Abstract
DNA repair should occur after cells sense DNA damage signals and undergo cell-cycle arrest to provide sufficient time for DNA repair, and suboptimal DNA repair capacity (DRC) in peripheral lymphocytes has been suggested as a cancer susceptibility marker. Numerous studies showed a functional link between DNA damage sensing, cell-cycle checkpoint and DNA repair. We hypothesized that in vitro cell-cycle checkpoint-related protein expression levels in stimulated lymphocytes predict DRC levels. To test this hypothesis, we performed the host-cell reactivation assay for DRC by transfecting stimulated peripheral blood lymphocytes from 120 normal donors with transient expression plasmids damaged by benzo[a]pyrene diol epoxide (BPDE). The same cells were assessed for protein expression induction of eight cell-cycle checkpoint-related genes using the reverse-phase protein lysate microarray assay. In multivariate linear regression analysis adjusting for age, sex, blastogenic rate, and sample storage duration, the association between DRC and expression levels of cell-cycle checkpoint-related proteins induced by BPDE-adducts was statistically significant for p27, CCND1, ATM, and MDM2 (P = 0.00, 0.03, 0.03, and 0.03, respectively), borderline for p73 and p21 (P = 0.07 and 0.09, respectively), but not for p53 and p16 (P = 0.13 and 0.18, respectively). Because the relative expression levels of all these eight proteins were highly correlated, we further performed the principal component analysis and identified ATM as the most important predictor of DRC, followed by MDM2 and p27. Our results provide population-based in vitro evidence demonstrating that cell-cycle checkpoint-related proteins play essential roles in regulating DNA repair, at least in unaffected human peripheral blood lymphocytes. Further studies are warranted to investigate the role of inter-individual variation in the expression levels of these proteins in cancer susceptibility.
Keywords: cell cycle checkpoint, DNA repair, microarray, molecular epidemiology, proteomics
Introduction
Human DNA is constantly assaulted by endogenous cellular metabolites and exogenous damaging agents.1, 2 Consequent lesions that are not properly repaired prior to cell division can be mutagenic, resulting in DNA sequence changes or mutation fixation that can be carcinogenic.3, 4 Normal cell-cycle arrest and DNA repair are sequential and integrated biological events in response to cellular stressors and are crucial for the maintenance of genomic integrity.
Inter-individual variation exists in human cellular response to carcinogens5 and in the resultant DNA repair capacity (DRC)6 in the general population. For example, on the extreme low end of the repair capacity continuum is xeroderma pigmentosum (XP), a rare recessive syndrome that is caused by genetic defects in nucleotide excision repair (NER) genes and characterized by an extraordinary hypersensitivity to ultraviolet light and a high risk of skin cancer.7 Research has shown that individual DRC differs widely and that suboptimal DRC is associated with the risk of developing various cancers.8–11 In addition to the DRC phenotype, variations in the mRNA12–17 and protein expression levels of DNA repair genes18 have been shown to be associated with cancer risk.
Although DNA repair is known to be controlled by cell-cycle checkpoint signals and cell-cycle arrest, this knowledge is supported mainly by in vitro studies using proliferating cell lines but not tested in population studies using primary cells such as non-proliferating peripheral lymphocytes.19 We hypothesized that in vitro cell-cycle checkpoint-related protein expression levels in stimulated lymphocytes predict DRC levels. We tested this hypothesis in a non-Hispanic white population through assessment of the DRC phenotype and the expression levels of proteins that participate in the repair of benzo[a]pyrene diol epoxide (BPDE)-induced DNA adducts in lymphocytes. We studied primary lymphocytes because stimulated lymphocytes in culture are as effective as other cell types in repairing various kinds of DNA damage and peripheral lymphocytes are accessible for epidemiological studies.8–11 BPDE-damaged plasmids were used as both a substrate for NER enzymes after transfection of lymphoblasts8–11 and a stimulant for the expression of cell cycle checkpoint-related proteins.18 The DRC phenotype was measured using the host-cell reactivation (HCR) assay,20, 21 and the expression levels of cell cycle checkpoint-related proteins were measured using the reverse-protein lysate microarray assay.18 These two assays were performed with the same cultured peripheral blood lymphocytes from 120 normal donors. For the relative protein expression, we used specific antibodies to detect eight proteins critical in cell cycle regulation, including the DNA damage sensors (ATM and p53), p53-negative regulator (MDM2), p53 family member p73, cell cycle regulator (CCND1), and cyclin dependent kinase (CDK) inhibitors (p16, p21, and p27). An additional protein, β-actin, was used as the assay control for the protein baseline expression to calculate the relative expression levels of other eight proteins. These proteins play key roles in the p53-mediated pathways22, 23 and are frequently studied in population-based case-control studies both for its expression levels and genetic variations.24–26 For example, p53 has a dual role in DNA damage sensing and cell-cycle regulation, MDM2 servers as the most important negative regulator of p53, p73 is a promising protein that participates in the p53-independent pathway, ATM is one of the earliest sensors in response to DNA damage, and expression levels of CCND1, p16, p21 and p27 were extensively studied in previous studies as regulators of cell-cycle progression.
Materials and Methods
Subject recruitment
We obtained previously cryopreserved viable peripheral blood lymphocyte samples from 120 cancer-free controls participating in an ongoing case-control study of squamous cell carcinoma of the head and neck.20 The controls had a mean age of 57.4 years (standard deviation 10.4; range 38–79) and included 108 men and 12 women. We selected samples that had a sufficient number of lymphocytes for successful cell culture, subsequent transfection by plasmids, DRC assay, and protein extraction for the reverse-protein lysate microarray assay. The University of Texas M. D. Anderson Cancer Center institutional review board approved the research protocol.
