Abstract
Background and Purpose: The primary purpose of this study was to report differences in calf intermuscular adipose tissue (IMAT), muscle strength (peak torque), power, and physical function in individuals with obesity, diabetes mellitus (DM), and peripheral neuropathy (PN) compared with those without these impairments. A secondary purpose was to assess the relationship between IMAT and muscle strength, power, and physical function.
Subjects and Methods: Six participants with obesity, DM, and PN (2 women, 4 men; mean age=58 years, SD=10; mean body mass index=36.3, SD=5; mean modified Physical Performance Test [PPT] score=22, SD=3) and 6 age- and sex-matched control subjects without these impairments were assessed and compared in muscle strength, muscle power, physical functioning, and muscle and fat volume, including IMAT in the calf muscles. Muscle, adipose tissue, and IMAT volumes of each calf were quantified by noninvasive magnetic resonance imaging. Muscle strength and power of the plantar-flexor and dorsiflexor muscles were quantified using isokinetic dynamometry. The modified PPT was used to assess physical function.
Results: Leg muscle and fat volumes were similar between groups, although IMAT volumes were 2.2-fold higher in the subjects with obesity, DM, and PN (X̄=120 cm3, SD=47) than in the control subjects (X̄=54 cm3, SD=41). Muscle strength, muscle power, ratio of leg muscle power to leg muscle volume, and modified PPT scores were lower in subjects with obesity, DM, and PN compared with the control subjects.
Discussion and Conclusion: The data indicate that excess fat infiltration in leg skeletal muscles is associated with low calf muscle strength, low calf muscle power, and impaired physical function in individuals who are obese with DM and PN.
The incidence and prevalence of obesity, diabetes mellitus (DM), and their attendant complications are rising to epidemic levels.1–3 Obesity is a condition characterized by excessive fat accumulation, resulting in metabolic impairments, and is associated with increased risk for DM, cardiovascular disease, disability, and mortality.4–6 Diabetes mellitus is a chronic disease of carbohydrate, fat, and protein metabolism that affects more than 20 million people (diagnosed and undiagnosed) in the United States and can result in significant progressive and permanent physical disability.7–13 In 2002, DM was reported as the sixth leading cause of death, and the risk of mortality for individuals with DM is twice that of individuals of the same age without DM.1 Several comorbid conditions are related to and accompany DM, including coronary heart disease,10,11,13 obesity,10,11 arthritis,10,11 stroke,10 depression,13 and visual impairments10,11 and have been identified as contributors to DM-related disability.14,15 Individuals who are obese with DM and its complications often are referred to physical therapists and other rehabilitation specialists to help manage or reverse these metabolic diseases and their accompanying impairments.16
The full extent of the combined effects of obesity and DM and their respective complications on alterations in body composition and muscular function has not been thoroughly explored. The major tissue affected by disturbances in glucose metabolism is skeletal muscle, and deficits in metabolic signaling in this tissue contribute to systemic insulin resistance.7,17,18 Intermuscular adipose tissue (IMAT) is defined as visible adipose tissue beneath the muscle fascia and between muscle groups.19,20 It is negatively associated with insulin sensitivity in individuals with type 2 DM,21,22 suggesting that minimal IMAT is beneficial in these individuals. Regional adiposity negatively affects the function of the insulin receptors within the muscle and is negatively associated with insulin sensitivity through cytokine mediated pathways.8,17 Understanding the composition of skeletal muscle tissue and exploring the relationships among body composition, muscle impairments, and physical limitations may yield new and potentially important insights for improving all of the affected metabolic pathways. These pathways may help explain the profound impairments in physical function that characterize chronic obesity and diabetes and their common complications and may aid physical therapists in optimizing therapeutic strategies and interventions for rehabilitation.14,15
Approximately 60% to 70% of people with DM have mild to severe forms of nervous system disease.1 It has been estimated that nearly 50% of all people with DM will develop somatic sensory, motor, or autonomic peripheral neuropathy (PN) or a combination of these types of neuropathy 25 years or later after diagnosis.23 Individuals with DM and PN develop rapid muscle weakness and motor dysfunction.24 Andersen et al25 reported strength (force-generating capacity) losses of ∼21% in the ankle plantar-flexor and dorsiflexor muscles of subjects with DM. The severity of PN has been shown to be associated with muscle weakness in both type 1 and type 2 phenotypes of DM.25,26
The combined effects of obesity, DM, and PN on muscle performance remain a topic of keen interest to physical therapists because many individuals with DM are obese and, based on clinical observation, appear to have larger muscle masses compared with individuals without DM. This large muscle mass often can mislead the rehabilitation specialist to assume that individuals who are obese with DM and PN have normal or enhanced muscle strength and that any physical functional limitations are not the result of muscle weakness.27 Therefore, we aim to demonstrate that middle-aged adults who are obese with DM and PN have significant deficits in muscle performance and physical impairments compared with control subjects.
