Abstract
Several polychlorinated biphenyls (PCBs) and their hydroxylated metabolites display axial chirality. Here we describe an enantioselective, gas chromatographic separation of methylated derivatives of hydroxylated (OH-)PCB atropisomers (MeO-PCB) using a chemically bonded β-cyclodextrin column (Chirasil-Dex). The atropisomers of several MeO-PCBs could be separated on this column with resolutions ranging from 0.42–0.87 under isothermal or temperature-programmed conditions. In addition, the enantiomeric fraction of OH-PCB 136 metabolites was determined in male and female rats treated with racemic PCB 136. The methylated derivatives of two OH-PCB 136 metabolites showed an enantiomeric enrichment in liver tissue, whereas PCB 136 itself was near racemic.
Keywords: polychlorinated biphenyls, metabolites, enantiomers, β-cyclodextrin
1. Introduction
Polychlorinated biphenyls (PCBs) are an important group of persistent environmental contaminants [1,2]. They were manufactured until the 1970s as complex mixtures containing over one hundred PCB congeners with different degrees of chlorination and substitution patterns. Seventy-eight of the 209 possible PCB congeners display axial chirality and exist as rotational isomers that are non-superimposable mirror images of each other [3]. Many chiral PCBs have been separated into their atropisomers by enantioselective HPLC at sub-ambient temperatures [4–6]; however, the pure atropisomers of most chiral PCBs racemize rapidly under ambient conditions because of the low energy barriers for rotation about the phenyl-phenyl bond [5]. Only nineteen PCB congeners with three or four ortho chlorine substituents exist as atropisomers that are stable at physiological temperature and under the temperature conditions employed for enantioselective gas chromatography. These chiral PCB congeners are not only of environmental relevance, but have also been associated with adverse (neuro-)developmental outcomes [3].
Similar to other chiral compounds, chiral PCBs can interact enantioselectively with biological macromolecules and, as a result of enantioselective biotransformation and/or transport processes, undergo enantiomeric enrichment in a variety of organisms, including fish, rodents and humans [3,7–19]. The processes responsible for the enantiomeric enrichment of PCBs in mammals are currently unknown, but enantioselective biotransformation by cytochrome P450 enzymes has been suggested a likely cause of the enantiomeric enrichment of PCBs. The corresponding hydroxyl (OH-) and methylsulfonyl (MeSO2-)PCB metabolites are also chiral and, in the case of MeSO2-PCBs, have been shown to be enantiomerically enriched in vivo. For example, MeSO2-PCB metabolites were significantly enriched in tissues and feces from rats [20,21]. Several studies have also reported the enantiomeric enrichment of MeSO2-PCB in wildlife [3,22–26].
A variety of stationary phases have been used to separate PCB and MeSO2-PCB atropisomers [3]. Initial studies employed triacetylcellulose and (+)-poly(tritylmethacrylate) as sorbents for the semi-preparative separation of PCB 88, 139 and 144 atropisomers. In subsequent studies, the semi-preparative, liquid chromatographic separation of many chiral PCB congeners has been accomplished using permethylated β-cyclodextrin, β-cyclodextrin and (+)-poly(diphenyl-2-pyridylmethyl methacrylate) chiral selectors. A number of functionalized β- and γ-cyclodextrin-based chiral selectors have been employed successfully for the gas chromatographic separation of PCB atropisomers. In addition, metabolites of chiral PCB congeners have been separated into their atropisomers using liquid or gas chromatography. For example, 4-OH- and 4-MeO-PCB 149 atropisomers have been separated using a permethylated β-cyclodextrin HPLC column [27]. Two commercially available β-cyclodextrin based columns, Chirasil-Dex [22] and BGB-172 [26], as well as several custom-made permethylated β-cyclodextrin columns with different concentrations of β-cyclodextrin and polarity of stationary phase [20,21,23–25,28] have been used for the separation of atropisomers of MeSO2-PCBs.
Unfortunately, little is known about the enantiomeric enrichment of OH-PCB metabolites in vivo. However, straightforward methods for the qualitative and quantitative analysis of OH-PCB atropisomers are of significant interest because of the potential (neuro-)developmental toxicity of these metabolites [29]. Herein we report first, the systematic investigation of the enantioselective separation of a series of methylated derivatives of chiral OH-PCBs; and second, the application of the optimized separation conditions to the analysis of the enantiomeric enrichment of selected metabolites in liver tissue samples.
2. Experimental
2.1. Reagents and materials
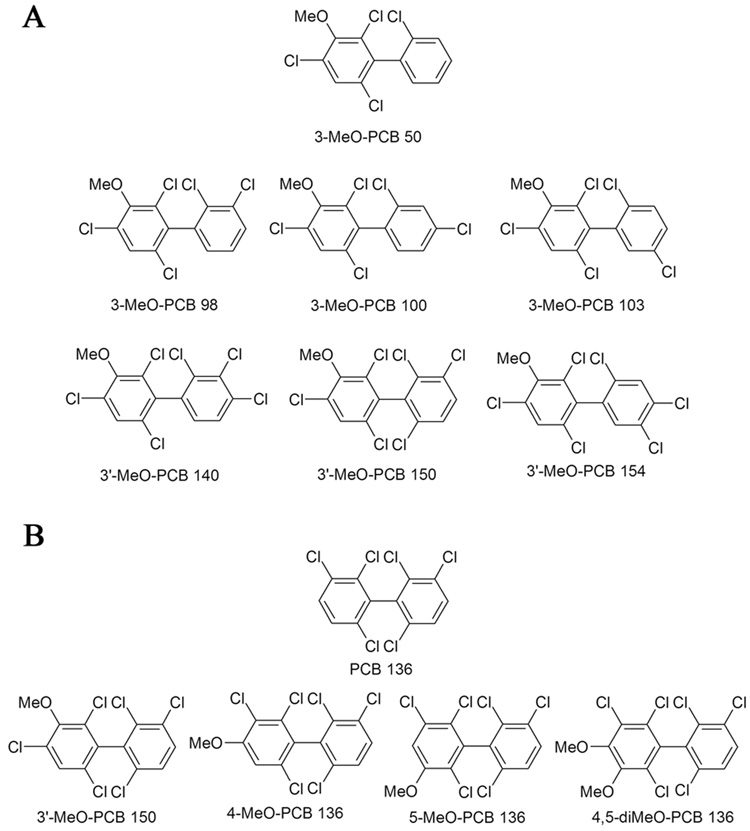
Florisil (60–100 mesh), absolute ethanol (200 proof, 99.5%), dimethylsulfoxide (anhydrous, 99.90%), hydrochloric acid, potassium hydroxide, potassium chloride, sodium sulfite, sulfuric acid, tetrabutylammonium sulfite and pesticide grade solvents were purchased from Fisher Scientific (Pittsburg, PA, USA). Diatomaceous earth (DE) was obtained from Dionex (Sunnyvale, CA, USA). The synthesis and characterization of the MeO-PCBs with a 3-MeO-2,4,6-trichloro substitution pattern (Fig. 1A) is described in the Supplementary Information [30,31]. The preparation of PCB 136 [32] and its mono- and di-OH metabolites as well as the corresponding methoxy (MeO-)derivatives [30] (Fig. 1B) has been described elsewhere. Diazomethane was synthesized from N-methyl-N-nitroso-p-toluenesulfonamide (Diazald) using an Aldrich mini Diazald apparatus (Milwaukee, WI, USA) following established procedures [33].
