Abstract
Objective
The purpose of this study was to determine whether a high-salt diet modulates physiological angiogenesis in skeletal muscle by altering angiotensin II (ANGII) and vascular endothelial growth factor (VEGF) levels.
Methods
Sprague-Dawley rats were placed on a control diet (0.4% NaCl by weight) or high-salt diet (4.0% NaCl) prior to treatment with the vasodilator prazosin in the drinking water. In addition, a group of animals fed high salt were infused intravenously with ANGII at a low dose to prevent ANGII suppression by high salt, and a group of rats fed control diet were treated with the angiotensin II type I (AT1) receptor blocker losartan and prazosin.
Results
Prazosin induced significant angiogenesis in the tibialis anterior muscle after 1 week of treatment. High-salt-fed rats demonstrated a complete inhibition of this angiogenic response. Maintenance of ANGII levels restored prazosin-induced angiogenesis in animals fed a high-salt diet. In addition, losartan treatment blocked prazosin-induced angiogenesis in animals on a control diet. Western blot analysis indicated that prazosin-induced angiogenesis was independent of changes in muscle levels of VEGF.
Conclusions
This study demonstrates an inhibitory effect of high salt intake on prazosin-induced angiogenesis. Further, these results indicate that ANGII acting through the AT1 receptor is a critical pathway in this model of angiogenesis.
Keywords: blood pressure, losartan, microcirculation
Angiogenesis, the growth of new capillaries in the microvasculature, is an important adaptation made by tissues in a variety of physiological and pathological states. This complex process commonly involves endothelial cell proliferation, migration, and tube formation and is impacted by various humoral, mechanical, and metabolic stimuli. An increase in metabolic demand, such as that seen in skeletal muscle during exercise training and electrical stimulation of muscle contraction, has been demonstrated to induce angiogenesis [1, 3, 21], presumably as an adaptation that increases both the delivery of critical metabolic substrates and the removal of metabolic waste products [13]. However, the growth of the microvasculature has also been linked to local hemodynamic stimuli, such as vessel stretch and shear stress at the vessel wall [6, 14, 20], independent of changes in tissue metabolism.
The role of capillary shear stress in angiogenesis has been elucidated with the use of experimental perturbations that increase capillary blood flow, an important determinant of shear stress, in the absence of increases in metabolism. Chronic treatment with the vasodilator prazosin, an α1-adrenergic receptor blocker, elevates capillary blood flow and shear stress [19, 20] and induces consistent increases in capillary density in a variety of tissues and species [6, 18, 19, 35]. Interestingly, the molecular [34] and morphological patterns [12] observed in prazosin-and muscle activity-induced models of angiogenesis appear to demonstrate important differences.
A number of recent studies have indicated a critical role for the renin–angiotensin system (RAS), an important endocrine regulator of cardiovascular function and structure, in regulating the processes involved in angiogenesis. These studies have demonstrated that pharmacological [1, 3, 15, 17, 24] and genetic [4, 28] manipulations resulting in reductions in the circulating levels on angiotensin II (ANGII), the most potent effector peptide of the RAS, or interruption in the signaling of the angiotensin type 1 (AT1) receptor dramatically attenuate the angiogenic response in a variety of models, including those dependent on muscle activity and muscle ischemia. In addition, evidence has demonstrated that a physiological modulator of RAS activity, a diet high in salt, inhibits the angiogenic response to muscle stimulation [4, 25]. However, the role of ANGII in models of angiogenesis primarily dependent on changes in hemodynamic variables remains untested.
The purpose of the current study was to directly examine the effect of a high-salt diet on the angiogenic response to chronic prazosin treatment. The results of the study suggest that a high-salt diet completely inhibits prazosin-induced angiogenesis in the rat, and that this inhibition is dependent on the effect of elevated salt consumption on the RAS, as maintenance of ANGII levels in high-salt-fed animals by intravenous infusion restores angiogenesis. These results clearly demonstrate a critical and necessary role for ANGII in prazosin-induced angiogenesis.
MATERIALS AND METHODS
Animal Surgery
All animal protocols were approved by the Medical College of Wisconsin (MCW) Institutional Animal Care and Use Committee. Animals were housed and cared for in the MCW Animal Resource Center and were given food and water ad libitum. Five days prior to surgical instrumentation, 7-week-old male Sprague-Dawley rats (n = 103) were randomly assigned to 8 treatment groups described in detail below. At this time, standard lab chow was replaced by either Dyets 0.4% NaCl chow (control diet, CD) or Dyets 4.0% NaCl chow (high-salt diet, HSD) for the duration of the protocol. On the day of the surgery, rats were anesthetized with an intramuscular injection of a mixture of ketamine (100 mg/kg), xylazine (50 mg/kg), and acepromazine (2 mg/kg). Under aseptic conditions, polyethylene catheters were placed in the left femoral artery and vein, tunneled subcutaneously, and exteriorized at the back of the neck. The catheters were then passed through a flexible spring that was secured to the rat subcutaneously. The spring was attached to a swivel above the cage, allowing the animal full range of movement in the cage while protecting the catheters.