BPDE-HCR assay
The HCR assay has been described in detail elsewhere.21, 27 Briefly, fresh peripheral blood lymphocytes were isolated by Ficoll gradient centrifugation within eight hours of collection and stored at −80°C until use. Purified pCMVcat plasmids were treated with BPDE (Midwest Research Institute, Kansas City, MO) at a final concentration of 60 µM.27 The cells were thawed and cultured in batches of 10–20 and stimulated by phytohemagglutinin to take up the plasmids with BPDE-induced DNA adducts. After stimulation, we determined the blastogenic rate by counting the number of viable, large lymphoblasts in the culture for each sample. The lymphoblasts from each subject were divided into four aliquots, each containing approximately 1 ×106 cells, for duplicate transfections with untreated plasmids (the baseline for comparison) and duplicate transfections with BPDE-treated plasmids. Because transcription of the reporter gene chloramphenicol acetyltransferase (CAT) can be blocked by a single unrepaired DNA adduct,28 any CAT activity provides a quantitative measurement of the DRC of the transfected cells that removed BPDE-induced adducts from their plasmids. We measured the activity of repaired CAT gene using a scintillation counter to detect [3H]monoacetylated and [3H]diacetylated chloramphenicols formed by the reaction between chloramphenicol and [3H]acetyl coenzyme A catalyzed by the CAT protein in the cell extract.27 Here, DRC is the ratio of the CAT activity of cells transfected with BPDE-treated plasmids to that of cells transfected with untreated plasmids, i.e., DRC = (CATBPDE60/CATBPDE0) ×100%.
Protein extraction and preparation of reverse-phase protein lysate microarrays (RPP)
The protocol for the RPP assay has been described elsewhere.18 Briefly, after stimulation and harvesting, the cells (~1 ×106) were transfected with BPDE-treated plasmids in duplicate, as done for the BPDE-HCR assay. Forty hours after transfection, the cells were collected and whole proteins were extracted, boiled, and stored at −80°C until use. Each sample was serially diluted by 1, 2, and 4 times by adding phosphate-buffered saline (PBS) buffer (pH 7.5) to final protein concentrations ranging from 1 mg/ml to 0.025 mg/ml accordingly. The serial dilutions were transferred to 384-well plates and spotted onto FAST™ slides (Scheicher & Schuell, Germany) using a SpotBot Arrayer (Telechem International, Cupertine, CA). Each sample was spotted in duplicate. The fabricated slides were treated for 15 min with Reblot (Chemicon, Temeula, CA) and subsequently washed two times for 10 min each with a washing buffer that contained 300 mM NaCL, 0.1% Tween 20, and 50mM Tris at pH7.6 (TBS). The protein arrays were then blocked for 30 min at room temperature using I-block (Applied Biosystems, Foster City, CA), incubated for 1 h with individual antibodies, and treated for 30 min with biotin-labeled secondary antibodies (Vector Laboratories, Burlingame, CA). We used the DAKO Catalyzed Signal Amplification (CSA) System (DAKO Corporation, Carpentaria, CA) according to manufacturer protocol (except for the final step) to enhance signals by incubating the protein arrays for 30 min with Cy5-conjugated streptavidin (1:1000 dilution) (Jackson Immuno Research Laboratories, West Grove, PA). Between each incubation step, the arrays were washed three times for 5 min each with TBS.
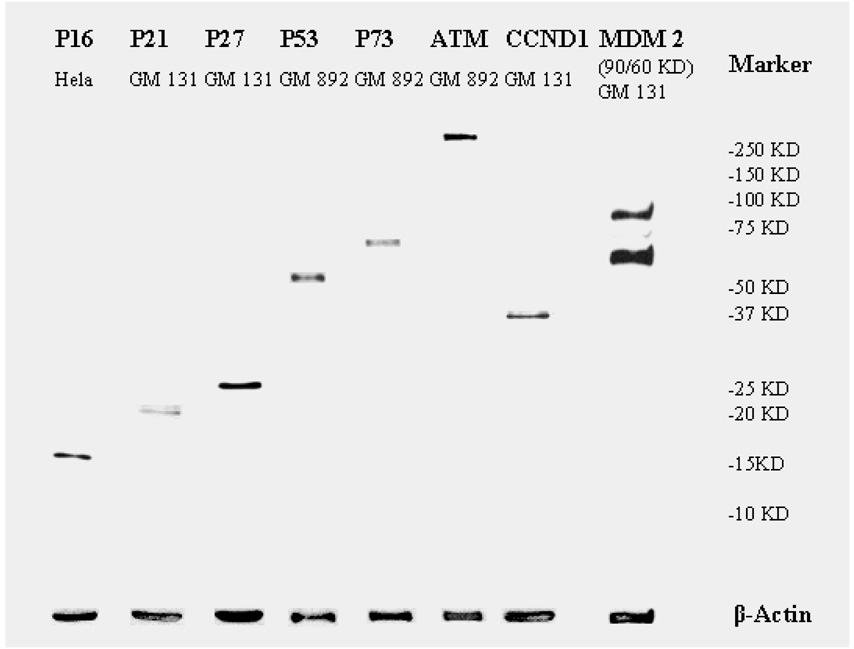
Antibodies against p16, p27, p73, CCND1, and MDM2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); ATM from Abcam (Cambridge, MA); p21 from BD Pharmigen (San Diego, CA); p53 from Lab Vision (Fremont, CA); and β-actin from Sigma (St. Louis, MO). These primary antibodies were diluted based on their affinities as determined in our preliminary testing (data not shown). The dilution ratios were 1:100 for p73; 1:200 for p16, p21, p53, CCND1, and MDM2; 1:250 for p27; 1:1500 for ATM; and 1:100,000 for β-actin. We also tested the antibodies by Western blotting of their specificities using human Hela (cervical cancer) cell line (American Type Culture Collection, Manassas, VA) and apparently normal peripheral lymphoblastoid cell lines GM131 and GM892 (Coriell Institute for Medical Research, Camden, NJ) (Figure 1). Signals on the protein microarrays were scanned on a ScanArray Lite microarray scanner (Perkin-Elmer Life Sciences, Boston, MA). The signal intensity of each spot and its background signal were analyzed using a ScanArray Express 2.0 microarray analysis system (Perkin-Elmer Life Sciences) using the “Run Easy Quant” protocol. As previously described,18 we treated any scan-reading value under 2000 as missing data and used the median value of the scan-reading data of each protein dot on the microarray to calculate the mean of the duplicates. Such scan-reading data of each protein relative to that of β-actin were used as the relative expression levels of each protein.
