Abstract
Metabolic rate and parameters associated with oxidative stress were compared in two strains of mice, one of which, C57BL/6, exhibits an extension of life span in response to caloric restriction while the other, DBA/2, shows no such effect. Metabolic rate was higher in the DBA/2 than in the C57BL/6 mice, when measured at 5–6 months of age as in vivo and in vitro rates of oxygen consumption or body temperature. There were no remarkable inter-strain differences in activities of the antioxidant enzymes, superoxide dismutase, catalase and glutathione peroxidase or in the rates of mitochondrial superoxide anion radical generation in heart or skeletal muscles. Comparison of glutathione redox state in the heart and skeletal muscles at 3 and 20 months of age indicated that the amount of glutathione (GSH) and the GSH:GSSG (glutathione disulfide) ratio were relatively higher in the young DBA/2 mice, but there were no inter-strain differences in the older mice. The age-related elevation in the level of oxidative stress reflected by GSH:GSSG ratio was greater in the C57BL/6 than DBA/2 mice. The energy balance, indicated by the gain/loss in body weight per unit of food consumed, is higher in C57BL/6 than DBA/2 mice. It is hypothesized that the genotype-specific extension of life span by caloric restriction may involve modulation of oxidative stress produced as a result of an interplay between metabolic rate and energy balance during aging.
1. Introduction
Several different experimental approaches have been pursued to understand the nature of the mechanisms by which caloric intake affects longevity of animals. Most often ad-libitum (AL)-fed animals are compared with those fed a relatively lesser amount and, in general, such studies have indicated that most of the age-related alterations occurring in the AL-fed animals are attenuated or delayed in the calorie-restricted (CR) animals. Because the age-related changes that are modulated by CR far outnumber those that remain unaffected, the nature of the specific alterations, that are most directly associated with the longevity prolongation effect of CR, has remained obscure. Thus it seems that experimental approaches, in addition to the direct comparisons between AL and CR animals, are needed to gain further understanding of the physiological mechanisms mediating the effects of CR on longevity.
Despite repeated claims in the literature that CR prolongs longevity in all genotypes tested, there are several documentations to the contrary; e.g. the life span of house flies decreases in proportion to the reductions in the quantity of food consumed by the AL-fed flies (Cooper et al., 2004; Mockett et al., 2006). Even within the same species, the longevity effect of CR may vary; e.g. the C57BL/6 mice exhibit an approximately > 25% increase in average life span in response to 40% CR, whereas the DBA/2 mice show no notable impact of CR on longevity (Fernandes et al., 1976; Forster et al., 2003; Turturro et al., 1999). Thus the question arises: what are the physiological/ biochemical differences between these two strains of mice that determine the varying effect of CR on longevity?
On the basis of an analysis of the studies in the literature, Sacher (1977) proposed that energy conservation, i.e. the balance between food intake and metabolic expenditure, may play a critical role in determining the length of survival of an organism. There is now considerable evidence that the rate of oxygen utilization also affects the generation of reactive oxygen species (ROS) and the level of oxidative stress/redox state of cells (Ku et al., 1993; Sohal and Weindruch, 1996). Moreover, CR decreases the rates of oxygen consumption and ROS generation as well as the severity of macromolecular oxidative damage (Ferguson et al., 2007; Lass, 1998; Sohal et al., 1994a; Sohal et al., 1994b). Accordingly, to understand the respective roles of energy balance, oxidative stress and responsiveness to CR, a comparison of metabolic rate and parameters associated with the tissue redox state was made between the C57BL/6 mice, which show a CR- induced extension of life span, and the DBA/2 mice, in which CR has no discernable effect on longevity. Metabolic rate was measured as in vivo and in vitro rates of oxygen consumption and the core body temperature. The parameters measured for the redox state were: amounts and ratios of glutathione (GSH) and glutathione disulfide (GSSG); activities of the antioxidant enzymes, superoxide dismutase, catalase and glutathione peroxidase; and rates of mitochondrial superoxide anion radical generation in the heart and hind leg skeletal muscles.
2. Materials and Methods
2.1 Animals
Male DBA/2J and C57BL/6J mice, 8–9 weeks of age, were obtained from Jackson Laboratories (Bar Harbor, Maine, USA) and housed one per cage in solid bottomed polycarbonate cages with air-filtered tops, in the vivarium of the University of Southern California (USC). For studies on the levels of GSH and GSSG, an additional group of 14-month-old mice was obtained from the National Institute on Aging (NIA) and housed in the USC vivarium until approximately 20 months of age. All animals had free access to food (Harlan #7912) and water, were housed under a 12-h light/dark cycle, and were treated according to the NIH Guide for Humane Care and Use of Laboratory Animals. For the measurement of colonic temperature, 7-month-old male DBA/2 and C57BL/6 mice were obtained from the NIA and housed in the vivarium of the University of North Texas Health Science Center (UNTHSC) under conditions similar to those at USC.
2.2. Measurement of oxygen consumption
The rate of resting oxygen consumption was measured as described previously (Ferguson et al., 2007). Single mice were placed in a cylindrical polycarbonate respiration chamber (713 cm3) and desiccated air, minus the carbon dioxide, was pumped through the chamber at the rate of 200 ml•min−1. Fluctuations in oxygen concentrations were determined at 1 s intervals using an Oxzilla Dual Absolute and Differential Oxygen Analyzer (Sable Systems International, Las Vegas, NV). The rate of oxygen consumption was calculated over a 10–30 min period when the mouse was asleep, as indicated by inactivity, decreased oxygen consumption and stability of the recording line. Body weights were measured immediately before the mice were placed in the respiration chamber. The rate of resting oxygen consumption was normalized per gram of body weight for each mouse. Two mice, one of each strain, were tested per day and the sequence of testing was alternated.