Experimental Protocols
Following a 2-day surgical recovery period, baseline blood pressure measurements were made via the femoral arterial cannula. Mean arterial pressure (MAP) was measured for at least 2 h at the same time of day for 2 baseline days and 6 days of treatment. MAP and heart rate were measured with Statham P23ID pressure transducers connected to a 4-channel BP display unit (Stemtech, Milwaukee, WI). The analog signal was low-pass-filtered at 100 Hz, sampled at 300 Hz, and processed with software of our own design. Acquired data were averaged in 1-min intervals throughout the measurement period.
Drug treatment and infusion was initiated following the baseline blood pressure monitoring period. During the treatment period, rats were continuously infused intravenously with ANGII (Sigma, St. Louis, MO) dissolved in sterile saline or saline vehicle, as indicated below, via the femoral venous catheter at a rate of 0.5 mL/h. Animals in the CD group (n = 15) were fed a control diet and given slightly acidified (pH 5.8) drinking water as vehicle. Rats fed a control diet were treated with acidified drinking water containing 50 mg/L of prazosin (Sigma) for 1 week (CD Praz 1wk, n = 16) or 2 weeks (CD Praz 2wk, n = 6). The slight acidification of the tap water facilitated dissolving of prazosin. High-salt-fed animals received either vehicle acidified water (HSD, n = 11) or water containing 25 mg/L prazosin for 1 week (HSD Praz 1 wk, n = 10) or 2 weeks (HSD Praz 2wk, n = 6). The dose of prazosin given to high-salt-fed animals was adjusted to 25 mg/L to account for increased daily water intake in high-salt-fed rats compared to animals on the control diet. Another group of animals received a high-salt diet, intravenous infusion with a subpressor dose of ANGII (3 ng·kg−1·min−1), and vehicle acidified water (HS+ANGII, n = 10) or prazosin at 25 mg/L (HS + ANGII + Praz 1wk, n = 11). A final group of rats were fed a control diet and received acidified tap water containing the AT1 receptor antagonist losartan (Merck, Rahway, NJ) at a concentration of 4 g/L, corresponding to a daily intake of approximately 50 mg/day, a dose previously shown to be effective by our laboratory [1, 23]. Losartan was administered without prazosin (CD+Los, n = 8) or simultaneously with prazosin at a dose of 50 mg/L (CD+Los Praz 1wk, n = 10). Individual water intake for each rat was measured daily to ensure that all groups received an identical dose of prazosin (average of 1.69 ± 0.04 mg/day) and losartan (44.7 ± 1.9 mg/day), where appropriate.
Tissue Harvest and Morphological Analysis of Vessel Density
After 7 days of prazosin treatment, the animals were euthanized by an overdose of pentobarbital sodium (Ovation Pharmaceuticals, Deerfield, IL) and the tibialis anterior (TA) muscle from the right hindlimb was removed and weighed. A 100-mg section was taken from the rostral portion of the TA muscle and frozen in liquid nitrogen for Western analysis. The remaining TA tissue was lightly fixed in a 0.25% formalin solution overnight. The muscles were sectioned with a microtome to a thickness of approximately 75 μm. The TA slices were then immersed in a solution of 30 μg/mL rhodamine-labeled Griffonia simplicifolia I (GS-I) lectin [26]. After several rinses, slices were mounted on microscope slides in a water-soluble mounting medium (SP ACCU-MOUNT 280, Baxter Scientific, McGraw Park, IL). Morphometric analysis of the scanned histochemical sections was performed as previously described [26]. Fifty representative fields were selected for analysis from each TA muscle.
Western Blot Analysis to Detect the Presence of Vascular Endothelial Growth Factor (VEGF) and VEGF Receptor Protein
Western blot analysis was performed as previously described [25]. Briefly, 100 mg TA muscle samples were homogenized and the cytosolic protein fraction suspended in 10 mM potassium phosphate buffer (pH 7.7) with 100 μM phenylmethylsulfonyl fluoride (PMSF, Sigma). The membrane protein fraction was separated by centrifugation (100,000g for 45 min) and suspended in a similar buffer (pH 7.25). For quantification of VEGF protein expression, 30 μg of protein (as determined by Bio-Rad DC protein assay kit, Bio-Rad, Hercules, CA) from the TA homogenate and 0.5 μg of human recombinant VEGF 165 standard (Panvera, Madison, WI) were separated on a 12% denaturing SDS–polyacrylamide gel. VEGF receptor (VEGFR) protein levels were determined by electrophoresis of 15 μg of membrane protein on an 8% SDS gel. Following transfer and overnight blocking, blots were incubated with a monoclonal antibody derived from the human VEGF sequence (1:2000 dilution, clone G143-850, Pharmingen, San Diego, CA) or polyclonal antibodies (1:1000 dilution, Santa Cruz Biotechnologies, Santa Cruz, CA) against the VEGF type 1 receptor (VEGFR1, clone SC-316) and VEGF type 2 receptor (VEGFR2, clone SC-315). Washed blots were then incubated with an appropriate secondary antibody conjugated to horse radish peroxidase and visualized with use of the SuperSignal West Dura chemiluminescence substrate detection system (Pierce, Rockford, IL, USA).
