Abstract
Relaxation and guided imagery are useful strategies for cancer pain; however, their effects vary from patient to patient. Patients’ perceptions of these treatments and factors that contribute to their effectiveness have not previously been described. Data from interviews conducted after a trial of guided imagery and progressive muscle relaxation (PMR) interventions were analyzed to compare patients’ perceptions of treatment effects with observed changes in pain scores, and to explore patients’ ideas about factors that contributed to the effectiveness of each intervention. Post-study interviews were conducted with 26 hospitalized patients with cancer-pain who had completed trials of guided imagery and PMR. In most cases, participants’ perceptions of treatment effects matched observed changes in pain scores. Participants described treatment and patient characteristics that influenced effectiveness of the interventions such as active involvement in the intervention, guided instructions, providing a source of distraction, stimulating relaxation, individual abilities and preferences, and pain qualities.
Keywords: Relaxation, Imagery, Pain, Neoplasms, Patient-Centered Care
INTRODUCTION
Cognitive-behavioral strategies, including guided imagery and relaxation interventions, are recommended as adjuvants to analgesic medications to maximize relief of cancer pain [1–2]. Although evidence generally supports the efficacy of such interventions, studies have demonstrated wide variation in their effects, with some patients achieving significant reductions in pain while others experience little or no improvement [3–5]. Moreover, changes in pain ratings described as statistically significant in clinical trials may not be perceived as meaningful improvement by individual patients. While researchers have speculated about patient characteristics that moderate the effects of cognitive-behavioral interventions, patients’ ideas about why a particular strategy works or fails to work have not been systematically investigated. This paper describes a secondary analysis of data collected during a trial of relaxation and imagery interventions for pain in hospitalized patients with cancer [5]. The purposes of these analyses were (1) to compare patients’ perceptions of the effectiveness of guided imagery and PMR interventions with observed changes in their pain scores after using each intervention, and (2) to explore patients’ ideas about factors that influenced effectiveness of the interventions.
BACKGROUND
Unrelieved pain is a frequent reason for hospitalization, particularly for patients undergoing complex and difficult cancer treatment regimens such as chemotherapy or bone marrow transplant, and for patients with advanced stages of the disease [6–8]. Estimates suggest that up to 79% of hospitalized cancer patients experience pain [8–11]. The primary treatment for cancer-related pain focuses on the use of analgesic and adjuvant medications; and while complementary therapies may be offered in some settings, they are widely considered to be underutilized [9,12–13].
Cognitive-behavioral strategies such as guided imagery and relaxation are recommended as adjuvant interventions to enhance pain relief and to increase patients’ perceptions of control over pain [1,14]. Guided imagery involves the use of one’s imagination to create mental images that distract attention away from pain or that alter the pain sensation itself. For example, patients may imagine themselves on a beach or on a mountainside to stimulate relaxation and distract attention from pain; or they may imagine the pain itself and manipulate that image to alter the pain sensation. Relaxation interventions involve physical or mental exercises to release muscle tension and to reduce emotional stress. This relaxation response relieves tension that can exacerbate pain and may also provide a temporary source of distraction from pain. Relaxation exercises such as deep breathing and progressive muscle relaxation are commonly recommended for pain management.
Systematic reviews of cognitive-behavioral strategies including guided imagery and relaxation have suggested that they are effective in reducing cancer pain [15–16]. However, investigators have reported variation in individual responses to cognitive-behavioral pain strategies. Donovan and Laack surveyed 163 chronic pain patients about their use of various nonpharmacologic pain interventions and found that none of the therapies were effective for all patients [3]. For example, relaxation was highly effective for some patients, yet resulted in increased pain for others. Kwekkeboom, Kneip and Pearson tested a guided imagery intervention in hospitalized patients with cancer pain and found that while the majority experienced benefit, 10% reported no change or an increase in pain [4]. And Rhiner and colleagues reported a wide range of treatment effectiveness ratings among elderly patients trying nonpharmacologic strategies for cancer pain [17].
Investigators conducting clinical trials of cognitive-behavioral pain strategies usually report only group data (e.g., means) to describe treatment effects and to determine statistical significance. Individual patient responses are often overlooked, including variation in individual responses and the magnitude or meaningfulness of treatment effects. Patients’ perceptions of how well the intervention worked for their pain may or may not match conclusions regarding effectiveness drawn from group data. In studies with large samples, very small differences in pain (e.g., 5 points on a 0 – 100 visual analogue scale) may reach statistical significance, but the change may be barely perceptible to patients. Thus, it appears that specific cognitive-behavioral strategies are only helpful to some patients and conclusions from studies testing these strategies may or may not match patients’ perceptions of benefit.
A number of pain researchers have recently turned their attention toward identifying patient-related factors that moderate the effectiveness of cognitive-behavioral interventions [18]. Variables including age, race, education, pain duration, number of pain sites, concurrent symptoms, depression, somatization, perceived stress, treatment expectancy, readiness to change, and cognitive aptitude for specific strategies have been suggested as potential moderators of treatment effects [4,14,19–21]. Patients’ perceptions of the factors that explain why specific cognitive-behavioral strategies work or fail to work have not been reported in previous literature.