2.3. Colonic temperature
Colonic temperature was measured at 4-h intervals over a 24-h period, using a Sensortech rectal probe (Thermalert model TH-6, Bailey Instr., Inc., Saddle Break, NJ). To minimize discomfort and stress and ensure accuracy of each measurement, only two of the time points were sampled within any 24-h period.
2.4. Preparation of tissue homogenates and isolation of mitochondria
Mice were euthanized by cervical dislocation and decapitation, and the organs were removed and placed in ice-cold antioxidant buffer (150 mM potassium phosphate, 2 mM EDTA, pH 7.4, 100 µM BHT). Heart and hind leg skeletal muscles were rinsed and finely chopped in antioxidant buffer and homogenized (5% w/v) in the appropriate isolation buffer (heart: 225 mM mannitol, 75 mM sucrose, 1 mM EGTA, pH 7.4; skeletal muscle: 120 mM KCl, 20 mM HEPES, 2 mM MgCl2, 0.5% (w/v) BSA, 1 mM EGTA, pH 7.4) with a Polytron (PT 1200) homogenizer. Mitochondria were isolated from the heart and hind leg skeletal muscle homogenates by differential centrifugation (Lash and Sall, 1993; Mela and Seitz, 1979). Briefly, heart homogenate was centrifuged at 700 g and the supernatant was centrifuged at 10,000 g for 10 min; the resultant mitochondrial pellet was re-suspended in the buffer. Skeletal muscle homogenate was centrifuged twice at 600 g for 10 min and the supernatants were filtered through 4 layers of cheesecloth and centrifuged at 17,000 g for 10 min; the resulting supernatant was discarded and the pellet suspended in a buffer containing 300 mM sucrose, 2mM Hepes and 0.1 mM EGTA, pH 7.4. This mixture was centrifuged at 1200 g for 10 min, pellet discarded, and the supernatant was centrifuged for another 10 min at 12,000 g; the resulting pellet was re-suspended in 300 mM sucrose, 2 mM HEPES, and 0.1 mM EGTA.
2.5. Cellular respiration
The rates of in vitro cellular respiration were determined using a Clark-type oxygen electrode connected to a computer-operated control unit (Hansatech Instruments, Norfolk, England). In all experiments, the temperature was maintained at 30°C and the total reaction volume was 1.0 ml. Tissue homogenate (400–700 µg protein) was added to the respiration buffer (120 mM KCl, 5 mM potassium phosphate, 3 mM HEPES, 1 mM EGTA, 1 mM MgCl2, and 0.2% BSA, pH 7.2), followed by the addition of substrates (10 mM glutamate plus 10 mM malate), allowing 1 min equilibration between each addition. The maximal rate of respiration was determined by adding 150 µM DNP (2,4-dinitrophenol) to the chamber after an initial recovery from an addition of 400 µM ADP. All measurements were made in triplicate within 1 h after death.
2.6. Activities of antioxidant enzymes
Enzyme activities were measured in supernatants of homogenates of tissues frozen at −80 C. To measure the activities of catalase and glutathione peroxidase (GSH-Px), tissues were homogenized (10% w/v) in 0.1% (v/v) triton x-100, and centrifuged at 3,000 and 18,000 g for 5 and 30 min, respectively. To measure superoxide dismutase (SOD) activity, tissues were homogenized (10% w/v) in 50 mM potassium phosphate buffer, pH 7.8, and centrifuged at 10,000 g for 10 min and the activity was determined according to Spitz and Oberley (1989). It involved the generation of O2·− by xanthine-xanthine oxidase and the reduction of nitroblue tetrazolium (NBT) to blue formazan. The inhibition of this reaction by the homogenates was monitored spectrophotometrically at 560 nm. The unit of SOD activity was defined as the amount of protein required for inhibition of NBT reduction by 50% in a reaction volume of 1 ml at 30°C. Catalase activity was measured by monitoring the disappearance of H2O2 at 240 nm, in 67 mM phosphate buffer, pH 7.0, , using an extinction coefficient of 0.036 µM −1cm−1 (Luck, 1965); unit of activity was defined as µmol of H2O2 consumed per min at 30°C. GSH-Px activity was determined by monitoring the oxidation of NADPH in a coupled reaction at 340 nm (Beutler, 1971); a unit of activity was defined as µmol of NADPH reduced per min at 37°C, using an extinction coefficient of 6.22 mM−1cm−1.
2.7. Mitochondrial superoxide anion radical production
The rate of superoxide anion radical (O2−.) generation was measured in submitochondrial particles (SMPs) as SOD-inhibitable reduction of acetylated ferricytochrome c, as described in Azzi et al. (1975) and Kwong et al. (2002). Mitochondria were prepared as described above, except that a modified buffer (300 mM sucrose, 30 mM nicotinamide, and 20 mM EDTA) was used for the isolation of heart mitochondria. The mitochondrial pellet was suspended prepared in a hypotonic buffer (30 mM potassium phosphate, pH 7.4) and sonicated (output power 40–60 watts, 30% duty cycle) using a Branson Sonifier 250 equipped with a microtip (Danbury, CT). The sonicated preparation was centrifuged at 5,000 g for 10 min and the pellet discarded; the supernatant was centrifuged at 100,000 g for 40 min and the resulting pellet was re-suspended in 30 mM potassium phosphate, pH 7.4.