Figure 1.
Quality evaluation by Western blotting of the antibody used in the reverse-protein microarray assay.
Statistical Analysis
We used Student’s t test or general linear model (GLM) to compare the differences in mean DRC between the high and low protein expression subgroups (dichotomized by the median). The blastogenic rate (percentage of lymphocytes that responded to phytohemagglutinin stimulation) and storage duration (in months) of cryopreserved lymphocytes were also compared between the high and low DRC subgroups (dichotomized by the median) and high and low protein expression subgroups. We calculated the Spearman correlation coefficient to estimate the correlations between two continuous variables of protein expression and fit the general linear regression model with adjustment for covariates to predict DRC by the expression levels of the proteins tested. Univariate and multivariate unconditional logistic regression analyses were performed to calculate the odds ratios (OR) and their 95% confidence intervals (CI) with and without adjustment for covariates. A principal component regression analysis29 was further performed to deal with the correlated structure of protein expression data, and the identified components were also fit into unconditional logistic regression models for their prediction of DRC. We used SAS software version 9.1.3 (SAS Institute Inc., Cary, NC) to perform all statistical analyses.
Results and Discussion
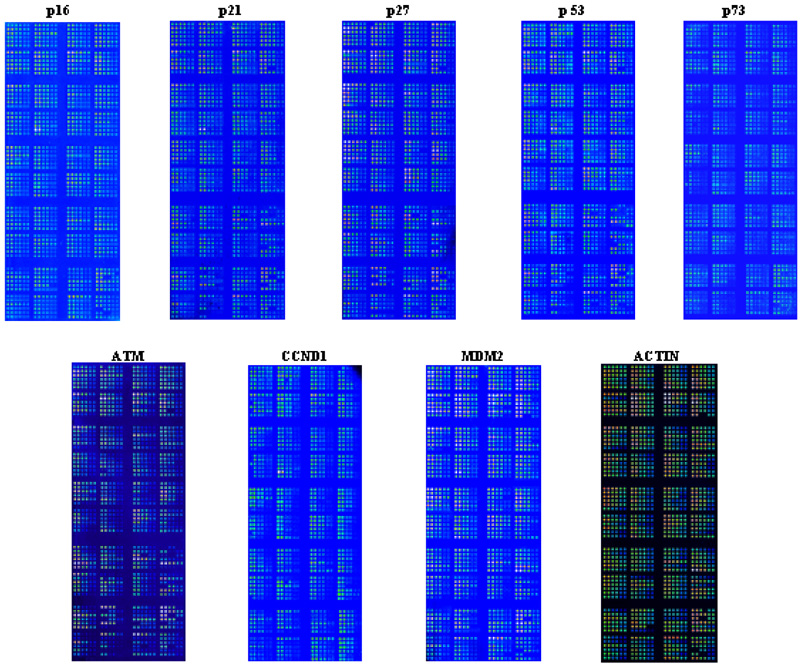
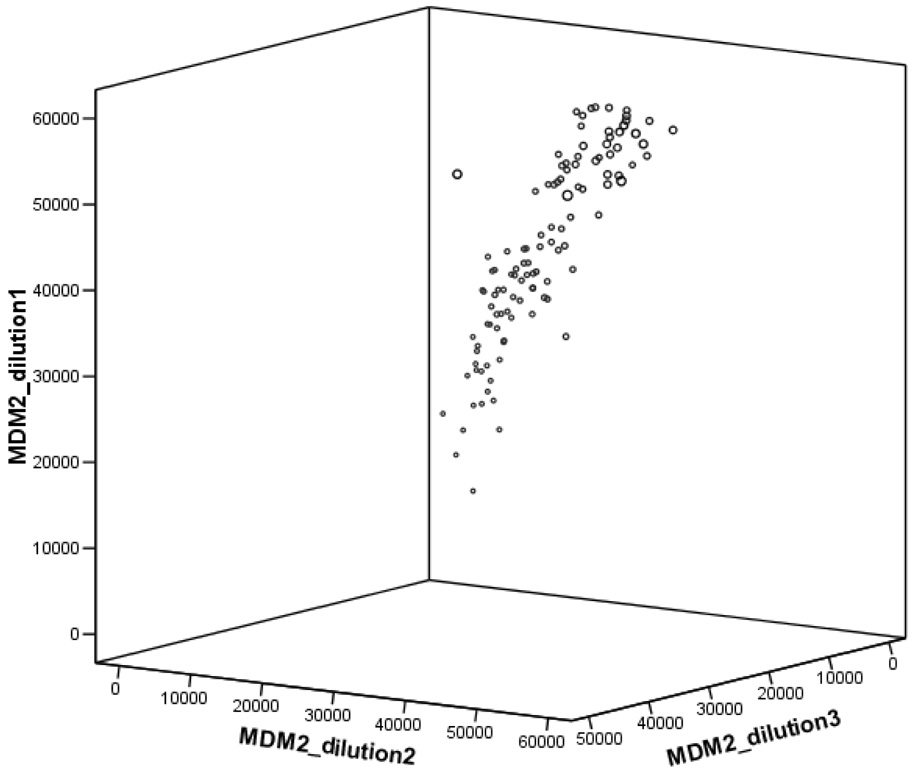
The expression levels of cells transfected with undamaged plasmids were not associated with DRC (data not shown) as expected, because no substrate was available for the repair enzymes in the cells; therefore, we focused on the association between the protein expression levels of cells transfected with damaged plasmids and DRC. As shown in Figure 2, we used three dilutions of the total cell lysate of each sample in duplicate. For statistical comparison, we used the average reading of the duplicates of the mean readouts to calculate the protein expression levels relative to that of β-actin. Because random mechanical errors occurred during plotting, a small number of protein spots were missed on the arrays, particularly for further diluted samples, and thus not usable. As expected, however, the readings of the three dilutions were highly correlated with each other. When we used MDM2 readings (a protein expressed relative low) as an example for quality control, Spearman correlations between the three dilutions were 0.94, 0.85, and 0.92 (P < 0.001 for all), respectively (Figure 3). However, as we reported previously, spots with the lower protein concentrations yielded larger variations in the readings, but spots with the highest protein concentration produced consistent, strong, and readable signals18 that were not saturated according to the scales of the machine readings and showed a roughly four-fold variation in the tested samples. Therefore, for further statistical comparison, we used the readings from the spots with the highest protein concentration (1 mg/ml) to calculate the relative protein expression levels for further analysis18. In this study, we also combined all dilution points that had been fit into the curves using the nonlinear regression models,30 from which we obtained very similar results as described in this study.
