Abstract
The purpose of this review is to provide information about the role of exercise in the prevention of skeletal muscle insulin resistance, that is, the inability of insulin to properly cause glucose uptake into skeletal muscle. Insulin resistance is associated with high levels of stored lipids in skeletal muscle cells. Aerobic exercise training decreases the amounts of these lipid products and increases the lipid oxidative capacity of muscle cells. Thus, aerobic exercise training may prevent insulin resistance by correcting a mismatch between fatty acid uptake and fatty acid oxidation in skeletal muscle. Additionally, a single session of aerobic exercise increases glucose uptake by muscle during exercise, increases the ability of insulin to promote glucose uptake, and increases glycogen accumulation after exercise, all of which are important to blood glucose control. There also is some indication that resistance exercise may be effective in preventing insulin resistance. The information provided is intended to help clinicians understand and explain the roles of exercise in reducing insulin resistance.
The purpose of this review is to provide information about the role of exercise in the prevention of insulin resistance. In particular, the review focuses on the association of insulin resistance with the storage of lipids in skeletal muscle cells and discusses the abilities of aerobic exercise to decrease the amounts of these lipid products and increase the lipid oxidative capacity of muscle cells. Additionally, the short-term effects of aerobic exercise that are important to blood glucose control—such as increased glucose uptake by muscle during exercise and the increased ability of insulin to promote the storage of glucose in muscle after exercise—are discussed. Finally, information about the possible role of resistance exercise in preventing insulin resistance is presented. The information provided is intended to help clinicians understand and explain the roles of exercise in reducing insulin resistance.
Diabetes mellitus is essentially the abnormal regulation of blood glucose concentrations. Elevated fasting blood glucose levels or extreme or prolonged increases in blood glucose levels during an oral glucose tolerance test (for which blood glucose concentrations are assayed before and periodically after a fasting individual drinks a beverage containing 75 g of glucose) lead to a diagnosis of diabetes. It is clear, then, that the control of blood glucose concentrations is the key to the prevention of diabetes.
Insulin robustly stimulates the transport of glucose out of the bloodstream and into tissues, such as skeletal muscle, that express glucose transporter 4 (GLUT4), the insulin-regulated glucose transporter. Because of the high responsiveness of skeletal muscle to insulin and the large overall mass of skeletal muscle, most glucose that is cleared from the blood in response to insulin in humans is stored as glycogen in skeletal muscle.1,2 When insulin-stimulated glucose transport into skeletal muscle is diminished—as it is in people with diabetes3—the result is an inability to keep blood glucose concentrations within normal ranges. Thus, skeletal muscle plays a primary role in the maintenance of normal blood glucose concentrations.
For a full understanding of the effects of exercise on insulin sensitivity, it is important to take into account the regulation of skeletal muscle fatty acid (FA) metabolism. Thus, the approach of this review is to discuss the regulation of FA metabolism in skeletal muscle and to use this information as a foundation to explore what is known about the ability of exercise to promote blood glucose clearance. (Also see the corresponding perspective article by Stehno-Bittel4 in this issue.)
Fatty Acid Metabolism and Insulin Resistance
Insulin resistance is frequently present in obesity and during the development of type 2 diabetes mellitus (T2DM) and is generally defined as a reduction in the ability of the body to clear a glucose load from the circulation in response to insulin. Because skeletal muscle is the most important tissue for insulin-stimulated glucose disposal, muscle insulin resistance is commonly viewed as the critical component of whole-body insulin resistance. In skeletal muscle, insulin resistance has been linked to lipid accumulation and, consequently, to defects in FA metabolism, which may include alterations in muscle FA uptake, triacylglycerol (TG) synthesis, TG breakdown (lipolysis), FA oxidation, or any combination of these.5–9 Here we summarize recently published data suggesting that alterations in the regulation of FA uptake and oxidation in skeletal muscle may contribute significantly to the development of T2DM. Although data have shown that alterations in liver and adipose tissue lipid metabolism are also involved in the development of insulin resistance and T2DM, here we focus solely on skeletal muscle FA uptake and oxidation. We also present evidence showing how exercise and regular physical activity can affect muscle FA metabolism and delay or prevent the development of insulin resistance. (Also see the article by Chen et al10 in this issue.)
Lipid Metabolism, Accumulation of Lipid Intermediates, and Insulin Resistance in Skeletal Muscle
Studies have shown that insulin resistance is associated with alterations in lipid metabolism. This process usually is manifested as elevated levels of circulating FAs and TGs and increased intracellular accumulation of lipid intermediates, such as TGs, diacylglycerols (DGs), ceramides, and long-chain FA coenzyme A (LC-CoA).11 The accumulation of these lipid intermediates, which is commonly seen for long-chain saturated FA species (such as palmitic, stearic, and arachidic esters) rather than for long-chain unsaturated FA species, in turn, has been linked to defects in the insulin signaling cascade.12,13 Ultimately, these changes in cellular signaling have been linked to a decrease in insulin-stimulated glucose uptake and metabolism, that is, insulin resistance.14 Thus, if the chain of events initiated by an accumulation of lipid intermediates is responsible for the development of insulin resistance in muscle, then the cellular mechanisms that lead to alterations in intracellular lipid accumulation need to be understood.
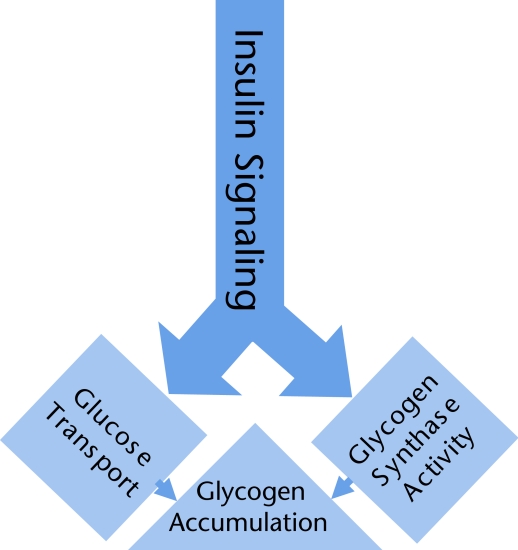
Data have shown that intramuscular TG content and insulin sensitivity are negatively correlated.8 This correlation has been shown to be dependent on fiber type expression. However, what has become more evident in recent years is that the accumulation of intracellular lipid intermediates other than TGs may be more harmful to the normal functioning of muscle cells. Indeed, the accumulation of LC-CoA, DGs, and ceramides has been shown to have a negative effect on insulin signaling in muscle.11,15,16 Insulin induces its effects in skeletal muscle by first binding to its receptor sites on the extracellular side of the plasma membrane. This binding initiates a series of phosphorylation reactions that lead to the phosphorylation of several signaling intermediates on tyrosine residues, including the critical insulin receptor substrate proteins (such as IRS1 and IRS2).17,18 This process is followed by the activation of phosphatidylinositol 3-kinase and of other downstream intermediates, including 3-phosphoinositide-dependent protein kinase 1, atypical protein kinase C- ζ, atypical protein kinase C-λ, and protein kinase B (Akt). In healthy insulin-sensitive skeletal muscle, the activation of these insulin-mediated signaling intermediates ultimately leads to an increase in glucose transport through the translocation of GLUT4 from intracellular vesicles to the plasma membrane and glycogen synthesis through the activation of glycogen synthase19 (Fig. 1). Recent studies5–7 have shown that the accumulation of LC-CoA, DGs, ceramides, or any combination of these negatively affects the activation of the insulin signaling cascades described above. This inhibitory action then leads to a reduction in insulin-stimulated glucose uptake and disposal into the muscle, and insulin resistance develops.14,20
Figure 1.
Insulin stimulates glucose transport and glycogen synthesis in skeletal muscle. Insulin stimulates the activation of a signaling cascade in skeletal muscle, which includes some of the proteins mentioned in the text. Insulin stimulates increased glucose uptake by skeletal muscle and activates glycogen synthase, the rate-limiting enzyme for the production of glycogen from intracellular glucose. Together, these actions of insulin contribute to increased glycogen accumulation. There is a growing body of evidence that the accumulation of lipid intermediates (such as diacylglycerols, ceramides, and long-chain fatty acyl coenzyme A molecules) attenuates the insulin signal, leading to glucose transport.
Ultimately, the important question is, “What are the cellular mechanisms that lead to the initial accumulation of intracellular lipid intermediates?” These mechanisms have not been completely elucidated, but data have shown that lipid accumulation may be predominantly attributable to lower rates of FA oxidation, higher rates of FA uptake, or both in insulin-resistant skeletal muscle. If this hypothesis is correct, then a mismatch between high rates of FA uptake and low rates of FA oxidation could easily result in an accumulation of lipid intermediates (Fig. 2).
Figure 2.
Schematic of mismatch between fatty acid (FA) uptake and FA oxidation with insulin resistance. In healthy muscle, the rate of FA uptake equals the rate of FA oxidation, leading to an equilibrium in the concentrations of lipid intermediates. In insulin-resistant muscle, the rate of FA uptake is higher and the rate of FA oxidation is lower, leading to an accumulation of lipid intermediates. After an exercise training program, the rate of FA oxidation increases in insulin-resistant muscle, leading to a new equilibrium. A horizontal arrow indicates that one physiological parameter leads to another. The size and boldness of the arrow indicate the level at which the action occurs. An up arrow indicates that the level of the physiological variable is increased because of an imbalance in the rates of the physiological processes leading into and out of the box.
Increase in Fatty Acid Uptake Into Muscle Cells
As mentioned above, high rates of FA uptake into muscle cells may be an important cellular mechanism leading to intracellular lipid accumulation. In insulin-resistant skeletal muscle of animals with obesity, T2DM, or both, the rates of FA uptake in oxidative or mixed muscle are markedly increased.21–24 Because higher rates of FA uptake have been measured at an early age, before T2DM is evident, in rats genetically predisposed to develop T2DM, this finding suggests that defects inherent in muscle tissue are present.21 In line with data obtained from animal studies, rates of FA uptake have been shown to be markedly higher in mixed muscle of people with insulin resistance and to be correlated with the associated increase in intracellular lipid accumulation.9 However, high correlations provide no insight into the cellular mechanisms that may be responsible for higher rates of FA uptake in insulin-resistant muscle. (Also see the articles in this issue by Hilton et al25 and Marcus et al26 describing fat infiltration into the muscle of people with diabetes mellitus.)
Fatty acid uptake is determined, in part, by plasma FA concentrations. When experimental conditions are kept constant, higher FA concentrations are associated with higher rates of FA uptake.27 The fact that plasma FA concentrations and thus availability tend to be higher in people who are obese and have insulin resistance, T2DM, or both, may partially explain the higher rates of FA uptake in insulin-resistant muscle. However, given similar FA delivery and availability, rates of FA uptake have been shown to be higher in insulin-resistant mixed muscle.23,24 Thus, even though reduced FA availability has been shown in some studies to improve insulin sensitivity in people with obesity or T2DM,28,29 it also is clear that factors intrinsic to skeletal muscle affect lipid accumulation.
