Abstract
The ATP-binding cassette (ABC) transporters constitute a family of large membrane proteins which transport a variety of substrates across membranes. The ABCA4 protein is expressed in photoreceptors and possibly functions as a transporter for N-retinylidene-phosphatidylethanolamine (N-retinylidene-PE), the Schiff base adduct of all-trans-retinal with PE. Mutations in the ABCA4 gene have been initially associated with autosomal recessive Stargardt disease. Subsequent studies have shown that mutations in ABCA4 can also cause a variety of other retinal dystrophies including cone rod dystrophy and retinitis pigmentosa. To determine the prevalence and mutation spectrum of ABCA4 gene mutations in non-Stargardt phenotypes we have screened 64 unrelated patients with autosomal recessive cone (arCD) and cone rod dystrophy (arCRD) applying the Asper Ophthalmics ABCR400 microarray followed by DNA sequencing of all coding exons of the ABCA4 gene in subjects with single heterozygous mutations.
Disease-associated ABCA4 alleles were identified in 20 of 64 patients with arCD or arCRD. In four of 64 patients (6%) only one mutant ABCA4 allele was detected and in 16 patients (25%), mutations on both ABCA4 alleles were identified. Based on these data we estimate a prevalence of 31% for ABCA4 mutations in arCD and arCRD, supporting the concept that the ABCA4 gene is a major locus for various types of degenerative retinal diseases with abnormalities in cone or both cone and rod function.
Introduction
The ATP-binding cassette (ABC) transporters constitute a family of large membrane proteins which transport a variety of substrates across membranes 1. One member of the family, the ABCA4 protein, is expressed in cone and rod photoreceptors 2 and possibly functions as a transporter for N-retinylidene-phosphatidylethanolamine (N-retinylidene-PE), the Schiff base adduct of all-trans-retinal with PE 3. It has been proposed that a failure in the transport of all-trans retinal from the luminal to the cytosolic side of photoreceptor discs may disrupt the visual cycle. Finally, it may lead to accumulation of N-retinylidene-PE and toxic lipofuscin A2-E in the RPE upon phagocytosis of photoreceptors. ABCA4 gene mutations have been initially identified in patients with autosomal recessively inherited Stargardt disease and fundus flavimaculatus (both OMIM ID 248200) 4,5 and subsequently also in autosomal recessively inherited cone rod dystrophy (arCRD, OMIM ID 604116) 6 and retinitis pigmentosa (OMIM ID 601718) 6–8. Moreover, single heterozygous ABCA4 sequence variants have been proposed as a susceptibility factor for age-related macular degeneration (OMIM ID 153800) 9. Consequently, a genotype-phenotype correlation based on the residual function of the ABCA4 protein has been suggested 10. Mutations in ABCA4 are the principal cause of Stargardt disease. However, predictions of the prevalence of ABCA4 gene mutations in arCRD have varied considerably between studies, ranging from 23 to 65% 11–14. Moreover, it has not yet been investigated whether ABCA4 mutation may also be involved in autosomal recessive cone dystrophy (arCD) with no detectable impairment of the rod system. In order to address these questions we investigated the prevalence of ABCA4 gene mutations in a large and clinically well defined group of patients affected by arCD or arCRD.
Methods
Mutation analysis
Patients diagnosed with CD or CRD and a family history consistent with an autosomal recessive mode of inheritance were included in the study and recruited in four different centers (Centre for Ophthalmology, University Tübingen, Tübingen, Germany; Scheie Eye Institute, Department of Ophthalmology, University of Pennsylvania, Philadelphia, USA; Department of Ophthalmology, Charité Campus Benjamin Franklin, Berlin, Germany; Department of Ophthalmology, Ludwig Maximilians University, Munich, Germany). The study was performed according to the tenets of the Declaration of Helsinki and approved by the ethical committees of the participating institutions. Informed consent was obtained from all patients.
Sixty-four patients met the inclusion criteria, forty-six patients had arCRD, eighteen patients had arCD. All patients were subsequently screened for ABCA4 gene mutations. The initial mutation screening was performed with the 10th version of the ABCR400 microarray (AsperBiotech, Tartu, Estonia) which queries >450 known sequence variants. The genotyping procedure included PCR amplification of all ABCA4 exons, fragmentation of amplification products, and solid-phase mini-sequencing, also termed arrayed primer extension technology (APEX), which has been described in detail elsewhere 15. Mutations identified by microarray genotyping were confirmed by conventional DNA sequencing of the relevant exon(s). For patients with only one mutation identified via chip analysis, all coding exons of the ABCA4 gene were amplified and sequenced employing the VariantSEQr Resequencing System (Applied Biosystems, Darmstadt, Germany) according to manufacturer’s instructions. DNA sequencing was done with BigDye Sequencing Chemistry and products were separated on a 3100 capillary sequencer (both Applied Biosystems).
