Abstract
This study examined whether adenosine 5’-triphosphate (ATP) modulated inhibitory glycinergic and GABAergic neurotransmission to cardiac vagal neurons. Inhibitory activity to cardiac vagal neurons was isolated and examined using whole-cell patch-clamp recordings in an in vitro brain slice preparation in rats. ATP (100 µM) evoked increases in the frequency of glycinergic and GABAergic miniature inhibitory postsynaptic currents (mIPSCs) in cardiac vagal neurons which were blocked by the broad P2 receptor antagonist pyridoxal-phosphate-6-azophenyl-2’,4’-disulphonic acid (100 µM). Application of the P2Y agonists uridine triphosphate (15 µM) and adenosine 5'-0-(Z-thiodiphosphate) (60 µM) did not enhance inhibitory neurotransmission to cardiac vagal neurons however, application of the selective P2X receptor agonist, α, β –methylene ATP (100 µM), increased glycinergic and GABAergic mIPSC neurotransmission to cardiac vagal neurons. The increase in inhibitory neurotransmission evoked by α, β–methylene ATP was abolished by the selective P2X receptor antagonist 2',3'-O-(2,4,6-Trinitrophenyl) adenosine 5'-triphosphate (100 µM) indicating P2X receptors enhance the release of inhibitory neurotransmitters to cardiac neurons. The voltage gated calcium channel blocker cadmium chloride did not alter the evoked increase in inhibitory mIPSCs. This work demonstrates that P2X receptor activation enhances inhibitory neurotransmission to parasympathetic cardiac vagal neurons and demonstrates an important functional role for ATP mediated purinergic signaling to cardiac vagal neurons.
Keywords: nucleus ambiguus, ATP, purinergic, glycine, GABA, heart rate
Introduction
The purine nucleotide ATP is widely accepted as a neurotransmitter in both the peripheral and central nervous system and has been shown to mediate neurotransmission throughout regions of the brain including the hippocampus (Bardoni et al., 1997), dorsal horn of the spinal cord (Sorimachi et al., 2001)and the hypothalamus (Edwards et al., 1997). ATP can act as an extracellular signaling molecule that mediates ionotropic and metabotropic P2 receptors, P2Y G protein-coupled receptors and P2X ionotropic ligand-gated receptors (North and Barnard, 1997); (Burnstock and Wood, 1996). One important target of P2 receptor modulation is inhibitory glycinergic and GABAergic neurotransmission.
ATP has been shown to enhance glycine release by activating P2X receptors in hypocretin/orexin neurons (Wollmann et al., 2005), neurons in the trigeminal nucleus pars caudalis (Wang et al., 2001), and facilitates both a presynaptic and postsynaptic mediated increase in glycinergic mIPSCs in spinal cord substantia gelatinosa neurons (Jang et al., 2001). Furthermore, ATP has been shown to both corelease and enhance the release of GABA via P2X receptors in lateral hypothalamus neurons (Jo and Role, 2002), and cultured rat spinal cord dorsal horn neurons (Jo and Schlichter, 1999). ATP has also induced the release of GABA in synaptosomes from rat midbrain (Gomez-Villafuertes et al., 2001).