Over the past decade, evidence has emerged to show that FA flux across the plasma membrane of cells occurs through a highly regulated, protein-mediated process that involves one or several FA transporter proteins30,31 and that FA uptake capacity may be inherently high in insulin-resistant muscle.21–24 In human and animal muscle, FABPPM and CD36 are among several key proteins that have been identified as FA transporter proteins.32–34 Under physiological conditions that are known to increase rates of FA uptake (such as insulin stimulation, exercise, or leptin stimulation), FABPPM and CD36 have been shown to translocate to the plasma membrane.35–37 Furthermore, the overexpression of skeletal muscle FABPPM or CD36 has been shown to enhance FA uptake in muscle, whereas a null mutation in skeletal muscle CD36 has been associated with decreased rates of FA uptake.38–40 These results suggest that defects leading to stimulation of the protein-mediated uptake system may cause higher rates of FA uptake and lead to the accumulation of lipid intermediates. In line with this notion, data have shown that high rates of FA uptake in people with insulin resistance are associated with an increased total protein content of FABPPM and with a permanent relocation of CD36 to the plasma membrane.9 Thus, a higher plasma membrane content of FABPPM, CD36, or both may provide a cellular mechanism through which rates of FA uptake are increased in insulin-resistant muscle.
Reduction of Fatty Acid Oxidative Capacity in Muscle Cells
Muscle oxidative capacity and, more specifically, the ability of muscle to oxidize FA appear to be good predictors of insulin sensitivity. Consistent with this suggestion, several groups have demonstrated that mitochondrial content, mitochondrial function, and oxidative capacity are reduced in obese people with insulin resistance, T2DM, or both.41–43 In fact, in people with diabetes, skeletal muscle oxidative capacity appears to be a better predictor of insulin sensitivity than either intracellular TG content or LC-CoA content.41,44 This finding suggests that the reduced oxidative capacity observed in people with obesity, T2DM, or both, may play a role in the development of insulin resistance. In line with the important role of skeletal muscle in the development of insulin resistance, evidence has shown that the reduction in whole-body FA oxidation observed in people with obesity, T2DM, or both, is attributable to metabolic defects at the muscle level. Indeed, low FA oxidation rates have been measured in primary myocytes isolated from obese subjects with insulin resistance and T2DM,45 in rectus abdominis muscle strips isolated from subjects with obesity,46 and in muscle homogenates isolated from subjects with obesity.47 These data show that the metabolic abnormalities observed at the whole-body level are inherent to the muscle cell. Furthermore, a recent report that mitochondrial density is lower in insulin-resistant muscle of children of people with T2DM suggests that reduced oxidative capacity may represent an early factor in the development of insulin resistance and that low oxidative capacity may be an inherited defect in these people.48 This conclusion is supported by the fact that rates of FA oxidation at rest have been shown to remain low in people who were formerly obese.49,50
Acetoacetate and β-hydroxybutyrate or ketone bodies are generally classified as freely soluble circulating lipid fuels,51 and they arise from partial hepatic FA oxidation. Circulating ketone body levels can be quite high in people with obesity and T2DM, reaching values that are 10 times higher than those in people who are healthy.52 Muscle FA oxidative capacity does not directly contribute to the production of ketone bodies by the liver. However, the reduction in FA oxidation usually observed in the muscle of people with obesity, T2DM, or both, may indirectly affect circulating ketone body levels by elevating circulating FA levels and thereby increasing the rate of ketone body production by the liver. Ultimately, however, even when circulating ketone body levels are high, the contribution of this lipid fuel to muscle oxidative metabolism during rest or exercise remains below 5%.53
Oxidative capacity is commonly assessed by measuring the content and activity of key mitochondrial enzymes. A number of studies have shown that the activities of carnitine palmitoyltransferase 1 (CPT1) and of other key mitochondrial enzymes, such as citrate synthase and β-hydroxyacyl-CoA dehydrogenase, are significantly reduced in the skeletal muscle of people with obesity, T2DM, or both.46,47,54 Furthermore, independent of fitness or fatness, people with insulin resistance have reduced expression of mitochondrial encoded genes such as cytochrome c oxidase and reduced protein expression of the subunits for complexes I and III of the electron transport chain.55 Together, these alterations lead to mitochondrial dysfunction. Mitochondrial dysfunction can be assessed by measuring electron transport chain activity in isolated mitochondria. With this method of measurement, it has been shown that electron transport chain activity is indeed lower in the muscle of people with obesity, T2DM, or both; this finding provides evidence for the existence of mitochondrial dysfunction with insulin resistance.56,57 Mitochondrial dysfunction also has been measured in insulin-resistant muscle of children of people with T2DM, indicating that an impairment in mitochondrial oxidative capacity may be an inherited defect.58
Mitochondrial dysfunction with insulin resistance also may be attributable to several other factors. It is well accepted that FA oxidation is regulated, in large part, by the capacity of the mitochondrion to carry FA across its membranes. Mitochondrial FA transport capacity, in turn, is dependent on several factors, including the functional activities of CPT1 and intracellular malonyl-CoA.59 The importance of functional CPT1 activity to intracellular lipid accumulation was demonstrated by studies in which CPT1 activity was manipulated pharmacologically or genetically. In rats given etomoxir (a covalent inhibitor of CPT1) for 4 weeks, intracellular lipid content increased and insulin-stimulated glucose uptake and metabolism decreased.60 Conversely, the overexpression of CPT1 in specific muscles through in vivo electrotransfer of a human CPT1-complementary DNA fragment was associated with increases in CPT1 activity and FA oxidation in both isolated mitochondria and muscle strips and with a decrease in intracellular lipid accumulation in muscle strips.61 These data agree with the data on oxidative capacity and insulin resistance in human subjects and demonstrate the importance of CPT1 expression for the regulation of FA oxidation and lipid accumulation and, ultimately, for the development of insulin resistance in muscle.61
Intracellular malonyl-CoA is also an important factor in the regulation of FA oxidation because malonyl-CoA is a potent inhibitor of CPT1 activity.62 Thus, in healthy muscle, malonyl-CoA levels are associated with reciprocal changes in FA oxidation.63,64 Because malonyl-CoA levels increase under conditions of hyperglycemia, hyperinsulinemia, or both,65,66 and because hyperglycemia and hyperinsulinemia typically are present with insulin resistance, it has been suggested that the reduction in muscle FA oxidation observed in people with these conditions may be attributable to high malonyl-CoA levels. Indeed, when the metabolic profile of insulin resistance was simulated by inducing physiological hyperglycemia (∼150 mg/dL) with hyperinsulinemia (∼35 μU/mL) in lean subjects, FA oxidation across the leg and at the whole-body level was inhibited and was associated with a significant increase in muscle malonyl-CoA levels, a normal metabolic response to an increase in the carbohydrate load.66,67 However, it was recently shown that the increases in muscle malonyl-CoA levels observed with the levels of hyperglycemia and hyperinsulinemia seen in people with T2DM were similar in lean people and people with T2DM, casting some doubt on the importance of this cellular factor as the mechanism for the lower rates of FA oxidation in people with T2DM.68 Thus, these data show that although both healthy muscle and insulin-resistant muscle react normally to hyperglycemic and hyperinsulinemic challenges, only healthy muscle is able to decrease FA oxidation under these conditions. To explain this difference, we suggest 2 possibilities. First, it is possible that the increase in muscle malonyl-CoA levels is not a critical factor regulating the consequent decrease in FA oxidation. Alternatively, it is possible that insulin-resistant muscle is less sensitive to a decrease in malonyl-CoA levels and, thus, that FA oxidation is not decreased adequately.
It also has been suggested that the content of the FA transporter protein CD36 in mitochondrial membranes may play a role in the regulation of FA oxidation.69–71 As explained above, CD36 is an FA transporter protein whose function is to carry long-chain FAs across membranes. Thus, it can be hypothesized that a higher mitochondrial CD36 protein content may be associated with higher rates of FA oxidation. In line with this hypothesis, mitochondrial CD36 content was found to be higher in exercised muscle than in resting muscle, and mitochondrial CD36 content was correlated with mitochondrial FA oxidation.71 Most importantly, specific inhibition of CD36 decreased mitochondrial FA oxidation, indicating the importance of this protein in the regulation of FA oxidation. Thus, the data suggest that changes in FA oxidation may be attributable, in part, to alterations in mitochondrial FA transport capacity through CD36 translocation. If this hypothesis is correct, low CD36 content in mitochondrial membranes, defects in the ability of muscle to translocate CD36 to mitochondrial membranes, or both, may be important factors regulating low FA oxidative capacity with insulin resistance. Only one study has been published on this topic and, contrary to expectations, mitochondrial CD36 content was not reduced in people with obesity.43 Thus, it is not clear whether mitochondrial FA transport through CD36 translocation is affected by insulin resistance.
Exercise and Insulin Resistance
So far, we have mentioned several factors that are inherent to skeletal muscle and that may play important roles in the development of insulin resistance. Among the factors that are at least partially determined genetically are a decreased ability to oxidize FA and an increased capacity to take up FA into muscle cells. Both of these factors, individually or together, can lead to a mismatch between rates of FA uptake and rates of FA oxidation, which appears to be causal to the accumulation of intracellular lipid intermediates that is observed with insulin resistance (Fig. 2). Conversely, there is good reason to believe that changes in lifestyle that include decreased physical activity and increased energy intake have contributed to the increased prevalence of obesity and T2DM. For people who have inherited genetic tendencies that promote the development of insulin resistance and for people who have adopted a lifestyle that promotes weight gain, the best strategy for better metabolic management is to include physical activity in their daily lives.72,73 Indeed, it was shown that a modest increase in energy output (brisk walking, 150 min/wk) along with a modest decrease in energy intake (∼1,883 kJ [450 kcal]) was more effective than drug therapy in preventing or delaying the development of insulin resistance and cardiovascular complications in people with obesity, T2DM, or both.72,74 Furthermore, it was shown that an improvement in insulin sensitivity could be measured for several hours and up to a few days after a single session of exercise in both people who are healthy and people with obesity and T2DM.75,76 Conversely, a few days without exercise could significantly decrease insulin sensitivity.77
In accordance with this evidence, the American Diabetes Association and the American College of Sports Medicine have made recommendations regarding physical activity, which include moderate-intensity aerobic physical activity for at least 150 min/wk or vigorous aerobic exercise for at least 90 min/wk.78,79 It also is recommended that people exercise at least 3 d/wk, with no more than 2 consecutive days without physical activity.78,79 Thus, physical activity, even if it is of shorter duration, is considered to be a critical component of any treatment modality, which may also include mild to moderate caloric restriction for people with obesity, T2DM, or both. Although the value of regular physical activity as a treatment modality is unquestioned, the exercise-induced cellular mechanisms that lead to an improvement in insulin sensitivity and metabolic function have not been clearly defined.
Exercise, Lipid Metabolism, and Intramuscular Lipid Intermediates
In contrast to the inverse relationship generally observed between intramuscular TG levels and insulin sensitivity in lean people, people with obesity, and people with T2DM who do not exercise, people who regularly exercise have paradoxically been shown to have high insulin sensitivity despite elevated intramuscular TG levels.80,81 This paradoxical relationship between intramuscular TG levels and insulin sensitivity has been observed not only in trained versus untrained people but also in previously untrained people after a 3-month training program.82 This observation suggests that high intramuscular TG levels may be a positive metabolic adaptation of regular physical activity.
In line with this notion, it has been suggested that higher intramuscular TG levels are beneficial because higher levels stimulate the use of intramuscular TG as an oxidizable fuel source.83 In fact, evidence has suggested that exercise may stimulate TG synthesis in muscle and reduce the accumulation of other, more labile lipid intermediates, such as ceramides and DAG.84 Evidence collected in animal studies has suggested that the stimulatory effects of regular exercise on muscle TG synthesis in oxidative fibers may occur, under some conditions, through an increase in the expression of a transcription factor, sterol regulatory element-binding protein 1c (SREBP-1c), and of lipogenic enzymes, such as FA synthase, acetyl-CoA carboxylase, and diacylglycerol acyltransferase 1.85,86
Because results of studies of the effects of insulin on SREBP-1c have not been consistent across experimental protocols, it is not clear whether the expected stimulatory effects of insulin on SREBP-1c expression and lipogenesis would be affected by obesity or T2DM.87–89 Nevertheless, in vivo evidence has suggested that there are upper limits to intramuscular TG accumulation above which healthy metabolic responses are compromised.90 Thus, when intramuscular TG levels are extremely high before a training program is initiated, regular exercise appears to improve insulin sensitivity and to reduce TG levels.91 Ultimately, these data suggest that whether or not a decrease in intramuscular TG levels will be measured after a training regimen is dependent on the intramuscular TG levels before training.
