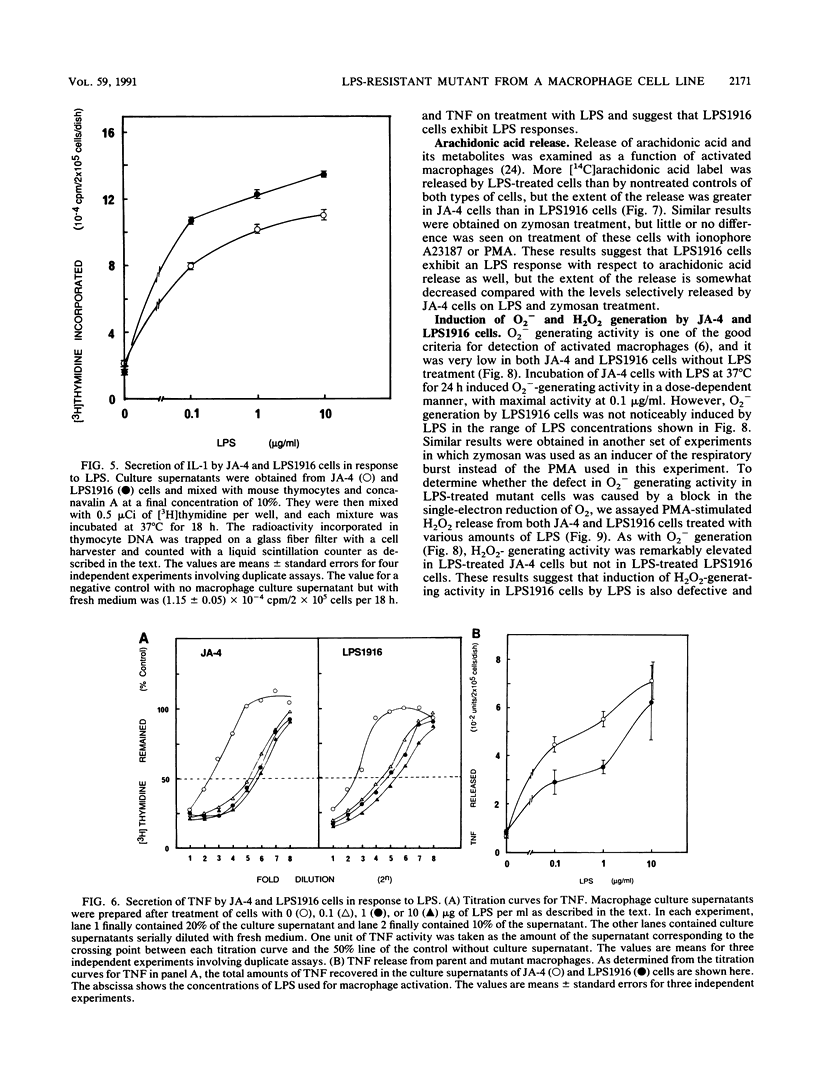
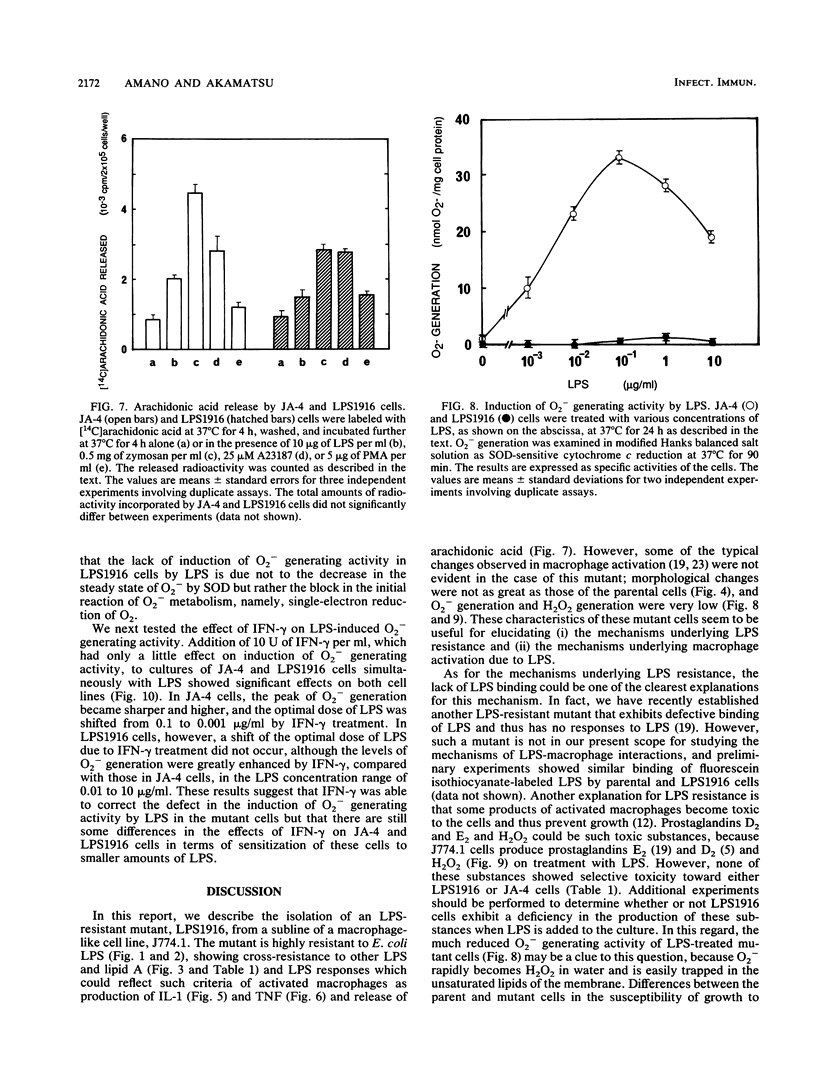
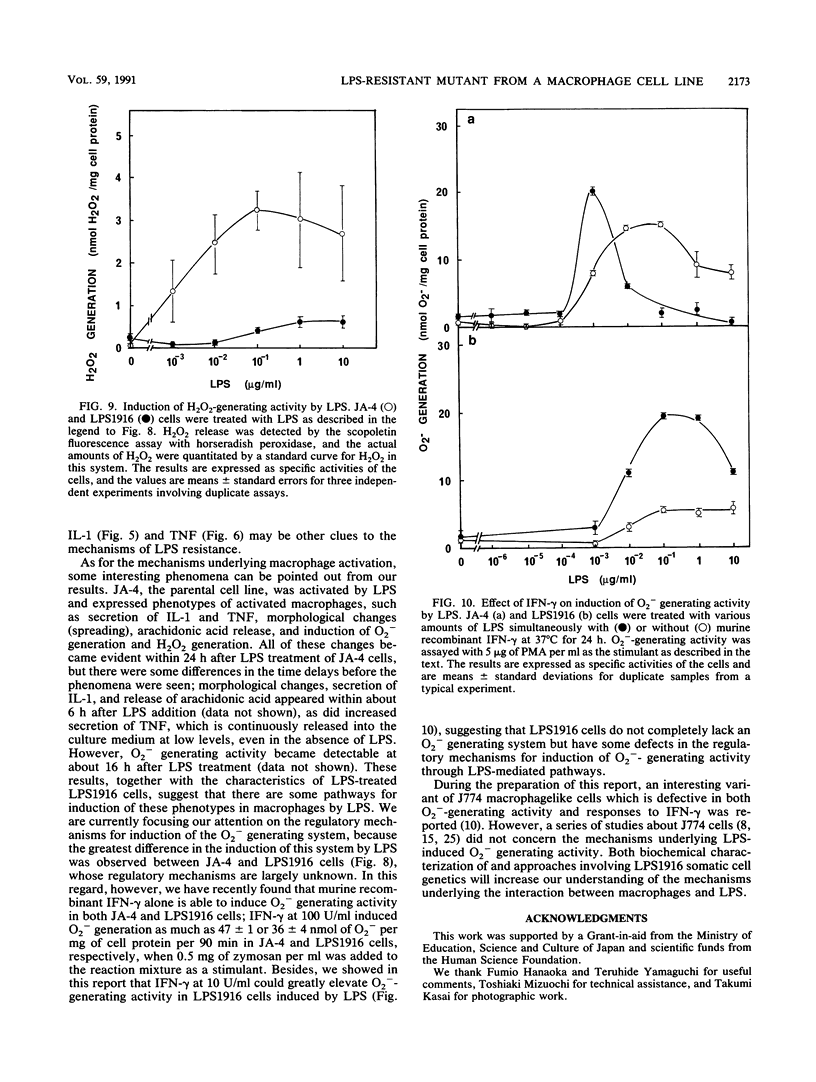
Abstract
A bacterial lipopolysaccharide (LPS)-resistant mutant was isolated from murine macrophagelike cell line J774.1. The mutant showed selective resistance to LPS and lipid A and was almost 10(5)- to 10(6)-fold more resistant than the parent; it grew even in the presence of 1 mg of Escherichia coli O55:B5 LPS per liter, whereas the parent did not grow with less than 10 ng of LPS per milliliter. We next examined the mutant for activation of various functions of macrophages on LPS treatment. This LPS-resistant mutant secreted interleukin-1 and tumor necrosis factor almost as effectively as the parent did. The mutant cells also changed transiently from a round to a spread form; however, they became round again afterwards. The mutant cells secreted less arachidonic acid in response to LPS. These results also suggest that this LPS-resistant mutant responds to LPS and shows activation of some macrophage functions. However, this mutant did not exhibit elevation of O2- generation or H2O2 generation after LPS treatment. Also, treatment of the mutant cells with murine recombinant gamma interferon was partly able to correct the defect in O(2-)-generating activity in response to LPS, suggesting that this defect is probably due to some of the LPS signal pathways. This implies that there is some correlation between O2- metabolism in LPS-activated macrophages and decreases in cell growth and viability.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akagawa K. S., Kamoshita K., Onodera S., Tokunaga T. Restoration of lipopolysaccharide-mediated cytotoxic macrophage induction in C3H/HeJ mice by interferon-gamma or a calcium ionophore. Jpn J Cancer Res. 1987 Mar;78(3):279–287. [PubMed] [Google Scholar]