Plasma ANGII Measurement
Arterial blood samples (1 mL) were drawn via the carotid arterial catheter into chilled tubes containing 50 μL/mL 0.125 mol/L Na2 EDTA, 0.025 mol/L phenanthroline, and 0.5 mM neomycin sulfate. Samples were immediately centrifuged and plasma separated and frozen at −80°C until extracted. ANGII levels were then measured by radioimmunoassay as previously described [27].
Data Analysis and Statistics
For each animal, the vessel counts of all the selected fields from the TA muscle were averaged to a single vessel density. Vessel density was expressed in terms of mean number of vessel–grid intersections per microscope field (0.224 mm2). The vessel density and relative VEGF expression were compared between corresponding vehicle drinking water and prazosin treatment groups. All values are presented as mean values ± SEM. The significance of differences in values measured between corresponding vehicle and prazosin treated groups was evaluated using an unpaired t test. Comparisons between groups were made by one-way analysis of variance followed by Tukey’s post hoc test. Blood pressure measurements were evaluated by a one-way analysis of variance. Significance was established at p < .05.
RESULTS
Table 1 displays rat and muscle weights. The initial weight of the CD+Los group was significantly greater than the initial weights of the HSD and HSD+ANGII groups (p < .05). There was no difference between groups in body weight at the completion of the prazosin treatment period. Prazosin treatment did not result in a change in the wet weight of the tibialis anterior (TA) muscle or the ratio between TA weight and total body weight at the end of the study in any groups compared to corresponding vehicle drinking water animals (data not shown).
Table 1.
Average body weight at the beginning of study and following the prazosin treatment period
| Body weight (g)
|
||||
|---|---|---|---|---|
| Start weight | Vehicle end weight | 1 wk prazosin end weight | 2 wk prazosin end weight | |
| Control diet (CD) | 216 ± 3.0 | 257 ± 4.1* | 260 ± 3.1* | 312 ± 4.8*# |
| High-salt diet (HSD) | 207 ± 5.7 | 261 ± 5.9* | 255 ± 5.9* | 285 ± 7.2* |
| High-salt diet + ANGII (HSD+ANGII) | 211 ± 4.5 | 255 ± 7.9* | 245 ± 6.8* | — |
| Control diet + losartan (CD+Los) | 231 ± 1.8 † | 261 ± 4.2* | 262 ± 3.9* | — |
Note. Values are given as means ± SEM. Significance:
p < .05 vs. corresponding start weight;
p < .05 vs. HSD and HSD + ANGII start weight;
p < .05 vs. corresponding 1 wk Praz end weight.
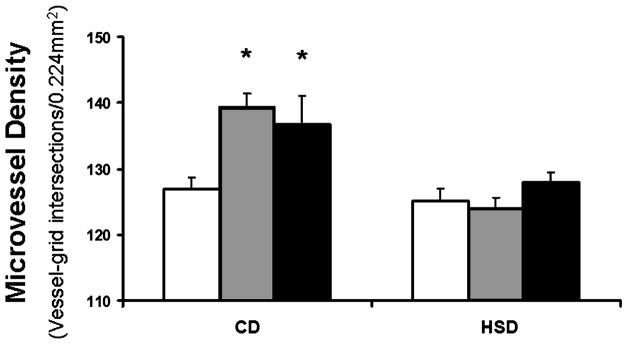
To initially determine whether a diet high in salt inhibited the angiogenic response to prazosin treatment, rats on control and high-salt diets were treated with prazosin for 1 or 2 weeks. As demonstrated in Figure 1, 1 week of prazosin treatment induced a significant increase in microvessel density in the TA muscle of rats on a control diet, from 127 ± 1.9 vessel–grid intersections in the animals receiving control diet and vehicle drinking water (CD) to 139 ± 2.1 in the CD Praz 1wk group (p < .05). An additional week of prazosin treatment did not result in further angiogenesis, as the vessel density of the CD Praz 2wk group (137 ± 4.2) was not different compared to 1 week of prazosin treatment. The angiogenic response to prazosin was completely inhibited by a high-salt diet, as the vessel densities in rats treated for 1 week (HSD Praz 1wk, 124 ± 1.7) and 2 weeks (HSD Praz 2wk, 128 ± 1.6) with prazosin were not different from high-salt animals receiving vehicle drinking water (HSD, 125 ± 2.0). These results agree with a previous report [6] indicating that the angiogenic response to prazosin occurs within 1 week of treatment. All remaining experiments were performed following 1 week of prazosin treatment.
