Abstract
We have previously shown that skeletal muscle angiogenesis induced by electrical stimulation is significantly attenuated when SS-13BN/Mcwi rats are fed a high-salt diet. This effect was associated with a large increase in endothelial cell (EC) apoptosis. We hypothesized that the low levels of ANG II during high-salt diet would increase EC apoptosis and consequently diminish the angiogenic response. To test this hypothesis, a series of in vitro and in vivo studies was performed. EC apoptosis and viability were evaluated after incubation with ANG II under serum-free conditions. After 24 h of incubation, ANG II increased EC viability and Bcl-2-to-Bax ratio along with a dose-dependent decrease in EC apoptosis. This effect was blocked by the ANG II type 1 receptor antagonist losartan. To confirm our in vitro results, ANG II (3 ng·kg−1·min−1) was chronically infused in rats fed a high-salt diet (4% NaCl). ANG II decreased EC apoptosis and produced a significant increase (40%) in skeletal muscle angiogenesis after electrical stimulation. These in vivo results were in agreement with our in vitro results and demonstrate that the attenuation of ANG II levels during a high-salt diet may induce EC apoptosis and consequently block the angiogenic response induced by electrical stimulation. Furthermore, under normal conditions, ANG II increases EC viability and protects EC from apoptosis possibly by inactivation of the mitochondrial apoptotic pathway.
Keywords: renin angiotensin system, endothelial cell apoptosis, angiogenesis
Angiogenesis consists of the formation of new capillaries from preexisting blood vessels (10) and is involved in physiological processes as well as in several pathological conditions such as tumor growth, diabetic retinopathy, atherosclerosis, and ischemic disease (9). The formation of new blood vessels is a complex process that involves several steps that include the stimulation of endothelial cells (ECs) by growth factors, degradation and invasion of the extracellular matrix, migration and proliferation of ECs, and finally the formation of new capillaries (10, 18). The permanency of the newly formed capillaries will be dependent on the balance between proangiogenic and/or prosurvival factors and antiangiogenic and/or prodeath factors (21, 18, 44). A number of drugs that interfere with the angiogenic balance have been studied extensively and are having a marked impact in a wide spectra of angiogenesis-related diseases.
ANG II, the main effector peptide of the renin-angiotensin system (RAS), has an important angiogenic property long-established in several in vivo systems, including the chick chorioallantoic membrane assay (27) subcutaneous sponge implant model (47), hindlimb ischemia (42), oxygen-induced retinopathy (31, 33), and tumor models (46, 19, 22). However, there are continuing efforts to understand the biological actions and expand knowledge of the mechanisms through which ANG II acts as a mediator of the angiogenic responses. Several related mechanisms have been proposed, including stimulation of EC proliferation (43) and increase in expression of several growth factors, including vascular endothelial growth factor and its receptors (13, 36). More recently a study has indicated that ANG II increased retinal angiogenesis, decreasing EC apoptosis by a mechanisms involving phosphatidylinositol 3-kinase (PI 3-kinase)/protein kinase B (Akt) activation, subsequent upregulation of survivin, and suppression of caspase-3 activity (35).
Over the past several years, we have provided experimental evidence showing the role of the RAS in the regulation of skeletal muscle angiogenesis induced by exercise or electrical stimulation (1, 2, 3, 37, 16) using genetic, pharmacological, and physiological approaches to dissect the mechanisms by which ANG II interferes with the angiogenic process. Recently, we have shown that transfer of chromosome 13, containing the renin gene, from the normotensive BN/Mcwi rat in the salt-sensitive hypertensive (SS/JrHsdMcwi) rat genetic background restored the plasma renin and angiogenesis response induced by electrical stimulation (16). However, this effect was significantly attenuated when the rats were fed a high-salt diet. To better understand the mechanisms involved in the inhibition of the angiogenic response by high-salt diet, we performed a microarray study that indicated upregulation of many genes related to apoptosis associated to a significant increase in EC death in the stimulated skeletal muscle (16). Because the RAS is significantly attenuated under high-salt conditions, we hypothesized that the modulation of ANG II levels may interfere with EC death pathways and consequently affect the angiogenic response in the skeletal muscle of rats fed a high-salt diet. To test this hypothesis, in the present study, we designed a series of in vitro and in vivo experiments to test the effects of ANG II on EC survival and apoptosis as well as its effect on the angiogenesis restoration in rats fed a high-salt diet.
MATERIALS AND METHODS
Induction and quantitative determination of EC apoptosis, proliferation, and viability in culture
To induce apoptosis in ECs, a serum deprivation method was used as described previously (26). Briefly, 20,000 primary ECs were plated on four-well chamber slides and incubated for 24 h in complete RPMI medium with 20% serum. After 24 h, cells were washed in complete or serum-free medium according to the experimental design, and the medium was changed to serum-free medium containing varying concentrations of ANG II, with or without losartan, as indicated in legends for Figs. 1–5. The control cells were maintained in complete RPMI medium and 20% serum during the 24-h incubation period. The number of apoptotic cells was determined using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL; Promega, Madison, WI) according to the manufacturer’s protocol. The slides were treated with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Molecular Probes) to quantify the total nuclei and to minimize fluorescence bleaching. Immunofluorescence microscopy was performed using a ×20 objective.
