG |
| Coding impact | SILENT | p.Leu94Phe | SILENT | p.Ala140Val | SILENT | p.Ala341Thr | p.Leu386Phe | SILENT |
| Incidence in disease cases | 25% (3/302)* | 1/302 | 1/302 | 9% (4%) | 3/302 | 2/302 (1/302) | 1/302 (1/302) | 4/302 |
| Incidence in controls | 18% | 0% | 0% | 27% | 0% | 0% | 0% | 0% |
| SNP databases | Y (rs11757217) | N | N | N | N | N | N | N |
| Causal? | No | ? | No | No | No | ? | ? | No |
The remaining three SNPs resulting in coding changes were rare among the disease samples, being present in only 1 or 2 patient samples (in all cases, in POF samples), and not identified in previous studies (Watkins et al., 2006). None were identified in controls, but this is not statistically significant, nor would analysis of additional disease samples be likely to be informative, given the rarity of these sequence variants. Thus, we subjected these three variants to further computational and functional analyses. Of the three variants, 2 were predicted by the Polyphen functional impact prediction algorithm to be benign, and 1 was possibly damaging, while the SIFT and SAPRED algorithms predicted all three variants to be neutral changes. All three variants had low Grantham distance scores, also consistent with neutral changes (Table 2).
Table 2.
Potentially disease-associated FOXO3 sequence variants. These correspond to the subset of sequence variants that are not silent or common in controls, and are indicated by a question mark in Table 1. Grantham scores (Grantham, 1974) were calculated using SAPRED (Ye et al., 2007). Other functional prediction methods were performed with SIFT and Polyphen algorithms (Ng and Henikoff, 2001) (Ramensky et al., 2002).
| Variant | SIFT prediction | SAPRED prediction | Polyphen Prediction | Grantham Score | Clinical Correlation | Zygosity |
|---|---|---|---|---|---|---|
| c.280C>T (p.Leu94Phe) | 0.13 (tolerated) | 0.19 (neutral) | 1.062 (benign) | 22 | PA (0) POF (1) | Het |
| c.1021G>A (p.Ala341Thr) | 0.54 (tolerated) | 0.46 (neutral) | 0.773 (benign) | 58 | PA (0) POF (1) | Het |
| c.1156C>T (p.Leu386Phe) | 0.08 (tolerated) | 0.24 (neutral) | 1.559 (possibly damaging) | 22 | PA (0) POF (1) | Het |
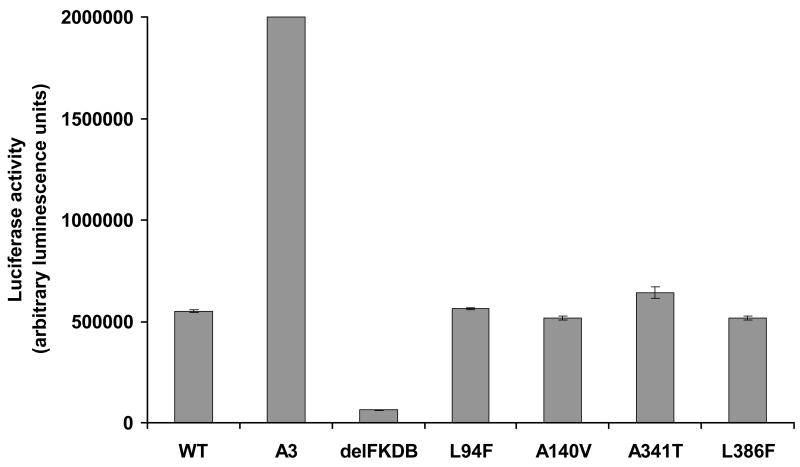
To more rigorously explore the potential functional impact of these amino acid variants, we performed site-directed mutagenesis of a wild-type FOXO3 cDNA and measured the activity of each variant through an in vitro system where a luciferase reporter is under the control of a promoter containing 8 tandem repeats of the FOXO DNA consensus binding element. In this reporter system, luciferase expression is dependent on FOXO activity (Brunet et al., 1999). Transfection of NIH3T3 cells with a control wild-type construct revealed baseline levels of activity, whereas a constitutively active variant in which the three AKT phosphorylation sites have been mutated (A3) showed dramatically increased activity. In contrast, a FOXO3 mutant bearing a deletion of the Forkhead DNA binding domain (delFKDB) showed negligible activity in this assay system. These controls thus fully validated this assay system. However, none of the four amino acid variants assayed (p.Leu94Phe, p.Ala140Val, p.Ala341Thr, or p.Leu386Phe) significantly affected FOXO3 activity, suggesting that these are rare, functionally normal coding variants. Taken together, this data suggest that FOXO3 coding sequence variants are not a common cause of POF or PA.