Figure 1.
Changes in TA muscle microvessel density following 1 or 2 weeks of prazosin treatment in rats on a control diet or a high-salt diet. * p < .05 vs. corresponding vehicle-treated rats. □, vehicle;
 , 1 wk Praz; ■, 2 wk Praz.
, 1 wk Praz; ■, 2 wk Praz.
To determine whether the inhibitory effect of a high-salt diet on prazosin-mediated angiogenesis was due to a suppression of angiotensin II (ANGII), animals on a high-salt diet were chronically infused with a subpressor dose of ANGII (3 ng·kg−1·min−1) to maintain ANGII at levels seen in animals on a control diet. This dose of ANGII has previously been demonstrated to maintain plasma ANGII at control levels during high salt intake [25]. As presented in Figure 2, infusion of ANGII levels restored angiogenesis, as the microvessel density in rats on a high-salt diet infused with ANGII and treated with prazosin for 1 week (HSD+ANGII Praz 1wk, 137 ± 4.8) was significantly greater than the vessel density in animals on high-salt diet receiving ANGII infusion and vehicle drinking water (HSD+ANGII, 124 ± 2.4, p < .05). The angiogenic response observed in the HSD+ANGII Praz 1wk group was similar in magnitude to that observed in the CD Praz 1wk group.
Figure 2.
Changes in TA muscle microvessel density following 1 week of prazosin treatment in rats on a control diet, a high-salt diet, a high-salt diet and intravenous ANGII infusion, and a control diet with oral losartan treatment. * p < .05 vs. corresponding vehicle treated rats. □, vehicle;
 , 1 wk Praz.
, 1 wk Praz.
To further investigate the mechanism of ANGII involvement in prazosin-induced angiogenesis, rats on a control diet were treated with losartan, a specific angiotensin type 1 receptor (AT1) antagonist, to determine if the angiogenic effects of ANGII were mediated by AT1 receptor signaling. As shown in Figure 2, losartan did not significantly alter microvessel density, as animals fed a control diet and treated with losartan (CD+Los) displayed a microvessel density of 130 ± 1.6, which was similar to that of animals on control diet receiving vehicle (CD). Additionally, losartan completely inhibited the angiogenic response to prazosin treatment, as the microvessel density in the CD+Los Praz 1wk group (129 ± 2.8) was similar to the CD+Los group.
Plasma ANGII levels were measured to confirm that prazosin treatment did not induce angiogenesis through changes in ANGII levels. The plasma ANGII levels of CD animals treated with vehicle drinking water (23 ± 5.2 pg/mL) were not different from CD animals treated with prazosin for 1 week (21 ± 4.1 pg/mL), suggesting that prazosin treatment has no significant impact on circulating levels of ANGII.
Blood pressure was measured daily to verify that prazosin did not stimulate angiogenesis by causing a reduction in mean arterial pressure (MAP). Table 2 demonstrates the results of baseline measurements before any drug treatment or infusion had begun and the average blood pressure of approximately 6 days of treatment. HS+ANGII and vehicle drinking water did not result in a change in MAP compared to the CD vehicle group. HS+ANGII Praz 1 wk resulted in a significant increase in MAP compared to the CD Praz 1wk group (p < .05). Both losartan treatment in drinking water and losartan combined with prazosin resulted in a reduction of MAP when compared to all other treatments. The MAP of prazosin-treated animals was not significantly reduced in any group when comparisons were made between corresponding vehicle drinking water-treated animals.
Table 2.
Mean arterial pressure prior to the initiation of drug treatment and throughout the treatment period in vehicle- and prazosin-treated rats
| Mean arterial pressure (mmHg)
|
|||
|---|---|---|---|
| Baseline | Vehicle | 1 wk prazosin | |
| Control diet | 116 ± 1.5 | 111 ± 1.8 | 111 ± 3.0 |
| High-salt diet | 118 ± 2.3 | 114 ± 4.0 | 118 ± 2.7 |
| High-salt diet + ANGII | 119 ± 2.7 | 120 ± 4.7 | 125 ± 3.0* |
| Control diet + losartan | 117 ± 1.7 | 76 ± 2.8* | 79 ± 1.9* |
Note. Values are given as means ± SEM. Significance:
p < .05 vs. CD vehicle.
VEGF has been previously shown to be an important angiogenic factor in skeletal muscle [1, 3]. Western blot analysis was performed to determine whether the inhibition of angiogenesis in animals on a high-salt diet occurred by a decrease in expression of VEGF protein in muscle tissue. Figure 3 shows a representative Western blot and the quantitative densitometry of six blots, represented as a percentage of a recombinant VEGF 165 standard. Whole muscle VEGF protein levels were unaffected by prazosin treatment regardless of treatment. There was no difference between groups in VEGF expression in muscles taken from either untreated or prazosin-treated animals. The expression of the two primary receptors of VEGF, VEGFR1 and VEGFR2, were similarly quantified, with no significant effect of high-salt diet or prazosin treatment identified (data not shown).
