Abstract
Background
Alcohol consumption is a common problem in HIV-infected individuals, and the effects of alcohol may alter the efficiency of the immune response, potentially aggravating the disease as well as affecting end organs, such as the brain. However, the elements of the virus-host interaction that are modulated by ethanol are poorly dissected.
Methods
Ethanol intake was conditioned in rhesus macaques prior to SIV infection, in order to mimic this common human behavior, and allow the evaluation of aspects of the virus-immune system interactions during acute time-points, when important facets of the infection are set up and when virus reproducibly enters the brain.
Results
Although ethanol had a limited effect on the acute plasma viral load, it resulted in reduced circulating memory CD4+ T cells and increased levels of monocytes expressing the viral coreceptor CCR5. In organs, ethanol consumption impacted immune cells in the liver as well as lymphoid and other nonlymphoid tissues, where CD4+ T cells were predominantly affected.
Conclusion
Overall, the consumption of alcohol causes immune cell alterations that can contribute to the generation of a disease susceptible environment upon SIV infection.
Introduction
Alcohol dependence is the most common form of drug abuse. About 7% of the adult population in United States meets the criteria for alcohol abuse and/or alcoholism (Grant, 1994), composing a population more likely to engage in risk behaviors that expose them to the possibility of HIV infection. Although alcohol abuse and HIV infection frequently occur in parallel, studies on the impact of alcohol consumption on the HIV / host interaction are sparse.
SIV infection in rhesus macaques replicates the human infection by HIV, producing similar symptoms and progressing to AIDS in infected animals. The SIV rhesus monkey model has previously been used to study the effects of alcohol on AIDS in two distinct systems, revealing possible interactions of SIV and alcohol. In the first, animals were given alcohol in a binge pattern (initial dose of 2.5 g/kg, followed by an additional 0.8 g/kg, over a four-hour period, 4 days a week) through an intragastric catheter, control animals received sucrose (Bagby et al., 2003; Bagby et al., 2006; Molina et al., 2008; Molina et al., 2006; Poonia et al., 2006; Winsauer et al., 2002). Following SIV inoculation, analysis of plasma revealed inconsistency in the time points where viral load differed between control and alcohol administered animals. Alcohol also induced changes in numbers or proportions of blood CD4 or CD8 T cells. Interestingly alcohol itself altered intestinal lymphocytes, inducing a higher proportion of central memory as well as naïve CD4 cells, and lower proportion of effector memory CD8 cells (Poonia et al., 2006). Although in one study alcohol accelerated the SIV-induced disease course (Bagby et al., 2006), this was not replicated in a subsequent experiment (Molina et al., 2008). Wasting disease is common as disease progresses in SIV-infected rhesus monkeys, and alcohol was found to significantly increase this disorder (Molina et al., 2008).
In a second model, animals drank alcohol provided in a sweetened solution (Kumar et al., 2005). Animals ingested 1–4 g/kg of alcohol over a daily three-hour period, and control animals drank a sweetened solution without alcohol. Animals were then inoculated with a mixture of SIV and SHIV viruses, which is known to lead to rapid CD4 T cell depletion. Beginning at 12 weeks p.i. in the plasma, and 10 weeks p.i. in the CSF, higher levels of virus were found in the alcohol-drinking group.
The central nervous system (CNS) is a target of both alcohol and HIV. Although the brain was not examined in either of the above models, the increase virus found in the CSF in the latter study indicates that there may be an interaction of alcohol with SIV. In addition, in the binge drinking model, behavioral testing indicated that the combination of SIV and alcohol resulted in cognitive deficits not found with either agent alone (Winsauer et al., 2002).
After infection with HIV or SIV, virus enters the brain early, in the first two weeks following inoculation. This early stage is a reproducible time in which virus enters in the CNS. Furthermore, events that occur in the acute phase of infection significantly affect the course of disease in both HIV infected people and SIV infected monkeys. Therefore, to study whether alcohol consumption affects this early phase, we developed a flavorant-fade protocol for oral self-administration of alcohol in rhesus monkeys. While determining the exact relationships of experimental animal studies to human alcohol consumption patterns is problematic, this method led to animals reaching a stable ad libitum daily intake of 2.7 ± 0.2 g/kg/day, sufficient to cause blood alcohol levels greater than the legal driving limit of 80 mg/dl (up to 269 mg/dl at the high end of the consumption range) and abnormalities in a motor skills task (Katner et al., 2004), modeling alcohol drinking that may lead to high-risk behavior. In the present study we analyzed the impact of this chronic alcohol consumption on viral and immune parameters during the acute phase of SIV, examining blood, CSF, lymphoid organs, the liver and brain.
Material and Methods
Animals and Infection
SIV, SRV-type D and Herpes B Virus-free rhesus macaques purchased from Labs of Virginia and Charles River Laboratory, were infected with a cell-free SIV stock derived from SIVmac251 (Burdo et al., 2005; Lane et al., 1995). All animal experiments were performed with approval from the Institutional Animal Care and Use Committee following NIH guidelines. Animals kept in containment were anesthetized with 10–15 mg/kg of ketamine intramuscularly prior to experimental procedures. Blood was serially drawn from the femoral vein, and plasma was obtained from EDTA-treated blood. At necropsy, performed after terminal anesthesia, animals were intracardially perfused with sterile PBS containing 1 U/ml heparin. Tissue samples were taken for cell isolation, virus quantification, and formalin fixation for histology.
Oral ethanol self-administration
Access to a palatable solution of 6% (w/v) ethanol / 6% (w/v) Tang® Kraft Foods Inc., orange flavored), limited to 3.0 g/kg, was offered in two 1 hr daily sessions. Drinking water was removed from the cage for 1 hr prior to, and was restored immediately after, each ethanol session. Oral ethanol self-administration was originally conditioned in these animals by a gradual increase in the concentration (%) and/or amount (g/kg/session) of ethanol in the Tang solution as previously described (Katner et al., 2004; Katner et al., 2007). For this current study, as opposed to once a day, animals were offered ethanol solutions twice daily for the 30 days preceding inoculation and then throughout SIV infection. Control animals were treated in parallel, receiving only vehicle.
Viral Quantitation
Plasma, liver, spleen and brain SIV RNA was calculated by using quantitative branched DNA (bDNA) signal amplification assay performed by Siemens Clinical Laboratory (Emeryville, CA).
Cells
Cells separated from EDTA-treated blood centrifugation, and 70̣µm nylon-mesh sieved cells from spleen, lymph nodes, and liver, were subjected to Histopaque (Sigma-Aldrich, St. Louis, MO) gradient for isolation of the mononuclear fraction. Gut cells were obtained from mid jejunum fragments, incubated with 1 mM EDTA in saline, followed by calcium-free HBSS containing 1% FBS, 1 mM EGTA, 1.5 mM MgCl2. The epithelial layer was carefully removed before incubation with 100 U/ml collagenase type IV (Sigma-Aldrich, St. Louis, MO) and 5U/ml DNAseI (Sigma-Aldrich). After digestion, gut fragments were pressed against a 40 m□□r□□ nylon sieve to generate a tissue-free cell suspension. This was resuspended to 1.033 g/ml Percoll (Sigma-Aldrich) and underlayed by a 1.088 g/ml Percoll solution, for gradient centrifugation. Cells harvested from the interface were washed and stained.
Brain cells
Following perfusion, the brain was removed and carefully freed of meninges. Brain immune cells were isolated by enzymatic digestion of minced tissue, followed by Percoll gradient, as previous described (Marcondes et al., 2001).