Figure 2.
Protein microarrays fabricated by using proteins extracted from activated peripheral T lymphocytes. Three dilutions were used for each sample in duplicate. Specific antibodies were used to detect levels of the corresponding proteins. The names of the proteins were shown on top of each microarray.
Figure 3.
Spearman correlations between the readings of the three dilutions (use MDM2 as an example): r = 0.94, P = 0.00 between dilution 1 and 2; r = 0.85, P = 0.00 between dilution 1 and 3; and r = 0.92, P = 0.00 between dilution 2 and 3. Each point represents a mean of duplicate experiments of MDM2 protein expression levels in three dilutions.
To determine whether age and sample cryopreservation duration adversely affected the cells, we compared age, blastogenic rate, and cell storage duration between subjects with either high or low DRC and high or low protein expression levels. As shown in Table 1, no differences existed in these three variables between dichotomized DRC and protein expression subgroups (see ATM and p27; similar data on other proteins not shown). However, because of considerable (roughly four-fold) interpersonal variation in protein expression levels, we adjusted for these potential confounders in subsequent multivariate general linear regression analyses.
Table 1.
Comparisons of selected covariates between high DRC or high protein expression subjects and low ones
| Variable | High DRC (N=60) Mean ± SD |
Low DRC (N=60) Mean ± SD |
P* |
|---|---|---|---|
| Age | 58.97 ± 10.65 | 55.77 ± 10.03 | 0.09 |
| Blastogenic rate (%)† | 33.37 ± 18.75 | 40.65 ± 23.65 | 0.06 |
| Cell storage time (months) | 3.68 ± 1.83 | 3.15 ± 2.42 | 0.18 |
| Variable | High ATM (N=58) Mean ± SD |
Low ATM (N=58) Mean ± SD |
P* |
| Age | 57.97 ± 10.75 | 57.05 ± 10.36 | 0.64 |
| Blastogenic rate (%)† | 36.03 ± 18.74 | 38.24 ± 24.11 | 0.58 |
| Cell storage time (months) | 3.67 ± 1.89 | 3.24 ± 2.43 | 0.28 |
| Variable | High p27 (N=58) Mean ± SD |
Low p27 (N=58) Mean ± SD |
P* |
| Age | 58.59 ± 10.63 | 56.22 ± 10.17 | 0.22 |
| Blastogenic rate (%)† | 39.46 ± 21.70 | 34.73 ± 21.33 | 0.24 |
| Cell storage time (months) | 3.54 ± 1.83 | 3.32 ± 2.48 | 0.58 |
Two-sided Student's t tests
Percentage of cells that were stimulated by phytohemagglutinin.
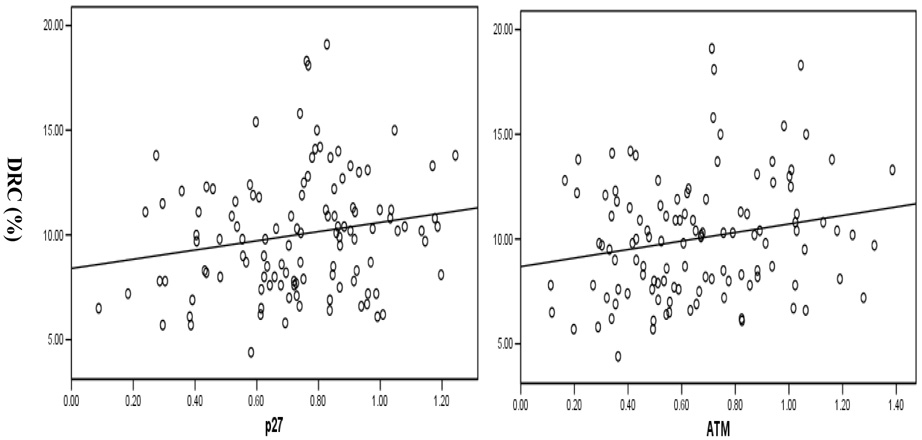
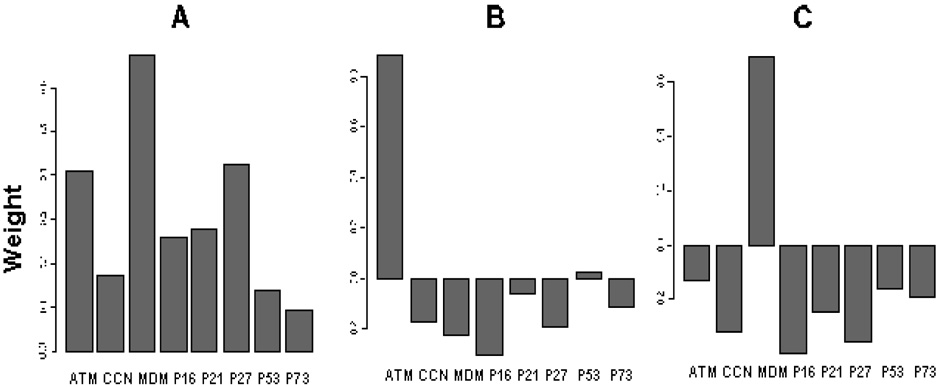
Table 2 shows the comparisons for the differences in the relative expression levels of cell-cycle checkpoint-related proteins and their associations with DRC levels. In the univariate analysis of each protein in the multivariate general linear regression model with adjustment for age, sex, blastogenic rate, and cell storage duration, the association between high DRC levels and high expression levels of cell-cycle checkpoint-related proteins induced by BPDE-adducts was statistically significant for p27, CCND1, ATM, and MDM2 (P = 0.00, 0.03, 0.03, 0.03, respectively), borderline for p73 and p21 (P = 0.07 and 0.09, respectively), but not for p53 and p16 (P = 0.13 and 0.18, respectively) (Table 2). In the correlation analysis, the relative expression levels of CCND1, p16, ATM, MDM2, and p27 were significantly correlated with DRC levels (P = 0.02, 0.02, 0.04, 0.03, and 0.05, respectively) (Table 2), but the correlation coefficients were rather weak (r = 0.19 to 0.22) due to large variations in the measurements as shown in the scatter plots in Figure 4, which depict the correlations between p27/ATM protein expression levels and DRC levels. Each point in the scatter plots represents a mean of duplicate experiments of ATM/p27 protein expression levels and the DRC measurement. Furthermore, the relative protein expression levels of all eight genes were highly correlated with each other (P < 0.001 for all pairs) (data not shown), suggesting that the effect of any given individual protein on DRC could be explained partly by its correlation with another protein that was also correlated with DRC. Given such a highly correlated data structure, not all data were suitable to be used to fit the multivariate general linear regression models requiring independent variables (Table 2). Instead, we used a principal component analysis to address the correlated data structure by reducing the data dimensions.31 As shown in Table 3, three major components explained roughly 88% of the variance, and the dominant proteins in these three components were ATM, MDM2, and p27 in the first component; ATM in the second component; and MDM2 in the third component (Figure 5). Therefore, using the first three principal components, we effectively resolved the co-linearity of the relative expression measurements of the eight proteins by reducing data dimension.