Figure 3.
A representative Western blot and results of quantitative densitometry of 6 separate Western blots comparing VEGF protein levels in the TA muscles of vehicle and prazosin-treated rats on a control diet, a high-salt diet, a high-salt diet with ANGII infusion, and a control diet with losartan treatment. □, vehicle;
 , 1 wk Praz.
, 1 wk Praz.
DISCUSSION
The results of this study suggest a critical and necessary role for circulating ANGII acting on the AT1 receptor in mediating prazosin-induced angiogenesis. A high-salt diet inhibited the angiogenic response to chronic prazosin treatment, and maintenance of ANGII completely restored physiological angiogenesis in high-salt-fed animals to a level similar to that of animals on a control diet. This study supports a number of findings demonstrating that a suppression of circulating ANGII inhibits angiogenesis, and is the first to demonstrate the critical role of ANGII in angiogenesis stimulated by chronic prazosin treatment.
The role of ANGII in mediating angiogenesis through activation of the AT1 receptor has been reported in several unique model systems. A number of studies have demonstrated the importance of ANGII acting on the AT1 receptor to facilitate reparative angiogenesis following femoral ligation. Pharmacological blockade of the AT1 receptor [15, 31] and disruption of the AT1a gene in the mouse [28] have been reported to inhibit ischemia-induced angiogenesis. In addition, the angiogenic response to chronic electrical stimulation of hindlimb muscle contraction has been shown to depend on ANGII acting through the AT1 receptor, as both pharmacologic blockade of the RAS [1, 3, 4] and suppression of circulating ANGII levels by high-salt diet [25] inhibit angiogenesis in this model. However, the angiogenic responses observed in both models of relative ischemia and increased muscle activity are thought to be stimulated primarily by metabolic stimuli. The current study is the first to demonstrate a role for ANGII in angiogenesis induced by prazosin, a model in which hemodynamic parameters are altered in the absence of metabolic changes.
Histological analysis of the microvasculature following prazosin treatment has shown that angiogenesis in response to shear stress occurs primarily by intra-luminal splitting of vessels [14, 35], as opposed to the abluminal sprouting pattern of angiogenesis seen following chronic muscle stimulation or muscle stretch [14]. This longitudinal splitting pattern appears to require relatively little endothelial cell proliferation [20] and different molecular pathways compared to abluminal sprouting [34]. While further research is necessary to define the molecular mechanisms mediating prazosin-induced angiogenesis, the results of the current study demonstrate that ANGII plays a central role in the pathways related to splitting as well as sprouting patterns of angiogenesis.
Numerous studies [16, 29, 30] have suggested that ANGII may stimulate angiogenesis via AT1 receptor-mediated upregulation of angiogenic growth factors, of which VEGF is considered to be the most critical. However, the current study does not support a role for ANGII in mediating angiogenesis through regulation of levels of VEGF in whole skeletal muscle tissue, as treatment with a high-salt diet and infusion with ANGII had no effect on VEGF protein levels. These data also suggest that an increase in VEGF is not necessary for prazosin to stimulate angiogenesis. However, it is possible that chronic increases in shear stress caused by prazosin treatment and ANGII signaling may regulate VEGF expression specifically in vascular endothelial cells, as has been demonstrated in culture conditions in several reports [11, 16]. Therefore, the current study cannot rule out the possibility that suppression of circulating ANGII by high dietary salt intake caused a decrease in VEGF in endothelial cells of muscle capillaries, which may have been undetectable by the techniques employed in these experiments. In addition, the results of the current study do not rule out the possibility that VEGF levels are altered at an early time point during the prazosin treatment period, as the reported data were collected at one time point only.
The critical nature of VEGF in the angiogenic pathway has previously been demonstrated by studies in which chronic injection of a VEGF-neutralizing antibody has completely eliminated angiogenesis in muscle in models relying primarily on angiogenesis by abluminal sprouting [1, 2, 30]. Importantly, recent studies have demonstrated that VEGF signaling is critical for intraluminal splitting angiogenesis as well, since treatment with a novel VEGF inhibitor (VEGF Trap) inhibited prazosin-induced angiogenesis [33]. We have previously demonstrated that ANGII appears to interact with VEGF to maintain susceptibility to angiogenic stimuli [25]. Given the lack of difference in VEGF and VEGF receptor protein levels, the current data appear to suggest that interruptions in signaling of ANGII, as demonstrated in HSD-fed and losartan-treated animals, may inhibit prazosin-induced angiogenesis by interfering with the signaling of VEGF downstream of VEGF receptor activation. The nature of this critical interaction between ANGII and VEGF requires further exploration.