Thus, the purposes of this secondary data analysis were:
To compare patients’ perceptions of the effectiveness of guided imagery and PMR interventions with observed changes in their pain scores after using each intervention, and
To explore patients’ ideas about factors that influenced effectiveness of the interventions.
MATERIALS AND METHOD
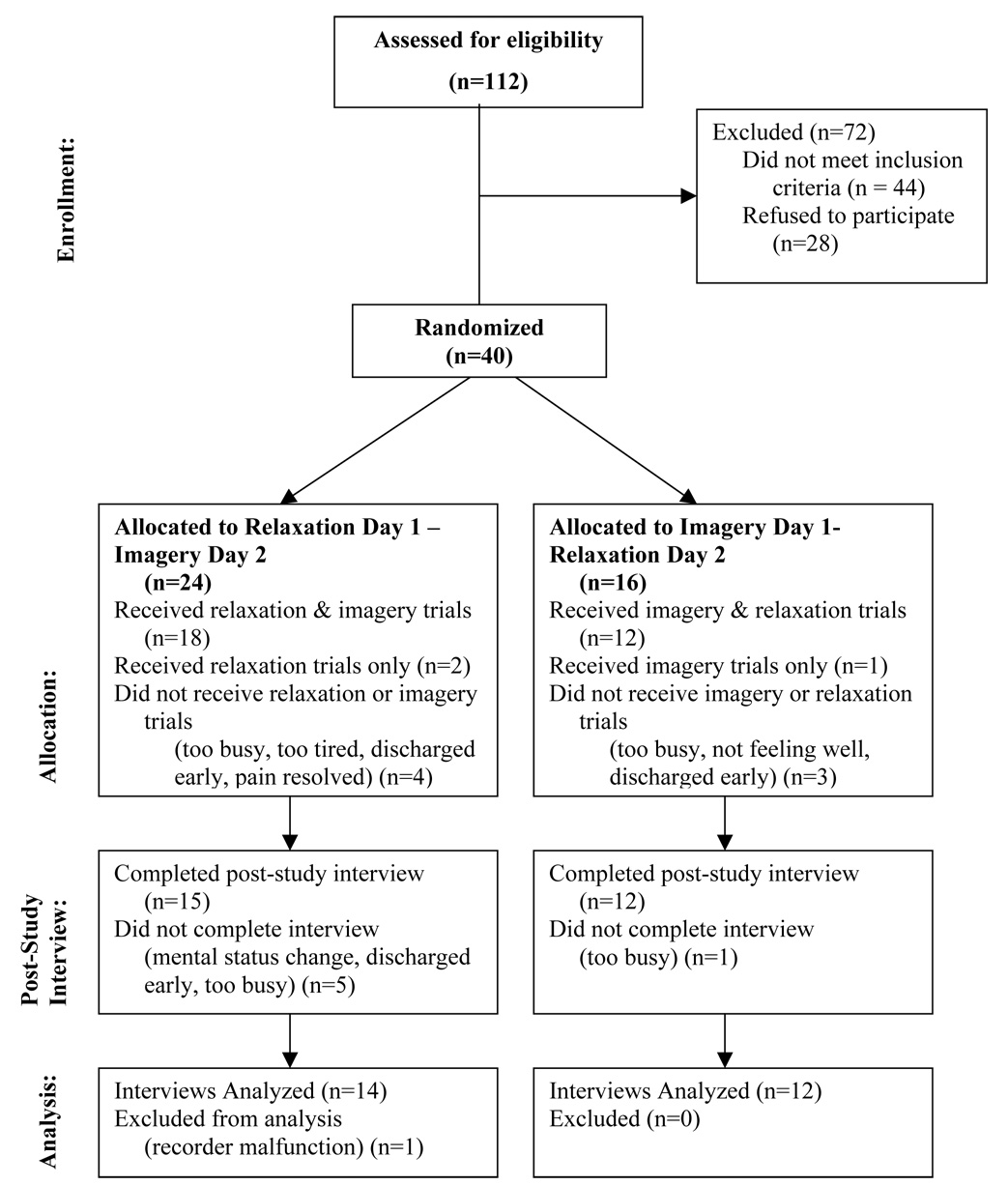
A secondary analysis was conducted using data from a trial of relaxation and guided imagery interventions [5]. The parent study used a two-day crossover design in which 40 hospitalized patients with cancer-related pain completed two trials of PMR and two trials of a guided imagery intervention. Data presented in this report are from 26 participants who completed a post-study interview regarding their perceptions of the interventions (Figure 1).
Figure 1.
Study Flow Diagram
A detailed description of procedures used in the parent study is provided in the primary study report [5]. All study procedures were reviewed and approved by the Health Sciences Institutional Review Board at the University of Wisconsin-Madison. Participants were hospitalized patients with average cancer pain rated ≥ 2 in the past 24 hours. Persons who agreed to participate signed a consent form and subsequently completed two trials each of guided imagery and PMR over a 2-day period. The order of interventions was counterbalanced so that some patients received imagery trials on day 1 and PMR trials on day 2, while others received PMR trials on day 1 and imagery trials on day 2.