It is important to realize that intracellular TG depots are largely inert and, in this sense, are less damaging than more labile lipid intermediates, such as ceramides, LC-CoA, and DGs. Thus, the more important question regarding the effects of exercise on intramuscular lipid intermediates is whether intramuscular levels of ceramides, DGs, or both, are reduced by regular exercise. Very few studies have examined the effects of short-term or regular exercise on the content of intracellular lipid intermediates, and the results have been conflicting. In line with the beneficial role of exercise in muscle insulin sensitivity, exercise training was shown to decrease intramuscular levels of DGs and ceramides in obese people.12 Conversely, short-term exercise was found to increase rather than decrease ceramide levels in lean people.92 Thus, whether changes in intracellular lipid accumulation are necessary to observe the beneficial role of short-term exercise, regular exercise, or both in muscle insulin sensitivity remains to be elucidated. However, regular physical activity is known to have other beneficial metabolic effects, including an increase in muscle oxidative capacity. Furthermore, as mentioned above, insulin resistance and intramuscular lipid accumulation are related to a mismatch between high rates of FA uptake and low rates of FA oxidation. Thus, it has been suggested that an exercise-induced increase in muscle oxidative capacity may be an important cellular mechanism through which regular exercise corrects the mismatch in FA kinetics and enhances the action of insulin in muscle.
Exercise and Fatty Acid Uptake
Short-term exercise has been shown to increase FA uptake across the working muscle in both isolated muscle preparations27,93 and exercising people.94 Thus, in subjects bicycling for 1 hour or performing 3 hours of knee extension resistance exercise, the level of FA uptake was higher during exercise than at rest and increased with exercise intensity up to 65% of maximal oxygen uptake.93,94 Because FA uptake is mediated by FA transporter proteins located at the plasma membrane, an increase in FA uptake should be accompanied by an increase in the content of CD36, FABPPM, or both, in the plasma membrane; this feature may be accompanied by changes in the total messenger RNA (mRNA) content or protein content of the FA transporter proteins, or both. In animal studies, evidence has shown that the plasma membrane content of CD36 increases with muscle contractions.35,95 However, in contrast to that of CD36, the plasma membrane content of FABPPM has been shown to remain unchanged after 75 minutes of running at moderate intensity.96
In human subjects, 1 hour of cycling exercise at moderate intensity did not change the mRNA or protein content of FABPPM or CD36.97 However, prolonged knee extension resistance exercise was shown to increase the mRNA content of CD36 and FABPPM in both trained and untrained subjects.34 The effects of exercise training on the mRNA and protein expression of CD36 and FABPPM in muscle also have been studied. In a cross-sectional study, FABPPM mRNA and protein levels were found to be higher in trained subjects than in untrained subjects.34 When subjects were trained with 3 weeks of intense one-legged endurance exercise, the FABPPM protein content was found to be higher in trained muscle than in untrained muscle.98 However, 9 days of cycling exercise at moderate intensity for 1 hour did not change the FABPPM mRNA or protein content.97 Conversely, this same training regimen was found to increase CD36 mRNA and protein levels.97
Together, these results show that careful attention will need to be paid to differences in exercise intensity and duration before any consensus on the cellular mechanisms regulating an exercise-induced increase in FA uptake can be reached. Furthermore, the effects of short-term or regular exercise on FA uptake and translocation of FABPPM, CD36, or both, to the plasma membrane have not been studied in insulin-resistant muscle. However, because the level of FA uptake and plasma membrane FA transporter protein content have been shown to be higher in insulin-resistant muscle, it is possible that the stimulatory effects of exercise on FA uptake and FA transporter protein translocation will be diminished in people with obesity, T2DM, or both. Furthermore, as mentioned above, FA uptake also is dependent on the plasma FA concentration (ie, availability)52 and thus, indirectly, on adipose tissue lipolysis. Because some studies have shown that regular exercise in subjects with obesity decreases the rate of basal adipose tissue lipolysis, it has been suggested that reduced plasma FA availability may be an important factor contributing to the accompanying improvement in insulin sensitivity.28 Alternatively, other studies have shown that regular exercise in subjects with obesity counteracts the decrease in adipose tissue lipolysis that often accompanies weight loss and thus helps in maintaining higher rates of FA oxidation.99 In line with this notion, it is possible that the well-known stimulatory effect of exercise on oxidative capacity may be the most beneficial adaptation for insulin-resistant muscle because it will increase FA oxidation rates and correct the imbalance between FA uptake and FA oxidation.
Exercise and Fatty Acid Oxidative Capacity
Rates of FA oxidation can increase 3- to 10-fold from resting values during exercise at mild to moderate intensity (commonly defined as exercise at 25%–65% of maximal oxygen consumption [V̇o2max]).100,101 As described above, insulin-resistant muscle is characterized by a reduction in oxidative capacity that is associated with lower rates of FA oxidation. This reduction in the ability of the muscle to use FA as oxidizable fuel extends to exercise conditions. Thus, FA oxidation has been shown to be impaired during exercise in people with obesity.49,50 When subjects with T2DM were compared with subjects with obesity and without diabetes and matched for fitness, rates of FA oxidation during submaximal exercise were found to be lower in the subjects with T2DM.102 Furthermore, rates of FA oxidation during submaximal exercise were found to be lower in patients who had undergone gastric bypass surgery that resulted in significant weight loss (∼50 kg), in subjects who were formerly obese than in weight-matched control subjects, and in subjects with glucose intolerance.49,50,103,104 These results emphasize the role of genetic defects in FA oxidative capacity and in the development of insulin resistance.103,104
After exercise training, the relative contribution of FA oxidation to total fuel demand increases in subjects who are healthy and performing moderate-intensity exercise. Data from cross-sectional93,105 and longitudinal94,106–108 studies have supported the notion that training reduces the reliance on carbohydrates as an energy source, thereby increasing FA oxidation during submaximal exercise. More importantly, the increased reliance on FA as a fuel source after exercise training has been observed in people with insulin resistance.109 Thus, an increase in FA oxidation rates during exercise may be critically important in eliminating the mismatch between FA uptake and FA oxidation that characterizes insulin-resistant muscle. In fact, an increase in oxidative capacity and an improvement in insulin sensitivity are highly correlated when people undergo a rigorous exercise training program.110
Because factors such as mitochondrial FA transport capacity through CD36 translocation, CPT1 activity, and mitochondrial oxidative capacity are known to play a role in the regulation of FA oxidation in muscle, training-induced changes in these parameters are usually recorded to determine their relative effects on training-induced alterations in FA oxidation. One of the key adaptations that takes place in skeletal muscle after training is an increase in the activity of the enzymes of the tricarboxylic acid cycle, the electron transport chain, and the β-oxidative pathway (necessary for FA oxidation).
In subjects who are healthy, 7 to 12 weeks of endurance training resulted in an increase in FA oxidation that was associated with an increase in the protein content of several enzymes, including the β-oxidative enzymes short-chain, medium-chain, and very long-chain acyl-CoA dehydrogenases and the tricarboxylic acid cycle enzyme citrate synthase.108,111 However, training effects have been measured with much shorter exercise programs. Seven sessions of high-intensity interval training over 2 weeks induced marked increases in whole-body and skeletal muscle capacity for FA oxidation during moderate-intensity exercise in moderately active women.112 Similarly, 9 days of cycling exercise at moderate intensity for 1 hour increased FA oxidation during exercise, and this effect was accompanied by an increase in CPT1 mRNA expression.97 In subjects who were sedentary and overweight or obese and who participated in a 4-month exercise training program that resulted in significant weight loss, the improvement in insulin sensitivity was accompanied by increases in oxidative enzyme activity and in mitochondrial functional capacity in muscle.113 After a training program of moderate-intensity exercise for a shorter duration (2 months), the improvement in glucose tolerance and metabolic function observed in subjects with obesity was accompanied by increases in mitochondrial FA oxidation and in CPT1 activity.12 More recent data have shown that multiple sessions of high-intensity exercise are better than a single, longer session of low-intensity exercise at improving blood glucose homeostasis in subjects with T2DM.114 Together, these results reinforce the notion that oxidative capacity is a critical factor that defines insulin sensitivity in muscle.
The effects of short-term or regular exercise on basal malonyl-CoA levels in the skeletal muscle of people who are healthy and people with obesity have not been studied extensively because it is difficult to accurately measure changes in malonyl-CoA levels in human muscle with the present technology. However, studies114,115 have shown that malonyl-CoA levels decrease during exercise and that basal malonyl-CoA levels are reduced after 12 weeks of exercise training. Because of the inverse relationship between malonyl-CoA levels and FA oxidation, lower malonyl-CoA levels are associated with higher rates of FA oxidation. These results suggest that a reduction in basal malonyl-CoA levels may be one of the cellular mechanisms through which regular exercise increases FA oxidation (Fig. 3). Interestingly, regular exercise has been shown to increase the association of CD36 with CPT1, suggesting that the translocation of CD36 to mitochondrial membranes may help in regulating the increase in FA oxidation.116 Combined with the beneficial effects of exercise training on oxidative capacity and malonyl-CoA levels, these results on the effects of regular physical activity on mitochondrial CD36 content suggest that all 3 factors may play important roles in raising the FA oxidative capacity of insulin-resistant muscle so that the mismatch between FA uptake and FA oxidation is corrected.
Figure 3.
Fatty acid (FA) uptake into skeletal muscle leads to accumulation or oxidation. Long-chain fatty acids (LCFA) are taken up into muscle cells through fatty acid transporters, including FABPPM and CD36. Once in the cell cytosol, FA are linked to coenzyme A (CoA), forming long-chain fatty acyl CoA (LCFA-CoA) molecules. The LCFA-CoA molecules may accumulate in the cytosol or serve as precursors for intracellular triacylglycerols (TG), diacylglycerols (DG), or ceramides, the accumulation of which is associated with insulin resistance. Alternatively, the LCFA-CoA molecules can be transported across mitochondrial membranes through the carnitine shuttle, which includes the enzyme carnitine palmitoyltransferase 1 (CPT1). The latter enzyme is potently inhibited (shown by the line with a rounded terminus) by malonyl coenzyme A (malonyl-CoA), the concentration of which is increased under many conditions, leading to insulin resistance, but decreased with exercise.183,184
Effects of Exercise on Glucose Metabolism
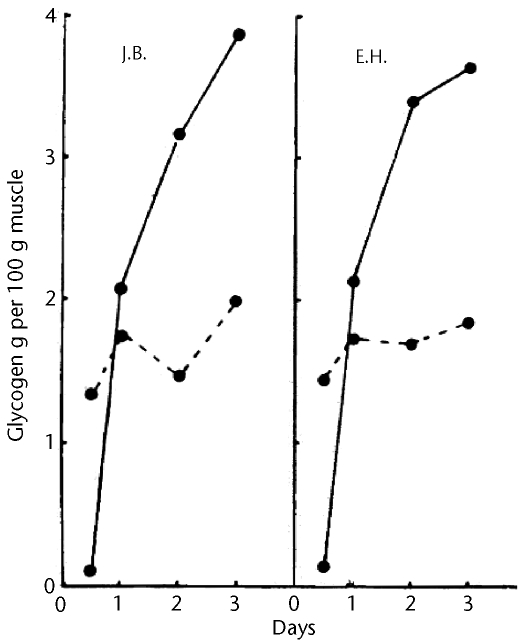
In 1966, Bergström and Hultman, who were already aware, from the work of others, that exercise decreased blood glucose concentrations and decreased the insulin requirement of people with diabetes, performed procedures to determine whether exercise would affect the rate of glycogen accumulation in skeletal muscle.117 Because there was a suggestion at the time that exercise released a blood-borne factor that stimulated glucose clearance from the bloodstream, Bergström and Hultman118 examined one-legged cycling performed until exhaustion so that the nonexercised leg could be used as a control. Immediately after exercise and each day for 3 days, quadriceps muscle biopsies were taken, and the glycogen content of the muscle was determined. Exercise depleted glycogen, but glycogen was completely replenished in the exercised leg by 1 day after exercise and, by 3 days after exercise, the glycogen content was twice that in the control leg (Fig. 4). Because the glycogen levels in the unexercised leg were essentially unchanged over the 3 days, the authors attributed the results to some effect within the exercised muscle—either an increase in glucose uptake or a direct stimulation of glycogen synthesis.