Discussion
Previous findings that Foxo3 inactivation in the mouse accelerates ovarian failure represent yet another intriguing connection between the Foxo forkhead transcription factors and aging, given the known roles of the Foxo factors in organismal stress responses, longevity, and survival (Castrillon et al., 2003; Tran et al., 2003). In considering the possibility of links between FOXO3 sequence variants and human disease, several observations pointed to the possibility that haploinsufficiency might have important phenotypic consequences. Haploinsufficiency for another forkhead transcription factor, FOXL2, results in the autosomal dominant BPES syndrome Type I, which is associated with eyelid anomalies and POF. Unlike ovaries from Foxo3 mice and most women with POF, biopsies of BPES ovaries show relatively normal numbers of growth arrested primordial follicles (Fraser et al., 1988), suggesting that these distantly related forkhead transcription factors have distinct roles in the control of primordial follicle growth. Mutations in FOXL2 have not been identified in idiopathic POF cases (De Baere et al., 2002). Nonetheless, from a broad perspective, these findings suggested that haploinsufficiency for strong FOXO3 mutations might similarly result in POF. Indeed, several hereditary, autosomal dominant syndromes are due to haploinsufficiency of forkhead transcription factors (Erickson, 2001). Furthermore, our demonstration of a small but significant Foxo3 haploinsufficiency effect in which Foxo3 heterozygous mice undergo accelerated reproductive senescence also lends credence to the notion that FOXO3 haploinsufficiency in women might accelerate the onset of ovarian insufficiency.
The absence of FOXO3 mutations in POF and PA, as documented in this and prior studies (Watkins et al., 2006) is somewhat surprising, particularly as the POF syndrome in Foxo3 null mice closely resembles human POF. It is possible that FOXO3 mutation has more severe phenotypic consequences in humans than mice (i.e. embryonic lethality). Perhaps more likely, functional redundancies among the human FOXOs mitigate against major phenotypes including ovarian failure. Although the human and mouse genomes share 3 FOXO orthologs (FOXO1/Foxo1, FOXO3/Foxo3, and FOXO4/Foxo4), it is also possible that these orthologs serve somewhat different, nonoverlapping functions in the two species. In mice, Foxo1 nullizygosity results in embryonic lethality (Hosaka et al., 2004; Paik et al., 2007), whereas Foxo4−/− mice are viable and fertile but exhibit defects in neointimal formation following aortic ligation (Li et al., 2007). In this regard, it is notable that Watkins et al. also screened POF patients for mutations in FOXO1 but failed to identify any definitively causal variants (Watkins et al., 2006), and disease-causing mutations of the FOXOs have not yet been reported (other than as reciprocal translocations in cancer) (Accili and Arden, 2004; Arden, 2007).
We note that some mutations might abrogate FOXO3 function in vivo yet not have a measurable impact on activity in our in vitro functional assay; for example, the FOXOs mediate numerous protein interactions not dependent on their function as transcriptional regulators (Greer and Brunet, 2005). Furthermore, our strategy would not detect FOXO3 copy number alterations that may result in POF/PA, a possibility suggested by our finding of Foxo3 haploinsufficiency in mice (Freeman et al., 2006). Future efforts to understand the hereditary basis of POF and PA will require systematic efforts to define the molecular components that regulate ovarian follicle formation and growth, followed by screening of large numbers of patients.
Figure 3.
Identified FOXO3 variants do not affect ability to transactivate reporter gene transcription. NIH3T3 cells were transiently co-transfected with FHRE-luc reporter construct (Brunet et al., 1999), a tk-Renilla luciferase reporter and the appropriate control or experimental FOXO3 cDNA. The cells were incubated for 36 hrs and luciferase activity assayed.