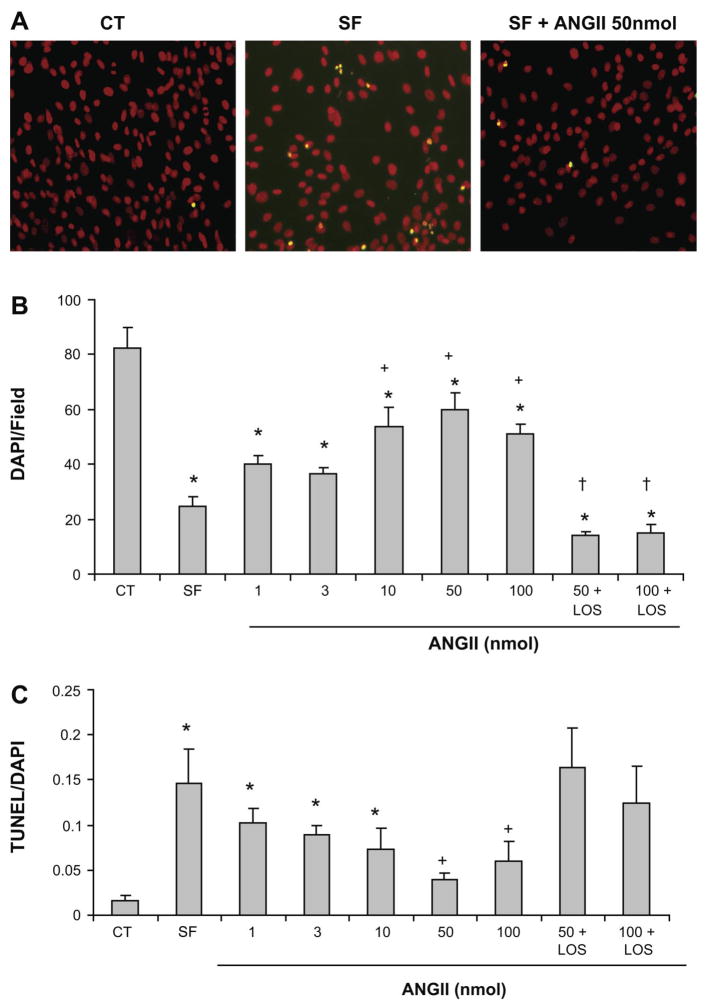
Fig. 1.
ANG II blocks serum deprivation-induced apoptosis in microvascular endothelial cells (ECs) through the angiotensin type 1 (AT1) receptor. Cells were treated with either serum-free medium or serum free + ANG II (1–100 nmol/l). A: representative fluorescence images of terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assays from cells in RPMI medium and 20% serum (CT), RPMI serum free (SF), and serum-free medium plus 50 nmol ANG II (SF + ANG II 50 nmol). Apoptotic cells are shown in yellow. Magnification, ×20. B: total number of cells stained by 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) after 24 h incubation period. C: dose-response analysis of the antiapoptotic effects of ANG II after 24 h incubation period. Values represent means ± SE. Each point is the mean of data from duplicate experiments. *P < 0.05 vs. CT; +P < 0.05 vs. SF; †P < 0.05 vs. respective ANG II dose.
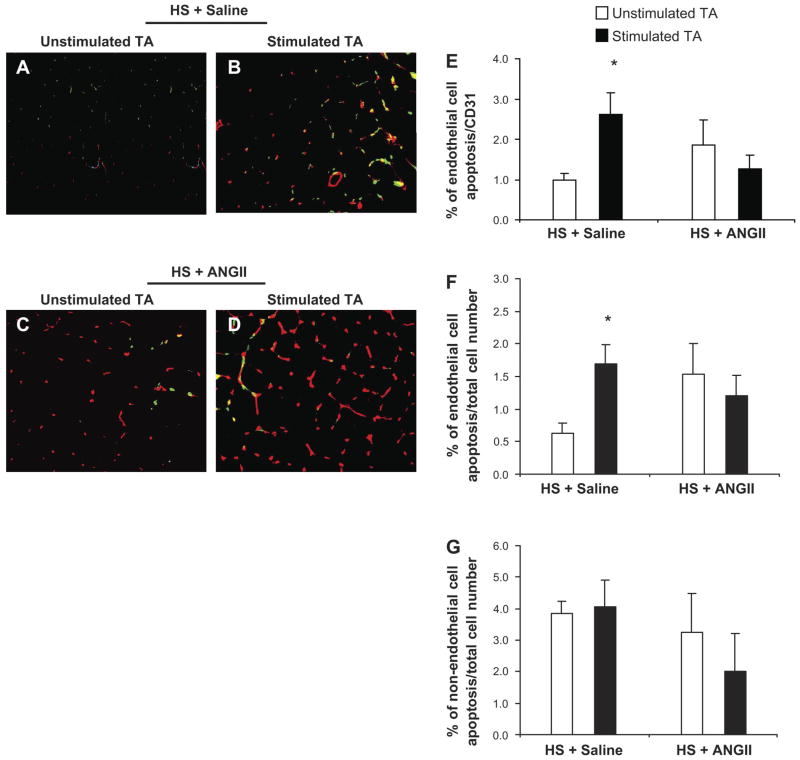
Fig. 5.
ANG II acts as a survival factor in skeletal muscle vascular ECs of SS-13BN fed a high salt diet. A–D: double immunofluorescent staining to detect apoptotic ECs in the TA muscle of SS-13BN rats fed a high-salt diet. A and B: representative images of unstimulated and stimulated TA of rats fed a high-salt diet and infused with saline, respectively. Red: ECs; green: apoptotic cells; yellow: apoptotic ECs. C and D: representative images of unstimulated and stimulated TA of rats fed a high-salt diet and infused with ANG II, respectively. E: percentage of EC apoptosis as a function of the total number of ECs. F: percentage of EC apoptosis as a function of the total number of cells. G: percentage of non-EC apoptosis as a function of the total number of cells. Values are expressed as means ± SE. *P < 0.05 vs. unstimulated TA; n = 9 in the HS + saline group and n = 6 in the HS + ANG II group. Values represent means ± SE. *P < 0.05 vs. unstimulated TA.
A CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega) kit containing the tetrazolium compound MTS was used to monitor EC viability and proliferation according to the manufacturer’s protocols. MTS color change was monitored by using a Tecan Spectrafluor Plus microplate reader (Tecan, Crailshaim, Germany) set at an absorbance reading of 492 nm. The quantity of formazan product as measured by the absorbance at 492 nm is directly proportional to the number of viable cells in proliferation.
Western blot for Bcl-2 and Bax
Primary ECs were washed with cold PBS and lysed in RIPA buffer (150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and 50 mM Tris base, pH 8.0) containing protease inhibitors (10 mmol/l sodium pyrophosphate, 100 mmol/l NaF, 1 mmol/l Na3VO4, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 2 mmol/l phenylmethylsulfonyl fluoride). Protein was separated in denaturing SDS-10–20% polyacrylamide gel (30 μg/lane) and then blotted on a nitrocellulose membrane. Membranes were incubated with a rabbit polyclonal antibody for Bax (dilution 1:500; Santa Cruz) and Bcl-2 (dilution 1:1,000; BD Pharmingen, San Diego, CA) for 2 h at room temperature and after serial washes (5 × 3 min in TBS-Tween 20) with the secondary antibody (anti-rabbit IgG, 1:3,000) for 1 h at room temperature. Immunoblots were visualized by chemiluminescence (Pierce, Rockford, IL), followed by autoradiography.
In vivo ANG II infusion
The Medical College of Wisconsin (MCW) Institutional Animal Care and Use Committee approved all animal protocols. Animals were housed and cared for in the MCW Animal Resource Center and were given food and water ad libitum. A consomic rat strain (SS-13BN/Mcwi) derived from BN/Mcwi rats and Dahl-SS/JrHsdMcwi rats was used in these studies; the origin of this strain has been described previously (15). Rats were placed on a high (4% NaCl)-salt diet 2 days before surgery and maintained throughout the entire experiment. Rats were anesthetized with intramuscular injection of a mixture of ketamine (100 mg/kg), xylazine (50 mg/kg), and acepromazine (2 mg/kg). Under aseptic conditions, a miniature stimulator was implanted subcutaneously in the thoracolumbar region of the rats, and a pair of electrodes was conducted under the skin from the stimulator to the lower hindlimb muscles to promote muscle contractions, as previously described (29). Next, a catheter was securely inserted in the left jugular vein, and the animals were allowed to recover for 24 h. Awake rats were then connected to a multisyringe pump to provide a continuous intravascular infusion of saline alone or saline containing ANG II at the subpressor dose of 3 ng·kg−1·min−1 at the rate of 0.5 ml/h. The electrical stimulators were subsequently activated to initiate the contractions of the extensor digitorum longus and tibialis anterior (TA) muscle for 8 h/day over a consecutive 7-day period, as previously described (29). After 7 days of stimulation, the animals were killed by an overdose of beuthanasia solution (Sigma, St. Louis, MO), and the stimulated and contralateral unstimulated TA muscles were removed and weighed.
Capillaries and myofibers ratio
At the end of the experiment, TA muscle from the unstimulated and stimulated hindlimbs were obtained for quantification of capillary density. Frozen transverse 8-μm tissue sections we were identified by red fluorescencere cut and stained with a CD31 antibody (BD Pharmingen) and a rhodamine-labeled secondary antibody (Invitrogen). Capillaries and myofibers were counted in 5–10 microscopic fields (×20 magnification) per muscle, with capillary density being expressed as capillaries per millimeter squared and the capillary-to-myofiber ratio as described earlier (38).
Quantitative determination of apoptosis in TA muscle
Frozen TA muscles were sectioned (8 μm), mounted on positively charged slides, air-dried for 30 min, and fixed in cold acetone for 5 min, 1:1 acetone-chloroform (vol/vol) for 5 min, and acetone for 5 min. Samples were washed three times with PBS, pH 7.2, and double stained with CD31, and TUNEL as previously described (16). The slides were treated with DAPI (Molecular Probes) to quantify the total nuclei and to minimize fluorescence bleaching. Immunofluorescence microscopy was performed using a ×20 objective. ECs were identified by red fluorescence (CD31), total cell number was detected by blue fluorescence (DAPI, DNA staining), and apoptosis was detected by green fluorescence (TUNEL). Apoptotic ECs were detected by colocalized red and green (displayed as yellow) fluorescence. Quantification of apoptotic ECs was expressed as an average of the ratio of apoptotic ECs per square millimeter to the total number of cells (DAPI staining) or total number of ECs (CD31 staining) per square millimeter at ×20 magnification.
To determine whether ECs in skeletal muscle underwent apoptosis after electrical stimulation, the number of apoptotic cells (TUNEL positive) was divided by the total cell number (DAPI staining) to determine the percentage of apoptotic cells in the muscle tissue. To determine the percentage of apoptotic ECs, the number of TUNEL/CD31 positive cells (apoptotic ECs) was normalized to the total EC number (CD31 staining).