Segregation analysis and exclusion of a novel sequence variant in healthy controls
Segregation analysis in family members was performed by direct DNA sequencing of the respective exon(s). The novel sequence variant c.4848+2T>C was evaluated in 123 healthy control individuals (246 chromosomes) by means of PCR/RFLP with the restriction enzyme Csp6I (Fermentas, Glen Burnie, Maryland). PCR products were digested overnight and the RFLP pattern evaluated by agarose gel electrophoresis.
Patients and evaluation of clinical phenotype
The clinical records of patients with one or two mutant ABCA4 alleles were analysed retrospectively. The diagnoses of arCD and arCRD were mainly based on the results of full field electroretinography (ERG). Patients with reduced cone ERGs and normal rod ERGs were diagnosed as arCD, whereas patients with reduced cone and rod ERGs were defined as arCRD. Characteristic symptoms and signs, fundus appearance and visual field results were used to corroborate the diagnosis 16–18. A standardised spreadsheet was used and the patient records were evaluated for the following information: onset age; disease duration; best corrected visual acuity (BCVA); color vision tested with saturated or desaturated Panel D15 test, or Arden color vision test; glare sensitivity; night vision symptoms; funduscopic appearance; kinetic and/or static perimetry and full field ERGs according to ISCEV standard (except for patient RCD63/3402 who received an ERG recording before introduction of ISCEV standards). Details of the ERG methodology and the normal data used by the contributing clinical authors have been published 18,19. Mean visual acuities were calculated by determining the logarithmic mean 20 with qualitative visual acuity measures scaled as follows: counting fingers - 0,0014; hand movements - 0,0052 according to 21; light perception - 0,0005 and no light perception - 0,0001 according to 22.
Results
Prevalence and mutation spectrum of ABCA4 mutations in patients with arCD and arCRD
Among the 64 unrelated patients, 46 patients with arCRD, 18 patients with arCD, who were screened with the ABCR400 gene chip, ABCA4 gene mutations were identified in 20 (31%) patients. Homozygous or compound heterozygous mutations were identified in 13 of the 64 (20%) patients, and single heterozygous mutations were identified in another 7 (11%) patients. All mutations identified by microarray analysis were confirmed by DNA sequencing. By design, the chip only detects known sequence variants and thus misses novel mutations which have not yet been integrated onto the chip. In order to identify the second mutant allele, we sequenced all coding exons of the ABCA4 gene in those 7 patients in whom the ABCR400 chip detected only a single disease associated allele. This resulted in the identification of three novel sequence variants (c.700C>T, c.3539_3554del, and c.4848+2T>C) in three unrelated patients.
Genotypes for all patients with one or two disease-associated alleles are given in Table 1. In total, microarray and the “all-exon-sequencing” approach together identified 36 mutant ABCA4 alleles in 64 patients, representing 24 different sequence variants, including the complex allele [c.1622T>C;c.3113C>T] (Table 2).
Table 1.
Genotypes of arCRD and arCD index patients screened for ABCA4 mutations.
| Family ID/Patient ID/Gender | Allele 1 | Allele 2 | Segregation in family | Diagnosis |
|---|---|---|---|---|
| RCD92/6809/F | c.731 T>C a | c.731 T>C | n.a. | CRD |
| RCD79/6184/M | c.735 T>G | c.735 T>G | yes | CRD |
| ZD289/781/F | [c.1622T>C;c.3113C>T] | c.4848+2T>C | yes | CRD |
| RCD157/10492/F | c.2588G>C | c.5196+1_c.5196+4del | (yes) | CRD |
| RCD51/4984/F | c.3608G>A | n.d. | n.a. | CRD |
| RCD9/1989/M | c.4139C>T | c.6229C>T | yes | CRD |
| RCD42/2509/M | c.4139C>T | c.1928T>G | n.a. | CRD |
| ZD291/5781/F | c.5285C>A | c.3539_c.3554del | yes | CD |
| ZD85/5862/F | c.5461-10T>C | c.5882G>A | yes | CD |
| RCD147/9136/F | c.5461-10T>C | c.700C>T | n.a. | CRD |
| RCD141/8594/M | c.5461-10T>C | n.d. | (yes) | CRD |
| RCD63/3402/M | c.5461-10T>C | n.d. | (yes) | CRD |
| RCD143/8805/M | c.5461-10T>C | c.5196+1G>A | yes | CRD |
| ZD146/10313/F | c.5461-10T>C | c.2588G>C | n.a. | CD, DD: CRD |
| RCD98/3474/F | c.5461-10T>C | c.4234C>T | n.a. | CRD |
| RCD194/10840/M | c.5461-10T>C | c.4457 C>T | n.a. | CRD |
| ZD290/6810/M | c.5714+5G>A | c.5819T>C | yes | CD |
| RCD113/3668/F | c.5917delG | c.5917delG | yes | CRD |
| RCD337/8019/F | c.5917delG | c.4462T>C | yes | CRD |
| RCD185/11721/F | c.6148G>C | n.d. | n.a. | CRD |
DD, differential diagnosis
Table 2.