- Akagawa K. S., Kamoshita K., Tomita T., Yasuda T., Tokunaga T. Regulatory mechanism of expression of LPS binding site(s) and signaling events by LPS in macrophages. Adv Exp Med Biol. 1990;256:467–480. doi: 10.1007/978-1-4757-5140-6_42. [DOI] [PubMed] [Google Scholar]
- Akagawa K. S., Tokunaga T. Lack of binding of bacterial lipopolysaccharide to mouse lung macrophages and restoration of binding by gamma interferon. J Exp Med. 1985 Nov 1;162(5):1444–1459. doi: 10.1084/jem.162.5.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander P., Evans R. Endotoxin and double stranded RNA render macrophages cytotoxic. Nat New Biol. 1971 Jul 21;232(29):76–78. doi: 10.1038/newbio232076a0. [DOI] [PubMed] [Google Scholar]
- Amano F., Nishijima M., Akagawa K., Akamatsu Y. Enhancement of O2- generation and tumoricidal activity of murine macrophages by a monosaccharide precursor of Escherichia coli lipid A. FEBS Lett. 1985 Nov 18;192(2):263–266. doi: 10.1016/0014-5793(85)80121-6. [DOI] [PubMed] [Google Scholar]
- Amano F., Nishijima M., Akamatsu Y. A monosaccharide precursor of Escherichia coli lipid A has the ability to induce tumor-cytotoxic factor production by a murine macrophage-like cell line, J774.1. J Immunol. 1986 Jun 1;136(11):4122–4127. [PubMed] [Google Scholar]
- Damiani G., Kiyotaki C., Soeller W., Sasada M., Peisach J., Bloom B. R. Macrophage variants in oxygen metabolism. J Exp Med. 1980 Oct 1;152(4):808–822. doi: 10.1084/jem.152.4.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Freudenberg M. A., Lüderitz O., Rietschel E. T., Westphal O. Chemical, physicochemical and biological properties of bacterial lipopolysaccharides. Prog Clin Biol Res. 1979;29:321–332. [PubMed] [Google Scholar]