The activation of purinergic receptors has been shown to alter many cardio respiratory functions in the brainstem. Purinergic receptors have been identified in brainstem cardiovascular and respiratory sites such as the nucleus of the solitary tract, rostroventrolateral medulla, ventral respiratory group and hypoglossal nucleus (Huang et al., 2007). Furthermore, recent work has shown that ATP plays a key role in modulating the excitatory neurotransmission to parasympathetic cardiac vagal neurons that dominate the neural control of heart rate (Griffioen et al., 2007a); (Jameson et al., 2008). The aim of the present study was to test whether ATP modulates inhibitory glycinergic and GABAergic neurotransmission to cardiac vagal neurons (CVNs).
Results
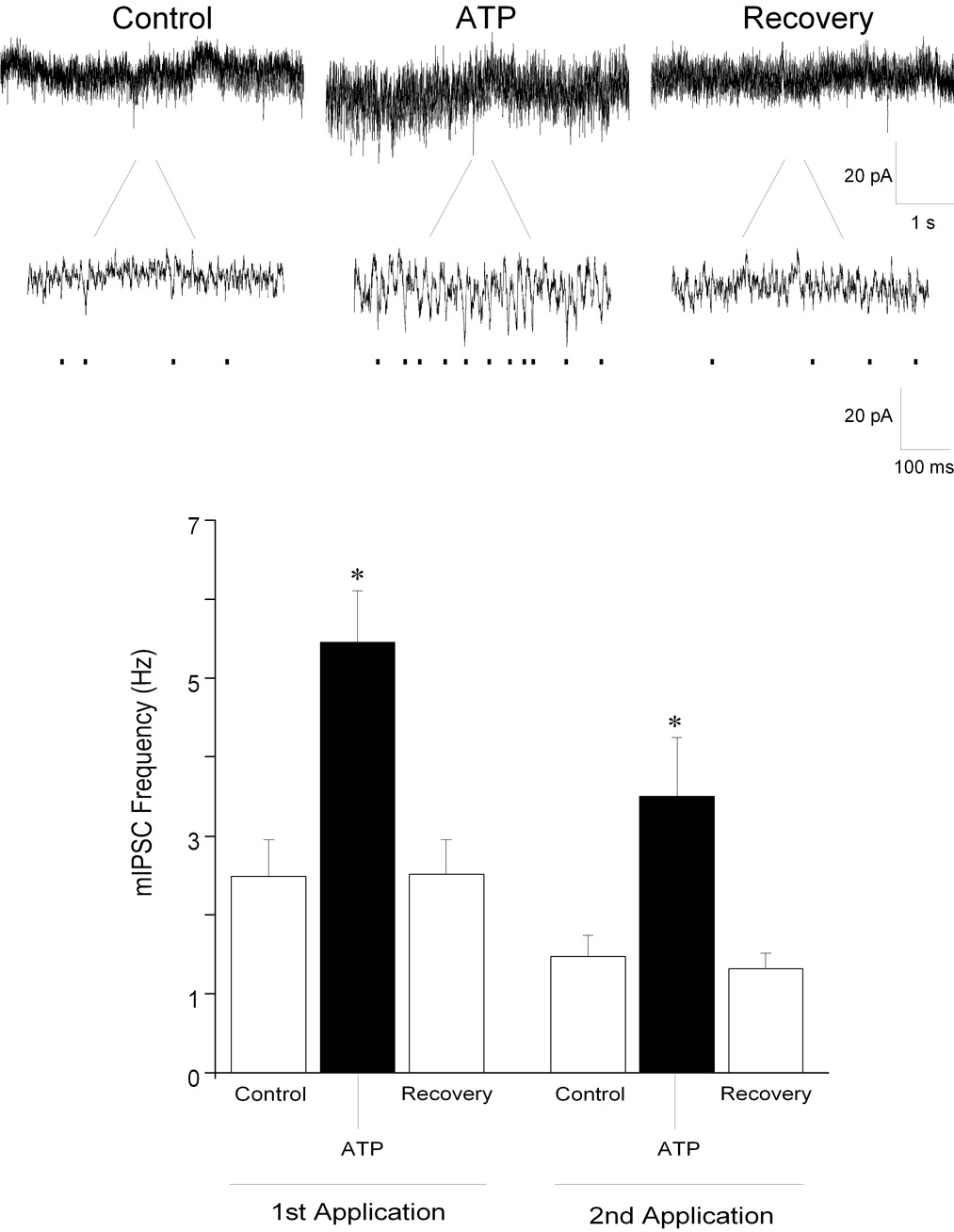
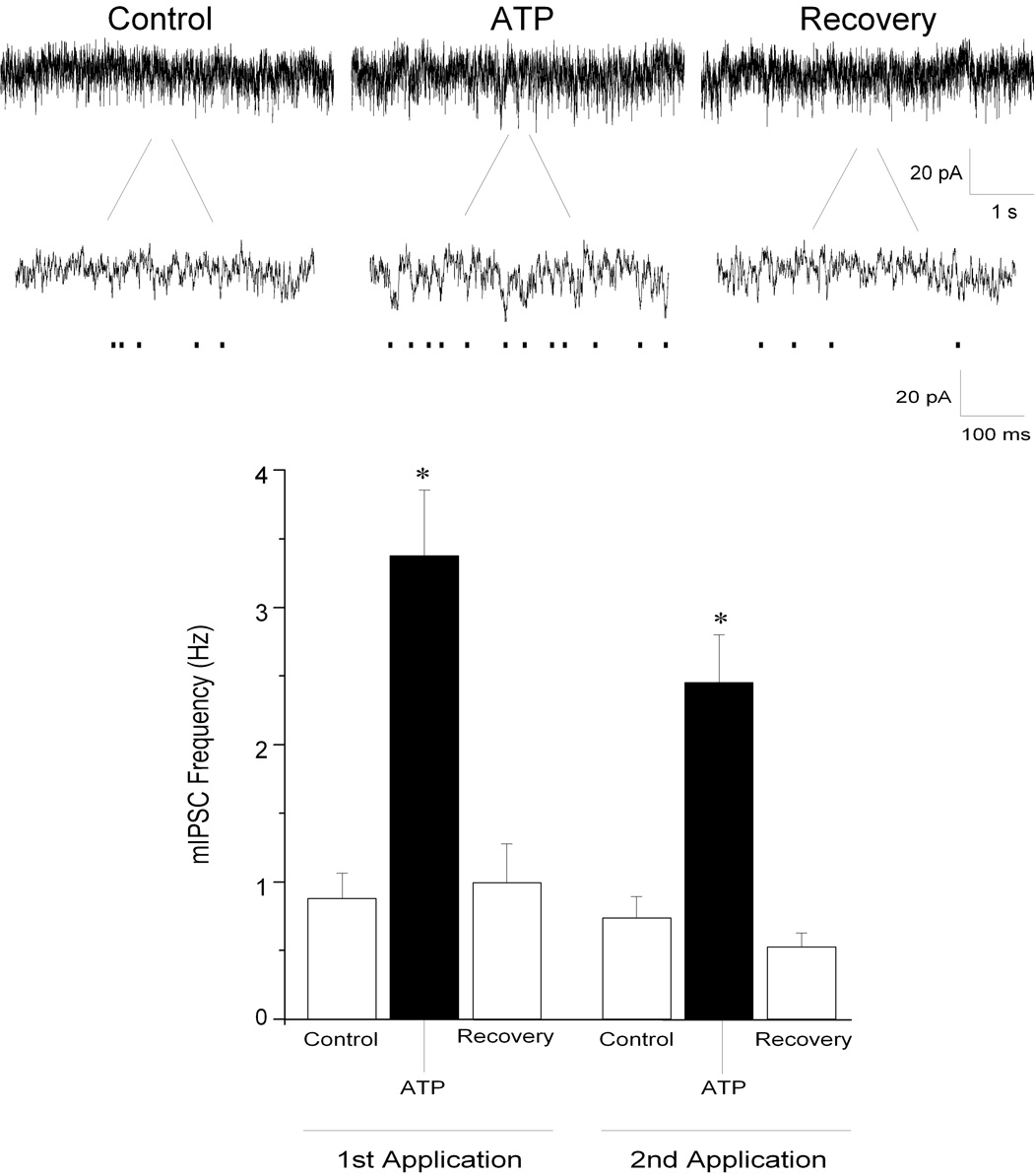
Focal application of ATP (100 µM) evoked a significant, repeatable and reversible increase in inhibitory mIPSCs in all CVNs tested. mIPSCs were isolated by inclusion of tetrodotoxin (TTX, 1 µM) in the perfusate, and this limited the actions of purinergic agonists and antagonists to the synapses surrounding CVNs and avoided any action potential dependent changes including diverse polysynaptic effects. In addition, examination of mIPSC frequency provides an assessment of changes in presynaptic release, whereas changes in mIPSC amplitude are generally indicative of alterations in postsynaptic receptor function. ATP elicited a significant increase in glycinergic mIPSC frequency from 2 ± 0.5 to 5 ± 0.6 Hz (See Fig. 1; n=12; P<0.05); glycinergic mIPSC amplitude also significantly (P<0.05) increased by 5 ± 0.5%. Furthermore, application of ATP significantly increased GABAergic mIPSC frequency from 0.9 ± 0.2 to 3 ± 0.5 Hz (See Fig. 2; n=8; P<0.05). Interestingly, ATP did not significantly alter the amplitude of GABAergic mIPSCs (control: 24 ± 2 pA; ATP: 24 ± 2 pA; P>0.05). Both the spontaneous glycinergic and GABAergic mIPSC responses, as well as those evoked by ATP, were blocked by application of strychnine (1 µM), a glycinergic receptor antagonist, and by gabazine (25 µM), a GABAA receptor antagonist, respectively (not shown).
Figure 1.
Miniature inhibitory glycinergic postsynaptic currents (mIPSCs) were isolated by bath application of gabazine (25 µM), CNQX (50 µM), AP5 (50 µM) and TTX (1 µM). Focal application of ATP (100 µM), significantly increased glycinergic mIPSC frequency in CVNs (top traces). A second application of ATP evoked responses that were not significantly different from the responses evoked by the first application. Results from a typical experiment are shown in the series of traces, including an expanded section of each trace to better visualize individual events (top), and the average results from 12 CVNs are shown in the bar graph, bottom. * denotes p≤ 0.05 as compared to the control values.