Figure 4.
Increase in glycogen accumulation after exercise. Glycogen was assessed in quadriceps muscle biopsies from both legs of each of 2 subjects after one-legged cycling exercise to exhaustion. Glycogen content is shown for exercised (solid lines) and control (dashed lines) legs. Reprinted with permission from Bergström J, Hultman E. Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature. 1966;210:309–310. Copyright 1966, Macmillan Publishers Ltd.
What was the underlying mechanism for the increased glycogen accumulation after exercise, as observed by Bergström and Hultman118 and later confirmed by others (see Holloszy et al119 for a discussion)? To answer this question, we discuss the effects of exercise on glucose metabolism during exercise, directly after exercise, and in the period from a few hours after exercise up to a few days after exercise. We generally confine our discussion to the effects of a single session of exercise on glucose metabolism, although it is clear that there is an additional effect of exercise training, that of increasing the action of insulin, which is independent of the effects of short-term exercise on insulin sensitivity.77,120 Long-term exercise training effects that increase the action of insulin are likely to include increased mitochondrial capacity, correction of a mismatch between FA uptake and FA oxidation, or both (as discussed above), but also may include increased activity, amount, or both, of key insulin signaling proteins, as discussed in a recent review by Hawley and Lessard.121
Exercise Stimulates Glucose Transport
Leg glucose uptake increases up to 20-fold during exercise in humans.122 The exercise-stimulated increases in leg glucose utilization123 and in the translocation of GLUT4 to the skeletal muscle cell membrane124 occur in people who are healthy and in patients with non–insulin-dependent diabetes. Likewise, whole-body glucose disposal increases during cycling exercise at 60% of V̇o2max in people who are healthy and in patients with T2DM.123 The increases in glucose transport into exercised muscle are transient, however. For example, in subjects with obesity (with and without T2DM), leg glucose uptake increased during cycling exercise (60% of V̇o2max) but returned to baseline values within about 0.5 hour of the end of exercise.125
Multiple factors in working skeletal muscle may be responsible for the insulin-dependent localization of GLUT4 at the cell membrane that underlies increased glucose transport during muscle contractions.126,127 A few of the leading candidates include increases in the cytosolic concentrations of calcium ions (Ca2+) and nitric oxide (NO), both of which have been found to be sufficient to stimulate glucose transport in muscle. In addition, a substantial increase in blood flow to working muscle during exercise contributes to glucose uptake in contracting muscle by boosting glucose availability to the muscle.128
Another factor that may be at least partially responsible for exercise-stimulated glucose transport is an increase in the ratio of adenosine monophosphate (AMP) to adenosine triphosphate (ATP). As ATP is hydrolyzed in working muscle (ATP→ADP+inorganic phosphate), an increase in the ADP level provides a substrate for a reaction that replenishes ATP but also produces AMP. The increase in the AMP/ATP ratio appears to work through the activation of a protein called AMP-activated protein kinase (AMPK) to stimulate glucose transport.63,129–132 5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR), a chemical that can be taken up by cells and converted to an AMP analog,133 also stimulates glucose transport in the skeletal muscle of rodents63,129 and humans.134,135 Although the activation of AMPK is sufficient to stimulate glucose transport, it appears from work with transgenic mice expressing inactive forms of AMPK or not expressing AMPK at all that AMPK is only partially necessary or is not required for the stimulation of glucose transport by muscle contractions.129,131,136 Most likely, AMPK stimulates glucose transport by inhibiting a protein called AS160, which generally prevents the distribution of GLUT4 to the cell membrane. Intriguingly, multiple signals that stimulate glucose transport (such as exercise, AICAR, and insulin) have in common the inhibition of AS160.137,138
Glycogen Synthase Activity Is Increased After Exercise
The insulin-dependent stimulation of glucose transport by exercise is transient; thus, it cannot explain the tremendous increase in muscle glycogen content that occurs after exercise. Interestingly, the activity of glycogen synthase (the rate-limiting enzyme in the formation of glycogen once glucose has entered the cell) is increased after cycling exercise in subjects who are healthy,139,140 in subjects with obesity and without diabetes,141 and in subjects with diabetes.141 This increase in glycogen synthase activity after exercise may be attributable, in part, to the decreased glycogen content of the exercised muscle, because there is an inverse relationship between the amount of glycogen in skeletal muscle and the activity of glycogen synthase.140 Glycogen synthase activity remains elevated after exercise (compared with the resting condition) for 48 hours.142 It has been suggested that a high level of glycogen synthase activity may itself be sufficient to promote increased glycogen synthesis,143 and this idea has been developed with regard to increased glycogen accumulation after exercise in a review by Christ-Roberts and Mandarino.144
Exercise Increases Insulin Sensitivity
The most important factor driving increased glucose disposal in skeletal muscle after a single session of exercise is a remarkable increase in insulin sensitivity, or an increase in the effectiveness of a given, submaximal (ie, physiological) concentration of insulin in stimulating glucose transport. Richter et al145 discovered a 2-fold increase in glucose transport stimulated by physiological insulin concentrations in perfused hind limbs of rats after treadmill running compared with that in resting rats. This astounding increase in insulin sensitivity after exercise was later confirmed in humans by Richter et al,146 who found a 2-fold increase in glucose uptake in response to low physiological insulin concentrations 4 hours after one-legged exercise in the exercised leg compared with the rested leg. That study by Richter et al was critical in establishing the site of increased action of insulin (ie, the exercised muscle), and the results were found to be reproducible147 (Fig. 5). However, it had been shown earlier that at 12 hours after glycogen-depleting exercise, whole-body insulin-stimulated glucose disposal was increased slightly (compared with the resting control condition).140 Similarly, insulin sensitivity (determined by using the concentration of insulin to obtain half of the maximal whole-body insulin-stimulated glucose disposal rate) was improved immediately after 60 minutes of cycling exercise in untrained men and remained elevated for 48 hours.142
Figure 5.
Leg insulin-stimulated glucose clearance is increased 4 hours after exercise. Four hours after one-legged cycling for 1 hour, leg glucose clearance (X̄±SE, n=7) was measured for rested (closed circles) and exercised (open circles) legs before (time 0), during (7–120 minutes), and after the infusion of insulin. Arterial insulin concentrations reached a plateau at ∼100 μU/mL by 15 minutes and returned to baseline values within 30 minutes after the cessation of the insulin infusion. During the insulin infusion, total glucose clearance was nearly 2-fold higher in the exercised leg than in the control leg (P<.005). Blood flow was similar at 0 minutes in the control and exercised legs but was slightly higher in the exercised leg than in the control leg during the insulin infusion (50±2 versus 42±3 mL/min/kg of muscle; P<.05). Reprinted with permission from Wojtaszewski JF, Hansen BF, Gade J, et al. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–331. Copyright 2000, American Diabetes Association.
The increase in insulin sensitivity in exercised muscle of humans reported by Richter et al146 was found in young men who were healthy and performed one-legged exercise for 1 hour at 75% of their one-legged work capacity. A variety of study designs have shown that increased insulin sensitivity also occurs in populations most likely to derive health benefits (eg, people with obesity, insulin resistance, or both) and after exercise of lesser intensity and duration. Cycling exercise sufficient to deplete glycogen led to increased insulin-stimulated nonoxidative glucose disposal when measured 12 hours later in men with non–insulin-dependent diabetes.76 Forty-five minutes of exercise at 65% of V̇o2max on a stair-climbing machine increased whole-body glucose disposal by greater than 20% and skeletal muscle glycogen synthesis by more than 60% when measured 48 hours after exercise in control subjects and subjects who were insulin-resistant.148 Insulin-stimulated glucose clearance was found to be increased (compared with the resting condition) 1 hour after treadmill exercise for 1 hour at an intensity that kept the heart rate at 150 to 160 beats per minute in subjects who were obese and did not have diabetes and in subjects who were obese and had diabetes.149 Interestingly, the intensity and duration of exercise were not sufficient to increase the action of insulin in lean control subjects.149 Similarly, a cycling exercise for 12 minutes (7 minutes at 60% of V̇o2max, 3 minutes at 100% of V̇o2max, and 2 minutes at 110% of V̇o2max) was sufficient to increase whole-body glucose disposal 24 hours after exercise in subjects with non–insulin-dependent diabetes but not in subjects who were healthy.150
Exercise training (ie, many exercise sessions over days, weeks, or months) provides the benefit of repeated increases in insulin-dependent glucose disposal (during and for a short time after exercise) and repeated periods of insulin sensitivity after exercise. However, an additional advantage of exercise training is that it increases the action of insulin even beyond what occurs after a single session of exercise. Because increases in the action of insulin after exercise are prolonged (up to 48 hours), measurements of the action of insulin would need to be obtained more than 2 days after the last exercise session of the training to make certain that improvements in the action of insulin were not attributable to the effects of a single session of exercise. In an example of a study designed to avoid the potential confounding effect of the last training session on the action of insulin (as opposed to a long-term training effect), DiPietro et al120 waited until 72 hours after the last exercise session of a 9-month endurance training program for previously inactive 73-year-old women to assess the action of insulin. Three days after the training had ceased, there was still a 20% increase in insulin-stimulated glucose disposal (compared with the pretraining measurement).
Dela et al77 demonstrated a training effect on insulin sensitivity. Their subjects performed one-legged cycling training (6 d/wk for 10 weeks, 30 min/d at 70% of V̇o2max), and leg glucose uptake was assessed 16 hours after the last exercise session. The subjects stopped training, and after 6 days, they performed one-legged exercise with the untrained leg. Comparing insulin-stimulated glucose uptake in the untrained leg after rest and 16 hours after a single session of exercise allowed the determination that the effect of exercise on the action of insulin at the intensity and duration used for these subjects had worn off by 16 hours after exercise. However, despite this finding, glucose uptake stimulated by a physiological concentration of insulin in the trained leg (16 hours after the last exercise session) was about 30% higher than that in the untrained leg (either with or without prior exercise). The use of the one-legged exercise model allowed the determination that the effect of training on insulin sensitivity was a local effect in the trained leg (versus the untrained leg) as opposed to a systemic effect (such as a reduction in adiposity).