Fig. 1.
Structure and abbreviation of chiral MeO-PCBs studied: (A) MeO-PCB derivatives with a 3-MeO-2,4,6-trichloro substitution pattern and (B) PCB 136 and its MeO-derivatives. The MeO-PCB derivatives correspond to putative OH-PCB metabolites of chiral PCB congeners and are named as suggested by Maervoet and co-workers [51].
2.2. Enantioselective gas chromatography
All analyses were performed on Agilent 6890N gas chromatograph equipped with a with a 63Ni micro-electron-capture detection (µECD) system and a Chirasil-Dex CP capillary column (β-cyclodextrin chemically bonded to dimethylpolysiloxane, 25 m × 0.25 mm I.D., 0.25 µm film thickness; Varian, Palo Alto, CA, USA). The injector and detector temperatures were set to 250°C. The injector worked in splitless mode and 2 µl of the authentic, racemic MeO-PCB standards (approximately 100 ng/ml in hexane) were injected. This concentration was selected based on previous studies reporting levels of OH-PCB in blood of wildlife [34,35].
The gas chromatographic conditions (e.g., temperature program and helium flow) investigated for the enantioselective analysis of the MeO-PCBs are outlined below. A detailed summary of the effects of initial temperature, heating rate and helium flow on the retention time, elution temperature and resolution (Rs) of MeO-PCBs is provided in Supplementary Table S1. The resolution was calculated using the formula Rs = (tR2- tR1)/0.5 (BW1+BW2), where Rs is the resolution, tR1 and tR2 are the retention times of the first and second eluting enantiomer, and BW1 and BW2 are the baseline width of the first and second eluting enantiomer [36]. The average resolution for PCB 136 was 0.66. The enantiomeric fraction (EF) was calculated as EF=Area(1)/(Area(1)+Area(2)) for MeO-PCBs with an unknown elution order of the atropisomers or as EF=Area(+)-PCB136/(Area(+)-PCB136+Area(−)-PCB136) for PCB 136. The elution order of PCB 136 enantiomers was reported previously [37].
2.3. Animal treatment
All animal experiments and procedures were approved by the Institutional Animal Care and Use Committee at the University of Iowa, USA. Seven male and seven female Sprague-Dawley rats were obtained from Harlan (Indianapolis, IN, USA). The rats were 9 weeks old, with an average weight of 285.4 ± 5.4 g (males) and 188.2 ± 4.5 g (females). The animals were allowed to acclimatize for one week and then randomly assigned to control or treatment groups. Rats in the treatment groups (n=6 per gender) received two intraperitoneal injection of PCB 136 (2 × 100 µmol/kg body weight, 2 × 36.1 mg/kg body weight) dissolved in corn oil (5 ml/kg body weight) on day 1 and 4. The control animals (n=1 per gender) received two injections of corn oil (5 ml/kg body weight) on day 1 and 4. The animals were sacrificed on day 7, the liver was excised en bloc, the wet weight was determined and the tissues were stored at −20°C in aluminum foil prior to PCB analysis.
2.4. Extraction of liver samples
The liver samples (0.45–0.84 g) were mixed thoroughly with diatomaceous earth (2 g, Dionex). An aliquot of this liver-diatomaceous earth mixture (0.10–0.28 g) was extracted using a pressurized liquid extractor (ASE 200, Dionex). The samples were placed in extraction cells over Florisil (12 g, Thermo Fisher Sci, Waltham, MA, USA), spiked with PCB 166 (2,3,4,4’,5,6-hexachlorobiphenyl, 200 ng) and 4’-OH-PCB 159 (4-OH-2’,3,3’,4,5,5’-hexachlorobiphenyl, 137 ng) as recovery standards, and extracted with hexane-dichloromethane-methanol (48:43:9 v/v) at 100°C and 1500 psi using one static cycle of 5 min and 60% of the cell flush volume. The extract was evaporated to dryness under a gentle stream of nitrogen and reconstituted in 2 ml of hexane.
2.5. Separation and clean-up of PCB metabolites
The extracts were separated into PCB 136, MeSO2-PCB and OH-PCB fractions based on the procedure described previously [38]. In short, the phenolic fraction containing the OH-PCBs was separated from the neutral fraction containing PCB 136 and MeSO2-PCBs by partitioning into potassium hydroxide (0.5 M, 50% ethanol in water, 2 ml). The aqueous phase was then acidified with hydrochloric acid (2M, 0.5 ml) and re-extracted twice with hexane-methyl tert-butyl ether (9:1 v/v, 3 ml). The OH-PCBs were derivatized with diazomethane (diethyl ether solution, 0.5 ml). PCB 136 was separated from the MeSO2-PCBs fraction by partitioning the MeSO2-PCBs into dimethyl sulfoxide (anhydrous, 0.5 ml). The PCB and MeO-PCB fractions were subjected to a sulfuric acid clean-up according to US Environmental Protection Agency methods before GC-ECD analysis [13,39].
2.6. Gas chromatography
The concentration of PCB 136 and OH-PCBs (as MeO-PCB derivatives) in liver samples was determined with an Agilent 6890N gas chromatograph equipped with a 63Ni µ-ECD detector and SLB-5MS capillary column (60 m × 0.25 mm I.D., 0.25 µm film thickness; Supelco, St Louis, MO, USA) [39]. The injector and detector temperatures were 280°C and 300°C, respectively. The following temperature program was used for quantification of PCB 136: 10°/min from 80°C to 280°C, hold for 15 min. The MeO-PCBs were analyzed using a slightly modified temperature program: 100°C for 1 min, 5°/min to 250°C, hold for 20 min. In addition, the concentrations and enantiomeric fractions of the MeO-PCBs were determined using enantioselective gas chromatography as described above. The following temperature program was used for the enantioselective analysis of PCB 136: 80°C for 2 min, followed by 15°/min to 130°C, 1°/min to 190°C, hold for 30 min, 1°/min to 220°C [13]. PCB and OH-PCB concentrations were determine using PCB 204 (2,2’,3,4,4’,5,6,6’-octachlorobiphenyl) as internal standard.
2.7. Quality control
The detection limit of all analytes was determined from the respective calibration curves [40]. The limits of detection for PCB 136 and its MeO-PCB metabolites ranged from 9 to 44 pg (IDLPCB 136 = 9 pg, with a linear calibration range of 1–510 ng/ml (r2 = 0.9996); IDL3’-MeO-PCB 150 = 44 pg, with a linear calibration range of 1–1000 ng/ml (r2 = 0.9980); IDL4-MeO-PCB 136 = 14 pg, with a linear calibration range of 1–990 ng/ml (r2 = 0.9998); IDL5-MeO-PCB 136 = 12 pg, with a linear calibration range of 1–1000 ng/ml (r2 = 0.9999); IDL4,5-diMeO-PCB 136 = 28 pg, with a linear calibration range of 1–1000 ng/ml (r2 = 0.9992)).
