Abstract
Background and Objective: Gait characteristics differ in individuals with diabetes compared with those without diabetes. Limited information regarding potential explanatory factors for this association exists. This study examined the association between diabetes and gait characteristics in older adults and explored potential explanatory factors.
Design: A cross-sectional, observational study design was used.
Methods: At the 1998–1999 clinic visit, 558 ambulatory older adults (mean age=79 years) from the Pittsburgh site of the Cardiovascular Health Study had an assessment of their gait characteristics, diabetes, health status, cognition, mood, lower-extremity circulation and sensation, vision, lower-extremity strength (force-producing capacity), physical activity, and body mass index (BMI). A series of linear regression models were developed to examine the association between diabetes and gait characteristics and to examine potential explanatory factors for the associations.
Results: Diabetes was related to gait speed (β=−.06 m/s); however, the association was partially explained by health status variables, cognition, mood, lower-extremity circulation and sensation, visual impairment, lower-extremity strength, physical activity, and BMI. Health status and lower-extremity strength each explained the greatest proportion of the association (β reduced 66% by each). Diabetes was related to step width (β=.02 m), and the association could not be explained by the examined factors.
Conclusions: Diabetes was associated with gait alterations in older adults. Slowed gait speed appears to be secondary to the peripheral effect of the disease on other body systems. The effect of diabetes on step width was not explained in the analyses and may be related to peripheral motor nerve function or central influences of the disease, which could not be assessed in this study.
Gait characteristics differ in individuals with diabetes compared with those who do not have diabetes.1–3 Individuals with diabetes walk slower and with shorter step lengths, a longer stance phase, a wider base of support, greater step time variability on irregular surfaces, and improper pressure distribution at the foot compared with individuals without diabetes.3–7 In a longitudinal study investigating parkinsonian-like signs in more than 800 adults, those with diabetes had a more rapid rate of decline regarding rigidity and gait measures (ie, shuffling, body bradykinesia, postural stability) compared with those without diabetes.8
Individuals with diabetes also have been shown to have less ankle mobility, ankle moment, and ankle power during walking compared with individuals without diabetes.1 Recently, an increased flexion/extension and lateral movement during linear walking and turning—as assessed by accelerometry—was found in the major joints (ankles, knees, hips, shoulders, and neck) in those with diabetes. This increased movement or greater movement error was partially a result of a tremor in both the 8- and 16-Hz bands, suggesting that some of this increased joint movement is likely central in origin and not just related to motor and sensory loss in the lower extremities.4
Factors such as sensory impairment (presence of neuropathic ulcer, impaired vibration and protective sensation), decreased lower-extremity strength (force-producing capacity), and central nervous system impairment are believed to contribute to this impaired gait.4–6,8,9 For instance, individuals with diabetes and peripheral neuropathy are 15 times more likely to report injuries associated with walking and to report less comfort walking in unfamiliar environments than individuals who have diabetes but not peripheral neuropathy.10 In addition, some research suggests that dysfunction of the vestibular and autonomic nervous systems may play a role in the gait changes experienced in individuals with diabetes, but the results are not conclusive.4 For additional information on the lower-extremity impairments associated with diabetes, see the articles by Hilton et al,11 Mueller et al,12 LeMaster et al,13 and Sinacore et al14 in this issue.
The majority of the work examining potential explanatory factors of impaired gait in individuals with diabetes have included a small number of subjects, have included primarily middle-aged subjects (mean age <60 years), have examined only 1 or 2 contributory factors, or did not adequately control for potential confounding factors. An understanding of the underlying mechanisms of gait alterations in individuals with diabetes may help to develop appropriate interventions to improve gait in people with diabetes. Therefore, the purposes of this study were: (1) to examine the cross-sectional association between diabetes and gait characteristics in older adults and (2) to examine potential explanatory factors (health status, cognition and mood, lower-extremity circulation and sensation, visual impairment, lower-extremity strength, and physical activity and body mass index [BMI]) of this association while controlling for age, sex, and race. We were interested in determining how much of the association between diabetes and gait was explained by these factors.