2.8. Measurement of GSH and GSSG by HPLC with electrochemical detection
Tissues were homogenized in 10 vol of freshly prepared, ice-cold 5% (w/v) meta-phosphoric acid (MPA), kept in ice for 30 min and centrifuged at 18,000 g for 20 min at 4°C. Supernatants were filtered using 0.45 µm PTFE Acrodisc® CR 4 mm syringe filters, obtained from Gelman Laboratory (Ann Arbor, MI); filtrates were transferred to sampling vials and either analyzed immediately or stored at −80°C. Pellets from the acid precipitation were washed 3 times in 5% MPA to remove the free (non-protein bound) GSH and GSSG.
The procedure for detection and quantification of GSH and GSSG used here included the previously described precautionary measures to minimize spontaneous GSH oxidation to GSSG (Rebrin et al., 2004; Rebrin et al., 2003). Briefly, GSH and GSSG were separated by HPLC, fitted with a Shimadzu Class VP solvent delivery system, using a reverse phase C18 Luna (II) column (3µ; 4.6 × 150 mm), obtained from Phenomenex (Torrance, CA). The mobile phase for isocratic elution consisted of 25 mM monobasic sodium phosphate, 0.5 mM of the ion-pairing agent 1-octane sulfonic acid, 1% (v/v) acetonitrile, pH 2.7, adjusted with 85% phosphoric acid. The flow rate was 0.7 ml/min. Under these conditions, the separation was completed in 35 min; GSSG was the last eluting peak, with a retention time of approximately 30 min. Calibration standards were prepared in 5% (w/v) MPA. GSH and GSSG were detected with a model 5600 CoulArray® electrochemical detector (ESA Inc., Chelmsford, MA), equipped with a four-channel analytical cell, using potentials of +400, +600, +750 and +875 mV. GSH was monitored at +600 mV and GSSG at +750 mV. Each sample was injected twice, and the average of the peak areas was used for quantification.
Potentials of the glutathione redox couple in homogenates were calculated by using experimentally determined concentrations of GSH and GSSG in the Nernst equation (Schafer and Buettner, 2001), as follows:
E0 is the standard potential of GSH (−270 mV at pH 7.4 in homogenate), R is the gas constant (8.31 J/deg mol), T is the absolute temperature (310 K), n is the number of electrons transferred (2) and F is the Faraday constant (96,406 J/V).
2.9. Statistical analysis
Data on oxygen consumption (in vivo and in vitro), average colonic temperature, antioxidant enzyme activities, superoxide anion radical generation, and body/organ weights were analyzed using separate one-way analyses of variance. Levels of GSH, GSSG, and the GSH: GSSG ratios were evaluated using separate two-way analyses of variance, with Strain and Age as the factors. Colonic temperature was also considered in a two-way analysis of variance, with strain as a between-groups factor, and time of day as a within-groups factor. The planned comparisons between strain and age groups were made using single degree of freedom F-tests within the overall analyses. The difference in redox potential from 3 to 20 months was calculated for each strain and considered in a 1-way analysis of variance for each tissue examined (heart or skeletal muscle). Statistical significance was defined as p < 0.05. in all analyses.
3. Results and Discussion
3.1. Comparison of body and organ weights
The body weights of C57BL/6 and DBA/2 mice, measured at 5–6 months of age, were found to be similar (Table 1). The hearts also had similar weights in the two strains of mice, whereas the skeletal muscles, stripped from the hind legs, weighed 23% less in the DBA/2 compared to C57BL/6 mice.
Table 1.
Body and Organ Weights of C57BL/6 and DBA/2 mice (g. ± SEM)
| Mouse Strain | Whole Body | Heart | Skeletal muscle |
|---|---|---|---|
| C57BL/6 | 26.4 ± 0.7 | 0.136 ± 0.003 | 2.66 ± 0.067 |
| DBA/2 | 25.9 ± 1.3 | 0.149 ± 0.008 | 2.06 ± 0.073 * |
indicates p<0.05 when compared with C57BL/6
In a previous study, we reported that the amount of food consumed, monitored from 2 to 16 months of age, by the C57BL/6 and DBA/2 mice, fed ad-libitum, was the same (3.99 ± 0.1 gm/day/animal), however, the pattern of changes in body weight differed in the two strains (Ferguson et al., 2007). From 3 to 8 months of age, the body weights of mice of both strains were found to increase by similar amounts, but thereafter the weight of DBA/2 mice remained stable until 13 months of age and then declined gradually. In contrast, C57BL/6 mice progressively gained more weight and were 25% heavier than the DBA/2 mice by15 months of age. The tendency of C57BL/6 mice to gain relatively more body weight, while consuming the same amount of food as the DBA/2 mice, was also observed under conditions of caloric restriction. At 15 months of age, i.e. 12 months after the imposition of 40% reduction in the amount of food intake, the DBA/2 mice weighed 13% less than the CB57BL/6 mice even though the mice of the two strains consumed the same amount of food under the CR regimen. Thus, a distinct difference between these two strains is that while they have similar levels of appetite, that is food consumed under AL conditions, the gain in body weight or ‘energy balance’ is greater in C57BL/6 than the DBA/2 mice. Since the food intake by these two strains of mice is similar, the primary cause for the difference in the energy balance can be inferred to be the variation in the expenditure of metabolic energy.