The PCB 136 level in extraction blanks was 0.78±0.11 ng (n=3) and in control (untreated) animal liver was 5.9±1.0 ng/g wet weight (n=1 per gender). The MeO-PCB background level in blanks was 0.59±0.61 ng (n=3) and in control animal livers was 1.0±1.4 ng/g wet weight (n=1 per gender). The mean recovery rates were 109±8% (PCB 166) and 100±18% (4’-OH-PCB 159), respectively. The concentrations were corrected for recovery rates below 100% [13].
2.8. Statistical analysis
Unless stated otherwise, data are presented as mean ± standard deviation. Differences in the EF values in liver tissue and the racemic standards (EF=0.5) were tested using a one-sample, one-sided t-test at the 95% confidence level. Differences in the EF values in male and female rats were tested using a two sample t-test at the 95% confidence level. R open-source statistical software (version 2.0.0, http://www.r-project.org/index.html) was used for all statistical analyses.
3. Results and discussion
3.1. Selection and synthesis of chiral MeO-PCB derivatives
OH-PCB metabolites are typically methylated immediately after extraction from the respective biological matrix and analyzed in form of their MeO-PCB derivatives. A series of chiral MeO-PCBs was synthesized to investigate the gas chromatographic separation of the atropisomers [30,31]. The series of MeO-PCBs was designed to investigate how the substitution patterns in the methoxylated and the non-methoxylated phenyl ring influence the enantioselective separation (Fig. 1). Several of the MeO-PCBs selected for this study contained a 3-MeO-2,4,6-trichloro substitution pattern in one phenyl ring and different degrees of chlorination and substitution patterns in the second phenyl ring (Fig. 1A). The corresponding OH-PCBs are putative NIH-shift metabolites [41] of PCBs 45, 84, 91, 132, 136 and 149, respectively. In addition, the MeO-derivatives of several known PCB 136 metabolites were included in this study (Fig. 1B) [30,42]. These include PCB 136 metabolites with a OH group in meta and para position, the 3,4-diOH metabolite and the NIH shift product (3’-MeO-PCB 150).
3.2. Selection of the enantioselective GC phase
Permethylated β-cyclodextrin (PMCD)-based stationary phases have been used to separate the atropisomers of several PCB congeners by gas chromatography or HPLC [4,43]. In addition, 4-OH- and 4-MeO-PCB 149 has been separated by HPLC using a permethylated β-cyclodextrin stationary phase [27]. Based on these earlier studies we selected the Chirasil-Dex CP capillary column to investigate the gas chromatographic separation of the MeO-PCBs shown in Fig. 1. The Chirasil-Dex CP capillary column is commercially available and contains a permethylated β-cyclodextrin chemically bonded to dimethylpolysiloxane. As a result, this column has a long life time compared to other, non-bonded chiral GC columns, better temperature stability and efficiency [44] and, thus, is highly suitable for routine analyses of a large number of biological samples. One disadvantage of the Chirasil-Dex CP column is the comparatively strong interaction of the MeO-PCBs with the stationary phases, which results in longer retention times compared to the corresponding parent PCBs.
3.3. Isothermal separation of MeO-PCB atropisomers
The separation of the MeO-PCBs (Fig. 1) was initially investigated using isothermal conditions, with temperatures ranging from 140 to 180 °C. The run time and the helium flow of the isothermal experiments were limited to 4 h and 3 ml/min to obtain a practical analysis time. Although the rotational energy barriers for OH- and MeO-PCBs with three and four ortho chlorine substituents are currently unknown, they are most likely similar to the rotational energy barriers reported for analogous PCB congeners (Δ‡GPCB > 180 kJ/mol) [45,46]. Therefore, it is unlikely that racemization of OH- and MeO-PCB atropisomers occurs in the time and temperature range investigated.
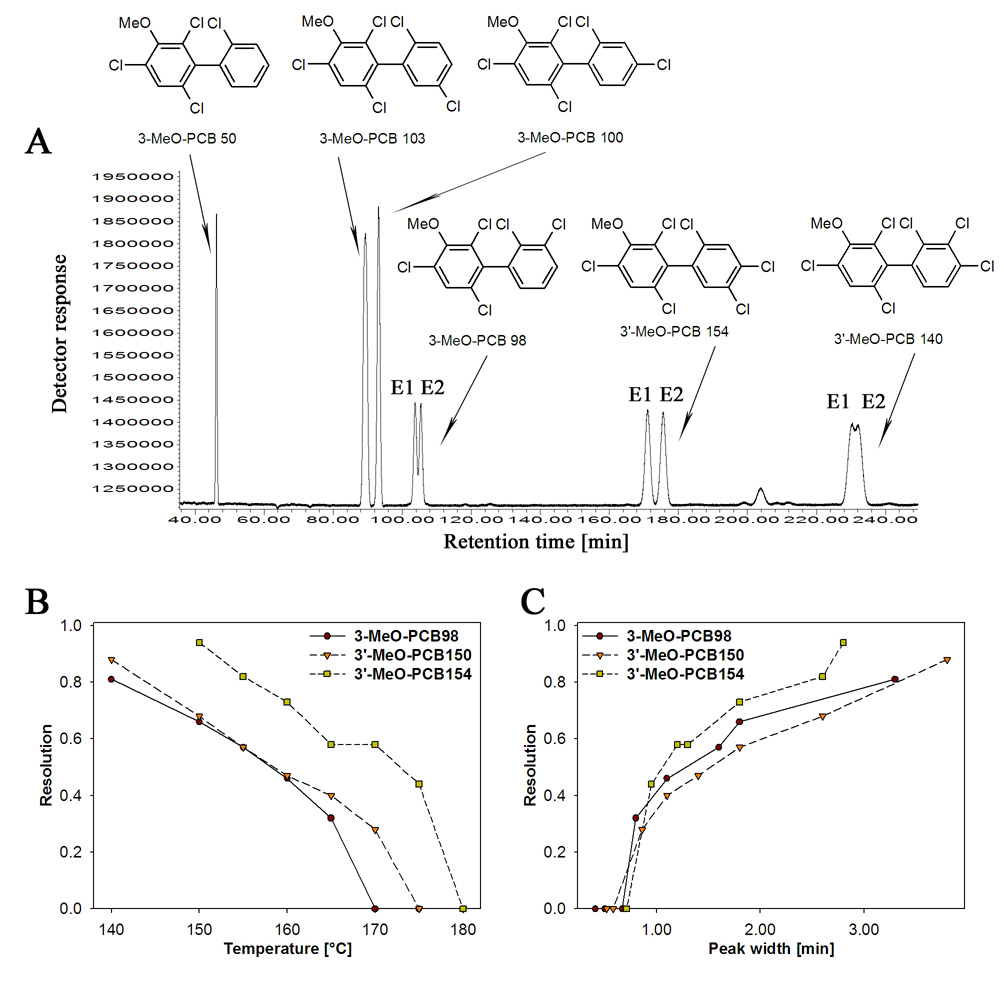
Several MeO-PCBs with a 3-MeO-2,4,6-trichloro substitution pattern (i.e., 3-MeO-PCB 98, 3’-MeO-PCB 140, 3’-MeO-PCB 150 and 3’-MeO-PCB 154) could at least be partially resolved on the Chirasil-Dex column (Fig. 2A and Fig.3A) at 150°C, whereas no enantiomeric separation was observed for 3-MeO-PCB 50, 3-MeO-PCB 103 and 3-MeO-PCB 100. The optimal resolution (i.e., maximal resolution with retention times < 4 h) for 3’-MeO-PCB 154 (Rs = 0.94), 3’-MeO-PCB 150 (Rs = 0.68), 3-MeO-PCB 98 (Rs = 0.66) and for 3’-MeO-PCB 140 (Rs = 0.39) was observed at this temperature. At 140°C, it was possible to partially resolve atropisomers of 3-MeO-PCB 103 (Rs = 0.44) and to obtain an improved resolution for 3-MeO-PCB 98 (Rs = 0.81) and 3-MeO-PCB 150 (Rs = 0.88). 3’-MeO-PCB 140 and 3’-MeO-PCB 154 did not elute at 140°C within 7 hours.