Table 2.
Comparisons of mean DRC between dichotomized proteins’ concentration subgroups and their correlation on a continuous scale
| Variable | No. (%) | DRC (Mean ± SD) | P value * | P value † | P value ‡ |
|---|---|---|---|---|---|
| p27 | 0.05 | ||||
| High (>0.745) | 58 (50.0) | 10.91 ± 3.00 | Ref. | Ref. | |
| Low (≤0.745) | 58 (50.0) | 9.12± 2.41 | 0.00 | 0.00 | |
| CCND1 | 0.01 | ||||
| High (>0.408) | 60 (50.0) | 10.67 ± 2.89 | Ref. | Ref. | |
| Low (≤0.408) | 60 (50.0) | 9.40 ± 2.65 | 0.01 | 0.03 | |
| ATM | 0.03 | ||||
| High (>0.623) | 58 (50.0) | 10.68 ± 3.10 | Ref. | Ref. | |
| Low (≤0.623) | 58 (50.0) | 9.37 ± 2.46 | 0.01 | 0.03 | |
| MDM2 | 0.04 | ||||
| High (>0.853) | 60 (50.0) | 10.51 ± 2.91 | Ref. | Ref. | |
| Low (≤0.853) | 60 (50.0) | 9.55 ± 2.70 | 0.07 | 0.03 | |
| p73 | 0.16 | ||||
| High (>0.242) | 60 (50.0) | 10.56 ± 2.76 | Ref. | Ref. | |
| Low (≤0.242) | 60 (50.0) | 9.50 ± 2.83 | 0.04 | 0.07 | |
| p21 | 0.08 | ||||
| High (>0.510) | 58 (49.6) | 10.52 ± 2.62 | Ref. | Ref. | |
| Low (≤0.510) | 59 (50.4) | 9.56 ± 3.01 | 0.07 | 0.09 | |
| p53 | 0.24 | ||||
| High (>0.423) | 58 (49.6) | 10.33 ± 2.88 | Ref. | Ref. | |
| Low (≤0.423) | 59 (50.4) | 9.75 ± 2.82 | 0.28 | 0.13 | |
| p16 | 0.02 | ||||
| High (>0.564) | 60 (50.0) | 10.34 ± 2.80 | Ref. | Ref. | |
| Low (≤0.564) | 60 (50.0) | 9.72 ± 2.86 | 0.23 | 0.18 |
Two-sided Student's t tests
Obtained from GLM with adjustment for age, sex, blastogenic rate and storage time
Obtained from Pearson correlation analysis for between the protein expression levels and DRC levels on a continuous scale.
Figure 4.
Correlation between p27 and ATM protein expression levels and DRC level in primary lymphoblasts from healthy subjects. Each point represents a mean of duplicate experiments of p27 or ATM protein expression levels and DRC measurements. (r = 0.19 and P = 0.05 for p27 vs. DRC and r = 0.20 and P = 0.03 for ATM vs. DRC)
Table 3.
Principal component analysis loadings
| Comp1 | Comp2 | Comp3 | Comp4 | Comp5 | Comp6 | Comp7 | Comp8 | |
|---|---|---|---|---|---|---|---|---|
| Standard Deviation | 0.448 | 0.212 | 0.191 | 0.151 | 0.093 | 0.050 | 0.038 | 0.026 |
| Proportion of variance (%) | 0.631 | 0.141 | 0.115 | 0.072 | 0.027 | 0.007 | 0.005 | 0.002 |
| Cumulative proportional variance (%) | 0.631 | 0.772 | 0.886 | 0.958 | 0.985 | 0.993 | 0.998 | 1.000 |
Note: The first three components contributed to 88.6% of the overall variance.
Figure 5.
Principal component analysis. A, first component; B, second component; C, third component. Each principal component is a weighted sum of the concentrations of the eight proteins. The weights (shown on y-axes) in each principal component form a unit-length vector, which means the sum of the squares of the weights is equal to one. These three principal components form three vectors that are perpendicular to each other in the eight-dimensional space, i.e., these vectors are linearly independent of each other.29
Using the general linear regression model (including age, sex, blastogenic rate, and cell storage duration) and the first three components, we found that only the second component was borderline significant in predicting the DRC levels (P = 0.07). Using the medians of these three components as the cutoff values in the unconditional logistic regression analysis adjusting for age, sex, blastogenic rate, and cell storage duration, the risk of first three components associated with suboptimal DRC was relatively higher, though not significant, for the second component (adjusted OR = 2.12 and 95% CI = 0.95–4.75) compared to other two components (adjusted OR = 0.58 and 95% CI = 0.25–1.36 for the first component and OR = 1.08 and 95% CI = 0.47–2.48 for the third component). As Figure 5 shows, the most weighted protein in the second was the ATM expression levels, which was most likely to explain the observed variations in the DRC phenotype. However, it appears this study did not have enough statistical power to detect a main component responsible for the observed variation of the protein expression levels in predicting DRC, and thus the finding is considered preliminary and needs to be validated in studies with large samples.