Figure 2.
Miniature inhibitory GABAergic postsynaptic currents (mIPSCs) were isolated by bath application of strychnine (1 µM), CNQX (50 µM), AP5 (50 µM) and TTX (1 µM). Focal application of ATP (100 µM), significantly increased GABAergic mIPSC frequency in CVNs (top traces). Responses with a second application of ATP were not significantly different from the responses evoked by the first application. Results from a typical experiment are shown in the series of traces, including an expanded section of each trace to better visualize individual events (top), and the average results from 8 CVNs are shown in the bar graph, bottom. * denotes p≤ 0.05 as compared to the control values.
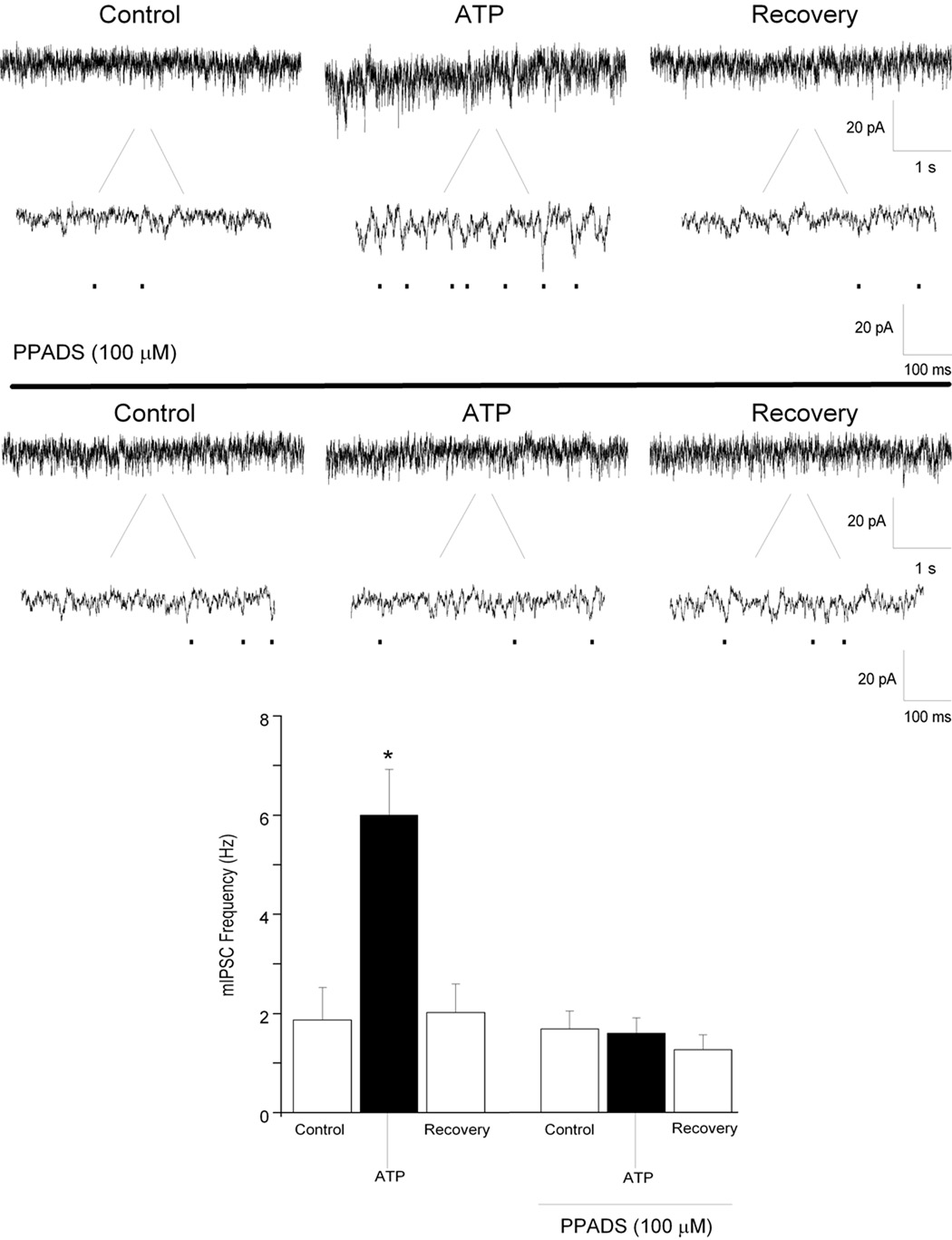
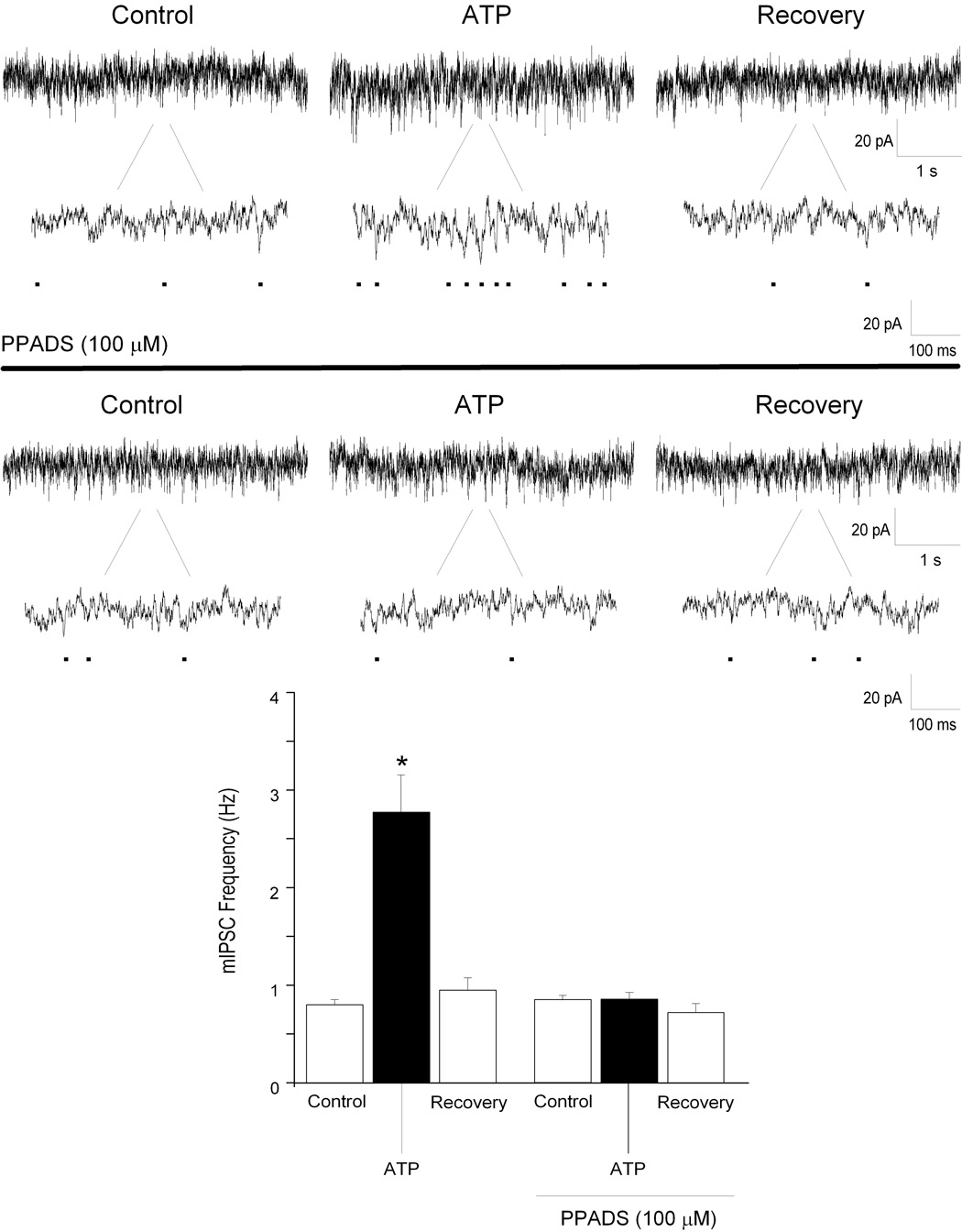
The broad P2 receptor antagonist PPADS (100 µM) was used to test whether ATP was acting on purinergic receptors to facilitate inhibitory neurotransmission to CVNs. There was no significant effect on endogenous glycinergic or GABAergic activity in CVNs upon application of PPADS alone. Application of PPADS however, prevented ATP mediated changes in both glycinergic mIPSC frequency (control: 2 ± 0.4 Hz; 6 PPADS: 2 ± 0.3 Hz; See Fig. 3; n=7; P>0.05) and amplitude (5 ± 0.5% to 5 ± 0.6%). PPADS abolished the evoked response of ATP in GABAergic mIPSC frequency (control: 0.9 ± 0.1 Hz; PPADS: 0.9 ± 0.1 Hz; See Fig. 4; n=7; P>0.05).
Figure 3.