Fig. 2.
The separation of MeO-PCB atropisomers with a 3-MeO-2,4,6-trichloro substitution pattern improved with decreasing temperature and increasing peak half-width during isothermal gas chromatographic analysis. (A) Representative gas chromatogram at 150°C (see Fig. 3A for 3’-MeO-PCB 150), (B) temperature-dependent decrease in resolution and (C) peak half-width-dependent increase in resolution of selected 3-MeO-2,4,6-trichloro substituted PCBs. No resolution was observed for 3-MeO-PCB 50, 3-MeO-PCB 100, 3-MeO-PCB 103 and 3’-MeO-PCB 140 at 150°C. The gas chromatographic conditions were as follows: helium flow: 65 cm/s (3 ml/min); inlet and detector temperature: 250°C.
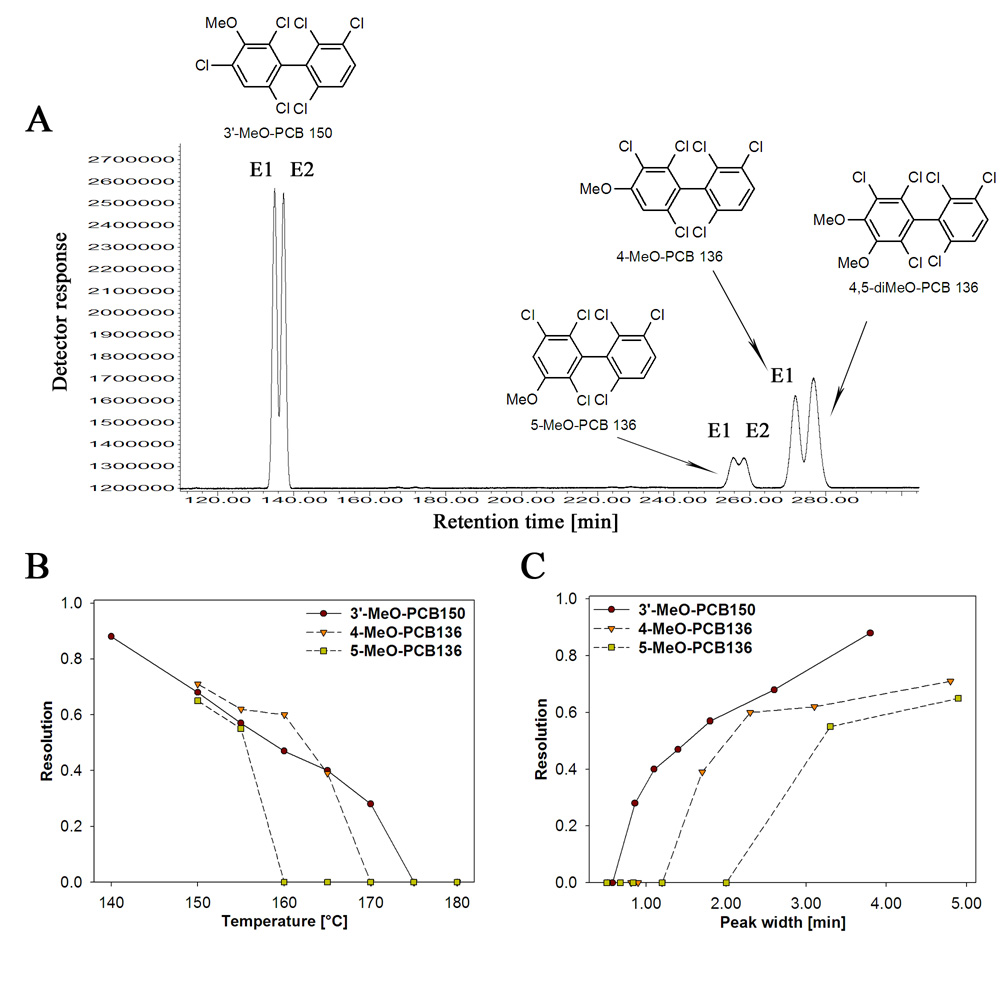
Fig. 3.
The enantioselective separation of chiral MeO-metabolites of PCB 136 improved with decreasing temperature and increasing peak half-width during isothermal gas chromatographic analysis. (A) Representative gas chromatogram at 150°C, (B) temperature-dependent decrease in resolution and (C) peak half-width-dependent increase in resolution of MeO-metabolites of PCB 136. Data for 3’-MeO-PCB 150 are also shown in Fig. 2. The data for non-resolved 4,5-diMeO-PCB 136 are omitted for clarity reasons in (B) and (C). The gas chromatographic conditions were as follows: helium flow: 65 cm/s (3 ml/min); inlet and detector temperature: 250°C.
The enantiomeric resolution increased linearly with decreasing temperature, with r2 ranging from 0.96 to 0.996 for the different MeO-PCBs (p≤0.001) (Fig. 2B). This inverse relationship between resolution and temperature is not surprising because the enantioselectivity of the separation is governed by the difference in the free enthalpy of the association complex between the chiral stationary phase and the PCB atropisomers and, thus, is a function of temperature [47]. The decrease in temperature was accompanied by elongated retention times and broadening of the peaks. The broadening of the peak was linearly correlated with increasing resolution (Fig. 2C, r2 from 0.86 to 0.96 at p≤0.01) over the concentration range investigated. It is important to note that this increase in peak half-width did not contribute to a decrease in resolution, even at the lowest temperature investigated (140°C). In contrast, some environmental contaminants, such as heptachlor, showed a decrease in resolution at lower temperatures due to increased peak broadening [47].