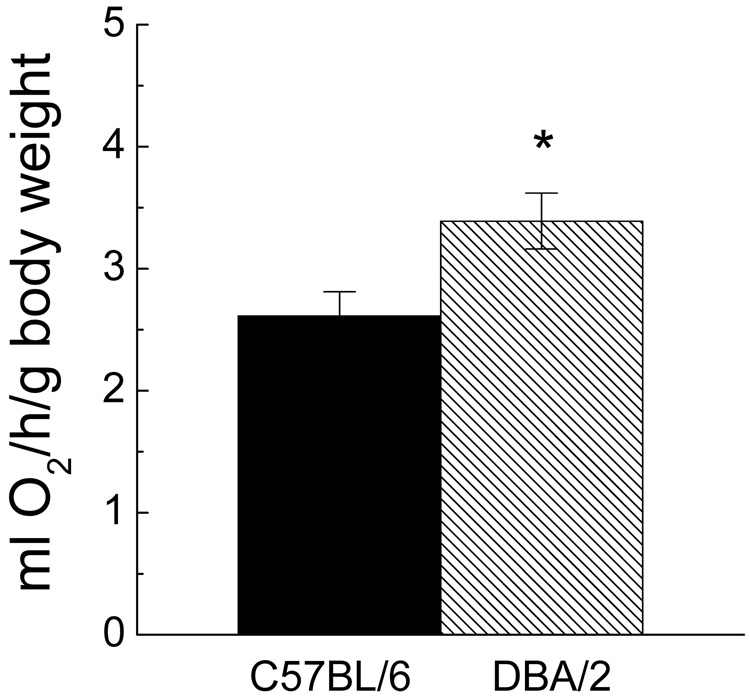
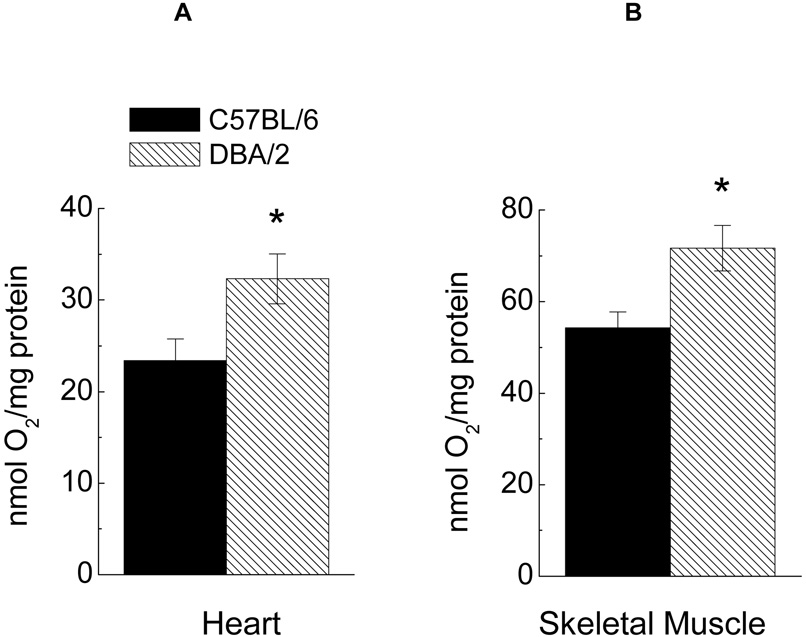
Accordingly, in the present study these two strains of mice were compared for their in vivo and in vitro rates of oxygen consumption and rectal temperature. The rate of resting oxygen consumption, measured at 5–6 months of age, was 30% higher in DBA/2 than the C57BL/6 mice (Fig. 1). The rates of cellular oxygen consumption in these mice were determined in homogenates of heart and hind leg skeletal muscles, with glutamate plus malate as the substrate and under conditions promoting the maximal potential rates, i.e. in the presence of ADP and/or DNP (Fig. 2). Initial studies indicated that activities of mitochondrial marker enzymes, citrate synthase and succinate dehydrogenase, were similar (data not shown), suggesting that the mitochondrial content of tissues was quite comparable in the two strains. Compared to C57BL/6 mice, the maximal rate of in vitro oxygen consumption in the DBA/2 was 24% and 27% higher in homogenates of the heart and skeletal muscles, respectively.
Fig. 1.
Resting rate of oxygen consumption per gram of body weight in 5 to 6-month-old DBA/2 and C57BL/6 mice. Data are presented as the mean ± SEM of 6 mice of each strain. (* Indicates p< 0.05 when compared with C57BL/6 in ANOVA).
Fig. 2.
Maximal rate of oxygen consumption measured in homogenates from heart (A) or hind limb skeletal muscle (B) of C57BL/6 or DBA/2 mice, after addition of 10 mM glutamate and 10 mM malate, 100 µM ADP and 150 µM DNP. Data are presented as the mean ± SEM of 4–7 mice of each strain.
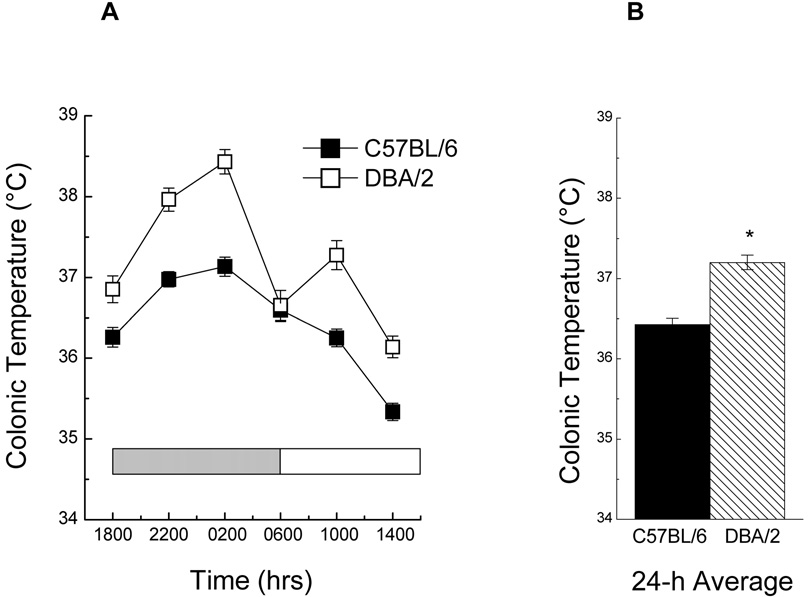
Measurements of rectal temperature, a surrogate indicator of the intensity of energy metabolism, were made at 4-h intervals over a 24-h period in 8-month-old mice (Fig. 3). Both strains showed circadian variations in temperature with a peak occurring between 2200 and 0200 h and a nadir at 1400 h. The average temperature of the DBA/2 mice over a 24-h period was 0.7°C higher than the C57BL/6 mice (Fig. 3B), a finding which accords with previous reports in the literature for mice of similar age (e.g., Rikke et al., 2003)
Fig. 3.