ANGII- and VEGF- mediated pathways appear to interact to regulate the activity of nitric oxide synthase (NOS) in the vasculature. A growing body of literature has developed suggesting that nitric oxide may play an important role in angiogenesis [6, 10, 30, 31], possibly by interacting with VEGF [5, 7]. Studies have indicated that blockade of the production of NO by the NOS inhibitor L-NAME significantly attenuated the angiogenic response to prazosin treatment; this finding was corroborated in the muscles of endothelial NOS (eNOS) knockout mice, in which prazosin treatment did not mediate angiogenesis [6]. The prazosin stimulus for angiogenesis appears to be particularly dependent on nitric oxide, as NOS inhibition in mice eliminates angiogenesis induced by prazosin, but not muscle overload [32]. Additional studies have demonstrated that inhibition of NOS in rat attenuates both the upregulation of VEGF and the angiogenic response normally associated with chronic electrical stimulation of muscle [21].
Importantly, reports suggest that ANGII may regulate the activity and expression of eNOS. Tamarat and colleagues reported that angiogenesis induced by intravenous ANGII infusion in an ischemic hindlimb model in wild-type mice was associated with upregulation of both VEGF and eNOS protein levels; identical ANGII treatment in eNOS deficient mice did not result in angiogenesis, suggesting a possible role for nitric oxide acting downstream of ANGII to mediate angiogenesis [31]. Additionally, studies by Boegehold [8, 9] and Zhu and colleagues [36] have suggested that high salt intake attenuates the bioavailability of nitric oxide, which may explain in part the inhibition of angiogenesis by high salt consumption reported in this study. Further research is necessary to elucidate the relationship between ANGII and nitric oxide, and the role this interaction may play in prazosin-mediated angiogenesis.
ANGII may also regulate the angiogenic response to prazosin treatment by activating key intracellular signaling pathways that maintain susceptibility to vascular remodeling. For instance, it has been suggested that angiogenesis induced by prazosin occurs by a process involving longitudinal splitting of microvessels into two distinct capillaries [14], a mechanism that requires relatively low levels of endothelial cell proliferation compared to sprouting of new capillaries, as occurs with activity-induced angiogenesis [20]. Cell migration is assumed to play an important role in the formation of new vessels, and ANGII has been shown to activate several kinase-mediated pathways resulting in increased endothelial cell migration [22]. Further characterization of the intracellular pathways regulated by ANGII will provide insight into the role of ANGII in directing cell migration and organization.
In summary, the present study indicates that high salt intake inhibits prazosin-induced angiogenesis in skeletal muscle by suppressing circulating ANGII, which signals through the AT1 receptor to mediate angiogenesis. These findings provide evidence for a critical role of ANGII in mediating important pathways involved in angiogenesis, and offer important insight into the mechanisms regulating the complex physiological process of angiogenesis.
Acknowledgments
The authors thank Christine Puza for expert technical assistance. This work was supported by NIH Grant HL 29587.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article maybe used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
References
- 1.Amaral SL, Linderman JR, Morse MM, Greene AS. Angiogenesis induced by electrical stimulation is mediated by angiotensin II and VEGF. Microcirculation. 2001;8:57–67. [PubMed] [Google Scholar]
- 2.Amaral SL, Maier KG, Schippers DN, Roman RJ, Greene AS. CYP4A metabolites of arachidonic acid and VEGF are mediators of skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol. 2003;284:H1528–H1535. doi: 10.1152/ajpheart.00406.2002. [DOI] [PubMed] [Google Scholar]
- 3.Amaral SL, Papanek PE, Greene AS. Angiotensin II and VEGF are involved in angiogenesis induced by short-term exercise training. Am J Physiol Heart Circ Physiol. 2001;281:H1163–H1169. doi: 10.1152/ajpheart.2001.281.3.H1163. [DOI] [PubMed] [Google Scholar]