The guided imagery intervention used in this study was a 15-minute recording of a pain-focused “glove anesthesia” imagery exercise. This intervention was based on images used in the glove anesthesia hypnotherapy technique [22–23], but did not use a hypnotic induction or encourage trance development. Rather, the instructions focused only on becoming comfortable and creating mental images. The guide asked participants to locate pain sensations in the body and replace them using images of more pleasant sensations, such as cooling or warmth. Participants were then guided in transforming any remaining pain using images of a numbing anesthetic agent.
The relaxation technique used in this study was a 14-minute recording of a PMR exercise. PMR was originally introduced by Jacobson (1929) and modified in 1973 by Bernstein and Borkovec [24–25]. Since that time, many forms of PMR have been described in textbooks, lay press, and the internet and the exercise has been recommended as a management strategy for pain [26–29]. For this study, the instructions led participants in tensing and relaxing 12 major muscle groups working from the hands and arms up to the head and down to the feet [26]. Participants were asked to focus on the contrast between sensations of muscle tension and relaxation. Specific instructions were included to avoid tensing muscles that felt sore or that triggered an increase in pain.
Audio-taped PMR and imagery exercises were used to enhance treatment integrity by assuring that all participants were exposed to the same instructions. No musical background was used on either of the recordings to avoid confounding the effects of music with those of PMR or imagery.
Background data regarding age, gender, race, education, diagnosis, cancer treatments, type of pain, and analgesic orders were obtained directly from participants and through medical record reviews. Participants rated pain intensity immediately before and after each PMR or guided imagery trial using a 0 – 10 numeric rating scale (0 = no pain, 10 = worst pain imaginable). Numeric ratings scales are widely used in pain research and acknowledged as reliable, valid measures of pain intensity [30].
At the end of the second study day a research nurse visited each patient to conduct a post-study interview. The research nurse used an interview guide to ask open-ended questions eliciting patients’ perceptions of the relaxation and guided imagery interventions (Table 1). Questions addressed participants’ enjoyment of each intervention, perceptions of whether or not the intervention worked (i.e., relieved pain), reasons it did or did not work, duration of effects, and preferences for intervention length. Interviews were conducted privately in the patient’s hospital room. The interviews were tape-recorded and later transcribed for analysis.
Table 1.
Interview Questions
| What did you think about the (guided imagery) (relaxation) treatment? |
| 1. Did you enjoy it? |
| 2. Do you think it worked for you? |
| a. If no: Do you have any ideas about why it didn’t work for you? |
| b. If yes: Do you have any ideas about why it worked for you? |
| How long did the effects last for you? |
| 3. Was the length okay? Would you have preferred longer, shorter, or the same? |
| 4. Did you feel like trying the (guided imagery) (relaxation) treatment two times was enough for you to know if it was going to be helpful to you or not? |
DATA ANALYSIS
Observed and Perceived Effect of the Interventions on Pain
Observed effectiveness of each intervention was determined based on improvement in pain intensity ratings. Change in pain intensity was calculated as a percentage to minimize bias associated with absolute (raw) change scores [31]. The posttest score was subtracted from the pretest score and the resulting value was divided by the pretest score. These scores were then averaged across the two PMR trials and across the two guided imagery trials, resulting in a single percent pain change score for each strategy. Participants were categorized as responders if their pain change score was ≥ 30%, which has been suggested as a clinically meaningful reduction in pain [32]. Participants who reported < 30% improvement or an increase in pain were categorized as non-responders. Perceived effectiveness of each strategy was determined from participants’ interview responses about whether or not PMR and guided imagery worked for their pain.
General Perceptions of the Interventions and Factors that Influenced their Effectiveness
Data from post-study interview questions that required only brief simple responses (e.g., yes or no) were analyzed by counting the frequency of specific responses. Data from interview questions about reasons that PMR and guided imagery did or did not work were less structured than responses to other interview questions, and were evaluated using basic content analysis [33–34]. A member of the research team reviewed the first 11 transcripts using emergent coding to identify main ideas or themes within the responses. Each theme described a factor perceived to have influenced the effectiveness of PMR or guided imagery. The remaining team members then reviewed the proposed themes, created categories of related themes, and discussed any disagreements until consensus was reached. Thus, a coding scheme was created which was used to analyze the full set of transcripts. Two team members reviewed the final coding and noted any disagreements. Percent agreement across questions ranged from 88–100%.
RESULTS
Demographic Characteristics
The 26 participants who completed post-study interviews ranged in age from 18 to 72 years (M=43, SD=16). Most were female (n=16, 62%), Caucasian (100%), and educated beyond high school (n=22, 85%). Twenty (77%) had hematologic malignancies. Eighteen (69%) were receiving chemotherapy or radiation treatment at the time of the study. The majority were experiencing somatic pain (n=18, 69%), and were receiving strong opioids (i.e., morphine, hydromorphone, fentanyl, or methadone) as pain treatment (n=22, 85%).