Focal application of ATP (100 µM), significantly increased glycinergic mIPSC frequency (top traces). Application of PPADS (100 µM), prevented the increase in glycinergic mIPSC frequency evoked by ATP (middle). Results from a typical experiment are shown in the 2 series of traces, including an expanded section of each trace to better visualize individual events (top, middle), and the average results from 7 CVNs are shown in the bar graph, bottom. * denotes p≤ 0.05 as compared to the control values.
Figure 4.
Focal application of ATP (100 µM), significantly increased GABAergic mIPSC frequency (top traces). Application of PPADS (100 µM), prevented the increase GABAergic mIPSC frequency evoked by ATP (middle). Results from a typical experiment are shown in the 2 series of traces , including an expanded section of each trace to better visualize individual events (top, middle), and the average results from 7 CVNs are shown in the bar graph, bottom. * denotes p≤ 0.05 as compared to the control values.
To examine the potential role of P2Y receptors in facilitating inhibitory neurotransmission to CVNs, the P2Y agonists uridine triphosphate ([UTP] 15 µM) and adenosine 5'-0-(Z-thiodiphosphate) (60 µM) were focally applied to determine whether the glycinergic and/or GABAergic inhibitory neurotransmission to CVNs was facilitated by P2Y receptors. Application of UTP and adenosine 5'-0-(Z-thiodiphosphate) did not significantly increase glycinergic mIPSC frequency (control: 2 ± 0.2 Hz; UTP and adenosine 5'-0-[Z-thiodiphosphate]: 2 ± 0.6 Hz; n=6; P>0.05); amplitude was also unaffected (12 ± 1 to 12 ± 1 pA; P>0.05). Similarly, application of the P2Y agonists did not significantly increase