Spectrum of ABCA4 gene mutations observed in patients with arCRD and arCD.
| Type of Mutation/Exon | Nucleotide change | Effect | No. of alleles | Reference |
|---|---|---|---|---|
| Missense: | ||||
| 6 | c.731T>C a | p.L244P | 2 | 28 |
| 12 | c.1622T>C b | p.L541P | 1 | 5 |
| 13 | c.1928T>G | p.V643G | 1 | 9 |
| 17 | c.2588G>C | p.G863A and p.G863del | 2 | 4 |
| 21 | c.3113C>T b | p.A1038V | 1 | 4 |
| 25 | c.3608G>A | p.G1203E | 1 | 32 |
| 28 | c.4139C>T | p.P1380L | 2 | 33 |
| 30 | c.4457C>T | p.P1486L | 1 | 33 |
| 30 | c.4462T>C | p.C1488R | 1 | 33 |
| 37 | c.5285C>A | p.A1762D | 1 | 32 |
| 41 | c.5819T>C | p.L1940P | 1 | 34 |
| 42 | c.5882G>A | p.G1961E | 1 | 9 |
| 45 | c.6148G>C | p.V2050L | 1 | 33 |
| 45 | c.6229C>T | p.R2077W | 1 | 33 |
| Nonsense: | ||||
| 6 | c.700C>T | p.Q234X | 1 | This study |
| 6 | c.735T>G | p.Y245X | 2 | 32 |
| 28 | c.4234C>T | p.Q1412X | 1 | 10 |
| Deletion: | ||||
| 24 | c.3539_3554del | p.S1181PfsX8 | 1 | This study |
| 43 | c.5917delG | p.V1973X | 3 | 23 |
| Splice site/intronic: | ||||
| 26 | c.5196+1G>A | splicing | 1 | 9 |
| 34 | c.4848+2T>C | splicing | 1 | This study |
| 36 | c.5196+1_5196+4del | splicing | 1 | 15 |
| 39 | c.5461-10T>C | unknown | 8 | 14 |
| 40 | c.5714+5G>A | splicing? | 1 | 6 |
In our study group missense mutations were most prevalent (17 of 37 mutations; 46%), followed by intronic and splice site mutations (12/37; 32%), nonsense mutations (4/37; 11%), and small deletions (4/37; 11%). Most sequence variants appeared only once. In contrast, the c.5461-10T>C allele was found in 8 patients and thus was the most prevalent sequence variant in this study.
The ABCA4 gene mutation prevalence was higher in arCRD (16/46, 35%) than in arCD (4/18, 22%).
Classification of newly identified sequence alterations
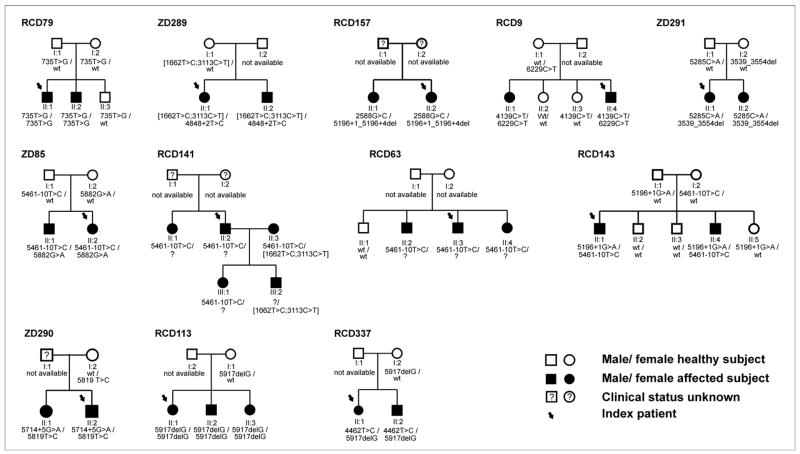
Based on our “all-exon-sequencing” strategy in subjects in whom only a single heterozygous mutation was detected by DNA chip analysis we identified three new sequence alterations that most likely represent disease-causing mutations. Two of the sequence variants are predicted to result in a truncated ABCA4 protein: c.700C>T constitutes an early stop mutation (p.Q234X), while the c.3539_3554del deletion leads to a frameshift and subsequent introduction of a premature termination codon after eight altered amino acid residues (p.S1181PfsX8). Furthermore, we observed a concordant segregation of c.3539_3554del in family ZD291 (Fig. 1). The third novel sequence alteration c.4848+2T>C affects the canonical GT-dinucleotide of the donor splice site of intron 34 and thus most likely impairs splicing of mutant transcripts. In addition, this sequence variant was not found in 246 chromosomes of healthy control individuals.