3.4. Isothermal separation of MeO-derivatives of PCB 136
The disposition of PCB 136 atropisomers has been investigated intensively in fish [7,17–19] and in mice [10,11,13,14]. However, little is know about the disposition of the OH-PCB 136 metabolites. Therefore, we investigated the separation of MeO-PCB 136 derivatives that, based on in vitro metabolism studies [30,42] correspond to OH-PCB 136 metabolites likely to be formed in vivo (Fig. 1B). In addition to 3’-MeO-PCB 150, the NIH-shift metabolite of PCB 136, the 4- and 5-MeO substituted metabolites of PCB 136 were resolved on Chirasil-Dex column (Fig. 3A), whereas 4,5-diMeO-PCB 136 was not resolved under any condition investigated. In addition, the diMeO-derivative co-elutes with the second eluting atropisomer of 4-MeO-PCB 136, making it impossible to analyze both compounds simultaneously using a single Chirasil-Dex CP column (Fig. 3). The optimal resolution was observed for 4-MeO-PCB 136 (Rs = 0.71) and 5-MeO-PCB 136 (Rs = 0.65) at 150°C. The resolution decreased with increasing temperature (Fig. 3B) and decreasing peak half-width (Fig. 3C). For 3’-MeO-PCB 136, these parameters were also well correlated with resolution (r2=0.997, p<0.001 for temperature and r2=0.96, p<0.001 for peak half-width). The correlations could not be calculated for 4-MeO-PCB 136 and 5-MeO-PCB 136 due to the small number of data points.
3.5. Temperature programmed atropisomer separation of MeO-PCBs
Although the best resolution of each MeO-PCB can be achieved under isothermal conditions [48] this approach is unlikely to result in an optimal resolution for a mixture containing several MeO-PCBs [47] – as would be the case during in vitro and in vivo metabolism studies. Also, isothermal separations at lower temperatures require long analysis times, which are not practical when many samples need to be analyzed. Therefore, we investigated different helium flow rates, temperature increments and initial temperatures in an effort to decrease the analysis time while ensuring the best resolution for each analyte.
Only a small effect of the flow rate on the resolution was observed for helium flow rates > 28 cm/s (see Supplementary Table S1 and Supplementary Figure S1). This is not surprising because the optimal flow rate for the enantioselective separation of PCBs is 30 to 40 cm/s [49]. However, the resolution decreased drastically at the lowest flow rate investigated (18 cm/s). Therefore, a flow rate of 65 cm/s was used for all subsequent experiments to obtain shorter retention times.
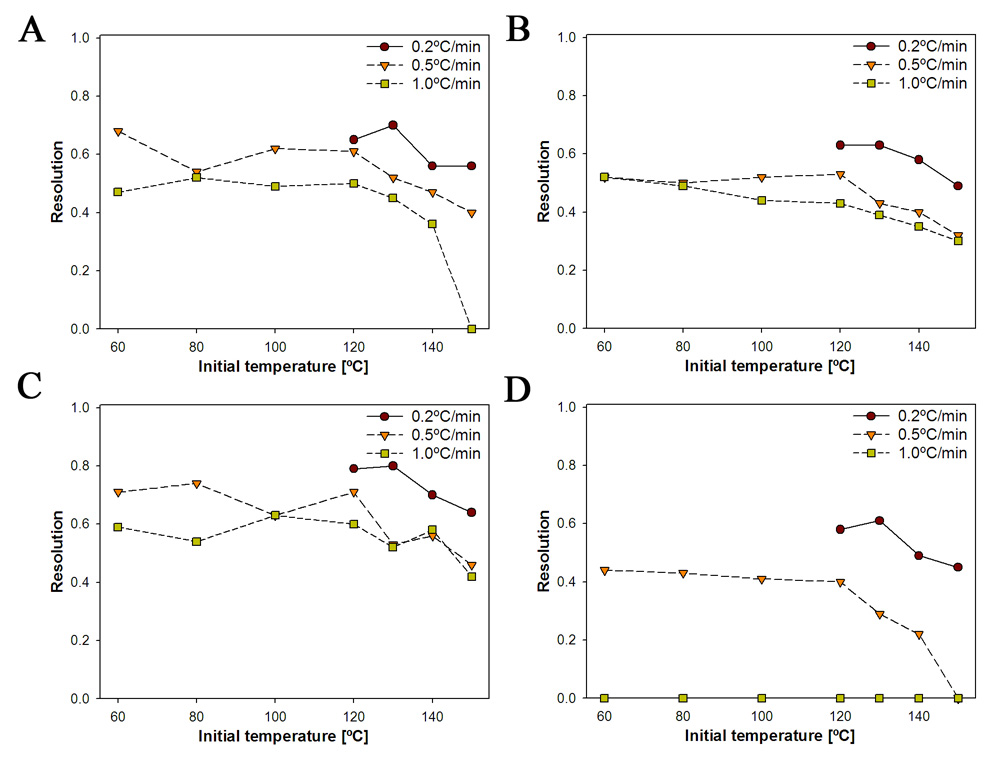
The resolution increased with a decrease in the temperature increment, with an increment of 0.2°/min giving the best resolutions for a given initial temperature (Fig. 4). The effect of the temperature increment appeared to depend on the chemical structure of the MeO-PCB. For example, 0.5°/min and 1.0°/min temperature increments had little effect on the resolution of 3-MeO-PCB 98, 3’-MeO-PCB 150 and 3’-MeO-PCB 154 (Fig. 4A–C), whereas the resolution of 4-MeO-PCB 136 dropped drastically with an increase in temperature increment at the same initial temperature (Fig. 4D).
Fig. 4.
The enantioselective separation of MeO-PCB atropisomers improved with decreasing initial temperature and smaller temperature increments. (A) 3-MeO-PCB 98; (B) 3’-MeO-PCB 150; (C) 3’-MeO-PCB 154; (D) 4-MeO-PCB 136. The gas chromatographic conditions were as follows: helium flow 65 cm/s (3 ml/min), inlet and detector temperature 250°C (Please see Supplementary Figure S2 for a plot of the resolution versus the temperature increment for different initial temperatures).
High initial temperatures (150°C) decreased the resolution, independent of the temperature increment employed (Fig. 4). However, the resolution increased with decreasing initial temperature and remained approximately constant at initial temperatures ≤ 120°C. The resolutions achieved with an initial temperature of 120°C and a temperature increment of 0.2°/min were comparable to the resolutions achieved under isothermal conditions. However, the retention times observed under these optimized conditions were longer compared to isothermal conditions.
3.6. Relationship between chemical structure and resolution of MeO-PCB atropisomers
PMCD-based chiral GC and HPLC columns, such as the Chirasil-Dex GC column and the Nucleodex β-PM HPLC columns, have been shown to separate several PCB congeners [5,37,43]. These studies suggest that there are some simple structural requirements that determine if a particular PCB congener is separated on a PMCD chiral selector. As a rule of thumb, the more lipophilic of the two phenyl rings of a PCB congener is thought to partition into the lipophilic cavity of the PMCD [5]. Assuming such a preferred PCB-PMCD interaction, a 2,3-substitution in the less lipophilic, free phenyl ring seems to be required for a PCB congener to be resolved [5].