(A) Mean colonic temperature of 7-month -old C57BL/6 and DBA/2 mice when sampled at 4-h intervals, over a 24-h period, while the mice were on a 12-h light : dark cycle (indicated by shaded bars). (B) 24-h average temperature for each strain. Data are represented as the mean ± SEM (n = 25). (* Indicates p< 0.05 when compared with C57BL/6 in ANOVA).
The data on in vivo and in vitro rates of oxygen consumption and the core body temperature together support the inference that at 5–6 months of age, the C57BL/6 mice, which exhibit a CR- induced extension of life span, have a relatively lower rate of metabolism than the DBA/2 mice, whose longevity is not affected by CR. A previous study showed that the rate of metabolism of DBA/2 mice, measured as body temperature or respiration/g body weight, was higher than the C57BL/6 mice also at 15 months of age (Ferguson et al., 2007). Since these two strains of mice consume similar amounts of food throughout life and because the C57BL/6 mice gain relatively more weight with age than the DBA/2 mice, it can be suggested that the inter-strain difference in the energy balance emanates from the difference in their metabolic rate. A number of physiological differences between these two strains of mice have been noted that may have an impact on rate of metabolism. For instance, the DBA/2 mice secrete more insulin in response to glucose administration than the C57BL/6 mice. The latter are also relatively more susceptible to diet- and age- associated diabetes (Andrikopoulos et al., 2005).
Thus the question arises: Is CR-related extension of life span of C57BL/6 mice dependent upon a positive energy balance? Although a cause-and-effect relationship cannot be established on the basis of this study, results of a study by Ross et al. (1976) are deemed to be relevant in addressing this important issue. They reported that in an AL-fed population of rats, animals that tended to gain relatively more weight than that expected solely on the basis of their food intake, had a correspondingly shorter life span. Furthermore, retrospective analysis indicated that the positive energy balance, expressed as the time taken to double the body weight, was a significant predictor of life span. Thus it can be reasoned that if a positive energy balance under AL feeding conditions tends to have a life-shortening effect in a particular genotype, then, conversely, a CR-induced decrease in energy balance in such a genotype could have an opposing effect on longevity. It must be emphasized that in the present context the relationship between energy balance and life span is suggested to be a genotype-specific rather than a generalized phenomenon.
3.2 Comparison of oxidative stress- associated factors
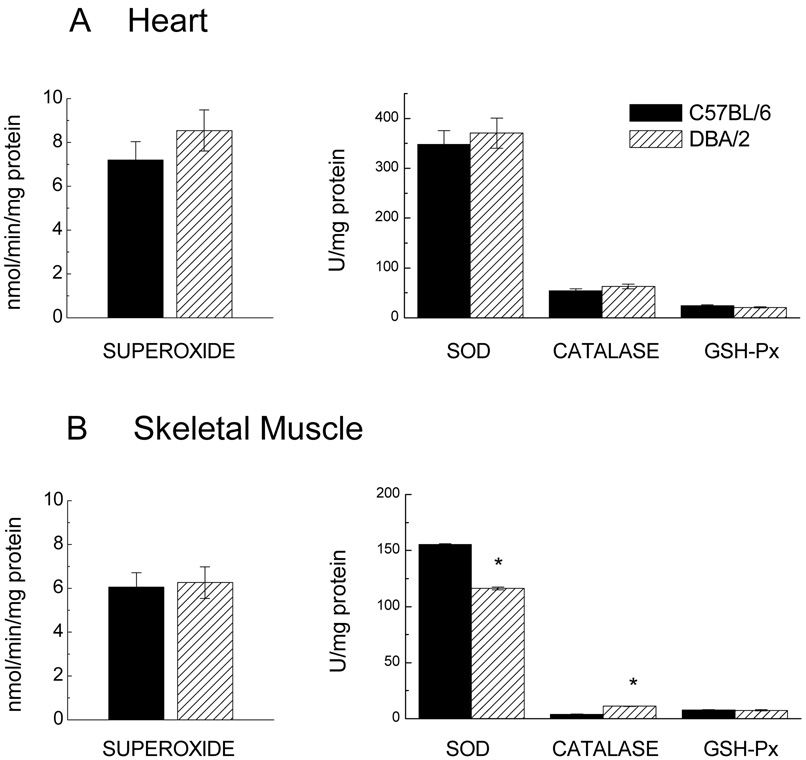
Besides metabolic rate, oxidative stress has also been implicated in the mechanisms by which CR modulates longevity in animals. Accordingly, an inter-strain comparison of activities of the antioxidant enzymes, superoxide dismutase (SOD), catalase and glutathione peroxidase (GSH-Px), and the rates mitochondrial superoxide anion radical generation was made in heart and hind leg skeletal muscles in 5–6 month old mice (Fig. 4). In addition, levels of glutathione (GSH) and glutathione disulfide (GSSG) were compared at 3 and 20 months of age. In heart homogenates, there were no significant inter-strain differences in the activities of any of the antioxidant enzymes (Fig. 4A). In the skeletal muscle homogenates of DBA/2 mice, SOD activity was 25% lower and catalase activity 181% higher than the C57BL/6 mice (Fig. 4B). There was no significant difference in GSH-Px activity between the two strains of mice in either of the two tissues.