Acknowledgments
This work was supported in part by the Intramural Research Program of NICHD/NIH/DHHS (L.M.N.) and by an extramural NICHD/NIH grant (HD048690) to D.H.C. We thank Anne Brunet for the FOXO luciferase reporter construct and the FOXO wild-type and mutant control cDNA constructs. L.M.N. is a commissioned officer in the United States Public Health Service.
References
- Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. doi: 10.1016/s0092-8674(04)00452-0. [DOI] [PubMed] [Google Scholar]
- Aittomaki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, Kaskikari R, Sankila EM, Lehvaslaiho H, Engel AR, Nieschlag E, Huhtaniemi I, de la Chapelle A. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–968. doi: 10.1016/0092-8674(95)90275-9. [DOI] [PubMed] [Google Scholar]
- Arden KC. FoxOs in tumor suppression and stem cell maintenance. Cell. 2007;128:235–237. doi: 10.1016/j.cell.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Bione S, Sala C, Manzini C, Arrigo G, Zuffardi O, Banfi S, Borsani G, Jonveaux P, Philippe C, Zuccotti M, Ballabio A, Toniolo D. A human homologue of the Drosophila melanogaster diaphanous gene is disrupted in a patient with premature ovarian failure: evidence for conserved function in oogenesis and implications for human sterility. Am J Hum Genet. 1998;62:533–541. doi: 10.1086/301761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bione S, Toniolo D. X chromosome genes and premature ovarian failure. Semin Reprod Med. 2000;18:51–57. doi: 10.1055/s-2000-13475. [DOI] [PubMed] [Google Scholar]
- Board JA, Redwine FO, Moncure CW, Frable WJ, Taylor JR. Identification of differing etiologies of clinically diagnosed premature menopause. Am J Obstet Gynecol. 1979;134:936–944. doi: 10.1016/0002-9378(79)90869-x. [DOI] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science (New York, NY) 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–606. [PubMed] [Google Scholar]
- Cramer DW, Xu H. Predicting age at menopause. Maturitas. 1996;23:319–326. doi: 10.1016/0378-5122(96)00992-9. [DOI] [PubMed] [Google Scholar]
- Cramer DW, Xu H, Harlow BL. Family history as a predictor of early menopause. Fertil Steril. 1995;64:740–745. doi: 10.1016/s0015-0282(16)57849-2. [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet. 2001;27:159–166. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- De Baere E, Lemercier B, Christin-Maitre S, Durval D, Messiaen L, Fellous M, Veitia R. FOXL2 mutation screening in a large panel of POF patients and XX males. J Med Genet. 2002;39:e43. doi: 10.1136/jmg.39.8.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RP. Forkhead genes and human disease. J Appl Genet. 2001;42:211–221. [PubMed] [Google Scholar]
- Ewing B, Hillier L, Wendl MC, Green P. Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome research. 1998;8:175–185. doi: 10.1101/gr.8.3.175. [DOI] [PubMed] [Google Scholar]
- Fraser IS, Shearman RP, Smith A, Russell P. An association among blepharophimosis, resistant ovary syndrome, and true premature menopause. Fertil Steril. 1988;50:747–751. doi: 10.1016/s0015-0282(16)60309-6. [DOI] [PubMed] [Google Scholar]
- Freeman JL, Perry GH, Feuk L, Redon R, McCarroll SA, Altshuler DM, Aburatani H, Jones KW, Tyler-Smith C, Hurles ME, Carter NP, Scherer SW, Lee C. Copy number variation: new insights in genome diversity. Genome research. 2006;16:949–961. doi: 10.1101/gr.3677206. [DOI] [PubMed] [Google Scholar]