GABAergic mIPSC frequency (control: 0.8 ± 0.1 Hz; UTP and adenosine 5'-0-[Z-thiodiphosphate]: 1 ± 0.3 Hz; n=7; P>0.05); amplitude was also unaffected (20 ± 1 to 19 ± 1 pA; P>0.05). These results suggest that P2Y receptors do not enhance glycinergic or GABAergic neurotransmission to CVNs.
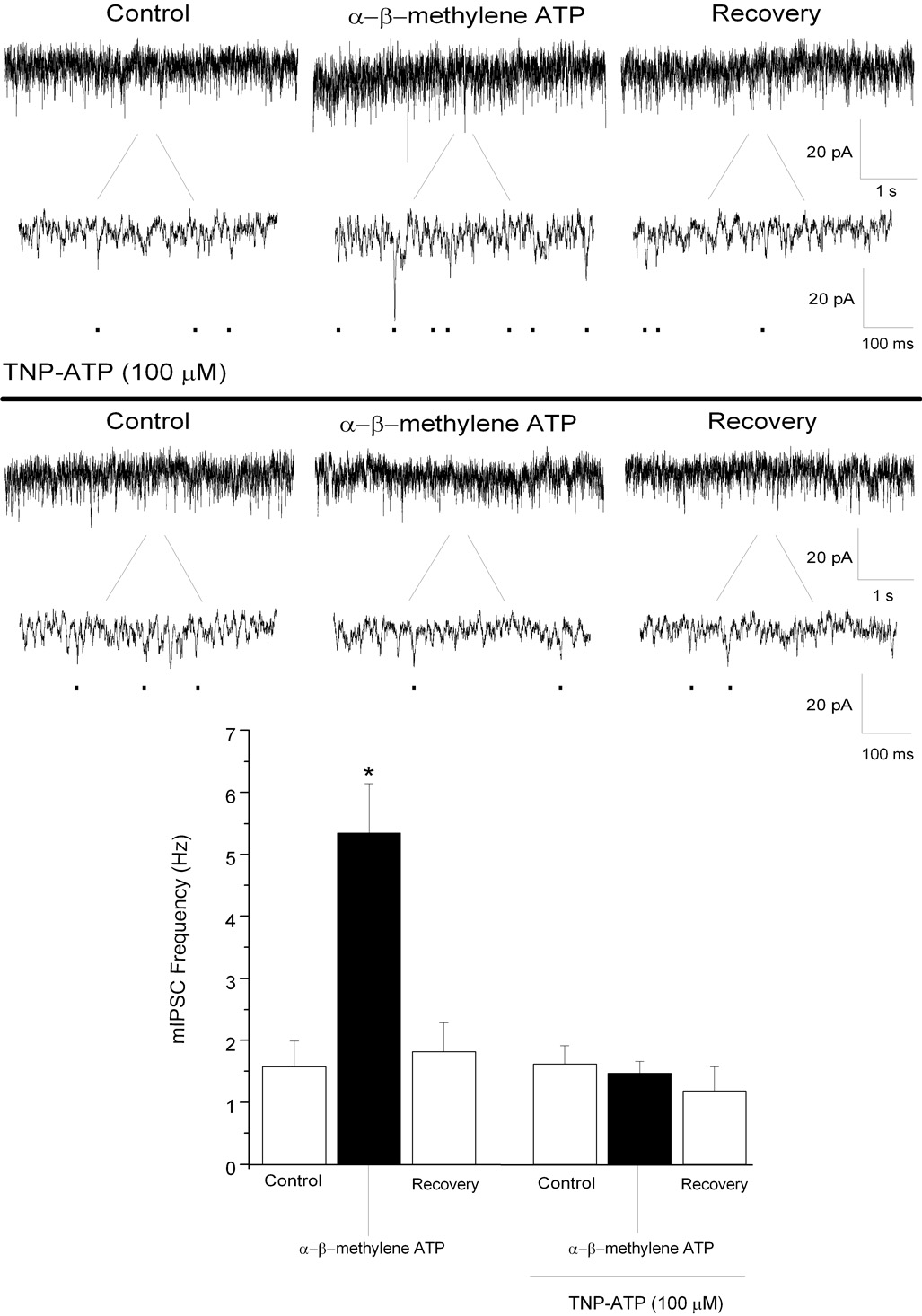
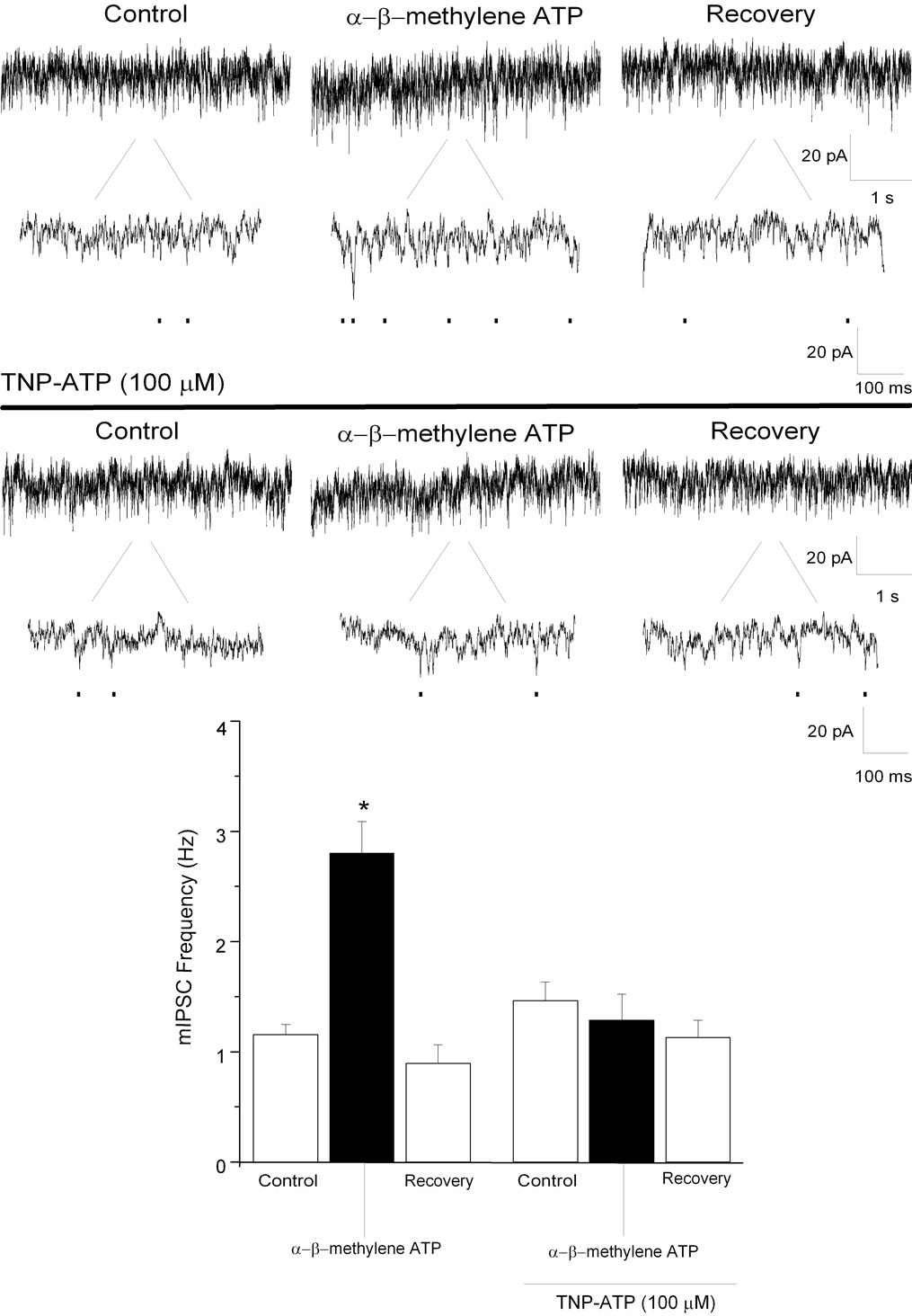
To further characterize the role of P2X receptors in facilitating inhibitory neurotransmission to CVNs, the selective P2X agonist α, β–methylene ATP (100 µM), was focally applied. α, β–methylene ATP significantly increased glycinergic mIPSC frequency from 2 ± 0.4 to 5 ± 0.8 Hz (See Fig. 5; n=7; P<0.05); glycinergic mIPSC amplitude also significantly increased. Bath application of TNP-ATP (100 µM), a selective P2X receptor antagonist, prevented the α, β–methylene ATP mediated changes in glycinergic mIPSC frequency (control: 2 ± 0.3 Hz; TNP-ATP: 1 ± 0.2 Hz; See Fig. 5; n=7; P>0.05) and changes in glycinergic mIPSC amplitude was also blocked. Focal application of α, β–methylene ATP significantly increased GABAergic mIPSC frequency from 1 ± 0.1 to 3 ± 0.2 Hz (See Fig. 6; n=7; P<0.05). α, β–methylene ATP did not alter GABAergic mIPSC amplitude (control: 24 ± 1 pA; α, β–methylene: 24 ± 2 pA; P>0.05). Bath application of TNP-ATP (100 µM) also abolished the ATP mediated changes in GABAergic mIPSC frequency (control: 1 ± 0.2 Hz; TNP-ATP: 1 ± 0.2 Hz; See Fig. 6; n=7; P>0.05). These results suggest that P2X but not P2Y receptors facilitate inhibitory neurotransmission to CVNs.