Flow cytometry
Isolated cells were stained with labeled antibodies in PBS containing 2% FCS and 0.01% NaN3. The antibodies used were anti-CD14-PE (clone M5E2, BD Pharmingen), anti-CD16-FITC (clone 3G8, BD Pharmingen), anti-monkey CD3-biotin (clone FN-18, Invitrogen Biosource, Carlsbad, CA) followed by Streptavidin-PerCP or APC (BD Pharmingen, San Diego, CA), anti-human CD8-PE, FITC or PeCy5 (clone DK25, Dako, Carpinteria, CA), anti-CD95-FITC (Dako), anti-human CCR5-APC (BD Pharmingen), anti-CCR2-PE (BD Pharmingen), anti-CD44v6-biotin (clone 2F10, Zymed, San Francisco, CA), anti-CD11a-FITC (clone 25.3.1, Immunotech), anti- CD69-PE (clone FN50, BD Pharmingen), anti-CD95-FITC (clone DX2, BD Pharmingen), or isotype controls (BD Pharmingen). Additionally, PE-labeled Tat and Gag-tetramers (Beckman-Coulter, Fullerton, CA) were employed for specific CD8 cells detection. (Burudi et al., 2002). Stained cells were acquired by a FACSCalibur (BD Biosciences, San Jose, CA) flow cytometer, and analyzed in FlowJo 6.2.1 software (Tree Star Inc., San Carlos, CA).
Quantitative real-time PCR
RNA was isolated, purified, quantified, and used as a template for cDNA as previously described (Burudi et al., 2002), with the addition of a phenol extraction and Qiagen RNeasy column purification for the liver RNA. Real-time PCR was performed using gene-specific primers and probes (Table 1). A dilutional analysis of the samples indicated a correlation coefficient for concentration and cycle threshold (Ct) of 0.99. Reactions were performed on a MX3000 (Stratagene) using Supermix UDG (Invitrogen Life Technologies). The delta Ct (dCt) method was performed to determine relative concentrations, using the average of the Ct of 18S, GAPDH, and TATA box-binding protein (TBP) as the normalizing value for calculation of relative values (2dCT).
Table 1.
Quantitative Real Time PCR primer/probe sequences. F – forward primer, R – reverse primer P – dual-labeled probe.
| Product | Template | Sequence |
|---|---|---|
| IFN alpha | IFNa F | GCCTGAAGGACAGACATGACTTT |
| IFNa R | GGATGGTTTGAGCCTTTTGG | |
| IFNa P | CCCCAGGAGGAGTTTGGCAACCA | |
| IFN beta | IFNb F | TGCCTCAAGGACAGGATGAAC |
| IFNb R | GCGTCCTCCTTCTGGAACTG | |
| IFNb P | CATCCCTGAGGAAATTAAGCAGCCGC | |
| IL6 | IL6 F | TGGCTGAAAAAGATGGATGCT |
| IL6 R | TTGCTCCTCACTACTCTCAAACCT | |
| IL6 P | TGATTTTCACCAGGCAAGTGTCCTCATTG | |
| CCL2 | CCL2 F | CAGCAGCAAGTGTCCCAAAAG |
| CCL2 R | TGTCCAGGTGGTCCATGGA | |
| CCL2 P | CTGTGCTGACCCCAAGCAGAAGTGG | |
| 18S | 18S F | CGGCTACCACATCCAAGGAA |
| 18S R | GCTGGAATTACCGCGGCT | |
| 18S P | TGCTGGCACCAGACTTGCCCTC | |
| TBP | TBP F | AAAGACCATTGCACTTCGTG |
| TBP R | GGTTCGTGGCTCTCTTATCC | |
| TBP P | TCCCAAGCGGTTTGCTGCAG | |
| GAPDH | GAPDH F | GCACCACCAACTGCTTAGCAC |
| GAPDH R | TCTTCTGGGTGGCAGTGATG | |
| GAPDH P | TCGTGGAAGGACTCATGACCACAGTCC | |
Statistics
Group comparisons were performed using the tests described in the text and figure legends. The difference between the means was considered significant at p < 0.05. Tests were performed using Excel (Microsoft Corporation, Redmond, WA) and Prism software (GraphPad Software, San Diego, CA) for Macintosh.
Results
Acute viral load and alcohol consumption
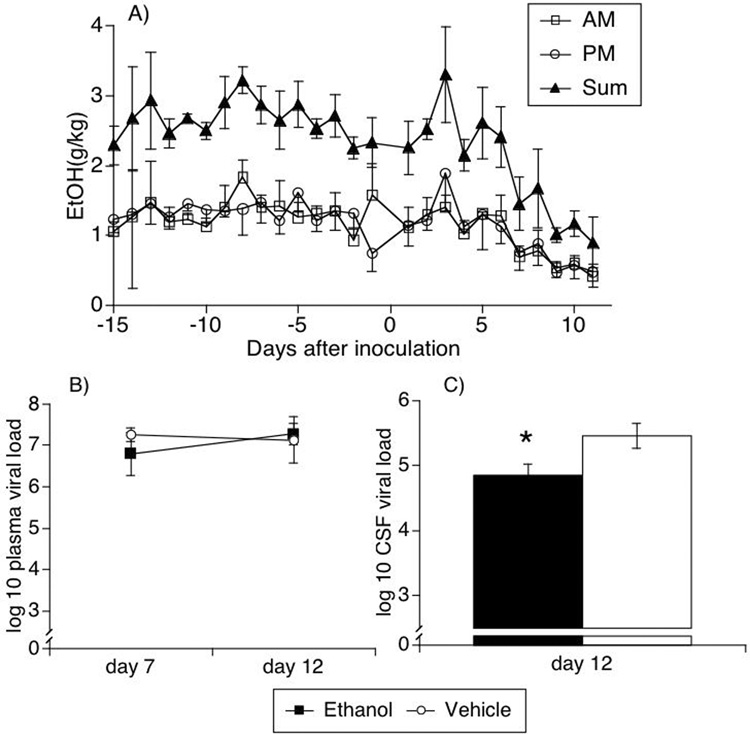
Two groups of animals were used. One group of 4 animals was ingesting alcohol, reaching a stable ad libitum daily consumption of 2.88 ± 0.62 g/kg/day. Average ethanol consumption was equivalent in each of the AM and PM drinking sessions (Figure 1A). The second group was the control group, also of 4 monkeys, which received vehicle during the same period. Both groups were intravenously inoculated with a derivative of the pathogenic stock SIVmac251, known to infect the CNS early after inoculation. In the alcohol-consuming group, the ethanol intake decreased during the second week following SIV inoculation (Figure 1A), in correlation with acute infection syndrome, which is characterized by malaise and fever. The animals were bled at day 7 and 12 post-inoculation, and sacrificed at day 12, with extensive perfusion with PBS to eliminate blood-borne cells from the organs.
Figure 1.
Alcohol intake (A) and viral load in the plasma (B) and CSF (C) of alcohol-treated and untreated SIV-infected rhesus macaques. Alcohol consumption was measured in two daily sessions as described. Results indicate the average ± SEM of 4 animals, in AM and PM sessions, as well as the sum of both sessions. Viral load was measured by bDNA in plasma collected on days 7 and 12, and in CSF collected at necropsy. Values represent the average ± SD of 4 animals in each group. * p=0.003, t test.
Two biofluids - blood and CSF - were sampled during the early post-inoculation period for determination of viral load. Alcohol consumption did not change the plasma viral load during the acute phase (Figure 1B). However, the viral load in the CSF was significantly (p=0.003, t test) lower in SIV plus ethanol animals than in SIV animals receiving the vehicle at day 12 p.i. (Figure 1C).