Potential Mechanisms for Insulin Sensitivity After Exercise
Several candidate mediators of insulin sensitivity after exercise have been suggested.151,152 For example, Wojtaszewski et al151 made a strong case that some factor brought about by a decrease in glycogen concentrations may be responsible for increased insulin sensitivity, and they cited numerous examples of the inverse relationship between muscle glycogen content and the action of insulin. Certainly, increased skeletal muscle GLUT4 protein content by 3 hours,153 8 hours,154 or 1 day154 after exercise would contribute to the increased action of insulin. However, this increase in GLUT4 content is not necessarily apparent a few hours after exercise, when robust insulin sensitivity is already observable,147 and the prevention of protein synthesis after exercise (and thus the prevention of an increase in GLUT4 content) does not prevent the increased action of insulin.155 The activation of p38 mitogen-activated protein kinase (p38) (which is activated during exercise) is sufficient to increase insulin sensitivity in skeletal muscle.156 However, chemical inhibition of p38 does not prevent insulin sensitivity after exercise.156 Similarly, the activation of AMPK is sufficient to increase the action of insulin,155,157–159 although it is unknown whether AMPK activation is required for the exercise-related enhancement of insulin sensitivity. The phosphorylation of AS160 remains increased for several hours after exercise in rats160 and humans,161 and perhaps this prolonged phosphorylation of AS160 mediates the increased action of insulin after exercise.
It seems likely that there are redundant mechanisms for the increased action of insulin after exercise. For example, it has been suggested that any stimulus that increases GLUT4 translocation will lead to an increase in the action of insulin a few hours later.162 Thus, changes in muscle during exercise, such as increased Ca2+ levels, activation of AMPK, and increased NO levels (all of which are sufficient to stimulate GLUT4 translocation), may be mediators of the increased action of insulin after exercise. On the other hand, the increased action of insulin after exercise may simply be a result of increased FA oxidation. This notion is consistent with recent findings in cultured myotubes and animal muscle, which showed an increased action of insulin under conditions of increased fat oxidation.163–166
Relevance to Clinical Practice
Aerobic exercise has been the subject of most studies examining the link between exercise and the improved action of insulin. However, because clinicians often recommend progressive resistance exercise and eccentric exercise training for their patients, it is important to delve into the effects of these types of exercise on insulin resistance.
A thread of the discussion in this review is that increases in mitochondrial function subsequent to endurance exercise may play a role in reducing insulin resistance in patients with diabetes. For example, subjects with diabetes showed increases in citrate synthase (a mitochondrial enzyme of the Krebs cycle) activity, NADH oxidase (a mitochondrial enzyme in the electron transport chain) activity, and mitochondrial density after 4 months of aerobic training, and the changes in these measurements were highly correlated with an increase in insulin-stimulated glucose disposal.167 In contrast, increases in mitochondrial density are not thought to be likely to occur as a result of heavy resistance training in young people who are healthy.168 On the other hand, it has been shown that in elderly subjects, resistance training results in a substantial increase in the oxidative capacity of skeletal muscle.169 Thus, it seems possible that resistance exercise may increase mitochondrial function in people with impaired mitochondrial activity, such as elderly people169 or potentially even people with diabetes. More work needs to be done in this area.
Findings regarding an effect of short-term resistance exercise on the action of insulin seem promising but are far from conclusive. In one study,170 a single session of resistance exercise actually caused a decrease in the glucose infusion rate during an insulin clamp 24 hours after exercise, although there was no such decrease (but also no increase) in the action of insulin when the subjects exercised 3 times over 1 week. On the other hand, resistance exercise (3 sets of the 10-repetition maximum [RM] of upper- and lower-body exercises) reduced the glucose area under the curve by 15% (compared with a control measurement without previous exercise) during an oral glucose tolerance test that was given 6 hours after exercise.171 Similarly, resistance exercise (8 sets, 10 repetitions, 75% of 1 RM; leg press and leg extension) led to a 13% higher (compared with a control measurement without previous exercise) calculated glucose disappearance rate after an intravenous insulin injection given 24 hours after exercise.172 Of greatest relevance to the current review, a single session of resistance exercise (3 sets, 8–12 repetitions, 8 exercises) decreased the glucose area under the curve during an oral glucose tolerance test by about 12% (compared with the value without previous exercise) 24 hours after exercise in women with T2DM.173
Long-term resistance exercise training appears to reduce insulin resistance. In middle-aged male subjects with obesity, some of whom had T2DM, 3 months of resistance exercise (3 d/wk, 12–15 repetitions per set, 60%–70% of 1 RM, with number of sets increased as fitness improved) led to a 24% increase in whole-body glucose disposal during a euglycemic-hyperinsulinemic clamp 48 to 72 hours after the last exercise session.174 A 6-month resistance exercise training program (3 d/wk, 8–12 repetitions, 80% of 1 RM) in elderly subjects who were overweight or obese led to improved whole-body glucose disposal during an insulin clamp 24 hours after the last exercise session.175 In children with obesity, 8 weeks of resistance training (3 d/wk, 55%–65% of 1 RM) caused a 20% increase in the insulin clamp glucose infusion rate 48 hours after the last training session.176 Circuit resistance training by subjects with impaired glucose tolerance (3 sets, 8–10 repetitions per set, 50%–60% of 1 RM, 8 exercises involving large muscle groups, 3 d/wk) caused a 23% increase in insulin clamp glucose disposal 5 to 7 days after the final exercise session.177 Finally, there was a 48% increase in the glucose disposal rate during a euglycemic-insulinemic clamp after a training program that included many repetitions with light loads (2 sets, 10–20 repetitions, 40%–50% of 1 RM, 9 exercises, 5 d/wk) for subjects with T2DM.178 (Also see the article in this issue by Gulve179 on the implications of pharmacology and exercise in people with diabetes.)
Clinicians should take special note that eccentric exercise actually has negative effects on the action of insulin. For example, 30 minutes of downhill running caused muscle soreness, an increased level of serum creatine kinase (a marker of muscle damage), and a 36% decline in insulin-stimulated glucose disposal 48 hours after exercise.180 Likewise, 2 days after intense one-legged eccentric exercise (4 sets each of knee extension and flexion, 5 minutes per set, maximally resisting a machine-driven motion), a decline in muscle GLUT4 content and a 15% decrease in the glucose infusion rate during an insulin clamp were observed.181 The intensity of eccentric exercise used in clinical practice would be unlikely to cause such deleterious effects. However, this information provides a further basis for clinicians to caution patients performing eccentric exercise training to avoid muscle damage. (Also see the article by Marcus et al26 on effects of eccentric exercise in people with diabetes mellitus.)
Conclusion
A single session of exercise and exercise training powerfully regulate fat and glucose metabolism and result in an increased action of insulin. Although the mechanisms for the increased action of insulin after exercise are likely to be different for aerobic exercise and anaerobic exercise, it appears that both aerobic exercise and resistance exercise have the potential to improve the action of insulin in patients with T2DM. Clinicians can play a key role in educating patients about the value of both aerobic exercise and resistance exercise (in addition, of course, to the well-accepted role of dietary restriction in preventing insulin resistance182) in the control of blood glucose concentrations.
Both authors provided concept/idea/project design and writing.
Dr Fisher was supported by National Institutes of Health grant K01 DK066330 and, subsequently, by National Institutes of Health grant R15 DK080437. Dr Turcotte was supported by the Women in Science and Engineering (WiSE) and Zumberge Research and Innovation Fund (ZRIF) programs of the University of Southern California as well as by a grant from the National Institutes of Health (AR-45168).
References
- 1.Jue T, Rothman DL, Shulman GI, et al. Direct observation of glycogen synthesis in human muscle with 13C NMR. Proc Natl Acad Sci U S A. 1989;86:4489–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shulman GI, Rothman DL, Jue T, et al. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–228. [DOI] [PubMed] [Google Scholar]
- 3.Cline GW, Petersen KF, Krssak M, et al. Impaired glucose transport as a cause of decreased insulin-stimulated muscle glycogen synthesis in type 2 diabetes. N Engl J Med. 1999;341:240–246. [DOI] [PubMed] [Google Scholar]
- 4.Stehno-Bittel L. Intricacies of fat. Phys Ther. 2008;88:1265–1278. [DOI] [PubMed] [Google Scholar]
- 5.Cooney GJ, Thompson AL, Furler SM, et al. Muscle long-chain acyl CoA esters and insulin resistance. Ann N Y Acad Sci. 2002;967:196–207. [DOI] [PubMed] [Google Scholar]
- 6.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. [DOI] [PubMed] [Google Scholar]
- 7.Powell DJ, Turban S, Gray A, et al. Intracellular ceramide synthesis and protein kinase Czeta activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J. 2004;382:619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan DA, Lillioja S, Kriketos AD, et al. Skeletal muscle triglyceride levels are inversely related to insulin action. Diabetes. 1997;46:983–988. [DOI] [PubMed] [Google Scholar]
- 9.Bonen A, Parolin ML, Steinberg GR, et al. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004;18:1144–1146. [DOI] [PubMed] [Google Scholar]
- 10.Chen CN, Chuang LM, Wu YT. Clinical measures of physical fitness predict insulin resistance in people at risk for diabetes. Phys Ther. 2008;88:1355–1364. [DOI] [PubMed] [Google Scholar]
- 11.Straczkowski M, Kowalska I, Baranowski M, et al. Increased skeletal muscle ceramide level in men at risk of developing type 2 diabetes. Diabetologia. 2007;50:2366–2373. [DOI] [PubMed] [Google Scholar]
- 12.Bruce CR, Thrush AB, Mertz VA, et al. Endurance training in obese humans improves glucose tolerance and mitochondrial fatty acid oxidation and alters muscle lipid content. Am J Physiol Endocrinol Metab. 2006;291:E99–E107. [DOI] [PubMed] [Google Scholar]
- 13.Houmard JA, Tanner CJ, Yu C, et al. Effect of weight loss on insulin sensitivity and intramuscular long-chain fatty acyl-CoAs in morbidly obese subjects. Diabetes. 2002;51:2959–2963. [DOI] [PubMed] [Google Scholar]
- 14.Hegarty BD, Furler SM, Ye J, et al. The role of intramuscular lipid in insulin resistance. Acta Physiol Scand. 2003;178:373–383. [DOI] [PubMed] [Google Scholar]
- 15.Ellis BA, Poynten A, Lowy AJ, et al. Long-chain acyl-CoA esters as indicators of lipid metabolism and insulin sensitivity in rat and human muscle. Am J Physiol Endocrinol Metab. 2000;279:E554–E560. [DOI] [PubMed] [Google Scholar]
- 16.Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3–L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys. 2003;419:101–109. [DOI] [PubMed] [Google Scholar]
- 17.Withers DJ, White M. Perspective: the insulin signaling system—a common link in the pathogenesis of type 2 diabetes. Endocrinology. 2000;141:1917–1921. [DOI] [PubMed] [Google Scholar]
- 18.Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000;106:165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. [DOI] [PubMed] [Google Scholar]
- 20.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. [DOI] [PubMed] [Google Scholar]
- 21.Chabowski A, Chatham JC, Tandon NN, et al. Fatty acid transport and FAT/CD36 are increased in red but not in white skeletal muscle of ZDF rats. Am J Physiol Endocrinol Metab. 2006;291:E675–E682. [DOI] [PubMed] [Google Scholar]
- 22.Luiken JJ, Arumugam Y, Dyck DJ, et al. Increased rates of fatty acid uptake and plasmalemmal fatty acid transporters in obese Zucker rats. J Biol Chem. 2001;276:40567–40573. [DOI] [PubMed] [Google Scholar]
- 23.Turcotte LP, Swenberger JR, Zavitz TM, Yee AJ. Increased fatty acid uptake and altered fatty acid metabolism in insulin-resistant muscle of obese Zucker rats. Diabetes. 2001;50:1389–1396. [DOI] [PubMed] [Google Scholar]
- 24.Todd MK, Yaspelkis BB III, Turcotte LP. Short-term leptin treatment increases fatty acid uptake and oxidation in muscle of high fat-fed rats. Metabolism. 2005;54:1218–1224. [DOI] [PubMed] [Google Scholar]
- 25.Hilton TN, Tuttle LJ, Bohnert KL, et al. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88:1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marcus RL, Smith S, Morrell G, et al. Comparison of combined aerobic and high-force eccentric resistance exercise with aerobic exercise only for people with type 2 diabetes mellitus. Phys Ther. 2008;88:1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turcotte LP, Petry C, Kiens B, Richter EA. Contraction-induced increase in Vmax of palmitate uptake and oxidation in perfused skeletal muscle. J Appl Physiol. 1998;84:1788–1794. [DOI] [PubMed] [Google Scholar]
- 28.Shojaee-Moradie F, Baynes KC, Pentecost C, et al. Exercise training reduces fatty acid availability and improves the insulin sensitivity of glucose metabolism. Diabetologia. 2007;50:404–413. [DOI] [PubMed] [Google Scholar]
- 29.Bajaj M, Suraamornkul S, Romanelli A, et al. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes. 2005;54:3148–3153. [DOI] [PubMed] [Google Scholar]
- 30.Turcotte LP. Skeletal muscle lipid metabolism during exercise. In: Hargreaves M, Spriet L, eds. Exercise Metabolism. 2nd ed. Champaign, IL: Human Kinetics Publishers; 2006:105–136.