This rule of thumb seems to analogously explain why some, but not all lower chlorinated MeO-PCBs can be separated on the Chirasil-Dex column. For example, in the case of tetra- and pentachloro MeO-PCBs, the 3-MeO-2,4,6-trichlorophenyl moiety is more lipophilic (assessed using the octanol-water partition coefficients of the corresponding benzene derivatives: log Kow(C6H2Cl3OCH3) = 4.0; log Kow(C6H3Cl3) = 3.9; log Kow(C6H4Cl2) = 3.3, as estimated using KOAWIN version 1.10 for EPI Suite version 3.20) and, therefore, expected to partition into the PMCD cavity. As a result, the substitution pattern of the non-methoxylated, free phenyl is expected to determine if a MeO-PCB can be separated by on PMCD selector. Consistent with this above mentioned structural requirements, tetra- and pentachloro MeO-PCBs without a 2,3-dichloro substitution pattern (i.e., 3-MeO-PCB 50, 3-MeO-PCB 100 and 3-MeO-PCB 103) were not resolved in our experiments, whereas 3-MeO-PCB 98 – the only tetra- or pentachloro MeO-PCB with such a substitution pattern - was resolved. However, further studies with a larger series of chiral MeO-PCBs are needed to confirm this explanation for the separation of some MeO-PCB atropisomers.
3.7. Enantioselective formation of MeO-metabolites of PCB 136
Several studies have reported an enrichment of one PCB atropisomer in vivo. For example, (+)-PCB 84 [15] and (+)-PCB 136 [10,11,13,14] was enriched in blood and tissues from mice, whereas (−)-PCB 136 was enriched in trout [18]. (+)-PCB 139 was also significantly enriched in liver from rats [16]. This enantiomeric enrichment is thought to be due to enantioselective metabolism of the parent compound to MeSO2- and OH-PCBs. This explanation is supported by several indirect observations. For example, PCB 136 atropisomers bind enantioselectively to hepatic cytochrome P450 enzymes [12]. Furthermore, several studies have shown that MeSO2-PCB metabolites are formed enantioselectively in rats [20,21]; however, it is unclear if the enantioselective formation of MeSO2-PCBs is indeed the result of enantioselective biotransformation in the liver. In contrast, the enantioselective formation of chiral OH-PCBs has not been demonstrated in vitro and in vivo.
Here we employ the enantioselective separation of MeO-PCBs, as described above, to investigate if OH-metabolites of PCB 136 (analyzed as their MeO-PCB derivative) are formed enantioselectively in the liver of rats treated with racemic PCB 136. In addition to PCB 136, we were able to detect and identify four OH-PCB 136 metabolites in the liver (Table 1). Based on earlier in vitro metabolism studies [30,42], these metabolites are expected to be formed in vivo. The levels of these OH-PCB metabolites were at least one order of magnitude lower compared to PCB 136 levels, and there were no significant differences in PCB and PCB metabolite levels between genders. This is not surprising based on earlier studies that showed no gender specific difference in the enantioselective disposition of PCB 136 [13].
Table 1.
Concentrations and enantiomeric fractions of PCB 136 and its OH-PCB metabolites in the liver of male and female ratsa,b
| PCB or OH-PCB metabolite | Male rats | Female rats | ||
|---|---|---|---|---|
| Concentrationc [ng/g wet weight] | Enantiomeric fractiond | Concentrationc [ng/g wet weight] | Enantiomeric fractiond | |
| PCB 136 | 5000±1600 | 0.49±0.01&& | 5000±1200 | 0.49±0.00&& |
| 3’-OH-PCB 150 | 19±3 | 0.41±0.03&&& | 14±3 | 0.23±0.02&&& |
| 4-OH-PCB 136 | 107±40 | n.d. | 61±12 | n.d. |
| 5-OH-PCB 136 | 530±230 | 0.36±0.03&&& | 341±120 | 0.38±0.03&&& *** |
| 4,5-diOH-PCB 136 | 104±30 | n.d. | 50±14 | n.d. |
Rats received two intraperitoneal injections of racemic PCB 136 (36 mg/kg body weight in corn oil, 100µmol/kg body weight) on day 1 and 4, and were sacrificed on day 7
OH-PCB 136 metabolites were quantified as the corresponding MeO-derivatives [38]
PCB 136 and its OH-PCB metabolites were quantified as described previously [39]
The enantiomeric fraction EF was calculated as EF=Area(1)/(Area(1)+Area(2)) for 3’-OH-PCB 150 and as EF=Area(+)-PCB136/(Area(+)-PCB136+Area(−)-PCB136) for PCB 136 [37]
n.d. = not determined due to coelution with another metabolite
different from racemic (EF = 0.50), one-sample, one-sided t-test, 95% confidence level, p<0.01
different from racemic (EF=0.50), one-sample, one-sided t-test, 95% confidence level, p<0.001
different from male animals, two sample t-test, 95% confidence level, p<0.001.
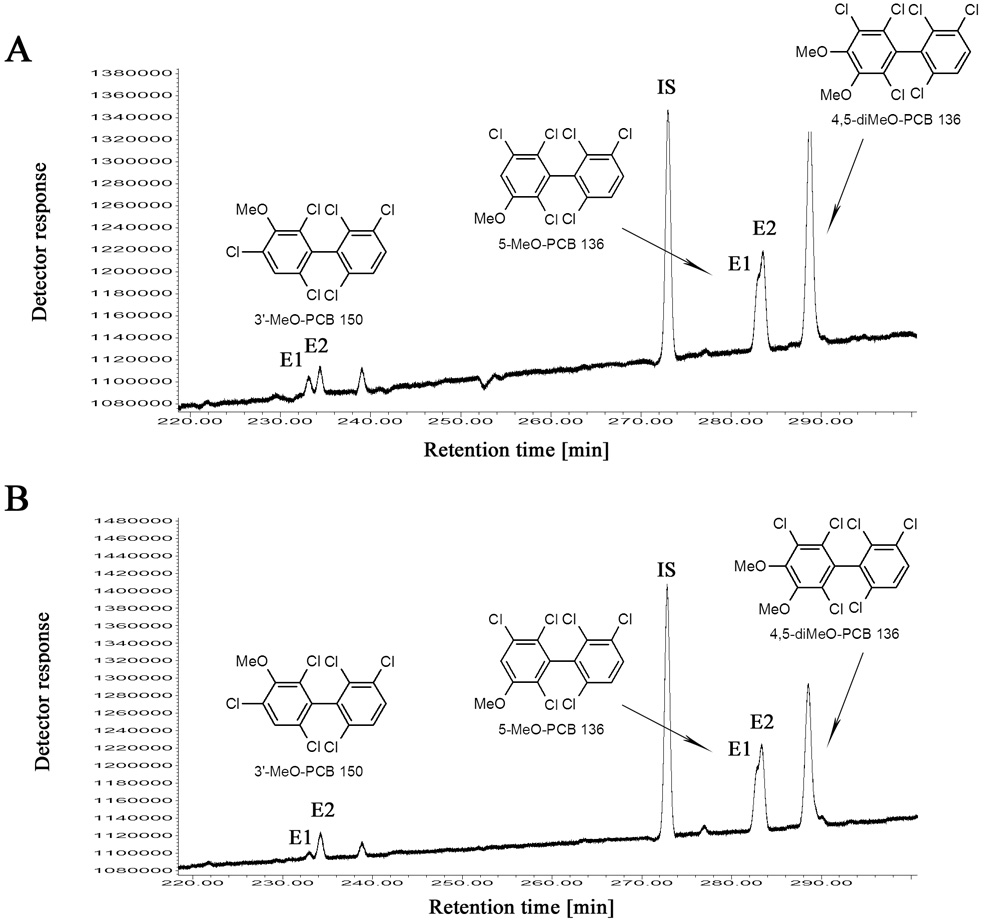
Enantioselective analysis showed that PCB 136 was near racemic in the liver of both male and female rats. Similarly, several studies administering racemic PCBs intraperitoneally to mice [13] or rats [9] show a less pronounced enantiomeric enrichment. Representative gas chromatograms of the OH-PCB metabolites (analyzed as their MeO-PCB derivative) from male and female rats are shown in Fig. 5. As discussed above, the methylated derivatives of 4-OH- and 4,5-diOH-PCB 136 co-elute on the Chirasil-Dex column. Therefore, we were not able to determine the EF of the methylated 4-OH-PCB 136 metabolite. However, the methylated 3-OH-PCB 136 and 3-OH-PCB 150 metabolites showed a clear enantiomeric enrichment (Fig. 5 and Table 1). There appears to be a small, gender specific difference in the EF values. These observations demonstrate for the first time that OH-PCB metabolites of PCB 136 are formed enantioselectively in the liver of male and female rats. Alternatively, the enantiomeric enrichment of the OH-PCB metabolites may be due to enantioselective transport processes and/or phase II metabolism of the OH-PCB. Similarly, Hühnerfuss and co-workers have reported that the enantiomeric enrichment of 3-MeSO2-PCB 149 in rats was due to enantioselective preferential transformation of an intermediate metabolite and not enantioselective phase I metabolism of PCB 149 [50].