Method
Study Population
Ambulatory older adults from the Pittsburgh site of the Cardiovascular Health Study (CHS) had an assessment of gait characteristics at the year 11 follow-up visit in 1998–1999. The CHS is an ongoing population-based, longitudinal, multicenter study of coronary heart disease and stroke risk in community-dwelling adults aged 65 years and older.15,16 At the initiation of the CHS in 1989–1990, individuals were identified from the Health Care Financing Administration sampling frame. Individuals were included in the study if they were 65 years of age or older, noninstitutionalized, expected to remain in the area for 3 years, and able to give informed consent. Individuals who were wheelchair-bound in the home or receiving hospice care, radiation therapy, or chemotherapy for cancer were excluded.15,16 In 1989–1990, an original cohort of 5,201 predominantly white (ie, >95% white) men and women were enrolled, and in 1992–1993 a cohort of 687 African Americans was added.
Participants in the current study included men and women who attended the 1998–1999 clinic visit at the Pittsburgh site, who could walk without the assistance of another person, and who could follow directions to complete the gait assessment (n=558). Individuals who used an assistive device were included if they could ambulate without the assistance of another person.
Diabetes
Diabetes status was defined based on medication information collected annually; serum glucose measured on blood samples drawn in 1988–1990, 1992–1993, and 1996–1997; and an oral glucose tolerance test (OGTT) conducted in 1996–1997. Participants were considered to have diabetes if any of the following conditions were met during the period from enrollment through the 1998–1999 assessment: (1) any use of diabetes medications, (2) fasting (≥8 hours) glucose ≥126 mg/dL, (3) nonfasting (<8 hours) glucose ≥200 mg/dL, or (4) OGTT ≥200 mg/dL.
Gait Characteristics
The GaitMat II system* was used for the gait analysis.17 The GaitMat II is an automated gait analysis system based on the opening and closing of pressure-sensitive switches, which are represented on the computer screen as footprints when the participant walks on the walkway. The GaitMat II consists of an approximately 4-m-long walkway and a computer system that controls the mat and analyzes the data. In addition to the 4-m-long walkway, the system has 1-m initial and final sections that are inactive to allow for acceleration and deceleration of the participant. After 2 practice passes, each participant completed 2 passes at their self-selected walking speed for data collection.
The following gait characteristics were examined: gait speed, step length, step width, stance time, and double-support time. Gait speed was determined by dividing the distance traversed by the time between the first and last step (eg, switch closure). Step length was determined as the distance between 2 consecutive footprints, measured from the heel of one footprint to the heel of the next footprint. Step width was determined as the distance between the outermost borders of 2 consecutive footprints. Stance time was determined as the time one foot was in contact with the floor (ie, from initial foot-floor contact until final foot-floor contact). Double-support time was determined as the time both feet were simultaneously in contact with the floor. The mean step width and double-support time were determined from all steps recorded over the 2 passes, whereas the mean of the unilateral gait characteristics (step length and stance time) were determined from only the right steps recorded over the 2 passes.
Potential Explanatory Variables
Items that have been shown to be related to both diabetes and gait were selected as potential explanatory variables. The following items were selected as potential explanatory factors: measures of health status, cognition, mood, lower-extremity circulation, lower-extremity sensation, visual impairment, lower-extremity strength, physical activity, and BMI.
Demographics.
At the clinic visit, data were collected on the following demographic factors: age, sex, and race.
Health status.
Health status was determined by self-report of health, the presence of 7 chronic conditions, and number of medications taken. General health status was self-reported as “excellent,” “very good,” “good,” or “fair/poor.” The chronic conditions included the following 7 self-reported conditions: arthritis, cancer, heart disease, congestive heart failure (CHF), claudication, stroke, and Parkinson disease. The presence of heart disease, CHF, and stroke were confirmed by medical records. The number of medications was recorded as the number of prescription medications the participant was currently taking.
Cognition and mood.
Measures of cognition included the modified Mini-Mental State Examination (3MS), the Digit Symbol Substitution Test (DSST), and Trail Making test Part B (Trails B).18–20 The 3MS, a global measure of cognition including attention, memory, and language, incorporates 4 added test items and more graded scoring and is scored on a 0 to 100 scale.18 The DSST, a subtest of the Wechsler Adult Intelligence Test, is a measure of information processing speed.19 For the DSST, the participants were given a coding key pairing 9 symbols to a list of digits from 1 to 9. Under the coding key were rows of randomly ordered numbers. Participants had 90 seconds to transcribe as many symbols as possible, based on the digit-symbol associations specified in the coding key. The number correctly completed was recorded. Trails B was used to evaluate visual scanning and mental flexibility.20
Depressed mood was assessed by a modified, validated, 10-item version of the Center for Epidemiologic Studies Depression Scale (CES-D).21,22 Scores on this modified scale range from 0 to 30, with a score of 10 or greater indicating significant depressive symptoms.22
Lower-extremity circulation and sensation.