- 31.Bonen A, Dyck DJ, Luiken JJ. Skeletal muscle fatty acid transport and transporters. Adv Exp Med Biol. 1998;441:193–205. [DOI] [PubMed] [Google Scholar]
- 32.Keizer HA, Schaart G, Tandon NN, et al. Subcellular immunolocalisation of fatty acid translocase (FAT)/CD36 in human type-1 and type-2 skeletal muscle fibres. Histochem Cell Biol. 2004;121:101–107. [DOI] [PubMed] [Google Scholar]
- 33.Turcotte LP, Swenberger JR, Tucker MZ, Yee AJ. Training-induced elevation in FABP(PM) is associated with increased palmitate use in contracting muscle. J Appl Physiol. 1999;87:285–293. [DOI] [PubMed] [Google Scholar]
- 34.Kiens B, Roepstorff C, Glatz JF, et al. Lipid-binding proteins and lipoprotein lipase activity in human skeletal muscle: influence of physical activity and gender. J Appl Physiol. 2004;97:1209–1218. [DOI] [PubMed] [Google Scholar]
- 35.Bonen A, Luiken JJ, Arumugam Y, et al. Acute regulation of fatty acid uptake involves the cellular redistribution of fatty acid translocase. J Biol Chem. 2000;275:14501–14508. [DOI] [PubMed] [Google Scholar]
- 36.Luiken JJ, Dyck DJ, Han XX, et al. Insulin induces the translocation of the fatty acid transporter FAT/CD36 to the plasma membrane. Am J Physiol Endocrinol Metab. 2002;282:E491–E495. [DOI] [PubMed] [Google Scholar]
- 37.Steinberg GR, Dyck DJ, Calles-Escandon J, et al. Chronic leptin administration decreases fatty acid uptake and fatty acid transporters in rat skeletal muscle. J Biol Chem. 2002;277:8854–8860. [DOI] [PubMed] [Google Scholar]
- 38.Clarke DC, Miskovic D, Han XX, et al. Overexpression of membrane-associated fatty acid binding protein (FABPpm) in vivo increases fatty acid sarcolemmal transport and metabolism. Physiol Genomics. 2004;17:31–37. [DOI] [PubMed] [Google Scholar]
- 39.Bonen A, Han XX, Habets DD, et al. A null mutation in skeletal muscle FAT/CD36 reveals its essential role in insulin- and AICAR-stimulated fatty acid metabolism. Am J Physiol Endocrinol Metab. 2007;292:E1740–E1749. [DOI] [PubMed] [Google Scholar]
- 40.Coburn CT, Knapp FF Jr, Febbraio M, et al. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275:32523–32529. [DOI] [PubMed] [Google Scholar]
- 41.Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, et al. Impaired in vivo mitochondrial function but similar intramyocellular lipid content in patients with type 2 diabetes mellitus and BMI-matched control subjects. Diabetologia. 2007;50:113–120. [DOI] [PubMed] [Google Scholar]
- 42.Kelley DE, He J, Menshikova EV, Ritov VB Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. [DOI] [PubMed] [Google Scholar]
- 43.Holloway GP, Thrush AB, Heigenhauser GJ, et al. Skeletal muscle mitochondrial FAT/CD36 content and palmitate oxidation are not decreased in obese women. Am J Physiol Endocrinol Metab. 2007;292:E1782–E1789. [DOI] [PubMed] [Google Scholar]
- 44.Bruce CR, Anderson MJ, Carey AL, et al. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab. 2003;88:5444–5451. [DOI] [PubMed] [Google Scholar]
- 45.Ukropcova B, McNeil M, Sereda O, et al. Dynamic changes in fat oxidation in human primary myocytes mirror metabolic characteristics of the donor. J Clin Invest. 2005;115:1934–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hulver MW, Berggren JR, Cortright RN, et al. Skeletal muscle lipid metabolism with obesity. Am J Physiol Endocrinol Metab. 2003;284:E741–E747. [DOI] [PubMed] [Google Scholar]
- 47.Kim JY, Hickner RC, Cortright RL, et al. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1039–E1044. [DOI] [PubMed] [Google Scholar]
- 48.Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ranneries C, Bulow J, Buemann B, et al. Fat metabolism in formerly obese women. Am J Physiol. 1998;274:E155–E161. [DOI] [PubMed] [Google Scholar]
- 50.Thyfault JP, Kraus RM, Hickner RC, et al. Impaired plasma fatty acid oxidation in extremely obese women. Am J Physiol Endocrinol Metab. 2004;287:E1076–E1081. [DOI] [PubMed] [Google Scholar]
- 51.Newsholme EA, Leech AR. Biochemistry for the Medical Sciences. New York, NY: John Wiley and Sons; 1983:246–300.
- 52.Turcotte LP, Richter EA, Kiens B. Lipid metabolism in exercise. In: Hargreaves M, ed. Exercise Metabolism. Champaign, IL: Human Kinetics; 1995:99–130.
- 53.Wahren J, Hagenfeldt L, Felig P. Splanchnic and leg exchange of glucose, amino acids, and free fatty acids during exercise in diabetes mellitus. J Clin Invest. 1975;55:1303–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol. 1997;83:166–171. [DOI] [PubMed] [Google Scholar]
- 55.Heilbronn LK, Gan SK, Turner N, et al. Markers of mitochondrial biogenesis and metabolism are lower in overweight and obese insulin-resistant subjects. J Clin Endocrinol Metab. 2007;92:1467–1473. [DOI] [PubMed] [Google Scholar]
- 56.Mogensen M, Sahlin K, Fernstrom M, et al. Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Diabetes. 2007;56:1592–1599. [DOI] [PubMed] [Google Scholar]
- 57.Ritov VB, Menshikova EV, He J, et al. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. [DOI] [PubMed] [Google Scholar]
- 58.Befroy DE, Petersen KF, Dufour S, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56:1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drynan L, Quant PA, Zammit VA. Flux control exerted by mitochondrial outer membrane carnitine palmitoyltransferase over beta-oxidation, ketogenesis and tricarboxylic acid cycle activity in hepatocytes isolated from rats in different metabolic states. Biochem J. 1996;317:791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dobbins RL, Szczepaniak LS, Bentley B, et al. Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes. 2001;50:123–130. [DOI] [PubMed] [Google Scholar]
- 61.Bruce CR, Brolin C, Turner N, et al. Overexpression of carnitine palmitoyltransferase I in skeletal muscle in vivo increases fatty acid oxidation and reduces triacylglycerol esterification. Am J Physiol Endocrinol Metab. 2007;292:E1231–E1237. [DOI] [PubMed] [Google Scholar]
- 62.McGarry JD, Mills SE, Long CS, Foster DW. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues: demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983;214:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–E1112. [DOI] [PubMed] [Google Scholar]
- 64.Merrill GF, Kurth EJ, Rasmussen BB, Winder WW. Influence of malonyl-CoA and palmitate concentration on rate of palmitate oxidation in rat muscle. J Appl Physiol. 1998;85:1909–1914. [DOI] [PubMed] [Google Scholar]
- 65.Sidossis LS, Stuart CA, Shulman GI, et al. Glucose plus insulin regulate fat oxidation by controlling the rate of fatty acid entry into the mitochondria. J Clin Invest. 1996;98:2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sidossis LS, Mittendorfer B, Chinkes D, et al. Effect of hyperglycemia-hyperinsulinemia on whole body and regional fatty acid metabolism. Am J Physiol. 1999;276:E427–E434. [DOI] [PubMed] [Google Scholar]
- 67.Rasmussen BB, Holmback UC, Volpi E, et al. Malonyl coenzyme A and the regulation of functional carnitine palmitoyltransferase-1 activity and fat oxidation in human skeletal muscle. J Clin Invest. 2002;110:1687–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bell JA, Volpi E, Fujita S, et al. Dysregulation of muscle fatty acid metabolism in type 2 diabetes is independent of malonyl-CoA. Diabetologia. 2006;49:2144–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bezaire V, Bruce CR, Heigenhauser GJ, et al. Identification of fatty acid translocase on human skeletal muscle mitochondrial membranes: essential role in fatty acid oxidation. Am J Physiol Endocrinol Metab. 2006;290:E509–E515. [DOI] [PubMed] [Google Scholar]
- 70.Campbell SE, Tandon NN, Woldegiorgis G, et al. A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem. 2004;279:36235–36241. [DOI] [PubMed] [Google Scholar]
- 71.Holloway GP, Bezaire V, Heigenhauser GJ, et al. Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J Physiol. 2006;571:201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conn VS, Hafdahl AR, Mehr DR, et al. Metabolic effects of interventions to increase exercise in adults with type 2 diabetes. Diabetologia. 2007;50:913–921. [DOI] [PubMed] [Google Scholar]
- 73.Katzmarzyk PT, Leon AS, Wilmore JH, et al. Targeting the metabolic syndrome with exercise: evidence from the HERITAGE Family Study. Med Sci Sports Exerc. 2003;35:1703–1709. [DOI] [PubMed] [Google Scholar]
- 74.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cartee GD, Young DA, Sleeper MD, et al. Prolonged increase in insulin-stimulated glucose transport in muscle after exercise. Am J Physiol. 1989;256:E494–E499. [DOI] [PubMed] [Google Scholar]
- 76.Devlin JT, Hirshman M, Horton ED, Horton ES. Enhanced peripheral and splanchnic insulin sensitivity in NIDDM men after single bout of exercise. Diabetes. 1987;36:434–439. [DOI] [PubMed] [Google Scholar]
- 77.Dela F, Mikines KJ, von Linstow M, et al. Effect of training on insulin-mediated glucose uptake in human muscle. Am J Physiol. 1992;263:E1134–E1143. [DOI] [PubMed] [Google Scholar]
- 78.American Diabetes Association. Standards of medical care in diabetes—2007. Diabetes Care. 2007;30(suppl 1):S4–S41. [DOI] [PubMed] [Google Scholar]
- 79.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–1434. [DOI] [PubMed] [Google Scholar]
- 80.Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761. [DOI] [PubMed] [Google Scholar]
- 81.Thamer C, Machann J, Bachmann O, et al. Intramyocellular lipids: anthropometric determinants and relationships with maximal aerobic capacity and insulin sensitivity. J Clin Endocrinol Metab. 2003;88:1785–1791. [DOI] [PubMed] [Google Scholar]
- 82.Pruchnic R, Katsiaras A, He J, et al. Exercise training increases intramyocellular lipid and oxidative capacity in older adults. Am J Physiol Endocrinol Metab. 2004;287:E857–E862. [DOI] [PubMed] [Google Scholar]
- 83.van Loon LJ, Goodpaster BH. Increased intramuscular lipid storage in the insulin-resistant and endurance-trained state. Pflugers Arch. 2006;451:606–616. [DOI] [PubMed] [Google Scholar]