Fig. 5.
The second eluting enantiomer (E2) of 3’-MeO-PCB 150 and 5-MeO-PCB 136 was enriched in the liver of male (A) and female (B) Sprague-Dawley rats. Rats received two intraperitoneal injection of racemic PCB 136 (2 × 100 µmol/kg body weight, 2 × 36.1 mg/kg body weight) dissolved in corn oil (5 ml/kg body weight) on day 1 and 4, and were euthanized on day 7. IS = internal standard (PCB 204).
4. Conclusions
Chiral OH-PCBs are analytes of interest for both environmental and toxicological studies. Because they are typically methylated with diazomethane prior to their gas chromatographic analysis, the present study investigated the enantioselective separation of a series of chiral MeO-PCB metabolites. Several hexachloro- and one pentachloro-MeO-PCB could be separated on the chemically bonded β-cyclodextrin Chirasil-Dex column, with resolutions ranging from 0.42–0.87 under isothermal and 0.42–0.79 under temperature programmed conditions. Similar to PCBs, the presence of a 2,3 substitution pattern in the less lipophilic phenyl ring (i.e., the ring that does not partition into the lipophilic cavity of the PMCD chiral selector) appeared to be a structural requirement for enantioselective separation on the Chirasil-Dex column. The enantioselective separation was used to study the enantiomeric enrichment of OH-PCB 136 in the liver of male and female rats. Two hydroxylated metabolites of PCB 136 were formed enantioselectively, whereas PCB 136 itself was near racemic. Overall, enantioselective gas chromatography with the Chirasil-Dex column can be used for further investigations of the enantioselective formation of OH-PCB metabolites in vitro and in vivo. One caveat of this approach is that the resolutions are relatively poor and the analysis is time consuming.
Supplementary Material
Acknowledgments
This work was supported by grants ES05605, ES013661 and ES012475 from the National Institute of Environmental Health Sciences, NIH. The OH- and MeO-metabolites of PCB 136 (3’-MeO-PCB 150, 4-MeO-PCB 136, 5-MeO-PCB 136) were a generous gift from E.A. Mash and S.C. Waller of the Synthetic Chemistry Facility Core of the Southwest Environmental Health Sciences Center, founded by NIH grant ES06694.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hansen LG. The Ortho Side of PCBs. Boston, MA: Kluwer; 1999. [Google Scholar]
- 2.Robertson LW, Hansen LG. PCBs: Recent Advances in Environmental Toxicology and Health Effects. Lexington, KY: University Press of Kentucky; 2001. [Google Scholar]
- 3.Wong CS. In: PCBs: Human and Environmental Disposition and Toxicology. Hansen LG, Robertson LW, editors. Urbana, IL: University of Illinois; 2008. [Google Scholar]
- 4.Haglund P. Chemosphere. 1996;32:2133. [Google Scholar]
- 5.Haglund P. J. Chromatogr. 1996;724:219. [Google Scholar]
- 6.Püttmann M, Oesch F, Robertson LW, Mannschreck A. Chemosphere. 1986;15:2061. [Google Scholar]
- 7.Buckman AH, Wong CS, Chow EA, Brown SB, Solomon KR, Fisk AT. Aquat. Toxicol. 2006;78:176. doi: 10.1016/j.aquatox.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Harrad S, Ren J, Hazrati S, Robson M. Chemosphere. 2006;63:1368. doi: 10.1016/j.chemosphere.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 9.Kania-Korwel I, Garrison AW, Avants JK, Hornbuckle KC, Robertson LW, Sulkowski WW, Lehmler H-J. Environ. Sci. Technol. 2006;40:3704. doi: 10.1021/es0602086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kania-Korwel I, Hornbuckle KC, Robertson LW, Lehmler H-J. Environ. Toxicol. Chem. 2007;27:299. doi: 10.1897/07-359R.1. [DOI] [PubMed] [Google Scholar]
- 11.Kania-Korwel I, Hornbuckle KC, Robertson LW, Lehmler H-J. Food Chem. Toxicol. 2008;46:637. doi: 10.1016/j.fct.2007.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kania-Korwel I, Hrycay EG, Bandiera S, Lehmler H-J. Chem. Res. Toxicol. 2008;21:1295. doi: 10.1021/tx800059j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kania-Korwel I, Shaikh N, Hornbuckle KC, Robertson LW, Lehmler H-J. Chirality. 2007;19:56. doi: 10.1002/chir.20342. [DOI] [PubMed] [Google Scholar]
- 14.Kania-Korwel I, Xie W, Hornbuckle KC, Robertson LW, Lehmler H-J. Arch. Environ. Contam. Toxicol. 2008 doi: 10.1007/s00244-007-9111-4. in press; [DOI] [PubMed] [Google Scholar]
- 15.Lehmler H-J, Price DJ, Garrison AW, Birge WJ, Robertson LW. Fresenius' Environ. Bull. 2003;12:254. [Google Scholar]
- 16.Püttmann M, Mannschreck A, Oesch F, Robertson L. Biochem. Pharmacol. 1989;38:1345. doi: 10.1016/0006-2952(89)90342-0. [DOI] [PubMed] [Google Scholar]
- 17.Wiberg K, Andersson PL, Berg H, Olsson P-E, Haglund P. Environ. Toxicol. Chem. 2006;25:1465. doi: 10.1897/05-329r.1. [DOI] [PubMed] [Google Scholar]
- 18.Wong CS, Lau F, Clark M, Mabury SA, Muir DCG. Environ. Sci. Technol. 2002;36:1257. doi: 10.1021/es0156791. [DOI] [PubMed] [Google Scholar]