The purposes of this study were: (1) to assess IMAT content in the distal extremities of individuals with obesity, DM, and PN and compare them with age- and sex-matched individuals without these impairments using noninvasive magnetic resonance imaging (MRI) and (2) to examine the relationship of IMAT infiltration in distal extremities to muscle performance measures (strength and power) and physical function.
Method
Subjects
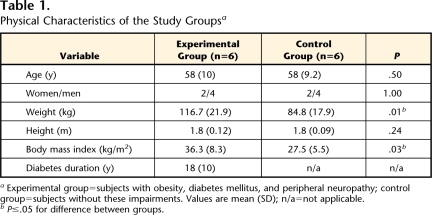
We studied 6 individuals with obesity, DM, and PN (4 men, 2 women; mean age=58 years, SD=10) and 6 individuals without these impairments (4 men, 2 women; mean age=58 years, SD=9.2). Participant physical characteristics are shown in Table 1. To be included in the study, subjects in the experimental group had to have a diagnosis of DM, had to have diminished or absent plantar sensation as assessed with Semmes-Weinstein monofilaments (inability to sense a 5.07 filament on at least one point on the plantar or dorsal surface of the foot) or vibration (inability to sense a 120-Hz tuning fork for at least 5 seconds), and had to be independent in ambulation without requiring use of an assistive device. Exclusion criteria for the experimental group were an active infection, a history of cerebrovascular accident or other neurological problems, and presence of non–MRI-compatible implants or devices or severe claustrophobia. The subjects in this group were compared with the age- and sex-matched control subjects. Subjects were not weight-matched for this exploratory study because we wanted to compare subjects with obesity, DM, and PN with subjects without these impairments to maximize visualization of fat infiltration. The exclusion criteria for the control group were a diagnosis of DM, an active infection, a history of cerebrovascular accident or other neurological problems, and presence of non–MRI-compatible implants or devices or severe claustrophobia. Study participants provided their written, informed consent to participate in the study. Study subjects were not remunerated for their participation.
Table 1.
Physical Characteristics of the Study Groupsa
Experimental group=subjects with obesity, diabetes mellitus, and peripheral neuropathy; control group=subjects without these impairments. Values are mean (SD); n/a=not applicable.
bP≤.05 for difference between groups.
Assessments
Isokinetic dynamometer measures of muscle performance.
All assessments were performed during one session throughout a single day. Several measures of muscle performance were used to compare the 2 groups. Concentric isokinetic and isometric ankle plantar-flexor and ankle dorsiflexor strength and power were assessed with a Biodex Multi-joint System 3 Pro isokinetic dynamometer.* Tests were performed at 0°/s (isometric) with the ankle fixed at 90 degrees (neutral) and at angular velocities of 60°/s and 120°/s over the available range of ankle motion (approximately 20° of dorsiflexion and 30° of plantar flexion).28 The mean values for muscle strength and power output of the 2 highest trials of 3 total trials were averaged. Peak torque (in newton-meters) was used as the measure of muscle strength. Average power (in watts) at each of the angular velocities was determined by the time-averaged integrated area under the curve at the constant velocity of movement in the available range of motion. Coefficients of variation for plantar-flexion peak torque and power in our laboratory were 6.9% and 7.9%, respectively.
Magnetic resonance imaging.
Distal lower-extremity muscle and adipose tissue volumes and IMAT were quantified using proton MRI. The adipose tissue volume includes subcutaneous fat and the visible adipose tissue beneath the muscle fascia and between muscle groups. The muscle and adipose tissue volumes were collected on both lower extremities for 5 subjects in each group and on the right lower extremity for 1 subject in each group. One subject with DM and PN had a below-knee amputation, so we acquired only data on one extremity for the matched control subject. The MRI scans were performed with the participants positioned supine. The MRI measurements were performed with a 1.5-T superconducting MRI instrument† with a conventional T1-weighted pulse sequence (echo time=12 milliseconds, repetition time=1,500 milliseconds, matrix=256 × 256) using a Siemens CP extremity coil. Thirty transverse images, 7 mm thick, were acquired beginning in the knee joint space and moving downward with no intersection gap between slices. Eight consecutive images were selected to calculate the muscle and adipose tissue volumes, and IMAT was quantified for each slice on a PC workstation using Analyze software (version 7.0),‡ which distinguishes muscle and adipose tissue based on pixel brightness.29 The IMAT interspersed between muscle was differentiated from the subcutaneous adipose tissue by drawing a line along the deep fascial plane surrounding the calf muscles.21 Slice-level volumes were summed for the 8 slices to represent total volumes in the longitudinal region of interest. Random-effects intraclass correlation coefficient values for repeated readings of the same scan by the same observer (interval: 7 days) averaged .98.