Data analysis and statistics
The results were expressed as means ± SD. In vitro data were analyzed by repeated-measures one-way ANOVA followed by Dunnett’s post hoc test. To evaluate the significance of differences in vessel density between stimulated and unstimulated limbs, a one-factor analysis of variance was performed. Significant differences were further investigated using a post hoc test (Tukeys).
RESULTS
Effect of ANG II on skeletal muscle microvascular EC apoptosis, proliferation, and viability
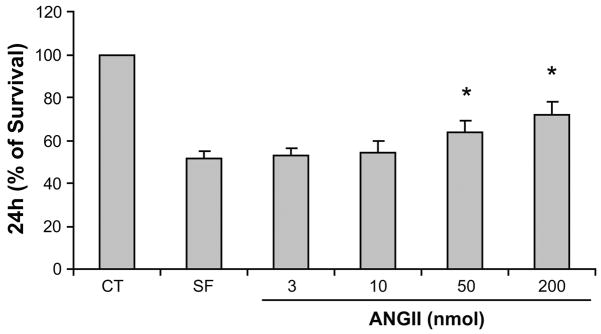
ECs underwent apoptosis after 24 h of serum deprivation, showing typical characteristics such as cell shrinkage, nuclear fragmentation, and chromatin condensation, which is consistent with apoptotic morphology (Fig. 1A). After 24 h, serum deprivation promoted a dramatic reduction in the number of attached ECs by ~70%. ANG II at doses of 10–100 nmol significantly increased the number of attached ECs. This effect was completely inhibited by the ANG II receptor I blocker losartan (1 μM; Fig. 1B). ANG II also promoted a dose-dependent decrease in EC apoptosis, becoming significantly lower than the serum-free condition at a dose of 50 and 100 nmol (Fig. 1C). Losartan (1μM) completely abolished the ANG II antiapoptotic effect. Because cell death is preceded by loss of cell viability and proliferation, we carried out a viability and proliferation assay for ECs treated with different ANG II concentrations (3, 10, 50, and 200 nmol). As shown in Fig. 2, EC viability decreased significantly by ~50% in serum-free conditions. ANG II restored EC viability in a dose-dependent manner, reaching a 64 to 72% increase at the doses of 50 and 200 nmol ANG II, respectively. This result was further validated by the dose-dependent increase in EC count after 24 h incubation with ANG II (Fig. 1B).
Fig. 2.
Dose-dependent effects of ANG II on EC survival. Cell viability in complete RPMI medium (20% serum) was considered to be 100%. EC viability was determined after 24 h incubation in serum-free medium or ANG II (3, 10, 50, and 200 nmol). Error bars represent SDs of 5 independent experiments performed in triplicate. *P < 0.05 vs. SF.
ANG II-induced EC survival is mediated by inactivation of the mitochondrial apoptotic pathway
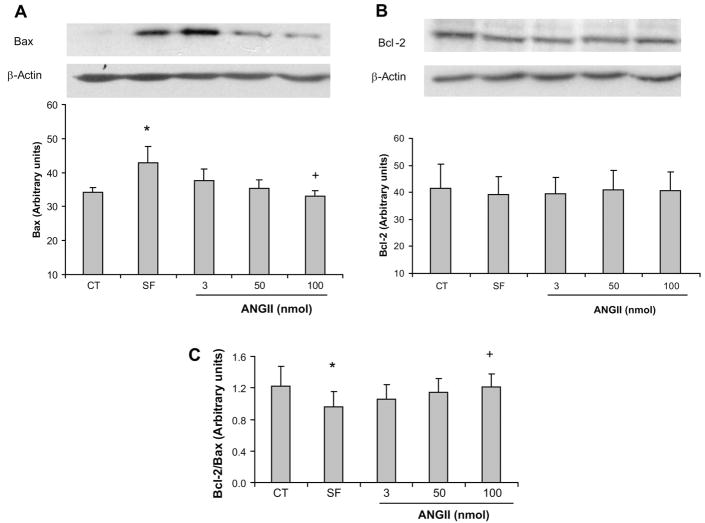
To further study the mechanisms underlying the antiapoptotic effect of ANG II in microvascular ECs, we investigated key regulatory pathways that control the cellular response to apoptosis. The Bcl-2 and Bax proteins are central modulators of apoptosis that operate predominantly within the mitochondrial pathway (17, 25). These proteins interact by functionally antagonizing each other: Bcl-2 suppresses and Bax promotes apoptosis (41). Serum deprivation (24 h) promoted a significant increase in Bax protein expression in ECs (34.1 ± 1.56 vs. 42.8 ± 4.87 arbitrary units; P < 0.05) (Fig. 3A). This effect was suppressed by ANG II, with a significant decrease at a dose of 100 nmol compared with the serum-free condition (42.8 ± 4.87 vs. 33.1 ± 1.48 arbitrary units; P < 0.05) (Fig. 3A). Bcl-2 expression did not change significantly in response to any treatment (Fig. 3B). However, The Bcl-2-to-Bax ratio was significantly decreased in serum-free conditions compared with control, and ANG II promoted a dose-dependent increase in the Bcl-2-to-Bax ratio, with a significant increase at the dose of 100 nmol compared with serum-free conditions (0.96 ± 0.20 vs. 1.21 ± 0.16; P < 0.05) (Fig. 3C).
Fig. 3.
A and B, top: effect of ANG II on Bax and Bcl-2 expression in ECs in culture. A–C, bottom: Bcl-2-to-Bax ratio. Values represent means ± SE. P < 0.05 vs. control (*) and vs. SF (+); n = 5 experiments.