Figure 5.
Focal application of α, β–methylene ATP (100 µM), significantly increased glycinergic mIPSC frequency in CVNs (top traces). Application of TNP-ATP (100 µM), prevented the increase in glycinergic mIPSC frequency evoked by α, β–methylene ATP (middle). Results from a typical experiment are shown in the 2 series of traces , including an expanded section of each trace to better visualize individual events (top, middle), and the average results from 7 CVNs are shown in the bar graph, bottom. * denotes p≤ 0.05 as compared to the control values.
Figure 6.
Focal application of α, β–methylene ATP (100 µM), significantly increased GABAergic mIPSC frequency (top traces). Application of PPADS (100 µM), prevented the increase in GABAergic mIPSC frequency evoked by α, β–methylene ATP (middle). Results from a typical experiment are shown in the 2 series of traces, including an expanded section of each trace to better visualize individual events (top, middle), and the average results from 7 CVNs are shown in the bar graph, bottom. * denotes p≤ 0.05 as compared to the control values.
To test whether the P2X facilitation of glycine and GABA release resulted from direct presynaptic actions of P2X receptors or indirectly via opening voltage-gated calcium channels the nonselective voltage-gated calcium channel blocker, CdCl2 (100 µM), was included in the perfusate. Bath application of CdCl2 did not significantly (P>0.05) alter the increase in glycinergic or GABAergic mIPSC frequency evoked by α, β–methylene ATP indicating that augmentation of glycine and/or GABA release by P2X receptors occurred independently of voltage-gated calcium channels.
Discussion
This report focuses on the functional role of P2X receptor activation in mediating inhibitory neurotransmission to CVNs. Focal application of ATP significantly increased the frequency of both glycinergic and GABAergic mIPSCs in CVNs, suggesting that the effect of ATP occurs both at glycinergic and GABA presynaptic terminals. These findings are consistent with other neurons as ATP increased glycine release in rat dorsal horn substantia gelatinosa neurons (Rhee et al., 2000) and GABA release from synaptic terminals isolated from rat midbrain (Gomez-Villafuertes et al., 2001).
It is likely that the purinergic modulation of GABAergic and glycinergic activity to CVNs plays an important role during and in recovery from hypoxia. Heart rate responses to hypoxia include a biphasic increase followed by a decrease in heart rate. Previous work has shown hypoxia evokes a biphasic augmentation followed by a reduction in GABAergic and glycinergic activity to CVNs, and this is likely is responsible for the initial tachycardia followed by a bradycardia in response to hypoxia (Neff et al., 2004). It is likely that the purinergic facilitation of GABAergic and glycinergic activity to CVNs examined in this study mediates the initial increase in inhibitory inputs to CVNs during hypoxia. After the initial increase in inhibitory inputs to CVNs and the resulting tachycardia, the inhibitory inputs to CVNs are reduced and a parasympathetic mediated bradycardia occurs. This increase in parasympathetic cardiac activity is likely not only mediated by disinhibition, a reduction of the GABAergic and glycinergic inputs to CVNs, but also by the recruitment of an excitatory neurotransmission to CVNs post hypoxia (Griffioen et al., 2007a; Griffioen et al., 2007b). Consistent with an important role of purinergic pathways and receptors in mediating the heart rate responses to hypoxia, previous work has shown that activation of purinergic pathways and activation of P2X receptors is responsible for the excitatory neurotransmission to CVNs upon recovery from hypoxia and/or hypercapnia (Griffioen et al., 2007a). ATP excites CVNs by increasing excitatory glutamatergic neurotransmission to CVNs via presynaptic P2X receptors, and that this facilitation of glutamate release occurs independently of voltage gated calcium channels (Jameson et al., 2008).
The nonselective P2 receptor antagonist PPADS inhibited the ATP facilitated increases in both glycinergic and GABAergic neurotransmission to CVNs, indicating ATP is likely facilitating this release by activating purinergic receptors on both presynaptic glycine and GABA synaptic terminals. Focal application of α, β–methylene ATP, a selective P2X receptor agonist, but not the P2Y agonists UTP and adenosine 5'-0-(Z-thiodiphosphate), significantly enhanced glycinergic and GABAergic mIPSCs in CVNs indicating P2X, but not P2Y receptors facilitate inhibitory neurotransmission to CVNs. The increases due to α, β–methylene ATP on inhibitory neurotransmission to CVNs was blocked by the P2X receptor antagonist TNP-ATP, further indicating facilitation of inhibitory neurotransmission to CVNs results from P2X but not P2Y receptors. This is congruent with the increased spontaneous IPSC frequency elicited by α, β–methylene ATP in lamina V neurons mediated by glycinergic/GABAergic inputs from dorsal horn interneurons (Nakatsuka et al., 2002).
These findings indicate that P2X receptors are responsible for the purinergic facilitation of glycinergic and GABAergic neurotransmission to cardiac vagal neurons. It is likely that P2X receptors may mediate the observed tachycardia during hypoxia in which there is a withdrawal of parasympathetic activity to the heart and respiratory activity is altered to short gasping breaths. Following the tachycardia, a bradycardia develops which serves to reduce the metabolic demand on cardiac and respiratory muscles and acts to extend survival by increasing parasympathetic outflow to the heart (Neff et al., 1998). The P2X receptor mediated increase in inhibitory glycinergic and GABAergic neurotransmission observed in this study may serve a vital role in the initial tachycardia, whereas a decrease in the purinergic facilitation of inhibitory neurotransmission, coupled with recruitment of an excitatory neurotransmission to CVNs is likely involved in the protective slowing of the heart (Griffioen et al., 2007a; Griffioen et al., 2007b).
P2X receptors are permeable to small cations such as sodium and potassium and have varying permeability to calcium (Evans et al., 1996). Both direct calcium entry through P2X receptors as well as activation of voltage gated calcium channels can facilitate neurotransmission to P2X receptors (Griffioen et al., 2007a). As a result, glycine and GABA release may be evoked in two ways: presynaptic terminal depolarization opening voltage-gated calcium channels or direct presynaptic calcium entry via P2X receptors. The results of this study show that α, β–methylene ATP enhances the release of glycine and GABA even in the presence of cadmium chloride indicating the P2X mediated facilitation of inhibitory neurotransmission to CVNs is independent of voltage-gated calcium channels and occurs likely via calcium influxes through ATP sensitive receptors. These results parallel those seen in mechanically dissociated rat spinal cord substantia gelatinosa neurons where both ATP and α, β–methylene ATP facilitated an increase in glycinergic mIPSC frequency in the presence of cadmium. However, the increase was abolished in a calcium-free external solution, suggesting glycine release is not due to the opening of voltage-gated calcium channels but most likely due to calcium influxes through purinergic receptors (Jang et al., 2001). Although this data indicates calcium entry into P2X receptors facilitates inhibitory neurotransmission to CVNs, further work is necessary to show whether purinergic receptors cease to facilitate inhibitory neurotransmission to CVNs in a calcium-free external solution. The results of such a study may further indicate the specific inhibitory mechanisms by which the heart responds to hypoxia.