Figure 1.
Pedigrees of arCD and arCRD families segregating ABCA4 gene mutations. Index patients used for the ABCA4 microarray screening and subsequent DNA sequencing are marked by an arrow. Genotypes of all available family members are listed below the respective subject. A suspected but not identified second mutation in the ABCA4 gene is indicated with a question mark. Pedigrees are arranged from left to right and top to bottom in the same order as patients are listed in Table 1. The pedigree number is given above left to each pedigree.
Segregation analysis in families affected by arCD and arCRD
We performed segregation analysis in all families in which samples from additional family members were available (Fig. 1). For an autosomal recessive mode of inheritance homozygous or compound heterozygous mutations in affected patients, and single heterozygous or no mutations in unaffected relatives is required, which we will call concordant segregation. Furthermore, independent segregation of mutations in trans (in subjects carrying two heterozygous mutations) is needed. A number of complex alleles (i.e. two sequence variants in cis) has been described in ABCA4 23,24. In total, independent and concordant segregation of the two mutations on different alleles could be demonstrated in 9 families: RCD79, ZD289, RCD9, ZD291, ZD85, ZD290, RCD113, RCD337, RCD143. Three families did not fulfill all of the above stated segregation criteria. In family RCD 157 both affected siblings were concordant in being heterozygous for two mutations but we could not prove independent segregation because no other family members were available for segregation analysis. Only one single heterozygous sequence variant was identified during screening of the index patient in RCD63 and could be followed in that family. However, the presence of this mutation in all three affected siblings at least supports a causal relationship with the ABCA4 locus in this family. Pedigree RCD141 involves the marriage of two affected subjects (II:2 and II:3) and affected offspring. For II:3 concordant and independent segregation of two identified mutations can be demonstrated. The second ABCA4 mutation in the pedigree remains unidentified. Yet the segregation pattern is fully compatible with an autosomal recessive inheritance and linkage with the ABCA4 locus.
Clinical description of patients with arCD and arCRD and ABCA4 gene mutations
The clinical data of the 20 index patients with at least one identified ABCA4 gene mutation were evaluated in detail (Table 3). Most patients had arCRD (16/20). Four patients had arCD, one of those with a differential diagnosis of arCRD. For both disease groups together, arCD and arCRD, the median age of onset was 9 years (range 2 to 27 years) and the mean visual acuity was 0,04 (range: light perception [digitised as 0.0005 according to 22] to 0,06) after a median disease course of 22 years (range: six months to 44 years). Color vision testing performed in 14 patients indicated minor to severe color vision abnormalities. Six patients showed minor confusions or confusions along the red-green or blue-yellow axis, while five patients, all affected by arCRD, had severe color vision impairment (i.e. chaotic arrangements in the PD15 test) or were unable to discriminate colors. In contrast, two patients, one of them with the diagnosis of arCD (RCD102/6810, RCD92/6809), had normal color vision with the color vision tests we used. Signs of retinal dystrophy such as pigment clumping and atrophy of RPE or choroid were present in almost all patients. All patients had a central scotoma. Peripheral visual field of patients with arCD was normal, whereas about half of the patients with arCRD had constricted visual fields. Two patients (RCD63/3402, RCD143/8805), both affected by arCRD, had extensive central and peripheral visual field defects with only islands of vision remaining. Patients affected by arCD had abnormal cone ERGs, and patients affected by arCRD had both abnormal rod and cone ERGs. Cone ERGs were not detectable in two patients (ZD289/781, RCD143/8805) and in another two patients (RCD185/11721, RCD147/9136) both rod and cone ERGs were abolished. Patients diagnosed with arCRD normally presented with both cone and rod photoreceptor dysfunction at their first visit. But in one patient (ZD289/781/F) who had a diagnosis of arCD even after 16 years of disease duration, a late occurring rod dysfunction was finally observed, resulting in the change of the diagnosis to arCRD.
Table 3.