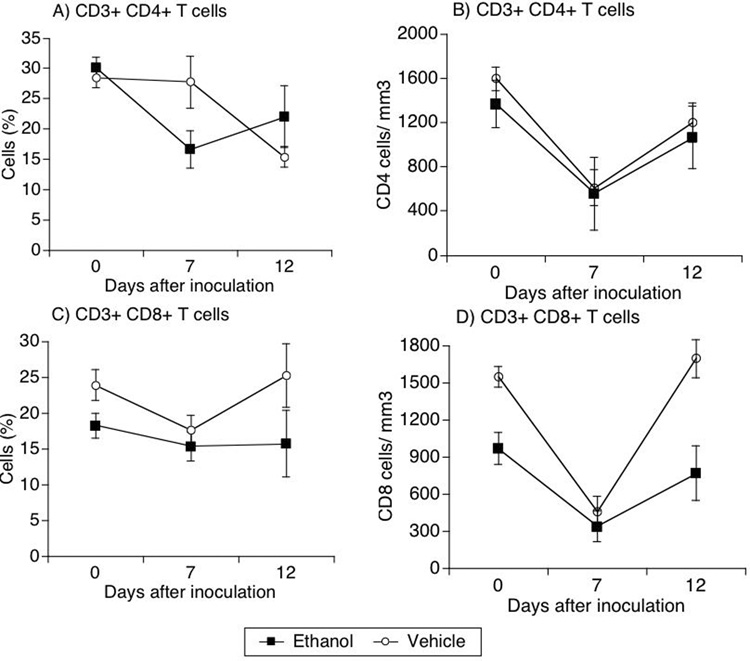
Longitudinal analysis of T cells and NK cells
We next evaluated the frequency of T cells in the blood of SIV-infected animals subjected or not to alcohol consumption. The frequency of CD4 cells in the blood significantly decreased in infected animals that consumed alcohol in comparison to the vehicle-consuming group at day 7 after viral inoculation (p=0.01, t-test) (Figure 2A). However, the absolute numbers of CD4 cells did not differ between groups (Figure 2 B). CD3+CD8+ T cell frequency was not different between the two groups or within each group compared to pre-infection levels (Figure 2C). The number of CD8 T cells in the blood of ethanol-treated animals was lower than vehicle-treated animals from the baseline (Figure 2D). Thus, both groups had an acute reduction on CD8 T cells at day 7 after infection, but at day 12 they returned to their respective baseline levels.
Figure 2.
Frequency and absolute numbers of T cells in the blood during acute SIV infection in alcohol-treated and untreated animals. Blood leukocytes were serially obtained from SIV-infected animals that received alcohol or vehicle. CD4 (A and B), and CD8 (C and D) T cells were identified in blood leukocytes by flow cytometry. Percentages (A and B) obtained upon analysis were applied on total lymphocyte numbers for determination of absolute numbers (C and D). Values represent average ± SD of 4 animals in each group. Comparison between groups was performed; * p=0.015.
The expression of phenotypic markers on CD4 and CD8+ T cells was also evaluated. The markers selected for this analysis correspond to surface molecules that correlate with activation and memory. However, following SIV infection, there was no difference in the expression of CD11a, CD69, CCR5 or CD95. We next investigated whether NK cells were affected by alcohol consumption during initial time-points after SIV inoculation. NK cells, assessed as CD3−CD8+ cells, did not differ between the two groups, either in relative or absolute values, and neither did NK subsets that expressed CD16 (comprising the majority of NK cells) nor the activated NK cells marked by CD69 (data not shown). Thus, alcohol consumption did not alter the frequency or subsets of NK cells at the early time-points of SIV infection.
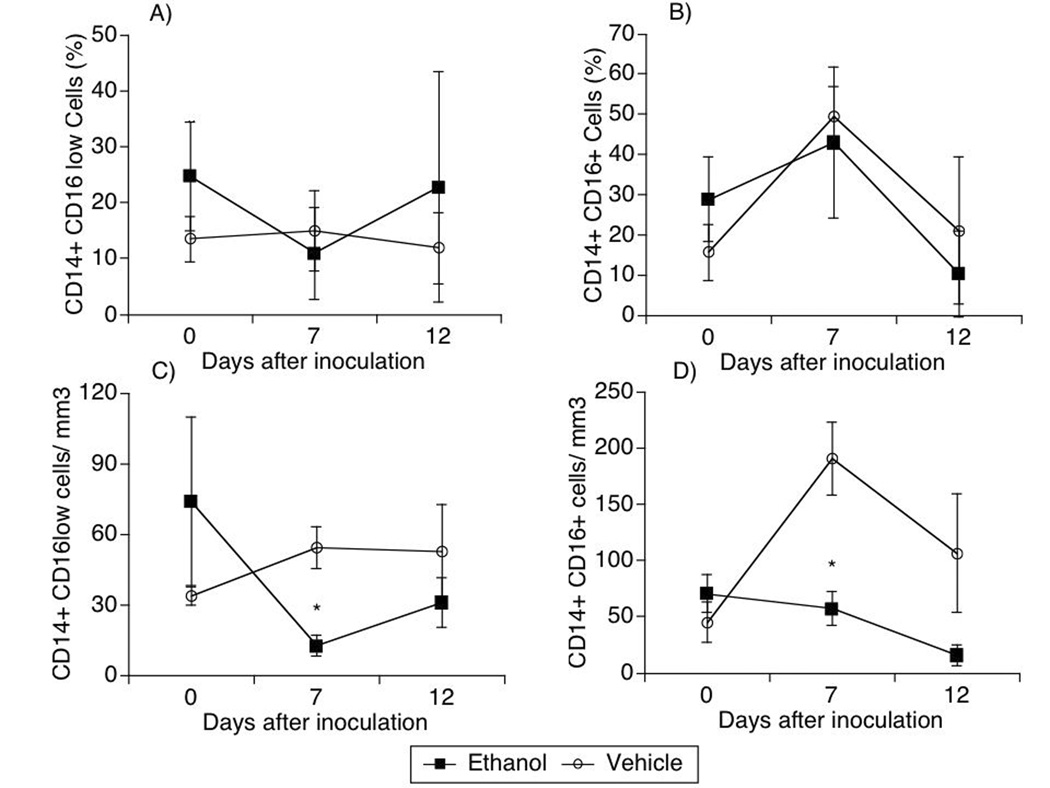
Longitudinal analysis of monocytes
To assess monocytes, cells expressing CD11b marker in the blood were gated in conjunction with forward and side scatter to identify monocytes, and were further characterized based on the expression of CD14 and CD16 markers. Although the monocyte subpopulations were not different in percentage between the groups (Figure 3A and Figure 3B), the absolute counts of these cells were distinctive following infection. Both CD14+ CD16low and CD14+CD16+ subpopulations were higher in animals that did not receive alcohol (open symbols), in comparison with animals that did (closed symbols, Figure 3) at day 7 after inoculation of the virus. CD14+ CD16low cells increased from baseline in animals that received vehicle, and decreased in animals that received alcohol (Figure 3C). Cells that were CD14+ CD16+ also increased at day 7 in vehicle-treated animals, and remained unchanged in the alcohol group (Figure 3D).
Figure 3.
Monocytic population kinetics during acute SIV infection in animals treated or not with ethanol. CD11b+ cells were further labeled with anti-CD14 and CD16 fluorescent antibodies and detected by flow cytometry. Populations were subdivided into CD14+ CD16low (A, C) and CD14+ CD16+ cells (B, D). The percentage (A, B) and the absolute numbers (C, D) were considered. Values represent the average ± SD of 4 animals in each group. *p<0.05, t test, between groups.
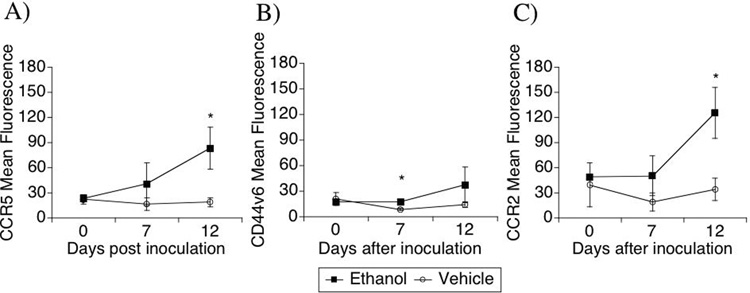
Considering that the CD16+ monocytes subset exhibits an inflammatory phenotype, which enables them to migrate into tissues (Kim et al., 2004), these cells were further analyzed for the expression of CCR5, CD44v6 and CCR2 (Figure 4). This analysis revealed that alcohol consumption affected the expression of surface molecules on these cells in response to SIV infection. For instance, the expression of the chemokine receptor and SIV/HIV coreceptor CCR5 was higher following SIV infection in the ethanol-consuming group, reaching statistical significance at day 12 after infection (Figure 4A). The expression of CD44v6, an adhesion molecule and one of the receptors for osteopontin, previously shown to correlate with brain-inflammatory phenotype, increased significantly on CD14+CD16+ cells (Figure 4B) in the alcohol-consuming group compared to the vehicle group at day 7 p.i. However at day 12, a high degree of variability was found in the alcohol-consuming group. Finally, CCR2 expression increased significantly on CD14+CD16+ cells after infection in animals treated with ethanol (Figure 4C) reaching statistical significance at day 12 p.i. Therefore, CD14+ CD16+ cells in animals treated with alcohol became more susceptible to the virus, and more prone to respond to osteopontin and chemokines, consistent with an increased inflammatory phenotype.