Both ATP and α, β–methylene ATP mediated a small yet significant increase in glycinergic mIPSC amplitude yet the purinergic agonists had no effect on the amplitude of GABAergic mIPSCs. The increase in glycinergic mIPSC amplitude maybe due to ATP and α, β–methylene ATP facilitating postsynaptic glycinergic receptors and is consistent with both the ATP and α, β–methylene ATP elicited presynaptic and postsynaptic responses of glycinergic mIPSC in mechanically dissociated rat spinal cord substantia gelatinosa neurons (Jang et al., 2001). While it has been shown previously that upon recovery from hypoxia/hypercapnia there is no change in the amplitude of excitatory neurotransmission to CVNs (Kamendi et al., 2008), further work in necessary to determine the implications of the increase in glycinergic amplitude seen here and what effects this may have upon recovery from hypoxia and/or hypercapnia. The absence of an increase in GABAergic mIPSC amplitude in the results of this study is consistent with that seen in cultured rat spinal cord dorsal horn neurons, where ATP modified the frequency but not the amplitude of GABAergic mIPSCs (Hugel and Schlichter, 2000).
In conclusion, ATP activates presynaptic P2X receptors that facilitate glycinergic and GABAergic neurotransmission to CVNs. The release of glycine and GABA is not dependent on voltage-gated calcium channels and as a result, suggests that glycine and GABA release is most likely a result of direct presynaptic calcium entry via P2X receptors. Further research is necessary to determine the endogenous role of ATP in modulating inhibitory neurotransmission to CVNs, particularly during challenges such as hypoxia and hypercapnia.
Experimental Procedures
In an initial surgery, Sprague-Dawley rats (post-natal: days 2 to 5) were anesthetized with hypothermia to slow the heart and aid in recovery. A right thoracotomy was performed to expose the heart and the retrograde tracer, rhodamine (XRITC, Molecular Probes, 2% solution, 20–50µL), was then injected into the pericardial sac to retrogradely label CVNs. Specificity of the cardiac vagal labeling was confirmed when injections of rhodamine, by either intravenous injections or injections into the chest cavity but outside the pericardial sac, failed to label any neurons in the medulla. On the day of the experiment, one to three days after the injection of the fluorescent tracer, the animal was anesthetized with isofluorane and sacrificed by cervical dislocation. The brain was rapidly removed and immersed in a cold HEPES buffer (4°C) with the following composition: NaCl (140 mM), KCl (5 mM), CaCl2 (2 mM), glucose (5 mM), HEPES (10 mM), continuously oxygenated with 100% O2. Using a dissection microscope, the hindbrain was isolated. The brain was glued to a stage and placed in the slicing chamber of a vibratome filled with the same buffer. Slices 500–600 µm in thickness were cut. The slices were then mounted in a perfusion chamber and submerged in a perfusate with the following composition: NaCl (125 mM), KCl (3 mM), CaCl2 (2 mM), NaHCO3 (26 mM), glucose (5 mM), HEPES (5 mM) oxygenated with 95% O2/5% CO2 gas mixture. The osmolarity of all solutions was 285–290 mosM, and the pH was maintained between 7.35 and 7.4. All animal procedures were performed in compliance with the institutional guidelines at The George Washington University, and were in accordance with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association and the NIH publication (85–23, revised 1996) ‘Guide for the Care and Use of Laboratory Animals’. The minimal number of animals was used and attention was given to minimize any possible discomfort.
Electrophysiological Recording
Individual CVNs located in the nucleus ambiguus were identified by the presence of the fluorescent tracer and imaged with differential contrast optics, infrared illumination and infrared-sensitive video detection cameras to gain better spatial resolution and to visually position the patch pipette onto the surface of the identified neuron. Pipettes were made with a puller (Narishige, Japan) and filled resistances were 2.5–5 MΩ. The pipettes were filled with a solution consisting of the following composition: KCl (150 mM), MgCl2 (4 mM), EGTA (2 mM), Na-ATP (2 mM), QX-314 (5 mM), and HEPES (10 mM) at pH 7.3. CVNs were studied using the whole-cell patch-clamp technique and were voltage clamped at a holding potential of −80 mV.
Glycinergic inhibitory postsynaptic currents (IPSCs) were isolated by adding gabazine (25 µM), a GABAA receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione ([CNQX] 50 µM), a non-NMDA antagonist and D-2-Amino-5 phosphonovalerate ([AP5] 50 µM), a NMDA receptor antagonist. Strychnine (1 µM), a glycinergic receptor antagonist, CNQX (50 µM) and AP5 (50 µM) were included in the perfusate to isolate GABAergic IPSCs. In order to block action potential dependent events and isolate both miniature glycinergic and GABAergic IPSCs (mIPSCs) tetrodotoxin ([TTX] 1 µM) was added to the perfusate. At the end of each experiment, both spontaneous GABAergic and glycinergic mIPSCs were blocked by gabazine (25 µM) and strychnine (1 µM), respectively. Only one concentration of drug was used per slice. In some experiments pyridoxal-phosphate-6-azophenyl-2',4'-disulfonic acid ([PPADS] 100 µM) a P2 purinergic receptor blocker or 2'(3')-O-(2,4,6-trinitrophenyl)adenosine 5'-triphosphate ([TNP-ATP] 100 µM) a purinergic P2X receptor blocker, were included in the perfusate for a period of 7 minutes. Both ATP (100 µM) and α, β–methylene ATP (100 µM) were focally applied (approximately 2 s) as well as, uridine triphosphate ([UTP] 15 µM) and adenosine 5'-0-(Z-thiodiphosphate) (60 µM) (approximately 10 s) using a WPI Pneumatic Picopump pressure delivery system using a patch pipette positioned within 30 µm from the patched cardiac vagal neuron. Each recording typically was 5 minutes long and contained a 2 minute control before focal application of ATP and a 3 minute period after application of ATP. MiniAnalysis (Synaptosoft version 4.3.1) was used to analyze all experiments. The threshold for glycinergic and GABAergic events was a threshold of 5 times the root mean square of noise. Results are presented as mean percentage of control ± S.E. and statistically compared with a paired Student's t-test (for significance of difference, *P<0.05).