Clinical data of arCD and arCRD patients with ABCA4 gene mutations.
| Family ID/ Patient ID/ Gender |
Diagnosis | Onset age |
Dis. dur. |
BCVA (OD/OS) |
Color vision |
Glare sensitivity |
Night vision |
RPE atrophy/ Choroidal atrophy/ RPE clumping |
Additional fundus findings |
Visual Field 1 | Rod GF-ERG |
Cone GF-ERG |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCD92/ 6809/ F |
CRD | 8 | 16 | 0.05 | PD15 sat: normal |
n.i. | Normal | M | X | C | central scotoma | abnormal | abnormal | |||
| 0.05 | P | P | slight concentric narrowing | |||||||||||||
| RCD79/ 6184/ M |
CRD | 5 | 27 | 0.05 | PD15 sat: chaotic |
increased | Normal | M | X | C | central scotoma | abnormal | abnormal | |||
| 0.05 | P | X | white dots | P | slight concentric narrowing | |||||||||||
| ZD289/ 781/ F |
CRD | 10 | 31 | 0.02 | PD15 sat: red-green defect |
increased | abnormal | M | X | X | X | C | n.i. | severely reduced | extinguished | |
| 0.02 | PD15 sat: scotopic defect |
P | X | chorioidal sclerosis | P | n.i. | ||||||||||
| RCD157/ 10492/ F |
CRD | 14 | 32 | 0.05 | PD15 desat: unable to discriminate colors |
increased | abnormal | M | X | X | X | C | central scotoma | abnormal | abnormal | |
| HM | P | P | concentric narrowing | |||||||||||||
| RCD51/ 4984/ F |
CRD | 2 | 20 | 0.05 | PD15 desat: unable to discriminate colors |
n.i. | n.i. | M | X | C | central scotoma | abnormal | abnormal | |||
| 0.05 | P | X | P | concentric narrowing | ||||||||||||
| RCD9/ 1989/ M |
CRD | 10 | 18 | 0.02 | PD15 sat: red-green defect |
increased | abnormal | M | X | X | X | C | central scotoma | amplitudes abnormal | abnormal | |
| 0.05 | P | X | X | P | normal | latencies normal | ||||||||||
| RCD42/ 2509/ M |
CRD | 13 | 44 | 0.02 | PD15 desat: unable to discriminate colors |
n.i. | n.i. | M | X | X | X | C | central scotoma | |||
| 0.05 | P | X | X | X | P | concentric narrowing | ||||||||||
| RCD94/ 5781/ F |
CD | 10 | 10 | 0.02 | PD15 sat: red-green defect |
increased | Normal | M | X | C | normal | abnormal | ||||
| 0.07 | P | X | P | normal | ||||||||||||
| ZD85/ 5862/ F |
CD | 6 | 8 | 0.05 | PD15 sat: red-green defect |
Normal | Normal | M | X | C | central scotoma | normal | abnormal | |||
| 0.1 | P | X | P | n.i. | ||||||||||||
| RCD147/ 9136/ F |
CRD | 13 | 24 | HM | subjectively abnormal |
increased | abnormal | M | X | X | X | bone spicules | widely extinguished visual | extinguished | extinguished | |
| C/ |
field with remaining central | |||||||||||||||
| 0.015 | P | X | X | X | bone spicules | P | and ring-shaped peripheral island | |||||||||
| RCD141/ 8594/ M |
CRD | 7 | 32 | 0.02 | subjectively abnormal |
increased | abnormal | M | X | X | X | C | n.i. | abnormal | abnormal | |
| 0.02 | P | X | X | bone spicules | P | n.i. | ||||||||||
| RCD63/ 3402/ M |
CRD | 12 | 43 | CF | n.i. | Normal | abnormal | M | X | X | X | widely extinguished visual | abnormal | abnormal | ||
| C/ |
field with remaining | |||||||||||||||
| CF | P | X | X | X | severe panretinal | P | peripheral island | |||||||||
| RCD143/ 8805/ M |
CRD | 9 | 34 | 0.02 | PD15 desat: unable to discriminate colors |
increased | abnormal | M | X | X | X | widely extinguished visual | abnormal | extinquished | ||
| C/ |
field with remaining central | |||||||||||||||
| LP | P | bone spicules | P | and sickle-shaped peripheral island | ||||||||||||
| ZD146/ 10313/ F |
CD | 18 | 0.5 | 0.6 | n.i. | increased | Normal | M | X | C | central scotoma | n.i. | abnormal | |||
| DD: CRD |
0.6 | P | P | n.i. | ||||||||||||
| RCD98/ 3474/ F |
CRD | 6 | 23 | 0.02 | PD15 sat: red-green defect |
increased | abnormal | M | X | X | C | central scotoma | abnormal | abnormal | ||