Physical Performance Test (modified PPT).
The severity of physical frailty in physical functioning was assessed using a modified PPT.30 The 9-item PPT31,32 consists of a combination of tests that correlate well with degree of disability, physical frailty, loss of independence, and mortality and with other objective measures of muscle strength, balance, and aerobic power.30–32 Each of the 9 items is timed and scored on a scale of 1 to 4 determined by the time required to complete the item (maximum score=36). Indexes of test-retest reliability for 3 physical therapists averaged .90 over a 7-day period in older adults.33
Data Analysis
The ratio of maximal muscle power output to muscle volume was calculated using the isokinetic power measures and the MRI volume values. Isometric peak torque and power at 60°/s and 120°/s of the ankle dorsiflexor and plantar-flexor muscles and PPT were compared between groups using multivariate analysis of variance. Pearson product moment correlations were used to assess the relationship among muscle performance measures, physical performance, and calf muscle, adipose tissue, and IMAT volumes.
Results
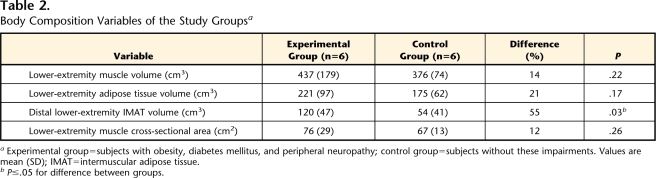
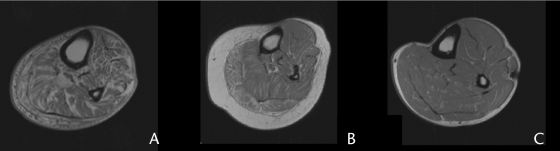
Total muscle volumes, fat volumes, IMAT volumes, values for muscle performance variables (peak torque and power), and PPT scores are reported in Tables 2 and 3. The IMAT volumes in the calf averaged 120 cm3 (SD=47) for the experimental group and 54 cm3 (SD=41) for the control group (P<.05). The IMAT volumes were 2.2-fold higher in the experimental group compared with the control group. Figure 1 illustrates the excessive IMAT volumes in 2 subjects with obesity, DM, and PN (Figs. 1A and 1B) compared with a control subject (Fig. 1C). Calf muscle volume was 14% greater in the experimental group compared with the control group. Despite the 14% increase, there was not a significant difference between groups. Calf adipose tissue volume was 1.3-fold higher in the experimental group compared with the control group, but this difference was not statistically significant. Distal lower-extremity muscle and adipose tissue volumes were similar, despite the higher body mass index in the subjects with obesity, DM, and PN, suggesting that excess fat accumulation in body regions other than the distal lower extremity account for greater whole-body adiposity.
Table 2.
Body Composition Variables of the Study Groupsa
Experimental group=subjects with obesity, diabetes mellitus, and peripheral neuropathy; control group=subjects without these impairments. Values are mean (SD); IMAT=intermuscular adipose tissue.
bP≤.05 for difference between groups.
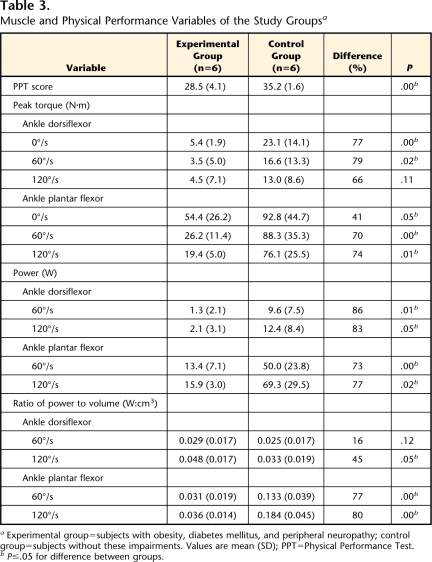
Table 3.
Muscle and Physical Performance Variables of the Study Groupsa
Experimental group=subjects with obesity, diabetes mellitus, and peripheral neuropathy; control group=subjects without these impairments. Values are mean (SD); PPT=Physical Performance Test.
bP≤.05 for difference between groups.