- Grantham R. Amino acid difference formula to help explain protein evolution. Science (New York, NY) 1974;185:862–864. doi: 10.1126/science.185.4154.862. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Hosaka T, Biggs WH, 3rd, Tieu D, Boyer AD, Varki NM, Cavenee WK, Arden KC. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2975–2980. doi: 10.1073/pnas.0400093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalantaridou SN, Nelson LM. Premature ovarian failure is not premature menopause. Ann N Y Acad Sci. 2000;900:393–402. doi: 10.1111/j.1749-6632.2000.tb06251.x. [DOI] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science (New York, NY) 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- Li H, Liang J, Castrillon DH, Depinho RA, Olson EN, Liu ZP. FoxO4 Regulates Tumor Necrosis Factor Alpha-Directed Smooth Muscle Cell Migration by Activating Matrix Metalloproteinase 9 Gene Transcription. Mol Cell Biol. 2007;27:2676–2686. doi: 10.1128/MCB.01748-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler JF, Stokes W, Mann PC, Takaoka M, Maronpot RR. Spontaneous lesions in aging FVB/N mice. Toxicologic pathology. 1996;24:710–716. doi: 10.1177/019262339602400606. [DOI] [PubMed] [Google Scholar]
- McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- Nakano R, Washio M, Hashiba N, Tojo S. Ovarian morphologic features and endocrine profile in amenorrheic patients. Gynecol Obstet Invest. 1982;14:19–31. doi: 10.1159/000299439. [DOI] [PubMed] [Google Scholar]
- Ng PC, Henikoff S. Predicting deleterious amino acid substitutions. Genome research. 2001;11:863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal L, Santoro N. Premature ovarian failure (POF): discordance between somatic and reproductive aging. Ageing Res Rev. 2002;1:413–423. doi: 10.1016/s1568-1637(02)00009-0. [DOI] [PubMed] [Google Scholar]
- Prueitt RL, Chen H, Barnes RI, Zinn AR. Most X;autosome translocations associated with premature ovarian failure do not interrupt X-linked genes. Cytogenet Genome Res. 2002;97:32–38. doi: 10.1159/000064052. [DOI] [PubMed] [Google Scholar]
- Ramensky V, Bork P, Sunyaev S. Human non-synonymous SNPs: server and survey. Nucleic Acids Res. 2002;30:3894–3900. doi: 10.1093/nar/gkf493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti F, Rizzolio F, Pramparo T, Sala C, Bione S, Bernardi F, Goegan M, Zuffardi O, Toniolo D. A susceptibility gene for premature ovarian failure (POF) maps to proximal Xq28. Eur J Hum Genet. 2004;12:829–834. doi: 10.1038/sj.ejhg.5201186. [DOI] [PubMed] [Google Scholar]
- Russell P, Bannatyne P, Shearman RP, Fraser IS, Corbett P. Premature hypergonadotropic ovarian failure: clinicopathological study of 19 cases. Int J Gynecol Pathol. 1982;1:185–201. doi: 10.1097/00004347-198202000-00006. [DOI] [PubMed] [Google Scholar]
- Starup J, Sele V. Premature Ovarian Failure. Acta Obstet Gynec Scand. 1973;52:259–268. [PubMed] [Google Scholar]
- Tilly JL. Commuting the death sentence: how oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2:838–848. doi: 10.1038/35099086. [DOI] [PubMed] [Google Scholar]
- Timmreck LS, Reindollar RH. Contemporary issues in primary amenorrhea. Obstet Gynecol Clin North Am. 2003;30:287–302. doi: 10.1016/s0889-8545(03)00027-5. [DOI] [PubMed] [Google Scholar]
- Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO’s road. Sci STKE 2003. 2003 doi: 10.1126/stke.2003.172.re5. [DOI] [PubMed] [Google Scholar]
- Watkins WJ, Umbers AJ, Woad KJ, Harris SE, Winship IM, Gersak K, Shelling AN. Mutational screening of FOXO3A and FOXO1A in women with premature ovarian failure. Fertil Steril. 2006;86:1518–1521. doi: 10.1016/j.fertnstert.2006.03.054. [DOI] [PubMed] [Google Scholar]
- Ye ZQ, Zhao SQ, Gao G, Liu XQ, Langlois RE, Lu H, Wei L. Finding New Structural and Sequence Attributes to Predict Possible Disease-Association of Single Amino Acid Polymorphism (SAP) Bioinformatics. 2007 doi: 10.1093/bioinformatics/btm119. [DOI] [PubMed] [Google Scholar]
- Zarate A, Karchmer S, Gomez E, Castelazo-Ayala L. Premature menopause. A clinical, histologic, and cytogenetic study. Am J Obstet Gynecol. 1970;106:110–114. [PubMed] [Google Scholar]
- Zinn AR, Ross JL. Molecular analysis of genes on Xp controlling Turner syndrome and premature ovarian failure (POF) Semin Reprod Med. 2001;19:141–146. doi: 10.1055/s-2001-15394. [DOI] [PubMed] [Google Scholar]