| 0.03 | P | X | X | P | concentric narrowing | |||||||||||
| RCD194/ 10840/ M |
CRD | 27 | 2 | 0.6 | PD15 desat: minor unspecific confusions |
slightly increased | Normal | M | fundus flavimaculatus | C | central scotoma | abnormal | abnormal | |||
| 0.6 | P | P | normal | |||||||||||||
| RCD102/ 6810/ M |
CD | 7 | 23 | 0.1 | PD15 sat: normal |
Normal | Normal | M | X | X | X | C | central scotoma | normal | abnormal | |
| 0.1 | P | X | P | normal | ||||||||||||
| RCD113/ 3668/ F |
CRD | 7 | 1 | 0.03 | Arden: red-green and blue-yellow defect |
increased | n.i. | M | X | X | central tapetoid C reflex | central scotoma | abnormal | abnormal | ||
| 0.03 | P | X | X | X | P | concentric narrowing | ||||||||||
| ZD124/ 8019/ F |
CRD | 6 | 13 | 0.02 | PD15 sat: red-green defect |
increased | abnormal | M | X | X | C | central scotoma | abnormal | abnormal | ||
| 0.02 | P | P | concentric narrowing | |||||||||||||
| RCD185/ 11721/ F |
CRD | 5 | 13 | 0.1 | subjectively normal | Normal | Normal | M | X | C | n.i. | extinguished | extinguished | |||
| 0.1 | P | X | X | X | P | concentric narrowing | ||||||||||
Discussion
Prevalence of ABCA4 gene mutations in arCRD and arCD
In this study we evaluated the prevalence and spectrum of ABCA4 gene mutations in 64 patients affected by arCD and arCRD, using a microarray-based approach and subsequent “all-exon-sequencing”. To our knowledge this is the largest number of arCD and arCRD patients assessed by a microarray-based ABCA4 mutation screening and the first time that ABCA4 gene mutations have been identified to cause arCD.
The reported prevalence of ABCA4 gene mutations in patients affected by arCRD varies among different studies, ranging from one fourth to almost two thirds of the studied populations 11,12,14,25–27. A recent investigation which combined previously published data with their own data calculated that ABCA4 gene mutations are found in 40% of the arCRD patients 13. Our findings are consistent with previous reports as we identified ABCA4 gene mutations in 31% of our cohort. The different prevalences observed between studies might be attributed to ascertainment bias due to the relatively small sample sizes in previous reports, ranging from 8 to 54 patients. The careful ascertainment of patients is also an important issue for exact determination of ABCA4 gene mutation prevalence in arCRD, since inclusion of patients with Stargardt disease, a phenotype with a relatively high ABCA4 gene mutation prevalence of 58 to 75 % 23,28, or arRP with an ABCA4 gene mutation prevalence of around 5%13, will bias the result.
In the current study we performed the “all-exon-sequencing” only in selected patients who already had one identified mutant allele after microarray analysis. Thus, the actual prevalence of ABCA4 mutations in patients with arCD and arCRD could be higher. We also cannot exclude that we might have missed genomic rearrangement mutations based on the applied methods. Taken together, the ABCR400 microarray is rapid, cost-effective and useful both in genetic diagnostics and research as a first screening tool.
The c.5461-10T>C mutation was the most common sequence variant in our patient sample, present both in patients with adCD and adCRD. It has been questioned whether this sequence variant is pathogenic on its own because heterologous expression of this mutation failed to show a splicing defect 23. Therefore it has been suggested that the c.5461-10T>C is in linkage disequilibrium with another yet unknown pathogenic mutation. We found that 6 of 8 patients carrying the c.5461-10T>C variant were also heterozygous for known pathogenic ABCA4 mutations, a much higher number than expected by chance.