Figure 1.
Magnetic resonance image of the distal lower extremity acquired at 1.5T in: (A) a 57-year-old male participant with diabetes mellitus, peripheral neuropathy, and a body mass index (BMI) of 27.3; (2) a 58-year-old female participant with diabetes mellitus, peripheral neuropathy, and a BMI of 45.4, and (C) a 50-year-old male participant without diabetes mellitus or peripheral neuropathy and a BMI of 24.5. Note the excessive intermuscular adipose tissue volume in images A and B.
Strength in the ankle dorsiflexor and plantar-flexor muscles was 41% to 79% lower (P<.05, except for no significant difference in ankle dorsiflexion at 120°/s) in the experimental group compared with the control group (Tab. 3). Muscle power was 83% to 86% lower in the ankle dorsiflexors and 73% to 77% lower in the ankle plantar-flexors (P<.05) in the experimental group compared with the control group (Tab. 3). The ratio of maximal plantar-flexor muscle power output to lower-extremity muscle volume was lower at both 60°/s (0.133 vs 0.031, P<.05) and 120°/s (0.184 vs 0.036, P<.05) for the control group and the experimental group, respectively (Tab. 3). This finding indicates the extent of the severely impaired muscle performance in the subjects with obesity, DM, and PN.
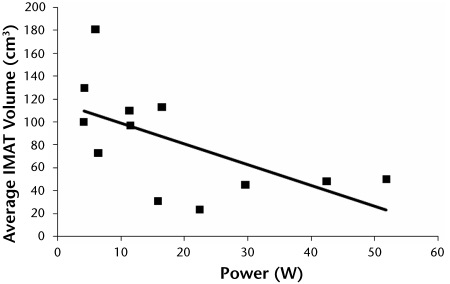
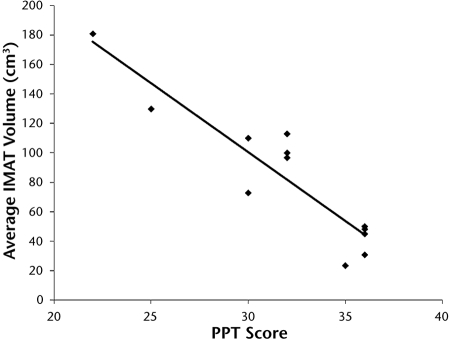
A higher volume of IMAT occurred in the lower-extremity muscles of the subjects with obesity, DM, and PN compared with the control subjects and was associated with weaker distal dorsiflexor and plantar-flexor muscle strength (r=−.13 to −.99). Figure 2 illustrates the relationship between average lower-extremity power at 60°/s and average IMAT volume (r=−.60). As indicated in Figure 3, a higher volume of IMAT in the lower-extremity muscles was associated with a lower PPT score (r=−.92).
Figure 2.
Relationship between average dorsiflexion and plantar-flexion muscle power at 60°/s and average bilateral distal lower-extremity intermuscular adipose tissue (IMAT) volume (cm3) (r=−.60).
Figure 3.
Relationship between Physical Performance Test (PPT) score and average bilateral distal lower-extremity intermuscular adipose tissue (IMAT) volume (cm3) (r= −.92).
Discussion
In this study, IMAT volume was 2 times higher and ratios of muscle power to muscle volume were lower in the subjects with obesity, DM, and PN compared with the age- and sex-matched control subjects. Muscle weakness in people with obesity, DM, and PN has been demonstrated previously. Andersen et al26 reported decreased strength and function in individuals with DM. However, their study did not examine body composition changes or specific muscle performance variables (eg, power or ratio of power to volume). Our data are consistent with the findings of Park et al,15 who found lower “muscle quality” in older adults with type 2 DM. Park et al utilized dual-energy x-ray absorptiometry to assess regional muscle appendicular mass and defined muscle quality as muscle strength per unit of regional muscle mass. They found that strength per unit of muscle mass was significantly lower in subjects with DM in both lower and upper extremities compared with subjects without DM.15
The neuropathic process also may help to explain the mechanism of and dramatic reductions in muscle strength, power, and function in individuals with obesity, DM, and PN. Andersen et al34 demonstrated that decreased muscle strength is a result of incomplete reinnervation after axonal loss in people with DM and PN. The results of our study also suggest that lower-extremity muscles (extrinsic muscles of the foot) may have decreased strength and power as a result of the neuropathy. Andersen et al24 used MRI to demonstrate that muscle atrophy underlies motor weakness of the distal extremities in people with type 1 DM and PN. In that study, ankle dorsiflexor and plantar-flexor muscle volumes were 32% lower in subjects with DM and PN compared with subjects without neuropathy.24 In an ensuing study,35 the volume of the intrinsic foot muscles was 50% lower in subjects with DM and PN compared with subjects with diabetes but without neuropathy and control subjects, suggesting more atrophy in distal muscles than in proximal muscles.