- 4.Amaral SL, Roman RJ, Greene AS. Renin gene transfer restores angiogenesis and vascular endothelial growth factor expression in Dahl S rats. Hypertension. 2001;37:386–390. doi: 10.1161/01.hyp.37.2.386. [DOI] [PubMed] [Google Scholar]
- 5.Babaei S, Stewart DJ. Overexpression of endothelial NO synthase induces angiogenesis in a co-culture model. Cardiovasc Res. 2002;55:190–200. doi: 10.1016/s0008-6363(02)00287-0. [DOI] [PubMed] [Google Scholar]
- 6.Baum O, Da Silva-Azevedo L, Willerding G, Wockel A, Planitzer G, Gossrau R, Pries AR, Zakrzewicz A. Endothelial NOS is main mediator for shear stress-dependent angiogenesis in skeletal muscle after prazosin administration. Am J Physiol Heart Circ Physiol. 2004;287:H2300–H2308. doi: 10.1152/ajpheart.00065.2004. [DOI] [PubMed] [Google Scholar]
- 7.Benoit H, Jordan M, Wagner H, Wagner PD. Effect of NO, vasodilator prostaglandins, and adenosine on skeletal muscle angiogenic growth factor gene expression. J Appl Physiol. 1999;86:1513–1518. doi: 10.1152/jappl.1999.86.5.1513. [DOI] [PubMed] [Google Scholar]
- 8.Boegehold MA. Effect of dietary salt on arteriolar nitric oxide in striated muscle of normotensive rats. Am J Physiol. 1993;264:H1810–H1816. doi: 10.1152/ajpheart.1993.264.6.H1810. [DOI] [PubMed] [Google Scholar]
- 9.Boegehold MA. Flow-dependent arteriolar dilation in normotensive rats fed low- or high-salt diets. Am J Physiol. 1995;269:H1407–H1414. doi: 10.1152/ajpheart.1995.269.4.H1407. [DOI] [PubMed] [Google Scholar]
- 10.Cai J, Jiang WG, Ahmed A, Boulton M. Vascular endothelial growth factor-induced endothelial cell proliferation is regulated by interaction between VEGFR-2, SH-PTP1 and eNOS. Microvasc Res. 2005 doi: 10.1016/j.mvr.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Chen BP, Li YS, Zhao Y, Chen KD, Li S, Lao J, Yuan S, Shyy JY, Chien S. DNA microarray analysis of gene expression in endothelial cells in response to 24-h shear stress. Physiol Genomics. 2001;7:55–63. doi: 10.1152/physiolgenomics.2001.7.1.55. [DOI] [PubMed] [Google Scholar]
- 12.Djonov VG, Kurz H, Burri PH. Optimality in the developing vascular system: branching remodeling by means of intussusception as an efficient adaptation mechanism. Dev Dyn. 2002;224:391–402. doi: 10.1002/dvdy.10119. [DOI] [PubMed] [Google Scholar]
- 13.Egginton S, Hudlicka O. Selective long-term electrical stimulation of fast glycolytic fibres increases capillary supply but not oxidative enzyme activity in rat skeletal muscles. Exp Physiol. 2000;85:567–573. [PubMed] [Google Scholar]
- 14.Egginton S, Zhou AL, Brown MD, Hudlicka O. Unorthodox angiogenesis in skeletal muscle. Cardiovasc Res. 2001;49:634–646. doi: 10.1016/s0008-6363(00)00282-0. [DOI] [PubMed] [Google Scholar]
- 15.Emanueli C, Salis MB, Stacca T, Pinna A, Gaspa L, Madeddu P. Angiotensin AT(1) receptor signalling modulates reparative angiogenesis induced by limb ischaemia. Br J Pharmacol. 2002;135:87–92. doi: 10.1038/sj.bjp.0704461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujiyama S, Matsubara H, Nozawa Y, Maruyama K, Mori Y, Tsutsumi Y, Masaki H, Uchiyama Y, Koyama Y, Nose A, Iba O, Tateishi E, Ogata N, Jyo N, Higashiyama S, Iwasaka T. Angiotensin AT(1) and AT(2) receptors differentially regulate angiopoietin-2 and vascular endothelial growth factor expression and angiogenesis by modulating heparin binding-epidermal growth factor (EGF)-mediated EGF receptor transactivation. Circ Res. 2001;88:22–29. doi: 10.1161/01.res.88.1.22. [DOI] [PubMed] [Google Scholar]
- 17.Hikichi T, Mori F, Takamiya A, Sasaki M, Horikawa Y, Takeda M, Yoshida A. Inhibitory effect of losartan on laser-induced choroidal neovascularization in rats. Am J Ophthalmol. 2001;132:587–589. doi: 10.1016/s0002-9394(01)01139-4. [DOI] [PubMed] [Google Scholar]
- 18.Ichioka S, Shibata M, Kosaki K, Sato Y, Harii K, Kamiya A. Effects of shear stress on wound-healing angiogenesis in the rabbit ear chamber. J Surg Res. 1997;72:29–35. doi: 10.1006/jsre.1997.5170. [DOI] [PubMed] [Google Scholar]
- 19.Ichioka S, Shibata M, Kosaki K, Sato Y, Harii K, Kamiya A. In vivo measurement of morphometric and hemodynamic changes in the microcirculation during angiogenesis under chronic alpha1-adrenergic blocker treatment. Microvasc Res. 1998;55:165–174. doi: 10.1006/mvre.1998.2069. [DOI] [PubMed] [Google Scholar]