Fig. 4.
Rate of generation of superoxide anion radicals and activities of antioxidant enzymes in heart (A) and skeletal muscle (B) of 5 to 6-month-old DBA/2 and C57BL/6 mice. Rates of superoxide anion radical generation (left) were measured in submitochondrial particles as the superoxide dismutase (SOD)-inhibitable reduction of acetylated ferricytochrome c. Data are presented as the mean ± SEM of 3 samples, each sample derived from tissues of two mice. Activity of SOD, catalase, and glutathione peroxidase (GSH-Px) (right) was measured in homogenates and presented as the mean ± SEM of 4–5 mice of each strain. (* Indicates p< 0.05 when compared with C57BL/6 in ANOVA).
The rate of superoxide anion radical production, determined in submitochondrial particles as SOD-inhibitable reduction of ferricytochrome, showed no significant inter-strain differences in the heart or skeletal muscles (Fig. 4).
The effects of age and mouse strain on GSH, GSSG, and GSH:GSSG ratio in the heart and skeletal muscle are depicted in Fig. 5. At 3 months of age, the GSH levels in the homogenates of both heart and skeletal muscles were about 15% lower in DBA/2 than the C57BL/6 mice (Fig. 5). At 20 months of age, a similar magnitude of difference in GSH level existed in the heart, but in skeletal muscles, the DBA/2 mice showed a slightly higher (6%) level of GSH than the C57BL/6 mice. This reflected the fact that only the C57BL/6 mice showed an age-related decline in skeletal muscle GSH; this effect was absent in the DBA/2 mice. A two-way analysis of variance indicated a significant main effect of Strain in both of the tissues, as well as a main effect of Age and a significant interaction between Age and Strain in the skeletal muscle (all ps<0.001).
Fig. 5.
Reduced glutathione (GSH), glutathione disulfide (GSSG) and the GSH:GSSG ratio in homogenates of heart (A) and skeletal muscle (B) of younger (3 mo) and older (20 mo) C57BL/6 and DBA/2 mice. Data are expressed as the mean ± SEM. of 5–8 mice of each strain. (* denotes p< 0.05 when compared with the young group of the same strain; ** indicates a significant difference from C57BL/6 of the same age. Individual comparisons were made within the Age × Strain interaction of each 2-way ANOVA).
The GSSG amounts increased with age by 11–16% in the heart and 100–105% in the skeletal muscles; however, there were no inter-strain differences at 3 or 20 months of age in either of these tissues. Analysis of variance indicated only a significant main effect of Age in both tissues (ps < 0.001).
The GSH: GSSG ratio is widely used as an indicator of the direction of the shift in the level of oxidative stress in tissues (de la Asuncion et al., 1996; Droge, 2002; Schafer and Buettner, 2001). GSH is oxidized to GSSG by direct interactions with ROS or by being a substrate for the enzymatic elimination of peroxides (reviewed in Dickinson and Forman, 2002; Reed, 1990; Schafer and Buettner, 2001). At 3 months of age, the GSH: GSSG ratio was found to be significantly lower (11–15%) in DBA/2 than the C57BL/6 mice in both heart and skeletal muscle; however, at 20 months of age, there were no significant inter-strain differences. This outcome reflected a relatively greater age-related decline in GSH: GSSG ratio in both tissues of C57BL/6 mice when compared with the DBA/2. Analysis of variance showed a significant main effect of Age and Strain in both tissues and a significant interaction between Age and Strain in the skeletal muscles (p < 0.05).
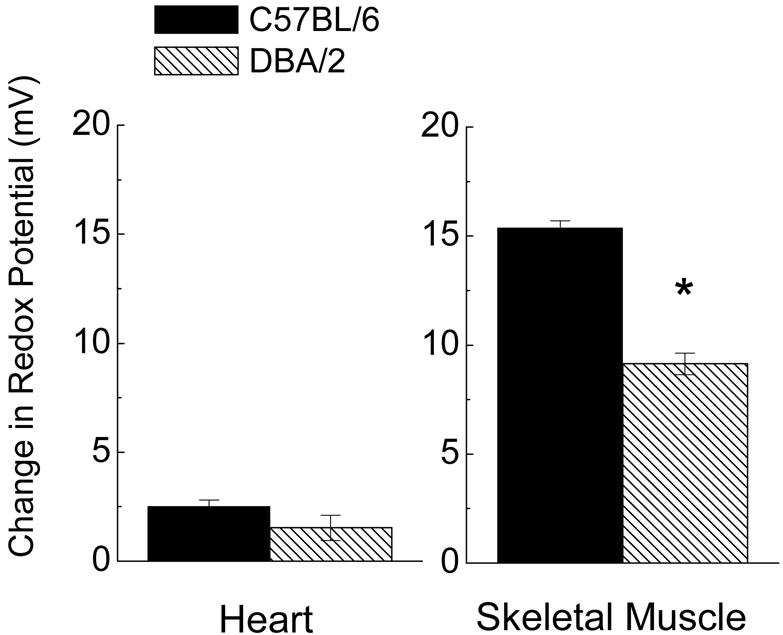
The glutathione redox potential was calculated for homogenates of heart and skeletal muscle to estimate the functional significance and relative magnitudes of the age-related pro-oxidizing shifts observed in the two mouse strains (Fig. 6). There was a distinct pro-oxidizing shift in the redox potential with age in both tissues. Overall, the age-related changes were several-fold greater in skeletal muscle (9–15 mV) when compared with the heart (< 3 mV). In the skeletal muscle homogenates, the age-related change in redox potential was 40% larger in C57BL/6 than in DBA/2 mice, a difference of 6.2 mV.