The Health, Aging, and Body Composition (Health ABC) study confirmed that both arm and leg muscle mass do not adequately represent the overall muscle strength in older adults with DM.15 In that study, 485 older adults with type 2 DM had larger arm and leg muscle mass than participants without DM. Despite the larger muscle mass, men with DM had lower muscle strength and the strength in women with DM was not significantly higher than in individuals without DM.15 The authors in that study concluded that DM is associated with lower strength and muscle quality (defined as muscle strength per unit of regional appendicular muscle mass as assessed by dual-energy x-ray absorptiometry) and may contribute to the development of disability in older adults with DM.15 An earlier Health ABC study was the first to report longitudinal data on changes in muscle mass and strength in people with type 2 DM.14 That study showed people with type 2 DM experience a more rapid and profound decline in muscle strength and muscle quality compared with people without DM.14 Despite the high prevalence of DM and accompanying PN and the potentially devastating effects on strength, power, and function, little is known about the additive influence of neuropathy on skeletal muscles and its impact on physical function.
We demonstrate several important physiological and performance impairments between people with obesity, DM, and PN compared with age- and sex-matched controls that physical therapists and rehabilitation specialists should recognize. Subjects who were obese with chronic DM and PN were much weaker (lower peak torque and power) in ankle dorsiflexor and plantar-flexor muscles than the control group. Subjects with obesity, DM, PN were 41% to 79% weaker in both groups of leg muscles at 0°/s, 60°/s, and 120°/s. The deficits in muscle power for subjects with obesity, DM, and PN were even larger than deficits in muscle strength and appeared to be magnified at higher limb velocities in the posterior calf muscles. These observations are consistent with type II muscle fiber atrophy that occurs in the presence of chronic motor PN.36 Further study is needed to determine whether exercise can help increase muscle strength in individuals with weakness secondary to obesity, DM, and PN.
The subjects with obesity, DM, and PN had significantly more IMAT volume in their distal lower extremities than the control subjects. Although body mass index was greater in the experimental group compared with the control group, there was no significant difference between groups in the estimated volume of muscle or adipose tissue in the distal lower extremities. Despite having similar volumes of muscle mass in the distal lower extremities, the experimental group had profoundly weaker dorsiflexor and plantar-flexor muscles as measured by isometric and concentric isokinetic torque and power values. These data are somewhat surprising because muscle mass and muscle cross-sectional area have been shown to be principal factors for determining muscle strength in older adults.15,21,37–39 Decreases in lean tissue and excessive IMAT infiltration may help explain the mechanism for decreased muscle strength, power, and function in individuals with obesity, DM, and PN.
In addition to playing a principal role in the metabolic disturbances associated with obesity, chronic DM, and PN, excess IMAT infiltration in and around key lower-extremity muscles may have a profound detrimental impact on muscle performance and, ultimately, the performance of instrumental and basic activities of daily living. We found a high inverse (negative) correlation between IMAT volumes and PPT scores in the subjects who were obese with DM and PN (Fig. 3). Whether this is a direct or indirect association cannot be fully ascertained by our preliminary observations, although Frimel et al40 observed similar strong associations between PPT scores and the content of lipid accumulation inside muscle cells (intramyocellular lipid [IMCL]) and immediately surrounding muscle cells (extramyocelluar lipid [EMCL]) in calf muscles with distinctly variable fiber types in elderly individuals with obesity but without DM or PN. They found that high IMCL and EMCL content in the medial gastrocnemius muscle contributes to the negative correlation between loss of muscle power and PPT scores, whereas IMCL and EMCL content in the soleus muscle does not have the same relationship to muscle power or PPT scores.