Observed and Perceived Effect of the Interventions on Pain
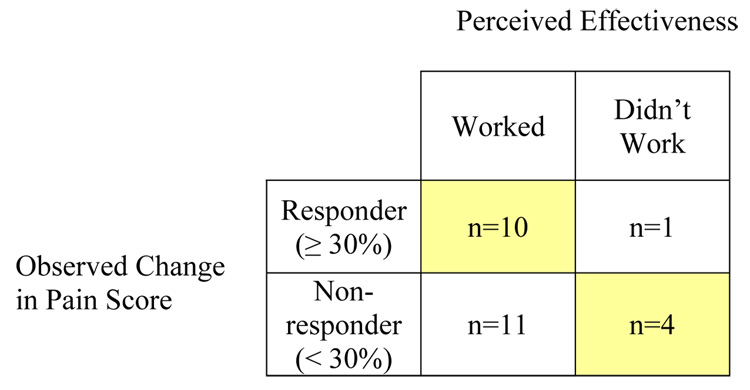
Observed responses to PMR and corresponding perceptions of effectiveness are summarized in Figure 2. Twenty-one participants (81%) reported that the PMR intervention worked to relieve their pain, but only ten of those were also categorized as responders based on observed pain scores. Eleven participants who perceived that PMR worked for their pain reported < 30% improvement in pain score after using the intervention. One of five participants who reported that PMR did not work for their pain actually had ≥ 30% improvement in pain scores. Overall, perceptions of effectiveness of PMR matched observed changes in pain in 14 cases (54%).
Figure 2.
Comparison of Observed Changes in Pain Score and Perceived Effectiveness of PMR
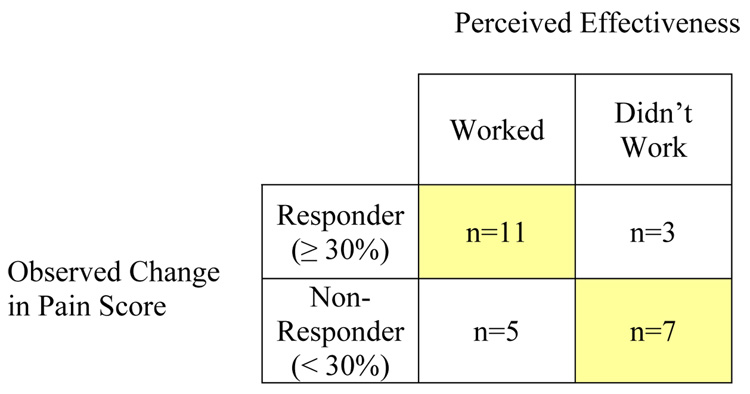
Observed responses to guided imagery and corresponding perceptions of effectiveness are summarized in Figure 3. Sixteen participants (62%) reported that the guided imagery intervention worked to relieve their pain, and eleven of those were also categorized as responders based on changes in their pain scores. Five participants who perceived that guided imagery worked for their pain reported < 30% improvement in pain score after using guided imagery. Three of ten participants who reported that guided imagery did not work for their pain actually reported ≥ 30% improvement in their pain scores. Overall, perceptions of effectiveness of guided imagery matched observed changes in pain in 18 cases (69%).
Figure 3.
Comparison of Observed Changes in Pain Score and Perceived Effectiveness of Guided Imagery
General Perceptions of the Interventions and Factors that Influenced their Effectiveness
Responses to simple post-study interview questions are described in Table 2. The majority of participants reported that they enjoyed both cognitive-behavioral strategies. Effects of the treatments lasted from a few minutes to more than an hour, with most participants reporting duration of 30–60 minutes. The 15-minute length of the interventions was acceptable to most participants, although a few noted that they would have preferred a longer session. Nearly all participants reported that two trials were enough to determine if the treatment was going to be effective for their pain.
Table 2.
Summary of Interview Responses
| Question | PMR | Guided Imagery |
|---|---|---|
| n (%) | n (%) | |
| Enjoyed the treatment | 21 (81%) | 14 (54%) |
| Perceived that it worked | 21 (81%) | 16 (62%) |
| Duration of effects | ||
| 0 (didn’t work) | 5 (19%) | 10 (38%) |
| 10 minutes or less | 5 (19%) | 3 (12%) |
| 11–29 minutes | 2 (8%) | 2 (8%) |
| 30–60 minutes | 11 (42%) | 7 (27%) |
| Longer than 60 minutes | 3 (12%) | 4 (15%) |
| Preference for length | ||
| Same length (15 min.) | 22 (85%) | 23 (88%) |
| Shorter | 0 (0%) | 1 (4%) |
| Longer | 4 (15%) | 2 (8%) |
| Two trials were enough to gauge effectiveness | ||
| Yes | 20 (76%) | 23 (88%) |
| No | 1 (4%) | 2 (8%) |
| Not sure | 3 (12%) | 0 (0%) |
| No answer | 2 (8%) | 1 (4%) |
Themes identified in participants’ comments about why the imagery and PMR interventions worked or did not work for their pain fell into two categories, (1) characteristics of the intervention itself, and (2) patient characteristics and preferences (Table 3).
Table 3.