Fig. 6.
Comparison of the magnitude of the age-related pro-oxidizing shift in glutathione redox potential (mV± SEM) calculated from homogenates of heart (left) and skeletal muscle (right) in C57BL/6 and DBA/2 mice. Data shown represent the difference in mV at 20 months relative to the average potential at 3 months for each strain. (* denotes p< 0.05 when compared with C57BL/6).
Results of a previous study on the brains of these two strains of mice are in accordance with the present observations in heart and skeletal muscles. Rebrin et al. (2007) reported that the GSH:GSSG ratio in most regions of the brain was significantly lower in young DBA/2 than in C57BL/6 mice of the same age, however, the magnitude of the age-related declines in GSH:GSSG ratios was markedly greater in the C57BL/6 than DBA/2 mice. It thus seems that although at the young age, the tissues of the DBA/2 mice display a higher level of oxidative stress than the C57BL/6 mice, the extent of the age-related rise in oxidative stress is greater in the latter than in the former.
4. Speculations
Results of the present and previous studies collectively suggest that the mechanisms by which caloric restriction affects longevity of animals may involve a combination of factors, such as energy balance, rate of metabolism and oxidative stress. Energy balance, i.e., the difference between food intake and energy expended that is reflected as a gain or loss in body weight, is inversely related to life span (Ross et al., 1976). Besides being a modulator of energy balance, the rate of metabolism is also linked to the mitochondrial generation of ROS and oxidative stress (Sohal and Weindruch, 1996). Rates of metabolism of a set of mammalian species have been shown to be directly related to rates of mitochondrial production of superoxide anion radical and hydrogen peroxide (Ku et al., 1993). Furthermore, increased rates of oxygen consumption have been shown to enhance oxidative stress/damage (Yan and Sohal, 2000). Thus, while an increase in metabolic expenditure would counter-act the gain in energy balance, it would also tend to elevate the level of oxidative stress. Oxidative stress/damage has been demonstrated to be affected by the amount of food intake. Both short- and long-term CR have been shown to decrease the rate of ROS generation and/or macromolecular oxidative damage (Dubey et al., 1996; Forster et al., 2000; Sohal et al., 1994b).
Previous studies on C57BL/6 and DBA/2 mice suggest that the ability of CR to lower the metabolic rate does not necessarily lead to an extension of the life span. For example, the two strains show an equivalent effect of CR on the rate of oxygen consumption (Ferguson et al., 2007), yet CR prolongs the life span only in the C57BL/6 mice (Forster et al., 2003). These observations led us to propose that the ability of the DBA/2 mice to maintain a neutral energy balance throughout much of their life span was the most obvious strain-specific correlate of their resistance to the CR effect on longevity. The current studies suggest that ability of the DBA/2 mice to sustain this neutral energy balance may be due to the fact that they maintain a relatively higher strain-specific metabolic rate and body temperature, a characteristic that is indeed also associated with the expected higher level of oxidative stress. In spite of their relatively higher level of oxidative stress at young age, the ability of DBA/2 mice to maintain a relatively greater metabolic rate and neutral energy balance may confer some resistance to age-related increases in oxidative stress in this strain. In contrast, the C57BL/6 mice exhibit a sustained positive energy balance and a relatively greater age-related increase in oxidative stress. The demonstrations that the age-associated increase in steady-state level of oxidative stress, expressed by GSH;GSSG ratios, is greater in C57BL/6 than DBA/2 mice accords with this view. The application of CR regimens would thus appear to be most efficacious in the extension of life span when correcting the energy balance/diet-related increase in oxidative stress, as observed in the C57BL/6 mice. To conclude, although a cause-and-effect relationship has yet to be established, the present and previous studies tend to point to oxidative stress, generated by a positive energy balance, as a key factor linking the amount of food intake and life span.