Effect of ANG II on skeletal muscle endothelial apoptosis and angiogenesis in vivo
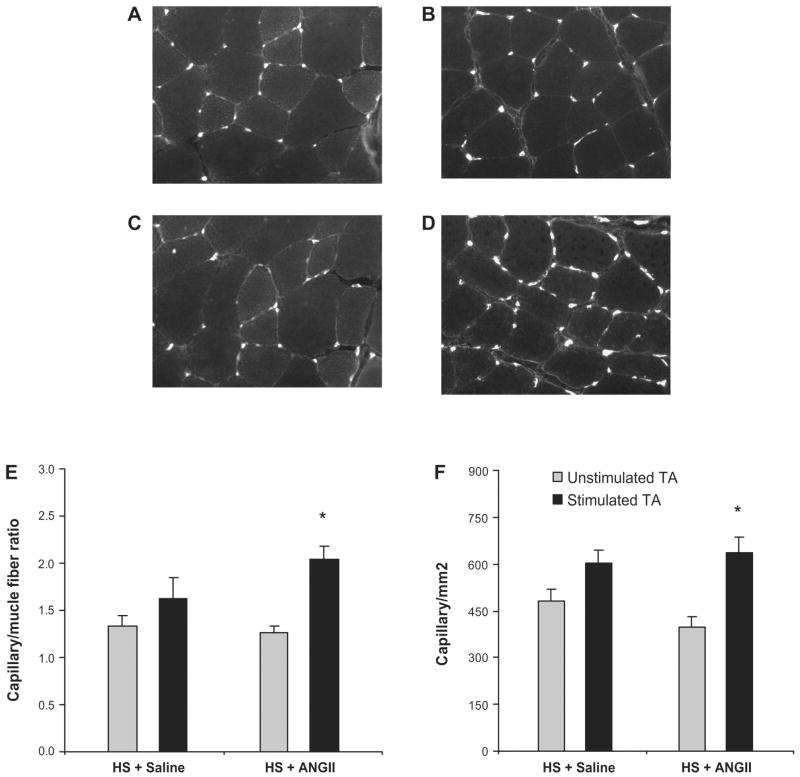
We have previously reported that high-salt diet significantly increases EC apoptosis and inhibits skeletal muscle angiogenesis (represented by a reduction in lectin-labeled microvessel density and capillary-to-fiber ratio), induced by electrical stimulation (16). Figure 4 revealed that, in contrast with the saline infusion group (Fig. 4, A and B), there was a significant increase in the capillary density represented by capillary per square millimeter and capillary-to-muscle fiber ratio in the stimulated hindlimb of rats that were fed with high-salt diet and received ANG II infusion, as depicted in examples of unstimulated and stimulated TA muscle sections (Fig. 4, C and D) and in the quantitative analysis (Fig. 4, E and F). The double immunostaining for TUNEL/CD31 (Fig. 5, A–D) confirm that ANG II acts as a survival factor for ECs in vivo, restoring the angiogenesis response after electrical stimulation and attenuating the increase in EC apoptosis induced by high-salt diet. ANG II infusion also blunted the increase in EC apoptosis in the stimulated TA muscle of rats fed a high-salt diet (Fig. 5, E and F). The number of nonendothelial TUNEL positive cells was not significantly changed in the stimulated vs. unstimulated TA muscle of rats that received saline or ANG II infusion (Fig. 5G).
Fig. 4.
Effect of ANG II on capillary density after 7 days of electrical stimulation. A and B: representative images of cross sections of the tibialis anterior (TA) muscle showing the capillaries and fibers in the unstimulated and stimulated TA muscle of SS-13BN/Mcwi rats fed a high-salt (HS) diet that received saline infusion, respectively. C and D: representative images of cross sections of the TA muscle showing capillaries and fibers in the unstimulated and stimulated TA muscle of SS-13BN/Mcwi rats fed a high-salt diet that received ANG II infusion, respectively. E: quantitative capillary-to-fiber ratio analysis. F: capillary/mm2; n = 8 in the HS + saline group and n = 7 in the HS + ANG II group. Values are expressed as means ± SE. *P < 0.05 vs. unstimulated TA.
DISCUSSION
Inhibition of EC apoptosis and consequent increase in EC survival is thought to be essential during the angiogenic process (21). In contrast, several lines of evidence suggest that the induction of EC apoptosis may counteract angiogenesis, and it is frequently associated with vessel quiescence or even vessel regression (11). Although several studies have been shown that ANG II can induce apoptosis in different cell types, including fibroblasts (28), cardiomyocytes (14), and vascular smooth muscle cells (8), little is known about whether ANG II plays either an anti- or a proapoptotic role in ECs, especially in microvascular ECs such as skeletal muscle vascular ECs. In the present study, we provide new insights into the effects of ANG II on EC apoptosis and the underlying mechanisms and its impact on skeletal muscle angiogenesis induced by electrical stimulation.
The present study shows that ANG II protects ECs against apoptosis induced by serum deprivation in a dose-dependent manner. In addition, ANG II at a dose of 10–100 nmol was able to maintain EC number even under serum-free conditions. This effect was dependent on ANG II receptor I activation (AT1 receptor), since losartan completely blocked the antideath and proliferative effect of ANG II on ECs. Depending on the cell type, AT1 receptor activation may have proapoptotic (8, 14, 24) or antiapoptotic (4, 30, 35, 39) effects. An antiapoptotic effect related to AT1 receptor activation was recently reported in retinal microvascular ECs showing that Candesartan, an AT1 antagonist, completely suppressed the antiapoptotic effect of ANG II on ECs, whereas PD-123319, an AT2 antagonist, had no effect (35). The survival effects of AT1 receptor activation were related to activation of PI 3-kinase and Akt, (45) which is a common feature in the signal transduction of the antiapoptotic effects of many growth factors.