Figure 4.
Kinetics of surface markers on CD14+ CD16+ cells along acute SIV infection in animals treated or not with ethanol. The surface marker expression intensity was analyzed by flow cytometry on gated CD14+ CD16+ cells. The expression of CCR5 (A), CD44v6 (B) and CCR2 (C) were evaluated. Values represent the average ± SD of 4 animals in each group. *p<0.05; t test between groups.
Acute tissue distribution of SIV with alcohol consumption
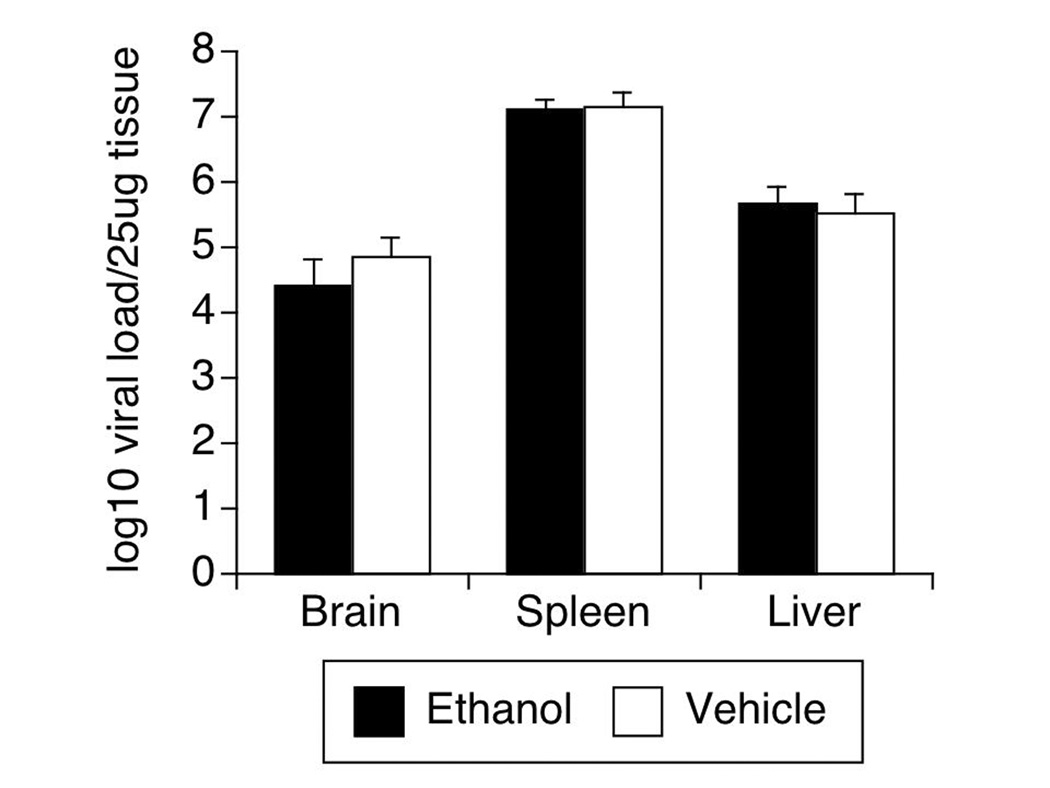
At 12 days after SIV was inoculated, the animals were sacrificed. In addition to obtaining the viral load in the plasma and CSF (Figure 1, above), RNA was isolated from the frontal lobe of the brain, the liver, and the spleen to investigate the effect of alcohol consumption on tissue No differences were found in tissue viral load between ethanol consuming and vehicle consuming groups (Figure 5).
Figure 5.
Tissue viral load in brain, spleen and liver of SIV-infected animals treated or not with ethanol. Virus was measured by bDNA on tissue samples. Values represent the average ± SD of 4 animals in each group.
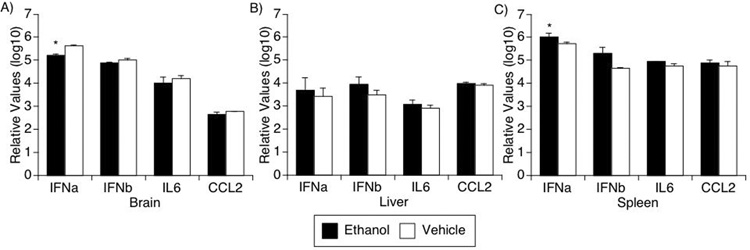
Tissue pro-inflammatory cytokine production
In the acute phase of infection, increased levels of interferon- and IL-6-induced genes are found in the brain (Katner et al., 2004). Furthermore, the chemokine CCL2 is expressed in the cerebrospinal fluid (Fox et al., 2000), correlating with the initial invasion of monocytes into the brain. We therefore examined the gene expression of IFNα, IFNβ, IL6, and CCL2 in the brain as well as the liver and spleen. In the brain, ethanol consumption decreased the level of expression of these molecules in SIV-infected animals (two-way ANOVA, p=0.0225) (Figure 6A), whereas in spleen (Figure 6C), the ethanol-consuming group had enhanced expression (two-way ANOVA, p=0.0029). In both organs, post-hoc Bonferroni’s testing revealed significant changes in IFNα̣p⪝01 for brain, p<0.05 for spleeṇ. Although higher levels of these cytokines were also found in the liver of the ethanol-consuming group (Figure 6B), these differences were not statistically significant.
Figure 6.
Pro-inflammatory cytokine gene expression. Type I IFNs: alpha(2) and beta, IL6 and CCL2 were detected using real-time PCR on RNA samples obtained from brain (A), liver (B) and spleen (C) of SIV-infected animals treated or not with ethanol. Values represent the average ± SD of 4 animals in each group. * p<0.05, two-way ANOVA.