- 20.Milkiewicz M, Brown MD, Egginton S, Hudlicka O. Association between shear stress, angiogenesis, and VEGF in skeletal muscles in vivo. Microcirculation. 2001;8:229–241. doi: 10.1038/sj/mn/7800074. [DOI] [PubMed] [Google Scholar]
- 21.Milkiewicz M, Hudlicka O, Brown MD, Silgram H. Nitric oxide, VEGF, and VEGFR-2: interactions in activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2005;289:H336–H343. doi: 10.1152/ajpheart.01105.2004. [DOI] [PubMed] [Google Scholar]
- 22.Montiel M, de la Blanca EP, Jimenez E. Angiotensin II induces focal adhesion kinase/paxillin phosphorylation and cell migration in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2005;327:971–978. doi: 10.1016/j.bbrc.2004.12.110. [DOI] [PubMed] [Google Scholar]
- 23.Munzenmaier DH, Greene AS. Chronic angiotensin II AT1 receptor blockade increases cerebral cortical microvessel density. Am J Physiol Heart Circ Physiol. 2005;290:H512–H516. doi: 10.1152/ajpheart.01136.2004. [DOI] [PubMed] [Google Scholar]
- 24.Munzenmaier DH, Greene AS. Opposing actions of angiotensin II on microvascular growth and arterial blood pressure. Hypertension. 1996;27:760–765. doi: 10.1161/01.hyp.27.3.760. [DOI] [PubMed] [Google Scholar]
- 25.Petersen MC, Munzenmaier DH, Greene AS. Angiotensin II infusion restores stimulated angiogenesis in the skeletal muscle of rats on a high salt diet. Am J Physiol Heart Circ Physiol. 2006;291:H114–H120. doi: 10.1152/ajpheart.01116.2005. [DOI] [PubMed] [Google Scholar]
- 26.Rieder MJ, O’Drobinak DM, Greene AS. A computerized method for determination of microvascular density. Microvasc Res. 1995;49:180–189. doi: 10.1006/mvre.1995.1014. [DOI] [PubMed] [Google Scholar]
- 27.Rieder MJRR, Greene AS. Reversal of microvascular rarefaction and reduced renal mass hypertension. Hypertension. 1997;30:120–127. doi: 10.1161/01.hyp.30.1.120. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki K, Murohara T, Ikeda H, Sugaya T, Shimada T, Shintani S, Imaizumi T. Evidence for the importance of angiotensin II type 1 receptor in ischemia-induced angiogenesis. J Clin Invest. 2002;109:603–611. doi: 10.1172/JCI13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shihab FS, Bennett WM, Isaac J, Yi H, Andoh TF. Angiotensin II regulation of vascular endothelial growth factor and receptors Flt-1 and KDR/Flk-1 in cyclosporine nephrotoxicity. Kidney Int. 2002;62:422–433. doi: 10.1046/j.1523-1755.2002.00452.x. [DOI] [PubMed] [Google Scholar]
- 30.Tamarat R, Silvestre JS, Durie M, Levy BI. Angiotensin II angiogenic effect in vivo involves vascular endothelial growth factor- and inflammation-related pathways. Lab Invest. 2002;82:747–756. doi: 10.1097/01.lab.0000017372.76297.eb. [DOI] [PubMed] [Google Scholar]
- 31.Tamarat R, Silvestre JS, Kubis N, Benessiano J, Duriez M, deGasparo M, Henrion D, Levy BI. Endothelial nitric oxide synthase lies downstream from angiotensin II-induced angiogenesis in ischemic hindlimb. Hypertension. 2002;39:830–835. doi: 10.1161/hy0302.104671. [DOI] [PubMed] [Google Scholar]
- 32.Williams JL, Cartland D, Hussain A, Egginton S. A differential role for nitric oxide in two forms of physiological angiogenesis in mouse. J Physiol. 2006;570:445–454. doi: 10.1113/jphysiol.2005.095596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams JL, Cartland D, Rudge JS, Egginton S. VEGF trap abolishes shear stress- and overload-dependent angiogenesis in skeletal muscle. Microcirculation. 2006;13:499–509. doi: 10.1080/10739680600785717. [DOI] [PubMed] [Google Scholar]
- 34.Williams JL, Weichert A, Zakrzewicz A, Da Silva-Azevedo L, Pries AR, Baum O, Egginton S. Differential gene and protein expression in abluminal sprouting and intraluminal splitting forms of angiogenesis. Clin Sci (Lond) 2006;110:587–595. doi: 10.1042/CS20050185. [DOI] [PubMed] [Google Scholar]
- 35.Zhou A, Egginton S, Hudlicka O, Brown MD. Internal division of capillaries in rat skeletal muscle in response to chronic vasodilator treatment with alpha1-antagonist prazosin. Cell Tissue Res. 1998;293:293–303. doi: 10.1007/s004410051121. [DOI] [PubMed] [Google Scholar]
- 36.Zhu J, Mori T, Huang T, Lombard JH. Effect of high-salt diet on NO release and superoxide production in rat aorta. Am J Physiol Heart Circ Physiol. 2004;286:H575–H583. doi: 10.1152/ajpheart.00331.2003. [DOI] [PubMed] [Google Scholar]