Study Limitations
There are several limitations to our study. The sample size was relatively small. The small sample size, however, can still be representative of the population at large of people with obesity, diabetes, and neuropathies. Using a cross-sectional study design, we demonstrated an association between increased IMAT volumes and decreased muscle strength, power, and physical function in individuals with multiple comorbidities that directly or indirectly affect skeletal muscles and physical functioning. Furthermore, with regard to the PPT scores being higher than 30 or lower than 25, even if we remove the 2 highest or 2 lowest performers, the correlations do not significantly differ compared with the current values with all subjects included. We interpret these results to indicate that excessive fat infiltration is associated with reduced physical performance. Although our design did not allow us to determine individual contributions from comorbidities, there are previous reports34,41–49 that obesity alone and neuropathy alone have shown excessive fat and reduced strength, power, mobility, and performance results, indicating a potential cumulative effect in those people with obesity and neuropathy. There is the possibility that increased amounts of IMAT may cause the development of insulin resistance that is the primary characteristic in muscles of people with DM and various forms of PN.18 Prospective studies are necessary to determine whether lower muscle strength and power are predictive of the onset of disability in people who are obese with DM and PN.15
Another limitation is that we did not assess glucose tolerance, glycemic control, or the severity of insulin resistance in our participants. Park et al15 demonstrated that poorer muscle quality in people with DM is associated with a longer duration of DM and poor glycemic control. Because the average length of time that our subjects were diagnosed with DM was 18 years, we suspect insulin resistance was a common pathological feature. Muscle atrophy and catabolism are metabolic consequences of uncontrolled hyperglycemia that can lead to muscle protein breakdown, inefficient energy use, and decreased muscle function. Poor glycemic control in people with DM also is related to increases in inflammatory cytokines that can cause decreases in muscle function.15,50–53 Therefore, in future studies, it would be useful to determine the isolated impact of severity of the diabetic state.
Even with the noted limitations, this study presents preliminary data so that we may begin to characterize the multiple impairments in skeletal muscle in people with obesity, chronic DM, and PN. A more comprehensive and thorough understanding of the muscular changes that occur due to obesity, DM, or PN and the impact of affected muscles on physical function is essential for the development of effective therapeutic interventions. Noninvasive MRI has the potential to increase our understanding of muscle dysfunction and to optimize rehabilitation strategies for individuals with metabolic impairments and their complications such as neuropathy. The mechanism for decreased physical function in people with DM is critical for physical therapists to elucidate so that we may determine the optimal rehabilitation strategies for the recovery of muscle function in individuals with DM and develop exercise therapies to prevent further disability (see the article by Marcus et al54 in this special issue for exercise intervention in people with DM).
Conclusions
The epidemic growth in the incidence and prevalence of obesity, DM, and their complications is of great concern to physical therapists and other rehabilitation specialists. Diabetes mellitus and obesity can result in serious medical complications, and when PN accompanies obesity and DM, severe decreases in physical functioning can drastically impair quality of life. An increase in IMAT content may be an important indicator of dysfunctional substrate metabolism in people with obesity, DM, and PN. Future studies aimed at assessing regional lipid accumulation in key skeletal muscles and how lipid content will change as a result of therapeutic interventions for the remediation of obesity, DM, and PN will become very relevant to physical therapists and rehabilitation specialists.
Dr Hilton and Dr Sinacore provided concept/idea/research design and data analysis. Dr Hilton, Ms Tuttle, Dr Mueller, and Dr Sinacore provided writing. Dr Hilton, Ms Tuttle, and Ms Bohnert provided data collection and clerical support. Dr Hilton, Ms Tuttle, and Dr Sinacore provided project management. Dr Mueller and Dr Sinacore provided fund procurement, subjects, and institutional liaisons. Dr Sinacore provided facilities/equipment. All authors provided consultation (including review of manuscript before submission).
The study protocol was approved by the Human Research Protection Office at Washington University School of Medicine.
Dr Hilton was supported by a New Investigator Fellowship Training Initiative from the Foundation for Physical Therapy and by National Center for Medical Rehabilitation Research/National Institutes of Health grant T32 HD007434-16. Ms Tuttle was supported by a Foundation for Physical Therapy Promotion of Doctoral Studies Scholarship (PODS I).
Biodex Medical Systems, 20 Ramsay Rd, Shirley, NY 11967-4704.
Siemens Corp, Citicorp Center, 153 E 53rd St, New York, NY 10022-4611.
Mayo Clinic, 200 First St SW, Rochester, MN 55905.
References
- 1.National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2005. Atlanta, GA: US Dept of Health and Human Services, Centers for Disease Control and Prevention; 2005.