- 84.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest. 2007;117:1690–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ikeda S, Miyazaki H, Nakatani T, et al. Up-regulation of SREBP-1c and lipogenic genes in skeletal muscles after exercise training. Biochem Biophys Res Commun. 2002;296:395–400. [DOI] [PubMed] [Google Scholar]
- 86.Nadeau KJ, Ehlers LB, Aguirre LE, et al. Exercise training and calorie restriction increase SREBP-1 expression and intramuscular triglyceride in skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291:E90–E98. [DOI] [PubMed] [Google Scholar]
- 87.Guillet-Deniau I, Mieulet V, Le Lay S, et al. Sterol regulatory element binding protein-1c expression and action in rat muscles: insulin-like effects on the control of glycolytic and lipogenic enzymes and UCP3 gene expression. Diabetes. 2002;51:1722–1728. [DOI] [PubMed] [Google Scholar]
- 88.Nadeau KJ, Leitner JW, Gurerich I, Draznin B. Insulin regulation of sterol regulatory element-binding protein-1 expression in L-6 muscle cells and 3T3 L1 adipocytes. J Biol Chem. 2004;279:34380–34387. [DOI] [PubMed] [Google Scholar]
- 89.Commerford SR, Peng L, Dube JJ, O'Doherty RM. In vivo regulation of SREBP-1c in skeletal muscle: effects of nutritional status, glucose, insulin, and leptin. Am J Physiol Regul Integr Comp Physiol. 2004;287:R218–R227. [DOI] [PubMed] [Google Scholar]
- 90.Aas V, Kase ET, Solberg R, et al. Chronic hyperglycaemia promotes lipogenesis and triacylglycerol accumulation in human skeletal muscle cells. Diabetologia. 2004;47:1452–1461. [DOI] [PubMed] [Google Scholar]
- 91.Bruce CR, Kriketos AD, Cooney GJ, Hawley JA. Disassociation of muscle triglyceride content and insulin sensitivity after exercise training in patients with type 2 diabetes. Diabetologia. 2004;47:23–30. [DOI] [PubMed] [Google Scholar]
- 92.Helge JW, Dobrzyn A, Saltin B, Gorski J. Exercise and training effects on ceramide metabolism in human skeletal muscle. Exp Physiol. 2004;89:119–127. [DOI] [PubMed] [Google Scholar]
- 93.Turcotte LP, Richter EA, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am J Physiol. 1992;262:E791–E799. [DOI] [PubMed] [Google Scholar]
- 94.Bergman BC, Butterfield GE, Wolfel EE, et al. Evaluation of exercise and training on muscle lipid metabolism. Am J Physiol. 1999;276:E106–E117. [DOI] [PubMed] [Google Scholar]
- 95.Turcotte LP, Raney MA, Todd MK. ERK1/2 inhibition prevents contraction-induced increase in plasma membrane FAT/CD36 content and FA uptake in rodent muscle. Acta Physiol Scand. 2005;184:131–139. [DOI] [PubMed] [Google Scholar]
- 96.Turcotte LP, Zavitz MD. Plasma membrane FABP content is increased during conditions of increased fat oxidation. Physiologist. 1996;39:A13. [Google Scholar]
- 97.Tunstall RJ, Mehan KA, Wadley GD, et al. Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E66–E72. [DOI] [PubMed] [Google Scholar]
- 98.Kiens B, Kristiansen S, Jensen P, et al. Membrane associated fatty acid binding protein (FABPpm) in human skeletal muscle is increased by endurance training. Biochem Biophys Res Commun. 1997;231:463–465. [DOI] [PubMed] [Google Scholar]
- 99.Nicklas BJ, Rogus EM, Goldberg AP. Exercise blunts declines in lipolysis and fat oxidation after dietary-induced weight loss in obese older women. Am J Physiol. 1997;273:E149–E155. [DOI] [PubMed] [Google Scholar]
- 100.Romijn JA, Coyle EF, Sidossis LS, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–E391. [DOI] [PubMed] [Google Scholar]
- 101.van Loon LJ, Greenhaff PL, Constantin-Teodosiu D, et al. The effects of increasing exercise intensity on muscle fuel utilisation in humans. J Physiol. 2001;536:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Blaak EE, Aggel-Leijssen DP, Wagenmakers AJ, et al. Impaired oxidation of plasma-derived fatty acids in type 2 diabetic subjects during moderate-intensity exercise. Diabetes. 2000;49:2102–2107. [DOI] [PubMed] [Google Scholar]
- 103.Guesbeck NR, Hickey MS, MacDonald KG, et al. Substrate utilization during exercise in formerly morbidly obese women. J Appl Physiol. 2001;90:1007–1012. [DOI] [PubMed] [Google Scholar]
- 104.Mensink M, Blaak EE, van Baak MA, et al. Plasma free fatty acid uptake and oxidation are already diminished in subjects at high risk for developing type 2 diabetes. Diabetes. 2001;50:2548–2554. [DOI] [PubMed] [Google Scholar]
- 105.Coggan AR, Raguso CA, Gastaldelli A, et al. Fat metabolism during high-intensity exercise in endurance-trained and untrained men. Metabolism. 2000;49:122–128. [DOI] [PubMed] [Google Scholar]
- 106.Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol. 1993;469:459–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Friedlander AL, Casazza GA, Horning MA, et al. Effects of exercise intensity and training on lipid metabolism in young women. Am J Physiol. 1998;275:E853–E863. [DOI] [PubMed] [Google Scholar]
- 108.Horowitz JF, Leone TC, Feng W, et al. Effect of endurance training on lipid metabolism in women: a potential role for PPARalpha in the metabolic response to training. Am J Physiol Endocrinol Metab. 2000;279:E348–E355. [DOI] [PubMed] [Google Scholar]
- 109.Aggel-Leijssen DP, Saris WH, Wagenmakers AJ, et al. Effect of exercise training at different intensities on fat metabolism of obese men. J Appl Physiol. 2002;92:1300–1309. [DOI] [PubMed] [Google Scholar]
- 110.Goodpaster BH, Brown NF. Skeletal muscle lipid and its association with insulin resistance: what is the role for exercise? Exerc Sport Sci Rev. 2005;33:150–154. [DOI] [PubMed] [Google Scholar]
- 111.Tarnopolsky MA, Rennie CD, Robertshaw HA, et al. Influence of endurance exercise training and sex on intramyocellular lipid and mitochondrial ultrastructure, substrate use, and mitochondrial enzyme activity. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1271–R1278. [DOI] [PubMed] [Google Scholar]
- 112.Talanian JL, Galloway SD, Heigenhauser GJ, et al. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. J Appl Physiol. 2007;102:1439–1447. [DOI] [PubMed] [Google Scholar]
- 113.Menshikova EV, Ritov VB, Toledo FG, et al. Effects of weight loss and physical activity on skeletal muscle mitochondrial function in obesity. Am J Physiol Endocrinol Metab. 2005;288:E818–E825. [DOI] [PubMed] [Google Scholar]
- 114.Eriksen L, Dahl-Petersen I, Haugaard SB, Dela F. Comparison of the effect of multiple short-duration with single long-duration exercise sessions on glucose homeostasis in type 2 diabetes mellitus. Diabetologia. 2007;50:2245–2253. [DOI] [PubMed] [Google Scholar]
- 115.Kuhl JE, Ruderman NB, Musi N, et al. Exercise training decreases the concentration of malonyl CoA and increases the expression and activity of malonyl CoA decarboxylase in human muscle. Am J Physiol Endocrinol Metab. 2006;290:E1296-E1303. [DOI] [PubMed] [Google Scholar]
- 116.Roepstorff C, Halberg N, Hillig T, et al. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am J Physiol Endocrinol Metab. 2005;288:E133–E142. [DOI] [PubMed] [Google Scholar]
- 117.Schenk S, Horowitz JF. Coimmunoprecipitation of FAT/CD36 and CPT I in skeletal muscle increases proportionally with fat oxidation after endurance exercise training. Am J Physiol Endocrinol Metab. 2006;291:E254–E260. [DOI] [PubMed] [Google Scholar]
- 118.Bergström J, Hultman E. Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature. 1966;210:309–310. [DOI] [PubMed] [Google Scholar]
- 119.Holloszy JO, Kohrt WM, Hansen PA. Regulation of carbohydrate and fat metabolism during and after exercise. Front Biosci. 1998;3:D1011–D1027. [DOI] [PubMed] [Google Scholar]
- 120.DiPietro L, Dziura J, Yeckel CW, Neufer PD. Exercise and improved insulin sensitivity in older women: evidence of the enduring benefits of higher intensity training. J Appl Physiol. 2006;100:142–149. [DOI] [PubMed] [Google Scholar]
- 121.Hawley JA, Lessard SJ. Exercise training-induced improvements in insulin action. Acta Physiol (Oxf). 2008;192:127–135. [DOI] [PubMed] [Google Scholar]
- 122.Wahren J, Felig P, Ahlborg G, Jorfeldt L. Glucose metabolism during leg exercise in man. J Clin Invest. 1971;50:2715–2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Martin IK, Katz A, Wahren J. Splanchnic and muscle metabolism during exercise in NIDDM patients. Am J Physiol. 1995;269:E583–E590. [DOI] [PubMed] [Google Scholar]
- 124.Kennedy JW, Hirshman MF, Gervino EV, et al. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes. 1999;48:1192–1197. [DOI] [PubMed] [Google Scholar]
- 125.Minuk HL, Vranic M, Marliss EB, et al. Glucoregulatory and metabolic response to exercise in obese noninsulin-dependent diabetes. Am J Physiol. 1981;240:E458–E464. [DOI] [PubMed] [Google Scholar]
- 126.Richter EA, Derave W, Wojtaszewski JF. Glucose, exercise and insulin: emerging concepts. J Physiol. 2001;535:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sakamoto K, Goodyear LJ. Invited review: intracellular signaling in contracting skeletal muscle. J Appl Physiol. 2002;93:369–383. [DOI] [PubMed] [Google Scholar]
- 128.Defronzo RA, Ferrannini E, Sato Y, et al. Synergistic interaction between exercise and insulin on peripheral glucose uptake. J Clin Invest. 1981;68:1468–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mu J, Brozinick JT Jr, Valladares O, et al. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. [DOI] [PubMed] [Google Scholar]
- 130.Hayashi T, Hirshman MF, Kurth EJ, et al. Evidence for 5′ AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. [DOI] [PubMed] [Google Scholar]
- 131.Fujii N, Hirshman MF, Kane EM, et al. AMP-activated protein kinase alpha2 activity is not essential for contraction- and hyperosmolarity-induced glucose transport in skeletal muscle. J Biol Chem. 2005;280:39033–39041. [DOI] [PubMed] [Google Scholar]
- 132.Hayashi T, Hirshman MF, Fujii N, et al. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527–531. [DOI] [PubMed] [Google Scholar]
- 133.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside: a specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–565. [DOI] [PubMed] [Google Scholar]