The maintenance of total cell number is constantly balanced by cell death and proliferation. Because cell death is preceded by a loss of cell viability and reduction in the proliferation rate, we also evaluated EC viability and proliferation after 24 h of serum starvation in addition to different doses of ANG II. This study showed that the reduction in EC apoptosis after 24 h of ANG II administration was also associated with a significant increase in EC viability and proliferation. ANG II promoted a dose-dependent increase in EC survival, reaching an 80% increase at the dose of 200 nmol. This result was further validated by the dose-dependent increase in EC count after 24 h incubation with ANG II. Other studies have indicated that ANG II may affect angiogenesis by promoting proliferation and increasing survival of several cell types (22, 5, 6, 40); however, little is known about the effect of ANG II in EC survival and the molecular mechanisms involved.
To investigate the apoptotic pathways affected by ANG II in microvascular ECs, we focused on the regulation of the mitochondrial proteins Bax and Bcl-2. These proteins are central modulators of apoptosis that operate predominantly within the mitochondria (17, 25). They interact by functionally antagonizing each other: Bcl-2 suppresses and Bax promotes apoptosis (41). The balance between cell viability and apoptosis depends on the ratio of Bcl-2 and Bax proteins. The present study shows that ANG II inhibited the increase in Bax expression induced by serum deprivation, leading to a dose-dependent increase in the Bcl-2-to-Bax ratio, which was significantly greater than the serum-free condition at a dose of 100 nmol. Several studies have shown that the Bcl-2-to-Bax ratio controls cell death, and an increase in this ratio may affect angiogenesis and the progression of numerous conditions, including different types of cancers (12, 32, 7), ischemic retinopathy (48), and the persistency of fetal ocular vasculature (20). Although previous studies in our laboratory have consistently demonstrated the effect of ANG II on angiogenesis induction after electrical stimulation (34, 1, 37), this finding brings new evidence on the mechanisms by showing that ANG II prevents activation of the mitochondrial apoptotic pathway, increasing EC survival, and that it may have an important impact on skeletal muscle angiogenesis after electrical stimulation.
Because the in vitro data strongly indicated an antiapoptotic function of ANG II, we decided to further investigate the potential role of ANG II in apoptosis in vivo by using a rat model of electrically stimulated skeletal muscle angiogenesis. Previously, we have shown that inhibition of the RAS with high-salt diet significantly attenuated angiogenesis induced by electrical stimulation, and this phenomenon was associated with a significant increase in EC apoptosis in the stimulated hindlimb (16). We have previously shown that ANG II levels are suppressed during high-salt diet and restored to the level on a low-salt diet after ANG II infusion (37). Therefore, we hypothesized that the decrease of ANG II levels may activate EC death pathways and consequently attenuate the angiogenic response in the skeletal muscle of rats fed a high-salt diet. This hypothesis is strongly supported in the present study, which shows that a subpressor dose of ANG II infusion was able to reestablish the angiogenesis response after electrical stimulation in animals fed a high-salt diet. In contrast to the saline infusion group, rats fed a high-salt diet and infused with ANG II had a dramatic increase of ~60% in the capillary density after electrical stimulation. Interesting, the ANG II infusion prevented the increase in EC apoptosis promoted by high-salt diet. Our in vitro results were further validated by the in vivo antiapoptotic effect of ANG II, suggesting that ANG II promotes the survival of microvascular cells and that suppression of ANG II signaling actually increases EC apoptosis and consequently reduces skeletal muscle vascular viability, having a significant impact in the angiogenesis response after electrical stimulation.
Acknowledgments
We thank Christine Puza and Erika Keyes for expert technical assistance and Hugo Metz for data analysis assistance.
GRANTS
This work was supported by National Institutes of Health Grants NIH HL-29587, N01 HV-28182, and HL-082798-02.