Lymphocyte and NK distribution in different organs: alcohol effects on target organs
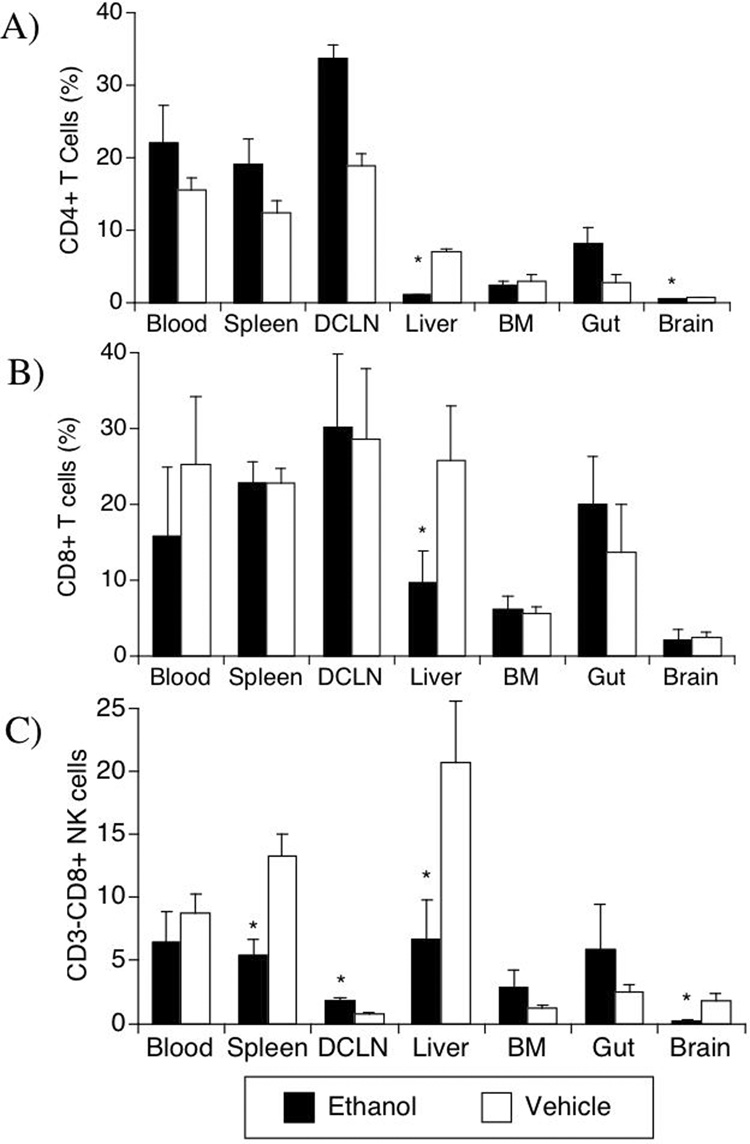
We next examined both the frequency of detection and surface marker expression on lymphocytes in a number of target organs during the acute phase (day 12) of infection. For the T cells, the CD4 cells were affected to a greater degree by alcohol treatment than CD8 cells following SIV infection (Figure 7). The SIV+EtOH group had a higher frequency of CD4 cells in deep cervical lymph nodes (p=0.001) than control SIV animals (Figure 7A). However, in the liver and in the brain, the frequency of CD4 cells was significantly lower in the SIV+EtOH group, comparatively to control SIV (p<0.001 and p=0.008, respectively). On the other hand, in the CD8 compartment a single difference was observed, a lower frequency of CD8 cells in the liver of alcohol treated monkeys (Figure 7B, p=0.004).
Figure 7.
Effect of ethanol on lymphocyte and NK cell distribution in different organs from SIV-infected animals. The percentage of CD3+CD8− CD4 T cells (A), CD3+CD8+ T cells (B), and CD3−CD8+ NK cells (C) were evaluated using flow cytometry, in cell suspensions obtained from blood, organs, deep cervical lymph nodes (DCLN) and bone marrow (BM). Values represent the average ± SD of 4 animals in each group. *p<0.05, t test.
The distribution of NK cells after SIV infection was also affected by ethanol (Figure 7C). A decreased percentage of NK cells in SIV+EtOH group was observed in the spleen (p=0.01), in the deep cervical lymph nodes (p=0.004), in the liver (p=0.043) and also in the brain (p=0.03). Thus following SIV infection, CD4 and CD8 T cells, as well as NK cells, were decreased in the liver in alcohol consuming animals relative to the vehicle consuming animals, whereas both CD4 and CD8 T cells were decreased in the brain.
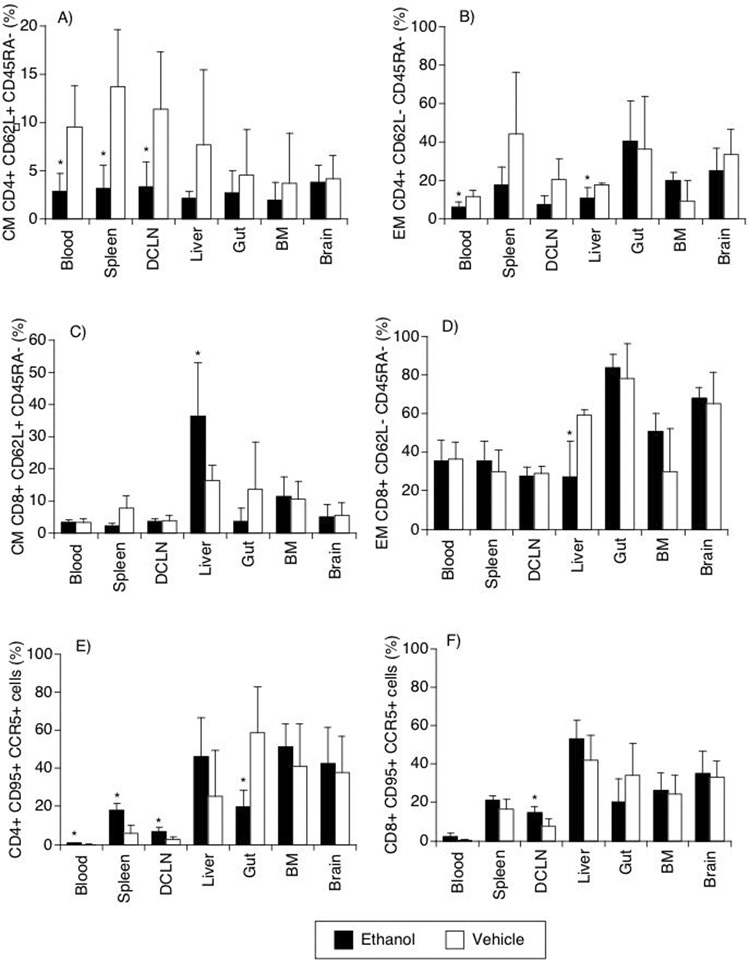
The surface markers CD62L and CD45RA can be used to separate naïve (CD62L±,CD45RA+), central memory (CM, CD62L+CD45RA−) and effector memory (EM, CD62L−CD45RA−) T cells. The blood, spleen and lymph nodes all had a significant lower frequency of CD4 T CM cells in alcohol-consuming animals (Figure 8A). The blood of alcohol-treated also had a lower CD4 T EM population (Figure 8B). In both CD4 and CD8 T cells, alcohol reduced the EM cells in the liver (Figure 8B and 8D).
Figure 8.
Central (CM) and Effector memory (EM) markers and activation markers on T cells is different organs. CM and EM were distinguished on CD4 (A, B) and CD8 (C, D), by the differential expression of CD62L and CD45RA (A, B, C, D), detected by flow cytometry. EM percentages were also checked by the detection of CD4 (E) and CD8 (F) T cells expressing simultaneously CD95 and CCR5 (E, F). Abbreviations are the same as Figure 7. Values represent the average ± SD of 4 animals in each group. *p<0.05, t test between groups.
The simultaneous analysis of CCR5 and CD95 expression allows the identification of cells which are frequent targets of the virus and exhibit memory phenotype. Within the CD4 compartment, cells expressing both CCR5 and CD95 were decreased in the gut of alcohol consuming animals in comparison to controls (p=0.024). Other sites, such as blood, lymph nodes and spleen had a higher proportion of CD4 cells expressing these markers (p=0.036, 0.021, and 0.005, respectively) (Figure 8E) in the alcohol group. In CD8 T cells CD95 and CCR5 expression was increased in cells from the lymph nodes of alcohol treated animals (p=0.023) (Figure 8F). This confirms that CD4 cells suffer the impact of alcohol consumption in a deeper way and in broader range of sites in the acute infection.