Both regression and principal component analyses appear to consistently identify ATM, MDM2, and p27 expression levels as potential predictors for DRC levels, and this identified association between in vitro expression levels of ATM, MDM2 and p27 and DRC levels in peripheral lymphocytes is likely to exist in vivo. ATM mutations are found in individuals with the autosomal recessive disorder Ataxia-telangiectasia (A–T), which is characterized by early-onset progressive cerebellar ataxia, skin, and ocular telangiectasia, immunodeficiency, chromosomal instability, and increased risk of malignancies.32 In mammals, ATM plays a central role in DNA-damage recognition to initiate checkpoint responses33 as well as in G1/S, G2/M, and intra S phase checkpoints.34
MDM2 promotes rapid degradation of p53, inhibits growth arrest or p53-mediated apoptosis, and masks the trans-activation domain of p53 that interacts with the transcriptional machinery, resulting in the inactivation of p53.35 It is likely that MDM2 may also have a role in regulating DNA repair, independent of p53.36 Furthermore, MDM2 is known to be a negative regulator of p21, independent of p53 as well.37 Therefore, given MDM2’s negative regulatory roles in the expression of p53 and p21, the greater contribution of MDM2 expression as an important component to the prediction model of DRC may weaken the correlation between the expression levels of p53 and p21 and DRC levels, as we observed in this in vitro study. The same mechanism may be true for the p73, because MDM2 also suppresses p73 functions.38
p27, a member of Cip/Kip family39–41, is a well-established universal inhibitor of CDKs and is among the first targets of environmental signaling.41 It can down-regulate not only cyclin E and cyclin A-CDK2 activity but also CDK1 activity, which is involved in G1/S, G2/M, and intra S phase checkpoints.42 Moreover, a mice knockout model showed “haploinsufficiency” of the p27 gene,43 suggesting a “dosage-dependent” tumor suppressor role of p27. Therefore, a reduced dosage of p27 rather than its absolute absence may also contribute to cancer susceptibility.44–46
Therefore, it is biologically plausible that p27, ATM and MDM2 have dual roles in the regulation of both cell-cycle checkpoints and DNA repair activity. In the present study, we showed that higher relative expression levels of two of eight cell-cycle checkpoint-related proteins were independently associated with higher DRC levels in both multivariate linear regression and principal component analyses. It is interesting to note the strong associations among the expression levels of these proteins, which may be regulated through various mechanisms. Although our study was not designed to be mechanistic, this finding from a population study does provide a rationale to further investigate regulation of these proteins in vivo. Because some of the peripheral blood lymphocytes might have been arrested at different phases of checkpoints in response to DNA damage, what we observed should be an average effect of the expression of checkpoint-related proteins on DRC level. Therefore, cell-cycle phase-specific assays with more related proteins are warranted to validate our results. Compared with the host-cell reactivation and RT-PCR assay, this protein microarray assay is rapid, has higher throughput, is more sensitive, and is reproducible, which is optimal for large-scale screening in molecular epidemiological studies.
One surprising finding was the lack of association between the p53 protein expression and DRC levels considering that p53 is regulated by stress, including exposure to the carcinogen used in this study, and plays a key molecular role in DNA repair. In addition to the possibility of negative relation by MDM2, another possible explanation for this finding is the limitation of our assay. Our assay cannot determine the functional activities of the protein because some of the p53 functions are based on its protein phosphorylation and genetic polymorphisms. Furthermore, other factors, particularly the p53 downstream proteins, may contribute to this complex repair system. In fact, p27 is one of the major p53 downstream proteins, and p27 expression levels were shown to be strongly associated with DRC levels, supporting such a possibility.
Our results provide some population evidence to support an essential role of p27, ATM and MDM2 proteins in maintaining effective DNA repair, at least in unaffected human peripheral blood lymphocytes. Because suboptimal DRC levels have been shown to be associated with increased cancer risk,8–11, 27, 47 it is likely that inter-individual variation in the expression levels of cell-cycle checkpoint-related proteins may also contribute to cancer susceptibility. This hypothesis needs to be tested further in future, larger molecular epidemiological studies.
Acknowledgements
We thank Margaret Lung for assistance in recruiting the subjects; Jianzhong He and Kejing Xu for laboratory assistance; Monica Domingue for manuscript preparation; and Margaret Newell for scientific editing.
Supported by National Institutes of Health Grant R01 ES11740, CA100264, PO1 CA106451, and CA16672 and Department of Defense Grant W81XWH-04-1-0142.