Factors Perceived to Have Influenced Effects of Cognitive-Behavioral Interventions
| Intervention | Categories and Themes |
|---|---|
| PMR | Characteristics of the Intervention |
| Active physical involvement in muscle tension / contraction | |
| Guided instructions | |
| Source of distraction | |
| Soothing tone of voice | |
| Facilitated a mind-body connection | |
| Produced other sensations – relaxation, energy | |
| Patient Characteristics / Preferences | |
| Previous experience with holistic therapies | |
| Awareness of personal control over pain | |
| Having a positive attitude | |
| Physical capacity / freedom of movement | |
| Energy level | |
| Guided Imagery | Characteristics of the Intervention |
| Source of distraction | |
| Provided uninterrupted quiet time | |
| Soothing tone of voice | |
| Stimulated relaxation | |
| Anesthetic images | |
| Guided instructions | |
| Pacing of instructions | |
| Relevance to pain | |
| No active physical involvement | |
| Patient Characteristics / Preferences | |
| Pain characteristics – type, intensity | |
| Individual imaging ability | |
| Preference for specific mental images | |
| Preferred coping style |
PMR: Characteristics of the Intervention
Participants who perceived that PMR was effective for their pain were asked why they thought the intervention worked. The most common themes in their responses related to characteristics of the PMR treatment itself. Eleven participants reported that awareness of muscle tension and instructions to tense and relax muscle groups contributed to the pain relieving effects of PMR. For example, one subject stated:
“I think just being aware that your muscles are tense, or they’re relaxed and that you can do something about that [made PMR work]”.
Another subject explained:
“The tensing thing, where you’re actually…focusing more attention on a particular part of your body, and then you feel more intense relaxation as a result [led to pain relief]”
Similarly, active involvement in performing the PMR exercise was described as contributing to the intervention’s effects (n=3). One participant stated that PMR worked:
“just because I was actively involved.”
Participants also noted the importance of having guided instructions (n=3), commenting that what made PMR work was:
“[The instructions] just guiding me though; ‘Use these muscles’, and the way you go about it”.
Two patients noted that PMR provided distraction from pain with comments like:
“you could blank your mind out…and concentrate on the person telling you to make fists, do your biceps, arch your back… You’re concentrating on that so much [that] you’re not really thinking about anything else.”
One participant commented that the soothing tone of voice on the recording made PMR effective and another participant noted that the PMR intervention facilitated a mind-body connection.
Participants who perceived that PMR was not effective for their pain also described characteristics of the intervention in explaining why the treatment did not work. Two participants suggested that the PMR produced sensations other than pain relief, with comments such as:
“It didn’t really relax me at the end. It actually energized me. So, it was counterproductive.”
“I don’t think it took the pain [away]…but it certainly helped me relax.”
PMR: Patient Characteristics and Preferences
In addition to comments about the PMR intervention, themes identified in participants’ responses revealed a number of personal characteristics and preferences as influencing the effects of the PMR intervention. Personal characteristics and preferences that positively influenced effects of the PMR interventions included previous experiences with holistic therapies (n=1), an awareness of personal control over pain (n=1), and having a positive attitude (n=1). Characteristics and preferences that negatively influenced effects of the PMR intervention were related to physical and mental capacity. One participant described the intervention as difficult to complete given her position lying in bed with IV lines:
“You start trying to move things; then it just gets awkward and you have to stop… [It was] a difficult combination of movements, depending on your position.”
Other participants described that feeling too tired (n=1) or feeling unable to focus or concentrate (n=1) prevented PMR from working for them.
Guided Imagery: Characteristics of the Intervention
Participants who perceived that guided imagery worked for their pain were asked why the intervention worked. The most common theme in their responses was that guided imagery provided a source of distraction from pain (n=4). Participants reported that:
“[The imagery] draws your attention away from the pain in your body, just sort of getting it out of your immediate environment.”
“If I can get my concentration someplace else, …it’s just not as painful.”
Four participants highlighted the importance of uninterrupted quiet time, noting:
“It actually focused quiet time and I didn’t have any disturbances, so I think it worked then.”
Three participants noted that the soothing voice guiding the imagery contributed to its effects. Two participants reported that imagery was effective because it stimulated relaxation.
Some participants described the specific images and instructions as influential factors in effectiveness. For example, two participants noted the value of the anesthetic images, citing:
“The anesthesia being on you and being able to use it where you are sore.”
Two participants described the guided instructions as important stating:
“it talked me through it, and told me how everything would work”.
Other positive aspects of the intervention included the pacing of instructions, providing sufficient time to create personal images (n=1), and relevance of the intervention to the patient’s pain (n=1).
One theme related to the guided imagery intervention itself was identified in the interview responses of persons who did not find the intervention to be effective. A single participant noted that the lack of physical involvement in performing guided imagery detracted from any impact on pain.
Guided Imagery: Patient Characteristics and Preferences
All of the themes related to patient characteristics and preferences were described by persons who reported that guided imagery was not effective for their pain. Three participants noted that characteristics of their pain (pain type or intensity) prevented the imagery from being effective. A participant with painful mucositis said:
“Once you start to talk, you irritate things…and it kinda brings the pain back.”
Another participant with severe pain reported that imagery didn’t work:
“just because I was in so much pain by then that I’d sort of gone over the brink.”