- Goldberg M., Belkowski L. S., Bloom B. R. Regulation of macrophage function by interferon-gamma. Somatic cell genetic approaches in murine macrophage cell lines to mechanisms of growth inhibition, the oxidative burst, and expression of the chronic granulomatous disease gene. J Clin Invest. 1990 Feb;85(2):563–569. doi: 10.1172/JCI114473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Kuge S., Amano F., Nishijima M., Akamatsu Y. Isolation of a lipopolysaccharide (LPS)-resistant mutant, with defective LPS binding, of cultured macrophage-like cells. J Biol Chem. 1990 Apr 25;265(12):6606–6610. [PubMed] [Google Scholar]
- Jackowski S., Rettenmier C. W., Rock C. O. Prostaglandin E2 inhibition of growth in a colony-stimulating factor 1-dependent macrophage cell line. J Biol Chem. 1990 Apr 25;265(12):6611–6616. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Godzik C. A., Cohn Z. A. Increased superoxide anion production by immunologically activated and chemically elicited macrophages. J Exp Med. 1978 Jul 1;148(1):115–127. doi: 10.1084/jem.148.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyotaki C., Peisach J., Bloom B. R. Oxygen metabolism in cloned macrophage cell lines: glucose dependence of superoxide production, metabolic and spectral analysis. J Immunol. 1984 Feb;132(2):857–866. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lyall R. M., Hwang J. L., Cardarelli C., FitzGerald D., Akiyama S., Gottesman M. M., Pastan I. Isolation of human KB cell lines resistant to epidermal growth factor-Pseudomonas exotoxin conjugates. Cancer Res. 1987 Jun 1;47(11):2961–2966. [PubMed] [Google Scholar]
- Morrison D. C., Ryan J. L. Bacterial endotoxins and host immune responses. Adv Immunol. 1979;28:293–450. doi: 10.1016/s0065-2776(08)60802-0. [DOI] [PubMed] [Google Scholar]