Myelomonocytic phagocytes in different organs
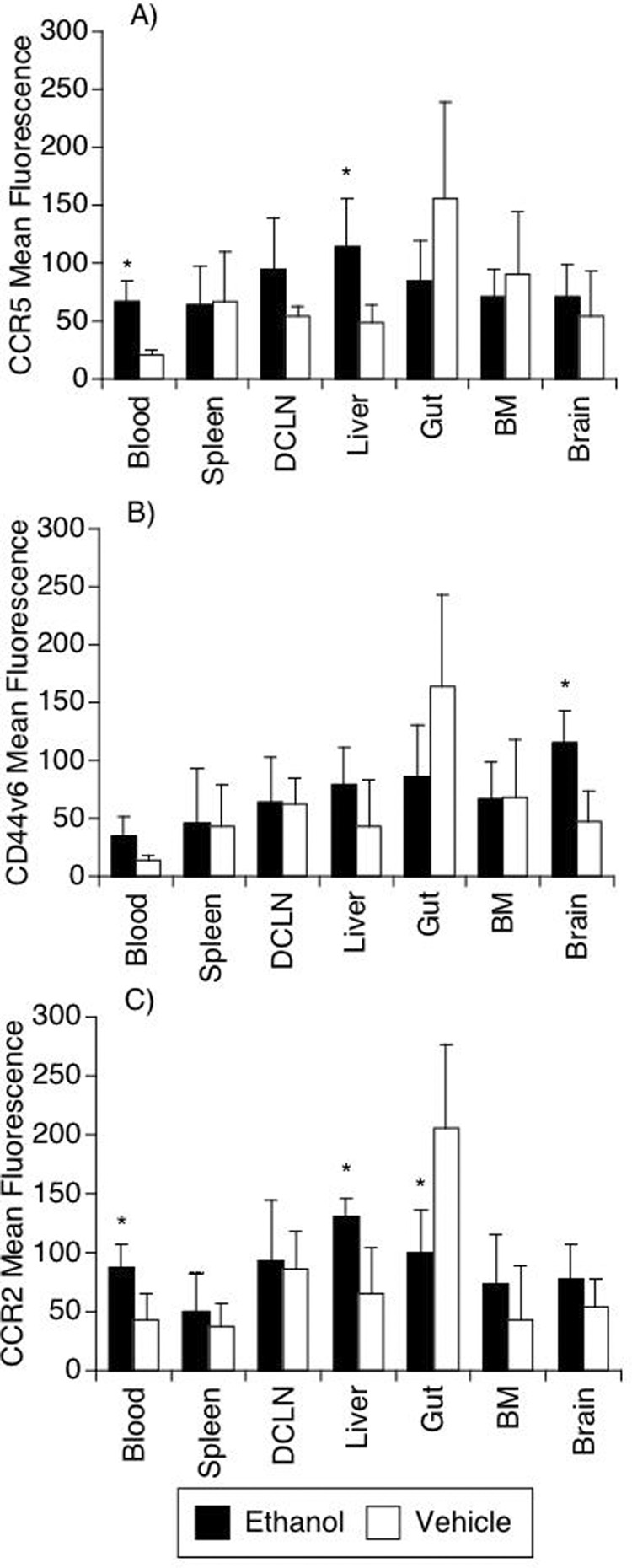
Macrophages (CD14+CD16+ cells) were evaluated in different organs in SIV-infected animals that did or did not receive alcohol. The only differences detected between the groups were in the liver and brain. In the liver, a significant increase in CCR5 expression was observed, similar to that found in the blood (Figure 9). In the brain, there was a significant increase in CD44v6 expression in the ethanol-consuming group (Figure 9B). Since virus is thought to enter the brain in infected monocytes/macrophages, we also quantified the number of macrophages in the brain. However there was no difference in the absolute number of macrophages (not shown).
Figure 9.
Surface markers on CD14+ CD16+ cells in different organs of SIV-infected animals treated or not with ethanol. The expression levels of CCR5 (A), CD44v6 (B) and CCR2 (C) were verified on CD14+ CD16+ gated cells using flow cytometry. Abbreviations are the same as Figure 7. Values represent the average ± SD of 4 animals in each group. *p<0.05, t test between groups.
Discussion
Our study was designed to investigate potential influences alcohol on acute aspects of SIV infection. Animals that consumed alcohol developed an acute decrease in CD4 T cell memory populations compared to vehicle treated-infected monkeys. We observed a decrease of central memory CD4 cells in the blood and lymphoid organs (spleen and lymph nodes), while effector memory CD4 cells decreased in the liver in addition to the blood.
We did not find an effect of alcohol ingestion on the early viral load following SIV infection in plasma, spleen, lymph nodes, or brain; a slight beneficial effect was found in CSF (with a lower viral load). Although CSF is thought to reflect changes occurring in the brain, we did not find a similar effect of alcohol on the CSF and brain viral load. While a lack of difference in brain viral load between animals of different groups could be a false negative result, given the sample size, the independence of CSF and brain viral dynamics has been suggested in both HIV and SIV infection (Demuth et al., 2000; Ellis et al., 2000).
Monocyte/macrophages were affected following SIV infection, as the acute increase in inflammatory blood monocytes after viral inoculation in control SIV infected animals did not did not occur in alcohol-treated animals. Interestingly, we have recently found that in animals that exhibited rapid disease progression and develop encephalitis, a similar lack of early increase of CD14+ CD16+ monocytes occurs (Marcondes et al., 2008). We note that although not increased in number following infection, these CD16+ monocytes in ethanol-consuming animals do have increased expression of both CCR2 and CCR5. Interestingly, the expression of CCR2 on inflammatory monocytes was recently shown to characterize a population with ability to migrate into the CNS and become microglia cells (Mildner et al., 2007), whereas CCR5 serves as the coreceptor for most SIV strains and HIV strains that dominate acute infection. Moreover, the expression of molecules on CD14+CD16+ macrophages was altered by the consumption of ethanol. Expression of CCR2 and CCR5 was increased by ethanol on CD16+ cells in blood and liver, whereas CCR2 expression was decreased by ethanol on CD16+ macrophages in the gut. Interestingly, in addition, expression of CD44v6 on these cells was increased in the brain. Since CD44v6 is an adhesion molecules as well as one of the receptors for osteopontin (Katagiri et al., 1999; Uede et al., 1997), found to be upregulated in brains with SIV and HIV encephalitis (Burdo et al., 2007; Roberts et al., 2003), as well as increased on monocytes in SIV-infected animals progressing rapidly to encephalitis (Marcondes, 2008). In combination, the increases in CCR2, CCR5 and CD44v6 on inflammatory monocytes and macrophages can provide the conditions for the accumulation of inflammatory macrophages susceptible to infection in the brain of alcohol-treated animals, increasing the susceptibility to later CNS disease.
It is important to note several differences between our studies and those of others on alcohol and SIV, specifically time course of study, alcohol dose, and viral inoculum. First, as already mentioned, we limited the study to the initial stages of infection in order to study the brain and other vital organs, and thus do not have information in longer term viral/immune interactions or disease course. Such studies would require a distinct increase in the number of animals per group to achieve the power required for definitive results, perhaps accounting for some of the variability in results already reported for the effects of alcohol on SIV even with the same virus stock (Bagby et al., 2003; Bagby et al., 2006; Molina et al., 2008; Molina et al., 2006; Poonia et al., 2006; Poonia et al., 2005; Winsauer et al., 2002). For example, for steady-state viral load, which highly predictive of disease course in both HIV infection of humans and SIV infection of monkeys (Mellors et al., 1997; Watson et al., 1997), studies with the commonly used pathogenic SIVmac251 stock would require 20 animals per group to assess whether a treatment such as alcohol administration could change viral load even if the effect size was 10-fold; smaller changes would require even larger groups sizes (Parker et al., 2001). Second, concerning alcohol dosing, the studies utilizing intragastric administration of alcohol achieved higher doses of ethanol in a binge pattern (Bagby et al., 2003; Bagby et al., 2006; Molina et al., 2008; Molina et al., 2006; Poonia et al., 2005), whereas the study with ad libitum self-administration (Kumar et al., 2005) was similar to ours, although the animals were not exposed to alcohol everyday. Third, the viral inoculum differed between the studies. While we used a derivative of SIVmac251 that induces high peak and steady-state viral loads (Burdo et al., 2005), the other study with alcohol self administration utilized a mixture of three SIV and SHIV stocks that induces rapid CD4 cell depletion and variable viral loads (Kumar et al., 2006), whereas the studies with intragastic alcohol administration utilized SIVdeltaB670, a stock yielding moderate peak and steady-state viral loads (Bagby et al., 2006).