Grading of ABCA4 mutations
A model which correlates phenotypes of retinal diseases with the residual activity of the mutant ABCA4 protein has been suggested 10. Depending on a frequent founder mutation, c.2588G>C, which has been assumed to be mild, a model has been proposed that categorises mutations as null, moderately severe, or mild. A combination of two null mutations should lead to retinitis pigmentosa, whereas a combination of a null and a moderately severe mutation should cause CRD. Our data, however, do not support such a clear cut genotype-phenotype correlation. For example, two of our arCRD patients (RCD79/6184 and RCD113/3668) are homozygous for putative null alleles (p.Y245X and p.V1973X). Another arCRD patient (RCD9/1989) harbours two mutations (p.P1380L and p.R2077W) which are both characterised by substantially impaired ATP-binding 29. A fourth arCRD patient (RCD92/6809) is homozygous for the missense mutation p.L244P. Interestingly, a patient who is also a homozygote for p.L244P, but suffers from Stargardt disease has been described in the literature 28. Moreover, there is no higher proportion of nonsense or truncating mutations in our sample of CRD and CD patients compared with that of a cohort comprised of Stargardt patients only 23. Yet, we noted that certain mutations which are highly prevalent in Stargardt patients (recruited from a comparable population 23), for example, the c.5882G>A, the c.3113C>T, and c.2588G>C mutations were rare in our sample; in contrast, the c.5461-10T>C variant is less common in Stargardt patients. Thus, although the model is attractive, it may not accurately predict the expression of the retinal disease. It has become clear in recent years that not only complex but also Mendelian diseases are shaped by modifying genetic or environmental factors 30,31. A model which includes the effect of modifying factors may increase understanding of the connection between genotype (at potentially various genetic loci), environment, and phenotype.
In conclusion, we performed mutation analyses and provided data on the prevalence of ABCA4 gene mutations in patients with arCD and arCRD. Our data confirm and extend previous investigations that identified the ABCA4 gene as the major locus responsible for autosomal recessive retinal dystrophies, including arCD and arCRD.
Acknowledgments
This work has been supported by grants of the German Research Council (KFO134 - Ko2176/1-1 and JA997/8-1), the EU (EVI-Genoret: LSHG-CT-2005-512036), NIH/NEI (EY13203), Tistou and Charlotte Kerstan Foundation, and the Macula Vision Research Foundation.
References
- 1.Borst P, Zelcer N, van Helvoort A. ABC transporters in lipid transport. Biochim Biophys Acta. 2000;1486:128–144. doi: 10.1016/s1388-1981(00)00053-6. [DOI] [PubMed] [Google Scholar]
- 2.Molday LL, Rabin AR, Molday RS. ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat Genet. 2000;25:257–258. doi: 10.1038/77004. [DOI] [PubMed] [Google Scholar]
- 3.Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 4.Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 5.Rozet JM, Gerber S, Souied E, et al. Spectrum of ABCR gene mutations in autosomal recessive macular dystrophies. Eur J Hum Genet. 1998;6:291–295. doi: 10.1038/sj.ejhg.5200221. [DOI] [PubMed] [Google Scholar]
- 6.Cremers FP, van de Pol DJ, van Driel M, et al. Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt’s disease gene ABCR. Hum Mol Genet. 1998;7:355–362. doi: 10.1093/hmg/7.3.355. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Mir A, Paloma E, Allikmets R, et al. Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet. 1998;18:11–12. doi: 10.1038/ng0198-11. [DOI] [PubMed] [Google Scholar]
- 8.Rudolph G, Kalpadakis P, Haritoglou C, Rivera A, Weber BH. Mutations in the ABCA4 gene in a family with Stargardt’s disease and retinitis pigmentosa (STGD1/RP19) Klin Monatsbl Augenheilkd. 2002;219:590–596. doi: 10.1055/s-2002-34425. [DOI] [PubMed] [Google Scholar]
- 9.Allikmets R, Shroyer NF, Singh N, et al. Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science. 1997;277:1805–1807. doi: 10.1126/science.277.5333.1805. [DOI] [PubMed] [Google Scholar]
- 10.Maugeri A, van Driel MA, van de Pol DJ, et al. The 2588G-->C mutation in the ABCR gene is a mild frequent founder mutation in the Western European population and allows the classification of ABCR mutations in patients with Stargardt disease. Am J Hum Genet. 1999;64:1024–1035. doi: 10.1086/302323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ducroq D, Rozet JM, Gerber S, et al. The ABCA4 gene in autosomal recessive cone-rod dystrophies. Am J Hum Genet. 2002;71:1480–1482. doi: 10.1086/344829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fishman GA, Stone EM, Eliason DA, Taylor CM, Lindeman M, Derlacki DJ. ABCA4 gene sequence variations in patients with autosomal recessive cone-rod dystrophy. Arch Ophthalmol. 2003;121:851–855. doi: 10.1001/archopht.121.6.851. [DOI] [PubMed] [Google Scholar]