- 2.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787. [DOI] [PubMed] [Google Scholar]
- 3.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA. 2007;298:2020–2027. [DOI] [PubMed] [Google Scholar]
- 6.Katzmarzyk PT, Janssen I, Ardern CI. Physical inactivity, excess adiposity and premature mortality. Obes Rev. 2003;4:257–290. [DOI] [PubMed] [Google Scholar]
- 7.Turcotte LP, Fisher JS. Skeletal muscle insulin resistance: roles of fatty acid metabolism and exercise. Phys Ther. 2008;88:1279–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stehno-Bittel L. Intricacies of fat. Phys Ther. 2008;88:1265–1278. [DOI] [PubMed] [Google Scholar]
- 9.Ryerson B, Tierney EF, Thompson TJ, et al. Excess physical limitations among adults with diabetes in the US population, 1997–1999. Diabetes Care. 2003;26:206–210. [DOI] [PubMed] [Google Scholar]
- 10.Gregg EW, Beckles GL, Williamson DF, et al. Diabetes and physical disability among older US adults. Diabetes Care. 2000;23:1272–1277. [DOI] [PubMed] [Google Scholar]
- 11.Gregg EW, Mangione CM, Cauley JA, et al. Diabetes and incidence of functional disability in older women. Diabetes Care. 2002;25:61–67. [DOI] [PubMed] [Google Scholar]
- 12.De Rekeneire N, Resnick HE, Schwartz AV, et al. Diabetes is associated with subclinical functional limitation in nondisabled older individuals: the Health, Aging, and Body Composition study. Diabetes Care. 2003;26:3257–3263. [DOI] [PubMed] [Google Scholar]
- 13.Von Korff M, Katon W, Lin EH, et al. Work disability among individuals with diabetes. Diabetes Care. 2005;28:1326–1332. [DOI] [PubMed] [Google Scholar]
- 14.Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the Health, Aging, and Body Composition study. Diabetes Care. 2007;30:1507–1512. [DOI] [PubMed] [Google Scholar]
- 15.Park SW, Goodpaster BH, Strotmeyer ES, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the Health, Aging, and Body Composition study. Diabetes. 2006;55:1813–1818. [DOI] [PubMed] [Google Scholar]
- 16.Kirkness CS, Marcus RL, LaStayo PC, et al. Diabetes and associated risk factors in patients referred for physical therapy in a national primary care electronic medical record database. Phys Ther. 2008;88:1408–1416. [DOI] [PubMed] [Google Scholar]
- 17.Wei Y, Chen K, Whaley-Connell AT, et al. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol. 2008;294:R673–R680. [DOI] [PubMed] [Google Scholar]
- 18.Sinacore DR, Gulve EA. The role of skeletal muscle in glucose transport, glucose homeostasis, and insulin resistance: implications for physical therapy. Phys Ther. 1993;73:878–891. [DOI] [PubMed] [Google Scholar]
- 19.Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35:9–16. [DOI] [PubMed] [Google Scholar]
- 20.Ruan XY, Gallagher D, Harris T, et al. Estimating whole body intermuscular adipose tissue from single cross-sectional magnetic resonance images. J Appl Physiol. 2007;102:748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. [DOI] [PubMed] [Google Scholar]
- 22.Goodpaster BH, Thaete FL, Kelley DE. Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 2000;71:885–892. [DOI] [PubMed] [Google Scholar]
- 23.National Diabetes Information Clearing House. Diabetes Statistics. Washington, DC: US Dept of Health and Human Services; 1995:96–3926. NIH publication.
- 24.Andersen H, Gadeberg PC, Brock B, Jakobsen J. Muscular atrophy in diabetic neuropathy: a stereological magnetic resonance imaging study. Diabetologia. 1997;40:1062–1069. [DOI] [PubMed] [Google Scholar]
- 25.Andersen H, Poulsen PL, Mogensen CE, Jakobsen J. Isokinetic muscle strength in long-term IDDM patients in relation to diabetic complications. Diabetes. 1996;45:440–445. [DOI] [PubMed] [Google Scholar]
- 26.Andersen H, Nielsen S, Mogensen CE, Jakobsen J. Muscle strength in type 2 diabetes. Diabetes. 2004;53:1543–1548. [DOI] [PubMed] [Google Scholar]
- 27.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–934. [DOI] [PubMed] [Google Scholar]
- 28.Villareal DT, Banks M, Siener C, et al. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913–920. [DOI] [PubMed] [Google Scholar]
- 29.Ory MG, Schechtman KB, Miller JP, et al. Frailty and injuries in later life: the FICSIT trials. J Am Geriatr Soc. 1993;41:283–296. [DOI] [PubMed] [Google Scholar]
- 30.Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000;55:M350–M355. [DOI] [PubMed] [Google Scholar]