As the administration of alcohol in our study was oral, it is absorbed from the gastrointestinal tract, where a decrease of CD4 cells expressing CD95 and CCR5 was found in alcohol consuming SIV infected animals. A depletion of CD4 T cells was previously found in the intestinal mucosa of SIV-infected macaques at between days 10 and 15 dpi (Burdo et al., 2005; Lifson et al., 2003). CM CD4 cells were deeply affected as a decrease on their frequency was observed in several sites corresponding to secondary lymphoid organs, while EM cell reduction was detectable in blood, and in the ethanol target organ, liver. As EM cells are very heterogeneous, effector phenotype was further explored based on the expression of other markers, CD95 and CCR5. Other studies show that CD95-expressing CD4 cells are the ones that are most drastically depleted from the GALT in SIV-infected animals (Pandrea et al., 2007). In our study, we observed that animals that consumed alcohol have fewer CD95+ CD4 T cells which simultaneously express CCR5 in the jejunum. Therefore it is possible that alcohol consumption accelerates or increases the severity of the depletion in the intestinal mucosa. In one hand, these cells are the primary targets of the virus, but also their depletion may impact secondary aspects of the local immune response in the gut mucosa. Although the GALT CD4 depletion does not seem to correlate with the virulence of the infection (Pandrea et al., 2007), it can contribute to produce a more susceptible environment to disease.
Perhaps not surprisingly, the liver, the source of alcohol metabolism, was a prime site for changes, with both CD4 and CD8 cells being lower in alcohol-treated than control animals following SIV infection. In liver CD8 cells, an increase of central memory and decrease of effector memory cells was found. A linear differentiation between these two stages is not clear (Schwendemann et al., 2005), but alcohol consumption could interfere with the final functional maturation of CD8 cells migrating to the liver, or perhaps affect the survival of cells with effector phenotype.
Alcohol consumption also impacted type I IFN production in the spleen, where IFNα̣ augmented in the alcohol group. Although type I IFNs are known to decrease viral replication, in SIV infected monkeys type I IFN expression have been shown to correlate with high viral RNA levels in tissues (Abel et al., 2002). As type I IFN stimulates CD4 T cells (Chen et al., 2006), it may also increase the availability of target cells. Type I IFN supports the clonal expansion and survival of antigen-specific CD8 T cells (Tough et al., 1996), thus the overall effects of this increase in the spleen are likely complex.
Interestingly, IFNα was expressed at lower levels in the brain of ethanol-treated animals in comparison to controls, which may represent a suppression of the brain’s innate immune response to infection. Ethanol was shown to suppress in vitro production of TNFα induced by LPS on bronchioalveolar macrophages from SIV-infected animals (Stoltz et al., 2000), as well as LPS-induced COX2, PGE2 and iNOS production by rat alveolar macrophages (Kato et al., 2004; Kato et al., 2005). The suppressive effect of acute administration of ethanol on innate immunity has been attributed to inhibition of TLR signaling pathways (Pruett et al., 2004), which are involved in the transcription of type I IFN genes (Colonna, 2006; Uematsu and Akira, 2007). Thus, ethanol may be involved in the observed suppression of type I IFN production, which is a functional aspect of brain infiltrating macrophages, microglia and astrocytes, that can aid to viral replication control. This seems to be a characteristic favored in the brain, as opposed to the periphery where IFNα was higher. Decreased production of anti-viral molecules, such as IFNα, from the main viral target cells in the brain, can contribute to produce a status of viral reservoir.
Gut tissue, liver and brain, therefore, are the sites where the impact of alcohol consumption has affected the host immune population during the acute phase of infection. Overall, these changes may contribute to the generation of microenvironments that are susceptible to infection. Alcohol consumption can indeed alter the phenotype of early infection, predominantly in blood and organ host cell composition. In addition to alcohol consumption altering risk-taking behavior and thus the risk of HIV infection, these changes may contribute to the eventual systemic and organ-specific pathologies found in AIDS.
Acknowledgements
We thank Nancy Delaney for administrative assistance, Stefani Von Huben, Sophia Davis and Christopher Lay for technical assistance. This is the manuscript #19379 of The Scripps Research Institute. This work was funded by grants AA13836, AA013972, MH062261, and MH073490 from the NIH.
References
- Abel K, Alegria-Hartman MJ, Rothaeusler K, Marthas M, Miller CJ. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-alpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J Virol. 2002;76(16):8433–8445. doi: 10.1128/JVI.76.16.8433-8445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby GJ, Stoltz DA, Zhang P, Kolls JK, Brown J, Bohm RP, Jr, Rockar R, Purcell J, Murphey-Corb M, Nelson S. The effect of chronic binge ethanol consumption on the primary stage of SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2003;27(3):495–502. doi: 10.1097/01.ALC.0000057947.57330.BE. [DOI] [PubMed] [Google Scholar]
- Bagby GJ, Zhang P, Purcell JE, Didier PJ, Nelson S. Chronic binge ethanol consumption accelerates progression of simian immunodeficiency virus disease. Alcohol Clin Exp Res. 2006;30(10):1781–1790. doi: 10.1111/j.1530-0277.2006.00211.x. [DOI] [PubMed] [Google Scholar]
- Burdo TH, Marcondes MC, Lanigan CM, Penedo MC, Fox HS. Susceptibility of Chinese rhesus monkeys to SIV infection. Aids. 2005;19(15):1704–1706. doi: 10.1097/01.aids.0000186823.76230.33. [DOI] [PubMed] [Google Scholar]
- Burdo TH, Wood MR, Fox HS. Osteopontin prevents monocyte recirculation and apoptosis. J Leukoc Biol. 2007;81(6):1504–1511. doi: 10.1189/jlb.1106711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burudi EM, Marcondes MC, Watry DD, Zandonatti M, Taffe MA, Fox HS. Regulation of indoleamine 2,3-dioxygenase expression in simian immunodeficiency virus-infected monkey brains. J Virol. 2002;76(23):12233–12241. doi: 10.1128/JVI.76.23.12233-12241.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Huang J, Zhang C, Huang S, Nunnari G, Wang FX, Tong X, Gao L, Nikisher K, Zhang H. Alpha interferon potently enhances the anti-human immunodeficiency virus type 1 activity of APOBEC3G in resting primary CD4 T cells. J Virol. 2006;80(15):7645–7657. doi: 10.1128/JVI.00206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M. Toll-like receptors and IFN-alpha: partners in autoimmunity. J Clin Invest. 2006;116(9):2319–2322. doi: 10.1172/JCI29879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth M, Czub S, Sauer U, Koutsilieri E, Haaft P, Heeney J, Stahl-Hennig C, ter Meulen V, Sopper S. Relationship between viral load in blood, cerebrospinal fluid, brain tissue and isolated microglia with neurological disease in macaques infected with different strains of SIV. J Neurovirol. 2000;6(3):187–201. doi: 10.3109/13550280009015822. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Gamst AC, Capparelli E, Spector SA, Hsia K, Wolfson T, Abramson I, Grant I, McCutchan JA. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000;54(4):927–936. doi: 10.1212/wnl.54.4.927. [DOI] [PubMed] [Google Scholar]
- Fox HS, Weed MR, Huitron-Resendiz S, Baig J, Horn TF, Dailey PJ, Bischofberger N, Henriksen SJ. Antiviral treatment normalizes neurophysiological but not movement abnormalities in simian immunodeficiency virus-infected monkeys. J Clin Invest. 2000;106(1):37–45. doi: 10.1172/JCI9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF. Alcohol consumption, alcohol abuse and alcohol dependence. The United States as an example. Addiction. 1994;89(11):1357–1365. doi: 10.1111/j.1360-0443.1994.tb03730.x. [DOI] [PubMed] [Google Scholar]