References
- 1.Amaral SL, Linderman JR, Greene AS. Angiogenesis induced by electrical stimulation is mediated by angiotensin II. Microcirculation. 2000;8:57–67. [PubMed] [Google Scholar]
- 2.Amaral SL, Papanek P, Greene AS. Angiotensin II and VEGF are involved in angiogenesis induced by short term exercise training. Am J Physiol Heart Circ Physiol. 2001;281:H1163–H1169. doi: 10.1152/ajpheart.2001.281.3.H1163. [DOI] [PubMed] [Google Scholar]
- 3.Amaral SL, Roman RJ, Greene AS. Renin Gene transfer restores angiogenesis and vascular endothelial growth factor expression in Dahl-S rats. Hypertension. 2001;37:386–390. doi: 10.1161/01.hyp.37.2.386. [DOI] [PubMed] [Google Scholar]
- 4.Amaya K, Ohta T, Kitagawa H, Kayahara M, Takamura H, Fujimura T, Nishimura G, Shimizu K, Miwa K. Angiotensin II activates MAP kinase and NF-kappaB through angiotensin II type I receptor in human pancreatic cancer cells. Int J Oncol. 2004;25:849–856. [PubMed] [Google Scholar]
- 5.Arafat HA, Gong Q, Chipitsyna G, Rizvi A, Saa CT, Yeo CJ. Anti-hypertensives as novel antineoplastics: angiotensin-I-converting enzyme inhibitors and angiotensin II type 1 receptor blockers in pancreatic ductal adenocarcinoma. J Am Coll Surg. 2007;204:996–1005. doi: 10.1016/j.jamcollsurg.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 6.Arrieta O, Guevara P, Escobar E, García-Navarrete R, Pineda B, Sotelo J. Blockage of angiotensin II type I receptor decreases the synthesis of growth factors and induces apoptosis in C6 cultured cells and C6 rat glioma. Br J Cancer. 2005;92:1247–1252. doi: 10.1038/sj.bjc.6602483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beenken SW, Bland KI. Biomarkers for breast cancer. Minerva Chir. 2002;57:437–448. [PubMed] [Google Scholar]
- 8.Bhaskaran M, Reddy K, Radhakrishnan N, Franki N, Ding G, Singhal PC. Angiotensin II induces apoptosis in renal proximal tubular cells. Am J Physiol Renal Physiol. 2003;284:F955–F965. doi: 10.1152/ajprenal.00246.2002. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 11.Chavakis E, Dimmeler S. Regulation of endothelial cell survival and apoptosis during angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:887–893. doi: 10.1161/01.atv.0000017728.55907.a9. [DOI] [PubMed] [Google Scholar]
- 12.Chew BP, Brown CM, Park JS, Mixter PF. Dietary lutein inhibits mouse mammary tumor growth by regulating angiogenesis and apoptosis. Anticancer Res. 2003;23:3333–3339. [PubMed] [Google Scholar]
- 13.Chua CC, Hamdy RC, Chua BH. Upregulation of vascular endothelial growth factor by angiotensin II in rat heart endothelial cells. Biochim Biophys Acta. 1998;1401:187–194. doi: 10.1016/s0167-4889(97)00129-8. [DOI] [PubMed] [Google Scholar]
- 14.Cigola E, Kajstura J, Li B, Meggs LG, Anversa P. Angiotensin II activates programmed myocyte cell death in vitro. Exp Cell Res. 1997;231:363–371. doi: 10.1006/excr.1997.3477. [DOI] [PubMed] [Google Scholar]
- 15.Cowley AW, Jr, Stoll M, Greene AS, Kaldunski ML, Roman RJ, Tonellato PJ, Schork NJ, Dumas P, Jacob HJ. Genetically defined risk of salt sensitivity in an intercross of Brown Norway and Dahl S rats. Physiol Genomics. 2000;2:107–115. doi: 10.1152/physiolgenomics.2000.2.3.107. [DOI] [PubMed] [Google Scholar]
- 16.de Resende MM, Amaral SL, Munzenmaier DH, Greene AS. Role of endothelial cell apoptosis in regulation of skeletal muscle angiogenesis during high and low salt intake. Physiol Genomics. 2006;25:325–335. doi: 10.1152/physiolgenomics.00253.2005. [DOI] [PubMed] [Google Scholar]
- 17.Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- 18.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 19.Fujita M, Hayashi I, Yamashina S, Fukamizu A, Itoman M, Majima M. Angiotensin type 1a receptor signaling-dependent induction of vascular endothelial growth factor in stroma is relevant to tumor-associated angiogenesis and tumor growth. Carcinogenesis. 2005;26:271–279. doi: 10.1093/carcin/bgh324. [DOI] [PubMed] [Google Scholar]
- 20.Hahn P, Lindsten T, Tolentino M, Thompson CB, Bennett J, Dunaief JL. Persistent fetal ocular vasculature in mice deficient in bax and bak. Arch Ophthalmol. 2005;123:797–802. doi: 10.1001/archopht.123.6.797. [DOI] [PubMed] [Google Scholar]
- 21.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 22.Ino K, Shibata K, Kajiyama H, Nawa A, Nomura S, Kikkawa F. Manipulating the angiotensin system–new approaches to the treatment of solid tumours. Expert Opin Biol Ther. 2006;6:243–255. doi: 10.1517/14712598.6.3.243. [DOI] [PubMed] [Google Scholar]
- 23.Ino K, Shibata K, Kajiyama H, Yamamoto E, Nagasaka T, Nawa A, Nomura S, Kikkawa F. Angiotensin II type 1 receptor expression in ovarian cancer and its correlation with tumour angiogenesis and patient survival. Br J Cancer. 2006;94:552–560. doi: 10.1038/sj.bjc.6602961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kajstura J, Cigola E, Malhotra A, Li P, Cheng W, Meggs LG, Anversa P. Angiotensin II induces apoptosis of adult ventricular myocytes in vitro. J Mol Cell Cardiol. 1997;29:859–870. doi: 10.1006/jmcc.1996.0333. [DOI] [PubMed] [Google Scholar]