- 13.Klevering BJ, Yzer S, Rohrschneider K, et al. Microarray-based mutation analysis of the ABCA4 (ABCR) gene in autosomal recessive cone-rod dystrophy and retinitis pigmentosa. Eur J Hum Genet. 2004;12:1024–1032. doi: 10.1038/sj.ejhg.5201258. [DOI] [PubMed] [Google Scholar]
- 14.Maugeri A, Klevering BJ, Rohrschneider K, et al. Mutations in the ABCA4 (ABCR) gene are the major cause of autosomal recessive cone-rod dystrophy. Am J Hum Genet. 2000;67:960–966. doi: 10.1086/303079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaakson K, Zernant J, Kulm M, et al. Genotyping microarray (gene chip) for the ABCR (ABCA4) gene. Hum Mutat. 2003;22:395–403. doi: 10.1002/humu.10263. [DOI] [PubMed] [Google Scholar]
- 16.Hamel C. Cone rod dystrophies. Orphanet Journal of Rare Diseases. 2007;2:7. doi: 10.1186/1750-1172-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szlyk JP, Fishman GA, Alexander KR, Peachey NS, Derlacki DJ. Clinical subtypes of cone-rod dystrophy. Arch Ophthalmol. 1993;111:781–788. doi: 10.1001/archopht.1993.01090060069025. [DOI] [PubMed] [Google Scholar]
- 18.Yagasaki K, Jacobson SG. Cone-rod dystrophy. Phenotypic diversity by retinal function testing. Arch Ophthalmol. 1989;107:701–708. doi: 10.1001/archopht.1989.01070010719034. [DOI] [PubMed] [Google Scholar]
- 19.Kellner U, Wissinger B, Tippmann S, Kohl S, Kraus H, Foerster MH. Blue cone monochromatism: clinical findings in patients with mutations in the red/green opsin gene cluster. Graefes Arch Clin Exp Ophthalmol. 2004;242:729–735. doi: 10.1007/s00417-004-0921-z. [DOI] [PubMed] [Google Scholar]
- 20.Bach M, Kommerell G. Determining visual acuity using European normal values: scientific principles and possibilities for automatic measurement. Klin Monatsbl Augenheilkd. 1998;212:190–195. doi: 10.1055/s-2008-1034863. [DOI] [PubMed] [Google Scholar]
- 21.Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47:1236–1240. doi: 10.1167/iovs.05-0981. [DOI] [PubMed] [Google Scholar]
- 22.Paunescu K, Preising MN, Janke B, Wissinger B, Lorenz B. Genotype-phenotype correlation in a German family with a novel complex CRX mutation extending the open reading frame. Ophthalmology. 2007;114:1348–1357. doi: 10.1016/j.ophtha.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Rivera A, White K, Stohr H, et al. A comprehensive survey of sequence variation in the ABCA4 (ABCR) gene in Stargardt disease and age-related macular degeneration. Am J Hum Genet. 2000;67:800–813. doi: 10.1086/303090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shroyer NF, Lewis RA, Yatsenko AN, Lupski JR. Null missense ABCR (ABCA4) mutations in a family with stargardt disease and retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2001;42:2757–2761. [PubMed] [Google Scholar]
- 25.Papaioannou M, Ocaka L, Bessant D, et al. An analysis of ABCR mutations in British patients with recessive retinal dystrophies. Invest Ophthalmol Vis Sci. 2000;41:16–19. [PubMed] [Google Scholar]
- 26.Birch DG, Peters AY, Locke KL, Spencer R, Megarity CF, Travis GH. Visual function in patients with cone-rod dystrophy (CRD) associated with mutations in the ABCA4(ABCR) gene. Exp Eye Res. 2001;73:877–886. doi: 10.1006/exer.2001.1093. [DOI] [PubMed] [Google Scholar]
- 27.Valverde D, Riveiro-Alvarez R, Aguirre-Lamban J, et al. Spectrum of the ABCA4 gene mutations implicated in severe retinopathies in Spanish patients. Invest Ophthalmol Vis Sci. 2007;48:985–990. doi: 10.1167/iovs.06-0307. [DOI] [PubMed] [Google Scholar]
- 28.Briggs CE, Rucinski D, Rosenfeld PJ, Hirose T, Berson EL, Dryja TP. Mutations in ABCR (ABCA4) in patients with Stargardt macular degeneration or cone-rod degeneration. Invest Ophthalmol Vis Sci. 2001;42:2229–2236. [PubMed] [Google Scholar]
- 29.Sun H, Nathans J. ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all-trans-retinal-mediated photooxidative damage in vitro. Implications for retinal disease. J Biol Chem. 2001;276:11766–11774. doi: 10.1074/jbc.M010152200. [DOI] [PubMed] [Google Scholar]
- 30.Nadeau JH. Modifier genes in mice and humans. Nat Rev Genet. 2001;2:165–174. doi: 10.1038/35056009. [DOI] [PubMed] [Google Scholar]
- 31.Weatherall DJ. Phenotype-genotype relationships in monogenic disease: lessons from the thalassaemias. Nat Rev Genet. 2001;2:245–255. doi: 10.1038/35066048. [DOI] [PubMed] [Google Scholar]