- Katagiri YU, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K, Chikuma S, Yagita H, Okumura K, Murakami M, Saiki I, Chambers AF, Uede T. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59(1):219–226. [PubMed] [Google Scholar]
- Katner SN, Flynn CT, Von Huben SN, Kirsten AJ, Davis SA, Lay CC, Cole M, Roberts AJ, Fox HS, Taffe MA. Controlled and behaviorally relevant levels of oral ethanol intake in rhesus macaques using a flavorant-fade procedure. Alcohol Clin Exp Res. 2004;28(6):873–883. doi: 10.1097/01.alc.0000128895.99379.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Von Huben SN, Davis SA, Lay CC, Crean RD, Roberts AJ, Fox HS, Taffe MA. Robust and stable drinking behavior following long-term oral alcohol intake in rhesus macaques. Drug Alcohol Depend. 2007;91(2–3):236–243. doi: 10.1016/j.drugalcdep.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Negoro M, Wakabayashi I. Inhibitory action of ethanol on cyclooxygenase-2 expression through suppression of the extracellular signal-related kinase-mediated pathway in rat alveolar macrophages. Basic Clin Pharmacol Toxicol. 2004;95(5):226–233. doi: 10.1111/j.1742-7843.2004.pto950505.x. [DOI] [PubMed] [Google Scholar]
- Kato H, Negoro M, Wakabayashi I. Effects of acute ethanol administration on LPS-induced expression of cyclooxygenase-2 and inducible nitric oxide synthase in rat alveolar macrophages. Alcohol Clin Exp Res. 2005;29 12 Suppl:285S–293S. doi: 10.1097/01.alc.0000191809.29775.41. [DOI] [PubMed] [Google Scholar]
- Kim WK, Corey S, Chesney G, Knight H, Klumpp S, Wuthrich C, Letvin N, Koralnik I, Lackner A, Veasey R, Williams K. Identification of T lymphocytes in simian immunodeficiency virus encephalitis: distribution of CD8+ T cells in association with central nervous system vessels and virus. J Neurovirol. 2004;10(5):315–325. doi: 10.1080/13550280490505382. [DOI] [PubMed] [Google Scholar]
- Kumar R, Orsoni S, Norman L, Verma AS, Tirado G, Giavedoni LD, Staprans S, Miller GM, Buch SJ, Kumar A. Chronic morphine exposure causes pronounced virus replication in cerebral compartment and accelerated onset of AIDS in SIV/SHIV-infected Indian rhesus macaques. Virology. 2006;354(1):192–206. doi: 10.1016/j.virol.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Kumar R, Perez-Casanova AE, Tirado G, Noel RJ, Torres C, Rodriguez I, Martinez M, Staprans S, Kraiselburd E, Yamamura Y, Higley JD, Kumar A. Increased viral replication in simian immunodeficiency virus/simian-HIV-infected macaques with self-administering model of chronic alcohol consumption. J Acquir Immune Defic Syndr. 2005;39(4):386–390. doi: 10.1097/01.qai.0000164517.01293.84. [DOI] [PubMed] [Google Scholar]
- Lane TE, Buchmeier MJ, Watry DD, Jakubowski DB, Fox HS. Serial passage of microglial SIV results in selection of homogeneous env quasispecies in the brain. Virology. 1995;212(2):458–465. doi: 10.1006/viro.1995.1503. [DOI] [PubMed] [Google Scholar]
- Lifson JD, Piatak M, Jr, Cline AN, Rossio JL, Purcell J, Pandrea I, Bischofberger N, Blanchard J, Veazey RS. Transient early post-inoculation anti-retroviral treatment facilitates controlled infection with sparing of CD4+ T cells in gut-associated lymphoid tissues in SIVmac239-infected rhesus macaques, but not resistance to rechallenge. J Med Primatol. 2003;32(4–5):201–210. doi: 10.1034/j.1600-0684.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- Marcondes MC, Burudi EM, Huitron-Resendiz S, Sanchez-Alavez M, Watry D, Zandonatti M, Henriksen SJ, Fox HS. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol. 2001;167(9):5429–5438. doi: 10.4049/jimmunol.167.9.5429. [DOI] [PubMed] [Google Scholar]
- Marcondes MC, Lanigan C, Burdo TH, Watry D, Fox HS. Increased Expression of Monocyte CD44v6 Correlates with the Development of Encephalitis in Rhesus Macaques Infected with Simian Immunodeficiency Virus. J Infect Dis in print. 2008 doi: 10.1086/588002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, Kingsley LA, Todd JA, Saah AJ, Detels R, Phair JP, Rinaldo CR., Jr Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126(12):946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10(12):1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Molina PE, Lang CH, McNurlan M, Bagby GJ, Nelson S. Chronic alcohol accentuates simian acquired immunodeficiency syndrome-associated wasting. Alcohol Clin Exp Res. 2008;32(1):138–147. doi: 10.1111/j.1530-0277.2007.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina PE, McNurlan M, Rathmacher J, Lang CH, Zambell KL, Purcell J, Bohm RP, Zhang P, Bagby GJ, Nelson S. Chronic alcohol accentuates nutritional, metabolic, and immune alterations during asymptomatic simian immunodeficiency virus infection. Alcohol Clin Exp Res. 2006;30(12):2065–2078. doi: 10.1111/j.1530-0277.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, Perelson AS, Douek DC, Veazey RS, Apetrei C. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol. 2007;179(5):3035–3046. doi: 10.4049/jimmunol.179.5.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RA, Regan MM, Reimann KA. Variability of viral load in plasma of rhesus monkeys inoculated with simian immunodeficiency virus or simian-human immunodeficiency virus: implications for using nonhuman primate AIDS models to test vaccines and therapeutics. J Virol. 2001;75(22):11234–11238. doi: 10.1128/JVI.75.22.11234-11238.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poonia B, Nelson S, Bagby GJ, Veazey RS. Intestinal lymphocyte subsets and turnover are affected by chronic alcohol consumption: implications for SIV/HIV infection. J Acquir Immune Defic Syndr. 2006;41(5):537–547. doi: 10.1097/01.qai.0000209907.43244.ee. [DOI] [PubMed] [Google Scholar]
- Poonia B, Nelson S, Bagby GJ, Zhang P, Quniton L, Veazey RS. Chronic alcohol consumption results in higher simian immunodeficiency virus replication in mucosally inoculated rhesus macaques. AIDS Res Hum Retroviruses. 2005;21(10):863–868. doi: 10.1089/aid.2005.21.863. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Zheng Q, Fan R, Matthews K, Schwab C. Acute exposure to ethanol affects Toll-like receptor signaling and subsequent responses: an overview of recent studies. Alcohol. 2004;33(3):235–239. doi: 10.1016/j.alcohol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Roberts ES, Zandonatti MA, Watry DD, Madden LJ, Henriksen SJ, Taffe MA, Fox HS. Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol. 2003;162(6):2041–2057. doi: 10.1016/S0002-9440(10)64336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwendemann J, Choi C, Schirrmacher V, Beckhove P. Dynamic differentiation of activated human peripheral blood CD8+ and CD4+ effector memory T cells. J Immunol. 2005;175(3):1433–1439. doi: 10.4049/jimmunol.175.3.1433. [DOI] [PubMed] [Google Scholar]
- Stoltz DA, Nelson S, Kolls JK, Zhang P, Bohm RP, Jr, Murphey-Corb M, Bagby GJ. In vitro ethanol suppresses alveolar macrophage TNF-alpha during simian immunodeficiency virus infection. Am J Respir Crit Care Med. 2000;161(1):135–140. doi: 10.1164/ajrccm.161.1.9905016. [DOI] [PubMed] [Google Scholar]
- Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272(5270):1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- Uede T, Katagiri Y, Iizuka J, Murakami M. Osteopontin, a coordinator of host defense system: a cytokine or an extracellular adhesive protein? Microbiol Immunol. 1997;41(9):641–648. doi: 10.1111/j.1348-0421.1997.tb01906.x. [DOI] [PubMed] [Google Scholar]
- Uematsu S, Akira S. Toll-like receptors and Type I interferons. J Biol Chem. 2007;282(21):15319–15323. doi: 10.1074/jbc.R700009200. [DOI] [PubMed] [Google Scholar]
- Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson PR, Hu SL, Haigwood NL. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71(1):284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsauer PJ, Moerschbaecher JM, Brauner IN, Purcell JE, Lancaster JR, Jr, Bagby GJ, Nelson S. Alcohol unmasks simian immunodeficiency virus-induced cognitive impairments in rhesus monkeys. Alcohol Clin Exp Res. 2002;26(12):1846–1857. doi: 10.1097/01.ALC.0000042171.80435.F1. [DOI] [PubMed] [Google Scholar]