Abbreviations
- NER
nucleotide excision repair
- DRC
DNA repair capacity
- BPDE
benzo[a]pyrene diol epoxide
- ORs
odds ratios
- CI
confidence interval
References
- 1.Garner RC. The role of DNA adducts in chemical carcinogenesis. Mutat Res. 1998;402(1–2):67–75. doi: 10.1016/s0027-5107(97)00283-2. [DOI] [PubMed] [Google Scholar]
- 2.Povey AC. DNA adducts: endogenous and induced. Toxicol Pathol. 2000;28(3):405–414. doi: 10.1177/019262330002800308. [DOI] [PubMed] [Google Scholar]
- 3.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411(6835):366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 4.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411(6835):342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 5.Pero RW, Bryngelsson C, Mitelman F, Kornfalt R, Thulin T, Norden A. Interindividual variation in the responses of cultured human lymphocytes to exposure from DNA damaging chemical agents: interindividual variation to carcinogen exposure. Mutat Res. 1978;53(3):327–341. doi: 10.1016/0165-1161(78)90005-5. [DOI] [PubMed] [Google Scholar]
- 6.Friedberg EC. Hot news: temperature-sensitive humans explain hereditary disease. Bioessays. 2001;23(8):671–673. doi: 10.1002/bies.1094. [DOI] [PubMed] [Google Scholar]
- 7.Neumann AS, Sturgis EM, Wei Q. Nucleotide excision repair as a marker for susceptibility to tobacco-related cancers: a review of molecular epidemiological studies. Mol Carcinog. 2005;42(2):65–92. doi: 10.1002/mc.20069. [DOI] [PubMed] [Google Scholar]
- 8.Wei Q, Matanoski GM, Farmer ER, Hedayati MA, Grossman L. DNA repair and aging in basal cell carcinoma: a molecular epidemiology study. Proc Natl Acad Sci U S A. 1993;90(4):1614–1618. doi: 10.1073/pnas.90.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei Q, Cheng L, Hong WK, Spitz MR. Reduced DNA repair capacity in lung cancer patients. Cancer Res. 1996;56(18):4103–4107. [PubMed] [Google Scholar]
- 10.Cheng L, Eicher SA, Guo Z, Hong WK, Spitz MR, Wei Q. Reduced DNA repair capacity in head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 1998;7(6):465–468. [PubMed] [Google Scholar]
- 11.Wei Q, Lee JE, Gershenwald JE, Ross MI, Mansfield PF, Strom SS, Wang LE, Guo Z, Qiao Y, Amos CI, Spitz MR, Duvic M. Repair of UV light-induced DNA damage and risk of cutaneous malignant melanoma. J Natl Cancer Inst. 2003;95(4):308–315. doi: 10.1093/jnci/95.4.308. [DOI] [PubMed] [Google Scholar]
- 12.Wei Q, Bondy ML, Mao L, Gaun Y, Cheng L, Cunningham J, Fan Y, Bruner JM, Yung WK, Levin VA, Kyritsis AP. Reduced expression of mismatch repair genes measured by multiplex reverse transcription-polymerase chain reaction in human gliomas. Cancer Res. 1997;57(9):1673–1677. [PubMed] [Google Scholar]
- 13.Soliman AS, Bondy ML, Guan Y, El-Badawi S, Mokhtar N, Bayomi S, Raouf AA, Ismail S, McPherson RS, Abdel-Hakim TF, Beasley RP, Levin B, Wei Q. Reduced expression of mismatch repair genes in colorectal cancer patients in Egypt. Int J Oncol. 1998;12(6):1315–1319. doi: 10.3892/ijo.12.6.1315. [DOI] [PubMed] [Google Scholar]
- 14.Wei Q, Eicher SA, Guan Y, Cheng L, Xu J, Young LN, Saunders KC, Jiang H, Hong WK, Spitz MR, Strom SS. Reduced expression of hMLH1 and hGTBP/hMSH6: a risk factor for head and neck cancer. Cancer Epidemiol Biomarkers Prev. 1998;7(4):309–314. [PubMed] [Google Scholar]
- 15.Cheng L, Spitz MR, Hong WK, Wei Q. Reduced expression levels of nucleotide excision repair genes in lung cancer: a case-control analysis. Carcinogenesis. 2000;21(8):1527–1530. [PubMed] [Google Scholar]
- 16.Strom SS, Spitz MR, Yamamura Y, Babaian RJ, Scardino PT, Wei Q. Reduced expression of hMSH2 and hMLH1 and risk of prostate cancer: a case-control study. Prostate. 2001;47(4):269–275. doi: 10.1002/pros.1071. [DOI] [PubMed] [Google Scholar]
- 17.Cheng L, Sturgis EM, Eicher SA, Spitz MR, Wei Q. Expression of nucleotide excision repair genes and the risk for squamous cell carcinoma of the head and neck. Cancer. 2002;94(2):393–397. doi: 10.1002/cncr.10231. [DOI] [PubMed] [Google Scholar]
- 18.Wei Q, Wang LE, Sturgis EM, Mao L. Expression of nucleotide excision repair proteins in lymphocytes as a marker of susceptibility to squamous cell carcinomas of the head and neck. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1961–1966. doi: 10.1158/1055-9965.EPI-05-0101. [DOI] [PubMed] [Google Scholar]
- 19.Moenkemann H, Flammer J, Wunderlich K, Breipohl W, Schild HH, Golubnitschaja O. Increased DNA breaks and up-regulation of both G(1) and G(2) checkpoint genes p21(WAF1/CIP1) and 14-3-3 sigma in circulating leukocytes of glaucoma patients and vasospastic individuals. Amino Acids. 2005;28(2):199–205. doi: 10.1007/s00726-005-0169-x. [DOI] [PubMed] [Google Scholar]
- 20.Li G, Sturgis EM, Wang LE, Chamberlain RM, Spitz MR, El-Naggar AK, Hong WK, Wei Q. Association between the V109G polymorphism of the p27 gene and the risk and progression of oral squamous cell carcinoma. Clin Cancer Res. 2004;10(12 Pt 1):3996–4002. doi: 10.1158/1078-0432.CCR-04-0089. [DOI] [PubMed] [Google Scholar]
- 21.Athas WF, Hedayati MA, Matanoski GM, Farmer ER, Grossman L. Development and field-test validation of an assay for DNA repair in circulating human lymphocytes. Cancer Res. 1991;51(21):5786–5793. [PubMed] [Google Scholar]
- 22.Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24(17):2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 23.Hussain SP, Harris CC. p53 biological network: at the crossroads of the cellular-stress response pathway and molecular carcinogenesis. J Nippon Med Sch. 2006;73(2):54–64. doi: 10.1272/jnms.73.54. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Wang LE, Wang L, Lu KH, Mills GB, Bondy ML, Wei Q. Methylation and messenger RNA expression of p15INK4b but not p16INK4a are independent risk factors for ovarian cancer. Clin Cancer Res. 2005;11(13):4968–4976. doi: 10.1158/1078-0432.CCR-04-2293. [DOI] [PubMed] [Google Scholar]