Two participants reported difficulty creating the visual images, stating:
“The images were hard for me to conjure up and maintain.”
Two participants noted a preference for different types of mental imagery (e.g., pleasant nature imagery). One participant shared that the guided imagery didn’t match her personal coping style, commenting:
“I think that I’m a different personality, that I need to see it to believe it.”
DISCUSSION
Perceptions of pain relief were consistent with observed changes in pain scores for approximately half of the participants when using PMR and for approximately two-thirds of the participants when using guided imagery. Approximately half of the subjects were categorized as responders to each intervention based on observed changes in pain scores. And while nearly the same number perceived that guided imagery was effective (n=16, 62%), a greater number of participants perceived that PMR was effective for their pain (n=21, 81%).
A large number of participants (n=11) reported that PMR worked for their pain, when in fact, their pain ratings decreased by less than 30%. Similarly five persons reported that guided imagery worked for their pain, when the change in their pain score had not met the criterion for clinical significance. Several studies have suggested that pain relief in the range of 30–33% constitutes a clinically meaningful improvement to patients [32,35], but perhaps, for at least a subsample of individuals, a meaningful change in pain does not require as much as 30% improvement. It is also possible that patients’ perceptions of the PMR intervention took into account more than simply reduction in pain intensity. Patients may have also considered a reduction in pain-related distress or general anxiety. Perhaps some patients were pleased with the outcome of the treatment if it stimulated relaxation or provided uninterrupted quiet time in the busy hospital environment. If this is the case, patients may be getting additional benefits from the cognitive-behavioral strategies and providers should consider offering treatment even if it produces only small reductions in pain intensity.
Only a small number of participants perceived that the interventions failed to work when, in fact, observed changes in pain would suggest otherwise. This experience was more frequent with the guided imagery intervention (n=3) compared to PMR (n=1). Guided imagery interventions may be less familiar than relaxation interventions, leading to uncertain expectations of benefit. The analgesic imagery may not have been what participants expected compared to the more traditional pleasant nature imagery, resulting in lower satisfaction with the imagery intervention.
Themes in participants’ interview responses revealed a number of reasons why the cognitive-behavioral strategies did or did not work for them. The themes represented characteristics of the treatment as well as patient characteristics and preferences. Factors that were consistent across both types of interventions included guided instructions, active involvement, a soothing tone of voice, and providing an engaging source of distraction as well as stimulating relaxation. Simple, easy to follow instructions are particularly important for patients who have little previous experience with the intervention. Active involvement when following instructions may facilitate patients’ development of a personal sense of control over pain and contribute to a therapeutic change in pain perception [14]. In addition to active involvement, participants suggested that providing a relaxing stimulus contributed to the effectiveness of the treatment. Thus, even imagery exercises that focus on creating images of the bothersome symptom itself could benefit from the inclusion of a brief relaxation component.
Participants identified factors unique to the perceived effectiveness of PMR including experience with holistic therapies, physical capacity, energy level, a positive attitude, awareness of personal control over pain, and facilitation of the mind-body connection. Factors unique to the effectiveness of guided imagery included the type and intensity of pain, uninterrupted quiet time, pacing of instructions, visual imaging ability, types of images, relevance to pain, and match with personal preferences for coping style. It would seem that many of these factors could apply to both interventions. For example, all cognitive-behavioral pain strategies should support a positive attitude and emphasize that one’s mind can contribute to how bodily sensations are experienced. Individuals’ mental and physical capacity to engage in a particular intervention should be taken into account, and their preferences for specific instructions and images should be solicited.
A few of the factors identified by participants have been previously described and studied. For example, previous experience with a holistic therapy (guided imagery) has been found to predict outcome expectancy about future uses of the intervention [36]. Imaging ability, the cognitive aptitude for creating and experiencing vivid mental images, has also been of interest in the research literature. Several investigators have found that response to imagery interventions is positively correlated with imaging ability [4,37–38]. The influence of type and intensity of pain on cognitive-behavioral strategies has received some attention, primarily in systematic reviews, but very little research has specifically compared strategies among different types of cancer pain or among persons with differing levels of pain intensity in a single study. Potential moderating factors identified by pain researchers that did not appear in our participants’ comments included demographic characteristics (age, race, education), concurrent symptoms (depression, stress, side effects of treatment), and readiness to change pain coping behaviors. Readiness to change, however, may have been implied by agreeing to participate in the study.
Participants’ responses to other interview questions yielded findings that have not previously been addressed in the literature. The duration of pain relief provided by both PMR and guided imagery interventions was typically described as 30–45 minutes or more. The length of the interventions, approximately 15-minutes, was considered desirable by nearly all participants. Fifteen minutes is a reasonable amount of time for a staff nurse to spend with an individual patient or to assure privacy, without interruption, in a busy inpatient setting. Patients indicated that they had a good idea of whether the interventions would be helpful to them after two trials. This suggests that in offering a range of cognitive-behavioral strategies, patients could complete brief trials and fairly quickly identify their preferred strategies.