Abbreviations
- AP5
D-2-Amino-5-phosphonovaleric acid
- ATP
adenosine 5’-triphosphate
- CNQX
6-Cyano-7-nitroquinoxaline-2,3-dione
- CVNs
parasympathetic cardiac vagal neurons
- GABA
gamma-aminobutyric acid
- mIPSC
miniature inhibitory postsynaptic currents
- NMDA
N-methyl-D-aspartate
- PPADS
pyridoxal-phosphate-6-azophenyl-2’,4’-disulphonic acid
- TNP-ATP
2',3'-O-(2,4,6-Trinitrophenyl) adenosine 5'-triphosphate
- TTX
tetrodotoxin
- UTP
uridine-5-triphosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardoni R, Goldstein PA, Lee CJ, Gu JG, MacDermott AB. ATP P2X receptors mediate fast synaptic transmission in the dorsal horn of the rat spinal cord. J Neurosci. 1997;17:5297–5304. doi: 10.1523/JNEUROSCI.17-14-05297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Robertson SJ, Gibb AJ. Properties of ATP receptor-mediated synaptic transmission in the rat medial habenula. Neuropharmacology. 1997;36:1253–1268. doi: 10.1016/s0028-3908(97)00127-5. [DOI] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Virginio C, Lundstrom K, Buell G, Surprenant A, North RA. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J Physiol. 1996;497(Pt 2):413–422. doi: 10.1113/jphysiol.1996.sp021777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Villafuertes R, Gualix J, Miras-Portugal MT. Single GABAergic synaptic terminals from rat midbrain exhibit functional P2X and dinucleotide receptors, able to induce GABA secretion. J Neurochem. 2001;77:84–93. doi: 10.1046/j.1471-4159.2001.t01-1-00228.x. [DOI] [PubMed] [Google Scholar]
- Griffioen KJ, Gorini C, Jameson H, Mendelowitz D. Purinergic P2X receptors mediate excitatory transmission to cardiac vagal neurons in the nucleus ambiguus after hypoxia. Hypertension. 2007a;50:75–81. doi: 10.1161/HYPERTENSIONAHA.106.086140. [DOI] [PubMed] [Google Scholar]
- Griffioen KJ, Kamendi HW, Gorini CJ, Bouairi E, Mendelowitz D. Reactive oxygen species mediate central cardiorespiratory network responses to acute intermittent hypoxia. J Neurophysiol. 2007b;97:2059–2066. doi: 10.1152/jn.00975.2006. [DOI] [PubMed] [Google Scholar]
- Huang ZG, Griffioen KJ, Wang X, Dergacheva O, Kamendi H, Gorini C, Mendelowitz D. Nicotinic receptor activation occludes purinergic control of central cardiorespiratory network responses to hypoxia/hypercapnia. J Neurophysiol. 2007;98:2429–2438. doi: 10.1152/jn.00448.2007. [DOI] [PubMed] [Google Scholar]
- Hugel S, Schlichter R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J Neurosci. 2000;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson HS, Pinol RA, Kamendi H, Mendelowitz D. ATP facilitates glutamatergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Brain Res. 2008;1201:88–92. doi: 10.1016/j.brainres.2008.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang IS, Rhee JS, Kubota H, Akaike N. Developmental changes in P2X purinoceptors on glycinergic presynaptic nerve terminals projecting to rat substantia gelatinosa neurones. J Physiol. 2001;536:505–519. doi: 10.1111/j.1469-7793.2001.0505c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo YH, Role LW. Cholinergic modulation of purinergic and GABAergic co-transmission at in vitro hypothalamic synapses. J Neurophysiol. 2002;88:2501–2508. doi: 10.1152/jn.00352.2002. [DOI] [PubMed] [Google Scholar]
- Jo YH, Schlichter R. Synaptic corelease of ATP and GABA in cultured spinal neurons. Nat Neurosci. 1999;2:241–245. doi: 10.1038/6344. [DOI] [PubMed] [Google Scholar]
- Kamendi HW, Cheng Q, Dergacheva O, Frank JG, Gorini C, Jameson HS, Pinol RA, Wang X, Mendelowitz D. Recruitment of excitatory serotonergic neurotransmission to cardiac vagal neurons in the nucleus ambiguus post hypoxia and hypercapnia. J Neurophysiol. 2008;99:1163–1168. doi: 10.1152/jn.01178.2007. [DOI] [PubMed] [Google Scholar]
- Nakatsuka T, Furue H, Yoshimura M, Gu JG. Activation of central terminal vanilloid receptor-1 receptors and alpha beta-methylene-ATP-sensitive P2X receptors reveals a converged synaptic activity onto the deep dorsal horn neurons of the spinal cord. J Neurosci. 2002;22:1228–1237. doi: 10.1523/JNEUROSCI.22-04-01228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff RA, Humphrey J, Mihalevich M, Mendelowitz D. Nicotine enhances presynaptic and postsynaptic glutamatergic neurotransmission to activate cardiac parasympathetic neurons. Circ Res. 1998;83:1241–1247. doi: 10.1161/01.res.83.12.1241. [DOI] [PubMed] [Google Scholar]
- Neff RA, Simmens SJ, Evans C, Mendelowitz D. Prenatal nicotine exposure alters central cardiorespiratory responses to hypoxia in rats: implications for sudden infant death syndrome. J Neurosci. 2004;24:9261–9268. doi: 10.1523/JNEUROSCI.1918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Barnard EA. Nucleotide receptors. Curr Opin Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Wang ZM, Nabekura J, Inoue K, Akaike N. ATP facilitates spontaneous glycinergic IPSC frequency at dissociated rat dorsal horn interneuron synapses. J Physiol. 2000;524(Pt 2):471–483. doi: 10.1111/j.1469-7793.2000.t01-1-00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorimachi M, Ishibashi H, Moritoyo T, Akaike N. Excitatory effect of ATP on acutely dissociated ventromedial hypothalamic neurons of the rat. Neuroscience. 2001;105:393–401. doi: 10.1016/s0306-4522(01)00192-0. [DOI] [PubMed] [Google Scholar]
- Wang ZM, Katsurabayashi S, Rhee JS, Brodwick M, Akaike N. Substance P abolishes the facilitatory effect of ATP on spontaneous glycine release in neurons of the trigeminal nucleus pars caudalis. J Neurosci. 2001;21:2983–2991. doi: 10.1523/JNEUROSCI.21-09-02983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollmann G, Acuna-Goycolea C, van den Pol AN. Direct excitation of hypocretin/orexin cells by extracellular ATP at P2X receptors. J Neurophysiol. 2005;94:2195–2206. doi: 10.1152/jn.00035.2005. [DOI] [PubMed] [Google Scholar]