- Nishijima M., Amano F., Akamatsu Y., Akagawa K., Tokunaga T., Raetz C. R. Macrophage activation by monosaccharide precursors of Escherichia coli lipid A. Proc Natl Acad Sci U S A. 1985 Jan;82(2):282–286. doi: 10.1073/pnas.82.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim J. J., Shneyour A., Kook A. I. Enhancement of DNA synthesis and cAMP content of mouse thymocytes by mediator(s) derived from adherent cells. J Immunol. 1976 May;116(5):1466–1472. [PubMed] [Google Scholar]
- Ralph P., Nakoinz I. Direct toxic effects of immunopotentiators on monocytic, myelomonocytic, and histiocytic or macrophage tumor cells in culture. Cancer Res. 1977 Feb;37(2):546–550. [PubMed] [Google Scholar]
- Suzuki H., Kurita T., Kakinuma K. Effects of neuraminidase on O2 consumption and release of O2 and H2O2 from phagocytosing human polymorphonuclear leukocytes. Blood. 1982 Aug;60(2):446–453. [PubMed] [Google Scholar]
- Takahashi I., Kotani S., Takada H., Tsujimoto M., Ogawa T., Shiba T., Kusumoto S., Yamamoto M., Hasegawa A., Kiso M. Requirement of a properly acylated beta(1-6)-D-glucosamine disaccharide bisphosphate structure for efficient manifestation of full endotoxic and associated bioactivities of lipid A. Infect Immun. 1987 Jan;55(1):57–68. doi: 10.1128/iai.55.1.57-68.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Amano F., Kishi H., Nishijima M., Akamatsu Y. Degradation of arachidonyl phospholipids catalyzed by two phospholipases A2 and phospholipase C in a lipopolysaccharide-treated macrophage cell line RAW264.7. Arch Biochem Biophys. 1989 Jul;272(1):210–218. doi: 10.1016/0003-9861(89)90212-9. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Tanowitz H., Bloom B. R. Growth of Trypanosoma cruzi in a cloned macrophage cell line and in a variant defective in oxygen metabolism. Infect Immun. 1983 Sep;41(3):1322–1331. doi: 10.1128/iai.41.3.1322-1331.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]