- 134.Koistinen HA, Galuska D, Chibalin AV, et al. 5-Amino-imidazole carboxamide riboside increases glucose transport and cell-surface GLUT4 content in skeletal muscle from subjects with type 2 diabetes. Diabetes. 2003;52:1066–1072. [DOI] [PubMed] [Google Scholar]
- 135.Cuthbertson DJ, Babraj JA, Mustard KJ, et al. 5-Aminoimidazole-4-carboxamide 1-beta-d-ribofuranoside acutely stimulates skeletal muscle 2-deoxyglucose uptake in healthy men. Diabetes. 2007;56:2078–2084. [DOI] [PubMed] [Google Scholar]
- 136.Jørgensen SB, Viollet B, Andreelli F, et al. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside but not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. [DOI] [PubMed] [Google Scholar]
- 137.Kramer HF, Witczak CA, Taylor EB, et al. AS160 regulates insulin- and contraction-stimulated glucose uptake in mouse skeletal muscle. J Biol Chem. 2006;281:31478–31485. [DOI] [PubMed] [Google Scholar]
- 138.Thong FS, Bilan PJ, Klip A. The Rab GTPase-activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes. 2007;56:414–423. [DOI] [PubMed] [Google Scholar]
- 139.Koval JA, Maezono K, Patti ME, et al. Effects of exercise and insulin on insulin signaling proteins in human skeletal muscle. Med Sci Sports Exerc. 1999;31:998–1004. [DOI] [PubMed] [Google Scholar]
- 140.Bogardus C, Thuillez P, Ravussin E, et al. Effect of muscle glycogen depletion on in vivo insulin action in man. J Clin Invest. 1983;72:1605–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cusi K, Maezono K, Osman A, et al. Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105:311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mikines KJ, Sonne B, Farrell PA, et al. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol. 1988;254:E248–E259. [DOI] [PubMed] [Google Scholar]
- 143.Lawrence JC Jr, Roach PJ. New insights into the role and mechanism of glycogen synthase activation by insulin. Diabetes. 1997;46:541–547. [DOI] [PubMed] [Google Scholar]
- 144.Christ-Roberts CY, Mandarino LJ. Glycogen synthase: key effect of exercise on insulin action. Exerc Sport Sci Rev. 2004;32:90–94. [DOI] [PubMed] [Google Scholar]
- 145.Richter EA, Garetto LP, Goodman MN, Ruderman NB. Muscle glucose metabolism following exercise in the rat: increased sensitivity to insulin. J Clin Invest. 1982;69:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Richter EA, Mikines KJ, Galbo H, Kiens B. Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol. 1989;66:876–885. [DOI] [PubMed] [Google Scholar]
- 147.Wojtaszewski JF, Hansen BF, Gade J, et al. Insulin signaling and insulin sensitivity after exercise in human skeletal muscle. Diabetes. 2000;49:325–331. [DOI] [PubMed] [Google Scholar]
- 148.Perseghin G, Price TB, Petersen KF, et al. Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335:1357–1362. [DOI] [PubMed] [Google Scholar]
- 149.Burstein R, Epstein Y, Shapiro Y, et al. Effect of an acute bout of exercise on glucose disposal in human obesity. J Appl Physiol. 1990;69:299–304. [DOI] [PubMed] [Google Scholar]
- 150.Kjaer M, Hollenbeck CB, Frey-Hewitt B, et al. Glucoregulation and hormonal responses to maximal exercise in non-insulin-dependent diabetes. J Appl Physiol. 1990;68:2067–2074. [DOI] [PubMed] [Google Scholar]
- 151.Wojtaszewski JF, Nielsen JN, Richter EA. Invited review: effect of acute exercise on insulin signaling and action in humans. J Appl Physiol. 2002;93:384–392. [DOI] [PubMed] [Google Scholar]
- 152.Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol. 2005;99:338–343. [DOI] [PubMed] [Google Scholar]
- 153.Kraniou GN, Cameron-Smith D, Hargreaves M. Acute exercise and GLUT4 expression in human skeletal muscle: influence of exercise intensity. J Appl Physiol. 2006;101:934–937. [DOI] [PubMed] [Google Scholar]
- 154.Greiwe JS, Holloszy JO, Semenkovich CF. Exercise induces lipoprotein lipase and GLUT-4 protein in muscle independent of adrenergic-receptor signaling. J Appl Physiol. 2000;89:176–181. [DOI] [PubMed] [Google Scholar]
- 155.Fisher JS, Gao J, Han D-H, et al. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2002;282:E18–E23. [DOI] [PubMed] [Google Scholar]
- 156.Geiger PC, Wright DC, Han DH, Holloszy JO. Activation of p38 MAP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol Endocrinol Metab. 2004;288:E782–E788. [DOI] [PubMed] [Google Scholar]
- 157.Fisher JS. Potential role of the AMP-activated protein kinase in regulation of insulin action. Cellscience. 2006;2:68–81. [PMC free article] [PubMed] [Google Scholar]
- 158.Ju JS, Gitcho MA, Casmaer CA, et al. Potentiation of insulin-stimulated glucose transport by the AMP-activated protein kinase. Am J Physiol Cell Physiol. 2007;292:C564–C572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Smith JL, Patil PB, Fisher JS. AICAR and hyperosmotic stress increase insulin-stimulated glucose transport. J Appl Physiol. 2005;99:877–883. [DOI] [PubMed] [Google Scholar]
- 160.Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes. 2005;54:41–50. [DOI] [PubMed] [Google Scholar]
- 161.Howlett K, Mathews A, Garnham A, Sakamoto K. The effect of exercise and insulin on AS160 phosphorylation and 14–3-3 binding capacity in human skeletal muscle. Am J Physiol Endocrinol Metab. 2007;294:E401–E407. [DOI] [PubMed] [Google Scholar]
- 162.Geiger PC, Han DH, Wright DC, Holloszy JO. How muscle insulin sensitivity is regulated: testing of a hypothesis. Am J Physiol Endocrinol Metab. 2006;291:E1258–E1263. [DOI] [PubMed] [Google Scholar]
- 163.Abu-Elheiga L, Oh W, Kordari P, Wakil SJ. Acetyl-CoA carboxylase 2 mutant mice are protected against obesity and diabetes induced by high-fat/high-carbohydrate diets. Proc Natl Acad Sci U S A. 2003;100:10207–10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Harwood HJ Jr, Petras SF, Shelly LD, et al. Isozyme-nonselective N-substituted bipiperidylcarboxamide acetyl-CoA carboxylase inhibitors reduce tissue malonyl-CoA concentrations, inhibit fatty acid synthesis, and increase fatty acid oxidation in cultured cells and in experimental animals. J Biol Chem. 2003;278:37099–37111. [DOI] [PubMed] [Google Scholar]
- 165.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. [DOI] [PubMed] [Google Scholar]
- 166.Patil PB, Minteer SD, Mielke AA, et al. Malonyl coenzyme A affects insulin-stimulated glucose transport in myotubes. Arch Physiol Biochem. 2007; 113:13–24. [DOI] [PubMed] [Google Scholar]
- 167.Toledo FG, Menshikova EV, Ritov VB, et al. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56:2142–2147. [DOI] [PubMed] [Google Scholar]
- 168.Luthi JM, Howald H, Claassen H, et al. Structural changes in skeletal muscle tissue with heavy-resistance exercise. Int J Sports Med. 1986;7:123–127. [DOI] [PubMed] [Google Scholar]
- 169.Jubrias SA, Esselman PC, Price LB, et al. Large energetic adaptations of elderly muscle to resistance and endurance training. J Appl Physiol. 2001;90:1663–1670. [DOI] [PubMed] [Google Scholar]
- 170.Howlett KF, Sakamoto K, Garnham A, et al. Resistance exercise and insulin regulate AS160 and interaction with 14–3-3 in human skeletal muscle. Diabetes. 2007;56:1608–1614. [DOI] [PubMed] [Google Scholar]
- 171.Venables MC, Shaw CS, Jeukendrup AE, Wagenmakers AJ. Effect of acute exercise on glucose tolerance following post-exercise feeding. Eur J Appl Physiol. 2007;100:711–717. [DOI] [PubMed] [Google Scholar]
- 172.Koopman R, Manders RJ, Zorenc AH, et al. A single session of resistance exercise enhances insulin sensitivity for at least 24 h in healthy men. Eur J Appl Physiol. 2005;94:180–187. [DOI] [PubMed] [Google Scholar]
- 173.Fenicchia LM, Kanaley JA, Azevedo JL Jr, et al. Influence of resistance exercise training on glucose control in women with type 2 diabetes. Metabolism. 2004;53:284–289. [DOI] [PubMed] [Google Scholar]
- 174.Klimcakova E, Polak J, Moro C, et al. Dynamic strength training improves insulin sensitivity without altering plasma levels and gene expression of adipokines in subcutaneous adipose tissue in obese men. J Clin Endocrinol Metab. 2006;91:5107–5112. [DOI] [PubMed] [Google Scholar]
- 175.Ferrara CM, Goldberg AP, Ortmeyer HK, Ryan AS. Effects of aerobic and resistive exercise training on glucose disposal and skeletal muscle metabolism in older men. J Gerontol Biol Sci. 2006;61:480–487. [DOI] [PubMed] [Google Scholar]
- 176.Bell LM, Watts K, Siafarikas A, et al. Exercise alone reduces insulin resistance in obese children independently of changes in body composition. J Clin Endocrinol Metab. 2007;92:4230–4235. [DOI] [PubMed] [Google Scholar]
- 177.Eriksson J, Tuominen J, Valle T, et al. Aerobic endurance exercise or circuit-type resistance training for individuals with impaired glucose tolerance? Horm Metab Res. 1998;30:37–41. [DOI] [PubMed] [Google Scholar]
- 178.Ishii T, Yamakita T, Sato T, et al. Resistance training improves insulin sensitivity in NIDDM subjects without altering maximal oxygen uptake. Diabetes Care. 1998;21:1353–1355. [DOI] [PubMed] [Google Scholar]
- 179.Gulve EA. Exercise and glycemic control in diabetes: benefits, challenges, and adjustments to pharmacotherapy. Phys Ther. 2008;88:1297–1321. [DOI] [PubMed] [Google Scholar]
- 180.Kirwan JP, Hickner RC, Yarasheski KE, et al. Eccentric exercise induces transient insulin resistance in healthy individuals. J Appl Physiol. 1992;72:2197–2202. [DOI] [PubMed] [Google Scholar]
- 181.Asp S, Daugaard JR, Kristiansen S, et al. Eccentric exercise decreases maximal insulin action in humans: muscle and systemic effects. J Physiol. 1996;494:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Toledo FG, Menshikova EV, Azuma K, et al. Mitochondrial capacity in skeletal muscle is not stimulated by weight loss despite increases in insulin action and decreases in intramyocellular lipid content. Diabetes. 2008;57:987–994. [DOI] [PubMed] [Google Scholar]
- 183.Ruderman N, Prentki M. AMP kinase and malonyl-CoA: targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3:340–351. [DOI] [PubMed] [Google Scholar]
- 184.Ruderman NB, Saha AK, Vavvas D, et al. Lipid abnormalities in muscle of insulin-resistant rodents: the malonyl CoA hypothesis. Ann N Y Acad Sci. 1997;827:221–230. [DOI] [PubMed] [Google Scholar]