- 25.Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–330. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- 26.Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 27.Le Noble FA, Schreurs NH, van Straaten HW, Slaaf DW, Smits JF, Rogg H, Struijker-Boudier HA. Evidence for a novel angiotensin II receptor involved in angiogenesis in chick embryo chorioallantoic membrane. Am J Physiol Heart Circ Physiol. 1993;264:H460–H465. doi: 10.1152/ajpregu.1993.264.2.R460. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Ye Y, Fu B, Wang J, Yu L, Ichiki T, Inagami T, Ichikawa I, Chen X. Genetic deletion of AT2 receptor antagonizes angiotensin II-induced apoptosis in fibroblasts of the mouse embryo. Biochem Biophys Res Commun. 1998;250:72–76. doi: 10.1006/bbrc.1998.9189. [DOI] [PubMed] [Google Scholar]
- 29.Linderman JR, Kloehn MR, Greene AS. Development of an implantable muscle stimulator: measurement of stimulated angiogenesis and poststimulus vessel regression. Microcirculation. 2000;7:119–128. [PubMed] [Google Scholar]
- 30.Liu WB, Wang XP, Wu K, Zhang RL. Effects of angiotensin II receptor antagonist, Losartan on the apoptosis, proliferation and migration of the human pancreatic stellate cells. World J Gastroenterol. 2005;11:6489–6494. doi: 10.3748/wjg.v11.i41.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lonchampt M, Pennel L, Duhault J. Hyperoxia/normoxia-driven retinal angiogenesis in mice: A role for angiotensin II. Invest Ophthalmol Vis Sci. 2001;42:429–432. [PubMed] [Google Scholar]
- 32.Mazurek A, Pierzynski P, Niklinska W, Chyczewski L, Laudanski T. Angiogenesis and Bcl-2 protein expression in patients with endometrial carcinoma. Neoplasma. 2002;49:149–154. [PubMed] [Google Scholar]
- 33.Moravski CJ, Kelly DJ, Cooper ME, Gilbert RE, Bertram JF, Shahinfar S, Skinner SL, Wilkinson-Berka JL. Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertension. 2000;36:1099–1104. doi: 10.1161/01.hyp.36.6.1099. [DOI] [PubMed] [Google Scholar]
- 34.Munzenmaier DH, Greene AS. Opposing actions of angiotensin II on microvascular growth and arterial blood pressure. Hypertension. 1996;27:760–765. doi: 10.1161/01.hyp.27.3.760. [DOI] [PubMed] [Google Scholar]
- 35.Ohashi H, Takagi H, Oh H, Suzuma K, Suzuma I, Miyamoto N, Uemura A, Watanabe D, Murakami T, Sugaya T, Fukamizu A, Honda Y. Phosphatidylinositol 3-kinase/Akt regulates angiotensin II-induced inhibition of apoptosis in microvascular endothelial cells by governing survivin expression and suppression of caspase-3 activity. Circ Res. 2004;94:785–793. doi: 10.1161/01.RES.0000121103.03275.EC. [DOI] [PubMed] [Google Scholar]
- 36.Otani A, Takagi H, Suzuma K, Honda Y. Angiotensin II potentiates vascular endothelial growth factor-induced angiogenic activity in retinal microcapillary endothelial cells. Circ Res. 1998;82:619–628. doi: 10.1161/01.res.82.5.619. [DOI] [PubMed] [Google Scholar]
- 37.Petersen MC, Munzenmaier DH, Greene AS. Angiotensin II infusion restores stimulated angiogenesis in the skeletal muscle of rats on a high-salt diet. Am J Physiol Heart Circ Physiol. 2006;291:H114–H120. doi: 10.1152/ajpheart.01116.2005. [DOI] [PubMed] [Google Scholar]
- 38.Pfosser A, Thalgott M, Buttner K, Brouet A, Feron O, Boekstegers P, Kupatt C. Liposomal Hsp90 cDNA induces neovascularization via nitric oxide in chronic ischemia. Cardiovasc Res. 2005;65:728–736. doi: 10.1016/j.cardiores.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Pollman MJ, Yamada T, Horiuchi M, Gibbons GH. Vasoactive substances regulate vascular smooth muscle cell apoptosis: countervailing influences of nitric oxide and angiotensin II. Circ Res. 1996;79:748–756. doi: 10.1161/01.res.79.4.748. [DOI] [PubMed] [Google Scholar]
- 40.Rivera E, Arrieta O, Guevara P, Duarte-Rojo A, Sotelo J. AT1 receptor is present in glioma cells; its blockage reduces the growth of rat glioma. Br J Cancer. 2001;85:1396–1399. doi: 10.1054/bjoc.2001.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki K, Murohara T, Ikeda H, Sugaya T, Shimada T, Shintani S, Imaizumi T. Evidence for the importance of angiotensin II type 1 receptor in ischemia-induced angiogenesis. J Clin Invest. 2002;109:603–11. doi: 10.1172/JCI13055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoll M, Steckelings UM, Paul M, Bottari SP, Metzger R, Unger T. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 1995;95:651–657. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stupack DG, Cheresh DA. Apoptotic cues from the extracellular matrix: regulators of angiogenesis. Oncogene. 2003;22:9022–9029. doi: 10.1038/sj.onc.1207110. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi T, Taniguchi T, Konishi H, Kikkawa U, Ishikawa Y, Yokoyama M. Activation of Akt/protein kinase B after stimulation with angiotensin II in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 1999;276:H1927–H1934. doi: 10.1152/ajpheart.1999.276.6.H1927. [DOI] [PubMed] [Google Scholar]
- 46.Vogt B, Frey FJ. Inhibition of angiogenesis in Kaposi’s sarcoma by captopril (Abstract) Lancet. 1997;349:1148. doi: 10.1016/S0140-6736(05)63025-5. [DOI] [PubMed] [Google Scholar]
- 47.Walsh DA, Hu DE, Wharton J, Catravas JD, Blake DR, Fan TP. Sequential development of angiotensin receptors and angiotensin I converting enzyme during angiogenesis in the rat subcutaneous sponge granuloma. Br J Pharmacol. 1997;120:1302–1311. doi: 10.1038/sj.bjp.0701062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang S, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization during oxygen-induced ischemic retinopathy in Bcl-2−/− mice. Dev Biol. 2005;279:205–219. doi: 10.1016/j.ydbio.2004.12.017. [DOI] [PubMed] [Google Scholar]