- 25.Harris SL, Gil G, Hu W, Robins H, Bond E, Hirshfield K, Feng Z, Yu X, Teresky AK, Bond G, Levine AJ. Single-nucleotide polymorphisms in the p53 pathway. Cold Spring Harb Symp Quant Biol. 2005;70:111–119. doi: 10.1101/sqb.2005.70.024. [DOI] [PubMed] [Google Scholar]
- 26.Landi S, Gemignani F, Canzian F, Gaborieau V, Barale R, Landi D, Szeszenia-Dabrowska N, Zaridze D, Lissowska J, Rudnai P, Fabianova E, Mates D, Foretova L, Janout V, Bencko V, Gioia-Patricola L, Hall J, Boffetta P, Hung RJ, Brennan P. DNA repair and cell cycle control genes and the risk of young-onset lung cancer. Cancer Res. 2006;66(22):11062–11069. doi: 10.1158/0008-5472.CAN-06-1039. [DOI] [PubMed] [Google Scholar]
- 27.Wei Q, Cheng L, Amos CI, Wang LE, Guo Z, Hong WK, Spitz MR. Repair of tobacco carcinogen-induced DNA adducts and lung cancer risk: a molecular epidemiologic study. J Natl Cancer Inst. 2000;92(21):1764–1772. doi: 10.1093/jnci/92.21.1764. [DOI] [PubMed] [Google Scholar]
- 28.Protic-Sabljic M, Kraemer KH. One pyrimidine dimer inactivates expression of a transfected gene in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1985;82(19):6622–6626. doi: 10.1073/pnas.82.19.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu RX, Kuang J, Gong Q, Hou XL. Principal component regression analysis with SPSS. Comput Methods Programs Biomed. 2003;71(2):141–147. doi: 10.1016/s0169-2607(02)00058-5. [DOI] [PubMed] [Google Scholar]
- 30.Mircean C, Shmulevich I, Cogdell D, Choi W, Jia Y, Tabus I, Hamilton SR, Zhang W. Robust estimation of protein expression ratios with lysate microarray technology. Bioinformatics. 2005;21(9):1935–1942. doi: 10.1093/bioinformatics/bti258. [DOI] [PubMed] [Google Scholar]
- 31.Riva A, Carpentier AS, Torresani B, Henaut A. Comments on selected fundamental aspects of microarray analysis. Comput Biol Chem. 2005;29(5):319–336. doi: 10.1016/j.compbiolchem.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 32.Shiloh Y. Ataxia-telangiectasia and the Nijmegen breakage syndrome: related disorders but genes apart. Annu Rev Genet. 1997;31:635–662. doi: 10.1146/annurev.genet.31.1.635. [DOI] [PubMed] [Google Scholar]
- 33.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15(17):2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 34.Khanna KK, Chenevix-Trench G. ATM and genome maintenance: defining its role in breast cancer susceptibility. J Mammary Gland Biol Neoplasia. 2004;9(3):247–262. doi: 10.1023/B:JOMG.0000048772.92326.a1. [DOI] [PubMed] [Google Scholar]
- 35.Freedman DA, Wu L, Levine AJ. Functions of the MDM2 oncoprotein. Cell Mol Life Sci. 1999;55(1):96–107. doi: 10.1007/s000180050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alt JR, Bouska A, Fernandez MR, Cerny RL, Xiao H, Eischen CM. Mdm2 binds to Nbs1 at sites of DNA damage and regulates double strand break repair. J Biol Chem. 2005;280(19):18771–18781. doi: 10.1074/jbc.M413387200. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Z, Wang H, Li M, Agrawal S, Chen X, Zhang R. MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53. J Biol Chem. 2004;279(16):16000–16006. doi: 10.1074/jbc.M312264200. [DOI] [PubMed] [Google Scholar]
- 38.Zeng X, Chen L, Jost CA, Maya R, Keller D, Wang X, Kaelin WG, Jr, Oren M, Chen J, Lu H. MDM2 suppresses p73 function without promoting p73 degradation. Mol Cell Biol. 1999;19(5):3257–3266. doi: 10.1128/mcb.19.5.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 40.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 41.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285(5430):1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 42.Belletti B, Nicoloso MS, Schiappacassi M, Chimienti E, Berton S, Lovat F, Colombatti A, Baldassarre G. p27(kip1) functional regulation in human cancer: a potential target for therapeutic designs. Curr Med Chem. 2005;12(14):1589–1605. doi: 10.2174/0929867054367149. [DOI] [PubMed] [Google Scholar]
- 43.Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396(6707):177–180. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catzavelos C, Bhattacharya N, Ung YC, Wilson JA, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, Franssen E, Pritchard KI, Slingerland JM. Decreased levels of the cell-cycle inhibitor p27Kip1 protein: prognostic implications in primary breast cancer. Nat Med. 1997;3(2):227–230. doi: 10.1038/nm0297-227. [DOI] [PubMed] [Google Scholar]
- 45.Ciaparrone M, Yamamoto H, Yao Y, Sgambato A, Cattoretti G, Tomita N, Monden T, Rotterdam H, Weinstein IB. Localization and expression of p27KIP1 in multistage colorectal carcinogenesis. Cancer Res. 1998;58(1):114–122. [PubMed] [Google Scholar]
- 46.Cordon-Cardo C, Koff A, Drobnjak M, Capodieci P, Osman I, Millard SS, Gaudin PB, Fazzari M, Zhang ZF, Massague J, Scher HI. Distinct altered patterns of p27KIP1 gene expression in benign prostatic hyperplasia and prostatic carcinoma. J Natl Cancer Inst. 1998;90(17):1284–1291. doi: 10.1093/jnci/90.17.1284. [DOI] [PubMed] [Google Scholar]
- 47.Shi Q, Wang LE, Bondy ML, Brewster A, Singletary SE, Wei Q. Reduced DNA repair of benzo[a]pyrene diol epoxide-induced adducts and common XPD polymorphisms in breast cancer patients. Carcinogenesis. 2004;25(9):1695–1700. doi: 10.1093/carcin/bgh167. [DOI] [PubMed] [Google Scholar]