Several limitations to this study should be noted. First, only a small sample of participants was available for this analysis. Opinions of those patients who were unable to complete or who dropped out of the parent study before the interview are unknown. In addition, our sample was entirely Caucasian, thus we don’t know how people of minority races might respond to the PMR and guided imagery interventions or what factors they would perceive as influencing treatment effects. Second, we selected a commonly identified criterion (30%) to categorize participants as treatment responders or non-responders. As previously described, this criterion may not be appropriate for all patients with pain. Third, imagery strategies like glove anesthesia that involve focusing attention on the painful stimulus, may be distressing and could actually intensify awareness of pain. A different type of imagery intervention that focuses attention away from pain, such as pleasant nature imagery, may have produced a different response and revealed alternative ideas about factors that influence effectiveness. The method of delivering interventions may have also influenced results. There is some evidence suggesting that live instruction in relaxation may be more effective than audio-taped instructions. Finally, there is the possibility that responses reflect demand characteristics. Participants may have guessed that the researchers wanted to see improvement in pain scores and hear, in post-study interviews, that the treatments worked. At least 38% of participants did, however, feel comfortable reporting that guided imagery did not work for them.
CONCLUSIONS
Cognitive-behavioral strategies like guided imagery and PMR are useful in treating cancer pain for some patients. A majority of participants perceived that the interventions worked for their pain and, in fact, many reported a clinically significant change in pain with the interventions. Individual patients have preferences for the way pain is managed and for the content and style of cognitive-behavioral interventions. Practitioners should inquire about these preferences prior to implementing interventions. Future research should explore treatment-related characteristics such as level of active involvement, degree of distraction / relaxation provided by various interventions, and environmental factors (e.g., uninterrupted quiet time). Patient-related variables including physical capacity, energy level, type and intensity of pain, coping style, and preferences for intervention content should also continue to be investigated. Health care providers may be able to use this information to help patients select specific cognitive-behavioral strategies and tailor their content to be most effective in managing an individual patient’s pain.
ACKNOWLEDGEMENTS
This work was supported by funding from the Center for Patient-Centered Interventions (Grant Number P20 NR008987 from NIH [PI: Sandra Ward, UW-Madison School of Nursing]), and the University of Wisconsin-Madison Graduate School. The sponsors had no involvement in study design, data collection and analyses, or manuscript preparation / submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.American Pain Society. Guideline for the management of cancer pain in adults and children. Glenview, IL: Author; 2005. [Google Scholar]
- 2.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Acute Cancer Pain (Version 1.2006) Available from: http://www.nccn.org.
- 3.Donovan M, Laack KD. Individually reported effectiveness of therapy for chronic pain. Clin Nurs Res. 1998;7:423–439. doi: 10.1177/105477389800700408. [DOI] [PubMed] [Google Scholar]
- 4.Kwekkeboom KL, Kneip J, Pearson L. A pilot study to predict success with guided imagery for cancer pain. Pain Manag Nurs. 2003;4:112–123. doi: 10.1016/s1524-9042(02)54213-2. [DOI] [PubMed] [Google Scholar]
- 5.Kwekkeboom KL, Wanta B, Bumpus M. Individual difference variables and the effects of progressive muscle relaxation and analgesic imagery interventions on cancer pain. J Pain Symptom Manage. doi: 10.1016/j.jpainsymman.2007.12.011. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fortner BV, Okon TA, Portenoy RK. A survey of pain-related hospitalizations, emergency department visits, and physician office visits reported by cancer patients with and without history of breakthrough pain. J Pain. 2002;3:38–44. doi: 10.1054/jpai.2002.27136. [DOI] [PubMed] [Google Scholar]
- 7.Goudas LC, Bloch R, Gialeli-Goudas M, Lau J, Carr DB. The epidemiology of cancer pain. Cancer Invest. 2005;23:182–190. [PubMed] [Google Scholar]
- 8.Wells N. Pain intensity and pain interference in hospitalized patients with cancer. Oncol Nurs Forum. 2000;27:985–991. [PubMed] [Google Scholar]
- 9.McMillan SC, Tittle M, Hagan S, Laughlin J. Management of pain and pain-related symptoms in hospitalized veterans with cancer. Cancer Nurs. 2000;23:327–336. doi: 10.1097/00002820-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 10.McNeill JA, Sherwood GD, Stark PL, Thompson CJ. Assessing clinical outcomes: Patient satisfaction with pain management. J Pain Symptom Manage. 1998;16:29–40. doi: 10.1016/s0885-3924(98)00034-7. [DOI] [PubMed] [Google Scholar]