- 19.Wong CS, Mabury SA, Whittle DM, Backus SM, Teixeira C, Devault DS, Bronte CR, Muir DCG. Environ. Sci. Technol. 2004;38:84. doi: 10.1021/es0346983. [DOI] [PubMed] [Google Scholar]
- 20.Norström K, Eriksson J, Haglund J, Silvari V, Bergman A. Environ. Sci. Technol. 2006;40:7649. doi: 10.1021/es061584t. [DOI] [PubMed] [Google Scholar]
- 21.Larsson C, Ellerichmann T, Hühnerfuss H, Bergman A. Environ. Sci. Technol. 2002;36:2833. doi: 10.1021/es025512n. [DOI] [PubMed] [Google Scholar]
- 22.Chu S, Covaci A, Haraguchi K, Voorspoels S, van de Vijver K, Das K, Bouquegneau J-M, de Coen W, Blust R, Schepens P. Environ. Sci. Technol. 2003;37:4573. doi: 10.1021/es034241t. [DOI] [PubMed] [Google Scholar]
- 23.Jorundsdottir H, Norström K, Olsson M, Pham-Tuan H, Hühnerfuss H, Bignert A, Bergman A. Environ. Poll. 2006;141:226. doi: 10.1016/j.envpol.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 24.Karasek L, Hajslova J, Rosmus J, Hühnerfuss H. Chemosphere. 2007;67:S22. doi: 10.1016/j.chemosphere.2006.05.081. [DOI] [PubMed] [Google Scholar]
- 25.Larsson C, Norström K, Athanansidais I, Bignert A, König WA, Bergman A. Environ. Sci. Technol. 2004;38:4950. doi: 10.1021/es049361v. [DOI] [PubMed] [Google Scholar]
- 26.Wiberg K, Letcher R, Sandau CD, Duffe J, Norstrom R, Haglund P, Bidleman TF. Anal. Chem. 1998;70:3845. doi: 10.1021/ac980064g. [DOI] [PubMed] [Google Scholar]
- 27.Pham-Tuan H, Larsson C, Hoffmann F, Bergman A, Froba M, Hühnerfuss H. Chirality. 2005;17:266. doi: 10.1002/chir.20158. [DOI] [PubMed] [Google Scholar]
- 28.Ellerichmann T, Bergman A, Franke S, Hühnerfuss H, Jakobsson E, König WA, Larsson C. Fres. Environ. Bull. 1998;7:244. [Google Scholar]
- 29.Kodavanti PRS. In: Molecular Neurotoxicology: Environmental Agents and Transcription-Transduction Coupling. Zawia NH, editor. Boca Raton, FL: CRC Press; 2004. p. 151. [Google Scholar]
- 30.Waller SC, He YA, Harlow GR, He YQ, Mash EA, Halpert JR. Chem. Res. Toxicol. 1999;12:690. doi: 10.1021/tx990030j. [DOI] [PubMed] [Google Scholar]
- 31.Bergman Å, Klasson Wehler E, Kuroki H, Nilsson A. Chemosphere. 1995;30:1921. [Google Scholar]
- 32.Shaikh N, Parkin S, Lehmler H-J. Organometallics. 2006;25:4207. [Google Scholar]
- 33.Black TH. Aldrichim. Acta. 1982;15:3. [Google Scholar]
- 34.Houde M, Pacepavicius G, Wells RS, Fair PA, Letcher RJ, Alaee M, Bossart GD, Hohn AA, Sweeney J, Solomon KR, Muir DCG. Environ. Sci. Technol. 2006;40:5860. doi: 10.1021/es060629n. [DOI] [PubMed] [Google Scholar]
- 35.Sandala GM, Sonne HC, Dietz R, Muir DCG, Valters K, Bennett ER, Born EW, Letcher RJ. Sci. Total Environ. 2004;331:125. doi: 10.1016/j.scitotenv.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 36.IUPAC Compendium of Chemical Terminology. International Union of Pure and Applied Chemistry. 1997 [Google Scholar]
- 37.Haglund P, Wiberg K, High Resol J. Chromatogr. 1996;19:373. [Google Scholar]
- 38.Hovander L, Linderholm L, Athanasiadu M, Bignert A, Fangstrom B, Kocan A, Petrick J, Trnovec T, Bergman A. Environ. Sci. Technol. 2006;40:3696. doi: 10.1021/es0525657. [DOI] [PubMed] [Google Scholar]
- 39.Bunaciu PR, Tharappel JC, Lehmler H-J, Kania-Korwel I, Robertson LW, Srinivasan C, Spear BT, Glauert HP. Toxicology. 2007;239:147. doi: 10.1016/j.tox.2007.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loconto PR. Trace Environmental Quantitative Analysis. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 41.Guroff G, Daly JW, Jerina DM, Renson J, Witkop B, Udenfriend S. Science. 1967;157:1524. doi: 10.1126/science.157.3796.1524. [DOI] [PubMed] [Google Scholar]
- 42.Schnellmann R, Putnam C, Sipes I. Biochem. Pharmacol. 1983;32:3233. doi: 10.1016/0006-2952(83)90209-5. [DOI] [PubMed] [Google Scholar]
- 43.Wong CS, Garrison AW. J. Chromatogr. A. 2000;866:213. doi: 10.1016/s0021-9673(99)01104-8. [DOI] [PubMed] [Google Scholar]
- 44.Schurig V. J. Chromatogr. 2001;906:275. doi: 10.1016/s0021-9673(00)00505-7. [DOI] [PubMed] [Google Scholar]
- 45.Harju MT, Haglund P. Fres. J. Anal. Chem. 1999;364:219. [Google Scholar]
- 46.Schurig V, Reich S. Chirality. 1998;10:316. [Google Scholar]
- 47.Vetter W. Food Rev. Int. 2001;17:113. [Google Scholar]
- 48.Vetter W, Klobes U, Luckas B, Hottinger G. J. Chromatogr. A. 1999;846:375. [Google Scholar]
- 49.Ali I, Aboul-Enein HY. Chiral Pollutants: Distribution, Toxicity and Analysis by Chromatography and Capillary Electrophoresis. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- 50.Hühnerfuss H, Bergman A, Larsson C, Peters N, Westendorf J. Organohalogen Compd. 2003;62:265. [Google Scholar]
- 51.Maervoet J, Covaci A, Schepens P, Sandau CD, Letcher R. Environ. Health Perspect. 2004;112:291. doi: 10.1289/ehp.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.