Acknowledgements
This study was supported by the grant RO1 AG 13563 from the National Institute on Aging-National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrikopoulos S, Massa CM, Aston-Mourney K, Funkat A, Fam BC, Hull RL, Kahn SE, Proietto J. Differential effect of inbred mouse strain (C57BL/6, DBA/2, 129T2) on insulin secretory function in response to a high fat diet. J. Endocrinol. 2005;187:45–53. doi: 10.1677/joe.1.06333. [DOI] [PubMed] [Google Scholar]
- Azzi A, Montecucco C, Richter C. The use of acetylated ferricytochrome c for the detection of superoxide radicals produced in biological membranes. Biochem. Biophys. Res. Commun. 1975;65:597–603. doi: 10.1016/s0006-291x(75)80188-4. [DOI] [PubMed] [Google Scholar]
- Beutler E. Red Cell Metabolism: A Manual of Biochemical Methods. New York: Grune and Stratton; 1971. [Google Scholar]
- Cooper TM, Mockett RJ, Sohal BH, Sohal RS, Orr WC. Effect of caloric restriction on life span of the housefly, Musca domestica. FASEB J. 2004;18:1591–1593. doi: 10.1096/fj.03-1464fje. [DOI] [PubMed] [Google Scholar]
- de la Asuncion JG, Millan A, Pla R, Bruseghini L, Esteras A, Pallardo FV, Sastre J, Vina J. Mitochondrial glutathione oxidation correlates with age-associated oxidative damage to mitochondrial DNA. FASEB J. 1996;10:333–338. doi: 10.1096/fasebj.10.2.8641567. [DOI] [PubMed] [Google Scholar]
- Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann. N. Y. Acad. Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- Droge W. The plasma redox state and ageing. Ageing Research Reviews. 2002;1:257–278. doi: 10.1016/s1568-1637(01)00008-3. [DOI] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch. Biochem. Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Ferguson M, Sohal BH, Forster MJ, Sohal RS. Effect of long-term caloric restriction on oxygen consumption and body temperature in two different strains of mice. Mech. Ageing Dev. 2007;128:539–545. doi: 10.1016/j.mad.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc. Natl. Acad. Sci. U. S. A. 1976;73:1279–1283. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Morris P, Sohal RS. Genotype and age influence the effect of caloric intake on mortality in mice. FASEB J. 2003;17:690–692. doi: 10.1096/fj.02-0533fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Sohal BH, Sohal RS. Reversible effects of long-term caloric restriction on protein oxidative damage. J. Gerontol. A. Biol. Sci. Med. Sci. 2000;55:B522–B529. doi: 10.1093/gerona/55.11.b522. [DOI] [PubMed] [Google Scholar]
- Ku HH, Brunk UT, Sohal RS. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free. Radic. Biol. Med. 1993;15:621–627. doi: 10.1016/0891-5849(93)90165-q. [DOI] [PubMed] [Google Scholar]
- Kwong LK, Kamzalov S, Rebrin I, Bayne AC, Jana CK, Morris P, Forster MJ, Sohal RS. Effects of coenzyme Q(10) administration on its tissue concentrations, mitochondrial oxidant generation, and oxidative stress in the rat. Free. Radic. Biol. Med. 2002;33:627–638. doi: 10.1016/s0891-5849(02)00916-4. [DOI] [PubMed] [Google Scholar]
- Lash LH, Sall JM. Mitochondrial isolation from liver and kidney: strategy, techniques, and criteria for purity. In: Lash LH, Jones DP, editors. Methods in Toxicology: Mitochondrial Dysfunction. San Diego: Academic Press; 1993. pp. 8–12. [Google Scholar]
- Lass A, Sohal BH, Weindruch R, Forster MJ, Sohal RS. Caloric restriction prevents age-associated accrual of oxidative damage to mouse skeletal muscle mitochondria. Free Radic. Biol. Med. 1998;25:1089–1097. doi: 10.1016/s0891-5849(98)00144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mela L, Seitz S. Isolation of mitochondria with emphasis on heart mitochondria from small amounts of tissue. Methods Enzymol. 1979;55:39–46. doi: 10.1016/0076-6879(79)55006-x. [DOI] [PubMed] [Google Scholar]
- Mockett RJ, Cooper TM, Orr WC, Sohal RS. Effects of caloric restriction are species-specific. Biogerontology. 2006;7:157–160. doi: 10.1007/s10522-006-9004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Bayne A-CV, Mockett RJ, Orr WC, Sohal RS. Free aminothiols, glutathione redox state and protein mixed disulphides in aging Drosophila melanogaster. Biochem. J. 2004;382:131–136. doi: 10.1042/BJ20040506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Forster MJ, Sohal RS. Effects of age and caloric intake on glutathione redox state in different brain regions of C57BL/6 and DBA/2 mice. Brain Res. 2007;1127:10–18. doi: 10.1016/j.brainres.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebrin I, Kamzalov S, Sohal RS. Effects of age and caloric restriction on glutathione redox state in mice. Free. Radic. Biol. Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DJ. Glutathione: Toxicological implications. Annu. Rev. Pharmacol. Toxicol. 1990;30:603–631. doi: 10.1146/annurev.pa.30.040190.003131. [DOI] [PubMed] [Google Scholar]
- Rikke BA, Yerg JE, 3rd, Battaglia ME, Nagy TR, Allison DB, Johnson TE. Strain variation in the response of body temperature to dietary restriction. Mech. Ageing Dev. 2003;124:663–678. doi: 10.1016/s0047-6374(03)00003-4. [DOI] [PubMed] [Google Scholar]
- Ross MH, Lustbader E, Bras G. Dietary practices and growth responses as predictors of longevity. Nature. 1976;262:548–553. doi: 10.1038/262548a0. [DOI] [PubMed] [Google Scholar]
- Sacher GA. Life table modifications and life prolongation. In: Finch E, Hayflick L, editors. Handbook of the Biology of Aging. New York: Van Nostrand; 1977. pp. 582–638. [Google Scholar]
- Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free. Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Agarwal S, Candas M, Forster M, Lal H. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech. Ageing Dev. 1994a;76:215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Ku HH, Agarwal S, Forster MJ, Lal H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994b;74:121–133. doi: 10.1016/0047-6374(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian homogenates. Anal. Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J. Gerontol. A. Biol. Sci. Med. Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Yan L-J, Sohal RS. Prevention of flight activity prolongs the life span of the housefly, Musca domestica, and attenuates the age-associated oxidative damage to specific mitochondrial proteins. Free Radic. Biol. Med. 2000;29:1143–1150. doi: 10.1016/s0891-5849(00)00423-8. [DOI] [PubMed] [Google Scholar]