- 11.Vainio A, Auvinen A. Prevalence of symptoms among patients with advanced cancer: An international collaborative study: Symptom prevalence group. J Pain Symptom Manage. 1996;12:3–10. doi: 10.1016/0885-3924(96)00042-5. [DOI] [PubMed] [Google Scholar]
- 12.Carulla Torrent J, Jara Sanchez C, Sanz Ortiz J, Batista Lopez N, Camps Herrero C, Cassinello Espinosa J, et al. Oncologists’ perceptions of cancer pain management in Spain: the real and the idea. Eur J Pain. 2007;11:352–359. doi: 10.1016/j.ejpain.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Gordon DB, Dahl JL, Miaskowski C, McCarberg B, Todd KH, Paice JA, et al. American pain society recommendations for improving the quality of acute and cancer pain management. Arch Intern Med. 2005;165:1574–1580. doi: 10.1001/archinte.165.14.1574. [DOI] [PubMed] [Google Scholar]
- 14.Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain. 2007;127:276–286. doi: 10.1016/j.pain.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Mundy EA, DuHamel KN, Montogery GH. The efficacy of behavioral interventions for cancer treatment-related side effects. Semin Clin Neuropsychiatry. 2003;8:253–275. [PubMed] [Google Scholar]
- 16.Roffe L, Schmidt K, Ernst E. A systematic review of guided imagery as an adjuvant cancer therapy. Psychooncology. 2005;14:607–617. doi: 10.1002/pon.889. [DOI] [PubMed] [Google Scholar]
- 17.Rhiner M, Ferrell BR, Ferrell BA, Grant MM. A structured nondrug intervention program for cancer pain. Cancer Pract. 1993;1:137–143. [PubMed] [Google Scholar]
- 18.Keefe FJ, Buffington ALH, Studts JL, Rumble ME. Behavioral medicine: 2002 and beyond. J Consult Clin Psychol. 2002;70:852–856. doi: 10.1037//0022-006x.70.3.852. [DOI] [PubMed] [Google Scholar]
- 19.Dijkstra A. The validity of the stages of change model in the adoption of the self-management approach in chronic pain. Clin J Pain. 2002;21:27–37. doi: 10.1097/00002508-200501000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Goossens MEJB, Vlaeyen JWS, Hidding A, Kole-Snijders A, Evers SMAA. Treatment expectancy affects the outcome of cognitive-behavioral interventions in chronic pain. Clin J Pain. 2002;21:18–26. doi: 10.1097/00002508-200501000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Kwekkeboom KL. A model for cognitive-behavioral interventions in cancer pain management. J Nurs Scholarsh. 1999;31:151–156. doi: 10.1111/j.1547-5069.1999.tb00456.x. [DOI] [PubMed] [Google Scholar]
- 22.Bassman SW, Wester WC. Hypnosis and pain control. In: Wester WC, Smith AH, editors. Clinical Hypnosis: A multidisciplinary approach. Philadelphia: JB Lippincott Company; 1984. pp. 236–287. [Google Scholar]
- 23.Bresler DE. Alpha Books, Inc: Malibu, CA; 1996. Free yourself from pain: Glove Anesthesia [audiocassette] [Google Scholar]
- 24.Jacobsen E. Progressive relaxation. Chicago: University of Chicago Press; 1929. [Google Scholar]
- 25.Bernstein D, Borkovec T. Progressive relaxation: A manual for the helping professions. Research Press: Champaign, IL; 1973. [Google Scholar]
- 26.McCaffery M, Beebe A. Pain: Clinical manual for nursing practice. St. Louis: Mosby; 1989. [Google Scholar]
- 27.Snyder M, Lindquist R. Complementary / alternative therapies in nursing. 3rd ed. New York: Springer Publishing Company; 1998. [Google Scholar]
- 28.Potter PA, Perry AG. Fundamentals of nursing. 6th ed. St. Louis: Mosby; 2005. [Google Scholar]
- 29.Mayo Clinic Staff. Relaxation techniques: Learn ways to calm your stress. 2007 Mar 7; Available from: http://www.mayoclinic.com/health/relaxation-technique/SR00007.
- 30.Jensen MP. The validity and reliability of pain measures in adults with cancer. J Pain. 2003;4:2–21. doi: 10.1054/jpai.2003.1. [DOI] [PubMed] [Google Scholar]
- 31.Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain. 2003;4:407–414. doi: 10.1016/s1526-5900(03)00716-8. [DOI] [PubMed] [Google Scholar]
- 32.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 33.Weber RP. Basic content analysis. 2nd ed. Newbury Park, CA: Sage Publications; 1990. [Google Scholar]
- 34.Stemler S. An overview of content analysis. Practical Assessment, Research & Evaluation. 2001;7 Available from: http://PAREonline.net/getvn.asp?v=7&n=17.
- 35.Farrar JT, Berlin JA, Strom BL. Clinically important changes in acute pain outcome measures: a validation study. J Pain Symptom Manage. 2003;25:406–411. doi: 10.1016/s0885-3924(03)00162-3. [DOI] [PubMed] [Google Scholar]
- 36.Kwekkeboom KL. Outcome expectancy and success with cognitive-behavioral interventions: The case of guided imagery. Oncol Nurs Forum. 2001;28:1125–1132. [PubMed] [Google Scholar]
- 37.Johnson EL, Lutgendorf SK. Contributions of imagery ability to stress and relaxation. Ann Behav Med. 2001;23:273–281. doi: 10.1207/S15324796ABM2304_6. [DOI] [PubMed] [Google Scholar]
- 38.Watanabe E, Fukuda S, Hara H, Maeda Y, Ohira H, Shirakawa T. Differences in relaxation by means of guided imagery in a healthy community sample. Altern Ther Health Med. 2006;12:60–6. [PubMed] [Google Scholar]