- 31.Binder EF, Birge SJ, Spina R, et al. Peak aerobic power is an important component of physical performance in older women. J Gerontol A Biol Sci Med Sci. 1999;54:M353–M356. [DOI] [PubMed] [Google Scholar]
- 32.Brown M, Sinacore DR, Ehsani AA, et al. Low-intensity exercise as a modifier of physical frailty in older adults. Arch Phys Med Rehabil. 2000;81:960–965. [DOI] [PubMed] [Google Scholar]
- 33.Host HH, Sinacore DR, Brown M, Holloszy JO. Reliability of the modified Physical Performance Test in older adults [abstract]. Phys Ther. 1996;76:S23–S24. [Google Scholar]
- 34.Andersen H, Stalberg E, Gjerstad MD, Jakobsen J. Association of muscle strength and electrophysiological measures of reinnervation in diabetic neuropathy. Muscle Nerve. 1998;21:1647–1654. [DOI] [PubMed] [Google Scholar]
- 35.Andersen H, Gjerstad MD, Jakobsen J. Atrophy of foot muscles: a measure of diabetic neuropathy. Diabetes Care. 2004;27:2382–2385. [DOI] [PubMed] [Google Scholar]
- 36.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roubenoff R. Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci. 2003;58:1012–1017. [DOI] [PubMed] [Google Scholar]
- 38.Hughes VA, Frontera WR, Wood M, et al. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci. 2001;56:B209–B217. [DOI] [PubMed] [Google Scholar]
- 39.Frontera WR, Hughes VA, Fielding RA, et al. Aging of skeletal muscle: a 12-year longitudinal study. J Appl Physiol. 2000;88:1321–1326. [DOI] [PubMed] [Google Scholar]
- 40.Frimel TN, Sinacore DR, Wright NR, et al. Sarcopenia in frail elderly obese subjects: role of excess lipid accumulation [abstract]. Journal of Geriatric Physical Therapy. 2007;30(3):A138. [Google Scholar]
- 41.Galanos AN, Pieper CF, Cornoni-Huntley JC, et al. Nutrition and function: is there a relationship between body mass index and the functional capabilities of community-dwelling elderly? J Am Geriatr Soc. 1994;42:368–373. [DOI] [PubMed] [Google Scholar]
- 42.Apovian CM, Frey CM, Wood GC, et al. Body mass index and physical function in older women. Obes Res. 2002;10:740–747. [DOI] [PubMed] [Google Scholar]
- 43.Jensen GL, Friedmann JM. Obeisty is associated with functional decline in community-dwelling rural older persons. J Am Geriatr Soc. 2002;50:918–923. [DOI] [PubMed] [Google Scholar]
- 44.Miller GD, Rejeski WJ, Williamson JD, et al. The Arthritis, Diet and Activity Promotion Trial (ADAPT): design, rationale, and baseline results. Control Clin Trials. 2003;24:462–480. [DOI] [PubMed] [Google Scholar]
- 45.Jensen GL. Obesity and functional decline: epidemiology and geriatric consequences. Clin Geriatr Med. 2005;21:677–687. [DOI] [PubMed] [Google Scholar]
- 46.Gutierrez E. Mild diabetic neuropathy affects ankle motor function. Clin Biomech (Bristol, Avon). 2001;16:522–528. [DOI] [PubMed] [Google Scholar]
- 47.Resnick HE, Stansberry KB, Harris TB, et al. Diabetes, peripheral neuropathy, and old age disability. Muscle Nerve. 2002;25:43–50. [DOI] [PubMed] [Google Scholar]
- 48.Padua L, Schenone A, Aprile I, et al. Quality of life and disability assessment in neuropathy: a multicentre study. J Peripher Nerv Syst. 2005;10:3–10. [DOI] [PubMed] [Google Scholar]
- 49.Richardson JK. Factors associated with falls in older patients with diffuse polyneuropathy. J Am Geriatr Soc. 2002;50:1767–1773. [DOI] [PubMed] [Google Scholar]
- 50.Temelkova-Kurktschiev T, Henkel E, Koehler C, et al. Subclinical inflammation in newly detected Type II diabetes and impaired glucose tolerance. Diabetologia. 2002;45:151. [DOI] [PubMed] [Google Scholar]
- 51.Helmersson J, Vessby B, Larsson A, Basu S. Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation. 2004;109:1729–1734. [DOI] [PubMed] [Google Scholar]
- 52.Visser M, Kritchevsky SB, Goodpaster BH, et al. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2002;50:897–904. [DOI] [PubMed] [Google Scholar]
- 53.Taaffe DR, Harris TB, Ferrucci L, et al. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–M715. [DOI] [PubMed] [Google Scholar]
- 54.Marcus RL, Smith S, Morrell G, et al. Comparison of combined aerobic and high-force eccentric resistance exercise with aerobic exercise only for people with type 2 diabetes mellitus. Phys Ther. 2008;88:1345–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]