The ankle arm index (AAI) is the ratio of the ankle-to-arm systolic blood pressure and is reduced when there is atherosclerotic obstruction from the heart to the leg. In adults without atherosclerotic obstruction, the ratio of the ankle-to-arm systolic blood pressure is greater than 1.0. A participant was classified as having a low AAI if either leg had an ankle-to-arm systolic blood pressure ratio of less than 0.90. An AAI of less than 0.90 is 95% sensitive and 99% specific for angiographically significant peripheral arterial disease.23 The AAI was measured by trained technicians according to a standard protocol that has been described previously.24 Briefly, the systolic pressures of the brachial and posterior tibial arteries were measured with a Doppler stethoscope (8 MHz).† Measurements have been shown to be reliable between observers, stable over time, and highly correlated between right and left legs.24 For more information on etiology, presentation, and clinical tests for vascular disease, see the article by Cade25 in this issue.
Sensory impairment was assessed using a measure of lower-extremity vibratory perception. Lower-extremity vibratory perception was assessed at the bilateral medial malleoli using a tuning fork. Vibratory sense groupings were as follows: vibratory sense intact at bilateral malleoli, diminished in at least one malleolus, or diminished in bilateral malleoli.
The history of a foot ulcer or sore and the presence of lower-extremity pain were determined by self-report. History of foot ulcers or sores was determined by asking the participants. Self-reported lower-extremity pain was recorded as being present if the participant reported having knee or foot pain for at least half of the days in a month; otherwise, it was recorded as being absent.
Visual impairment.
Visual impairment was assessed by self-report. Visual impairment was coded as present if the participant reported an inability to see to drive, to watch television, or to recognize someone across a room with or without corrective lenses.
Lower-extremity strength.
Repeated chair stand time was used as a surrogate measure of lower-extremity strength.26 Participants were timed as they stood from a standard chair 5 times without using their upper extremities for assistance. The time to complete the 5 chair stands was recorded.
Physical activity and BMI.
Physical activity was assessed as self-reported number of blocks or miles walked outside of their home in the past week. Body mass index was based on measured height and weight and was calculated as body weight in kilograms divided by height in meters squared.
Data Analysis
Differences in potential explanatory factors and gait characteristics between individuals with diabetes and those without diabetes were described and tested using the chi-square test for proportions and Student t tests for means. A series of linear regression models were developed to determine the association between diabetes (independent variable) and the gait characteristics (dependent variables) and to examine potential explanatory factors of the association between diabetes and gait. The measures of gait were normally distributed so ordinary least squares regression methods were used. The first model examined the association between diabetes and either gait speed or step width adjusted for basic demographics (age, sex, and race). Models 2 through 7 examined potential explanatory factors of this association. Model 2 examined measures of health that were related to diabetes in bivariate analyses (heart disease, CHF, stroke, and number of medications), with the majority of the factors representing cardiovascular health. Although significantly related to diabetes in bivariate analysis, general health status was not included in the model because of its strong association with number of medications (ie, to avoid issues with multicollinearity). Model 3 examined measures of cognition and mood, model 4 examined measures of lower-extremity circulation and sensation, model 5 examined visual impairment, model 6 examined lower-extremity strength, and model 7 examined measures of physical activity and BMI.
Gait speed was highly correlated with step length, stance time, and double-support time (all correlations: Pearson r>.75, P<.0001); therefore, to avoid redundancy, only gait speed and step width were examined in the regression models. The association between gait speed and step width was minimal (Pearson r=−.34, P<.0001). Analyses were performed using SAS version 9.1 for Windows.‡
Results
Table 1 provides the characteristics of the study cohort, stratified by the presence of diabetes. Of the 558 subjects, 119 (21%) were classified as having diabetes. Several characteristics were associated (P<.05) with diabetes status in unadjusted, bivariate analyses (Tab. 1). Individuals with diabetes were more likely to classify their race as black, to have a greater BMI, and to be less active than individuals without diabetes. Individuals with diabetes, in general, had poorer health (they were more likely to report health as fair or poor; they were more likely to have heart disease, CHF, stroke, depression, foot ulcers, an AAI <0.90, or lower-extremity pain; and they took a greater number of medications) than individuals without diabetes. Likewise, individuals with diabetes performed more poorly on measures of cognition than individuals without diabetes. Compared with individuals who did not have diabetes, individuals with diabetes walked more slowly, took shorter steps with a wider base of of support, and spent more time in stance and in more support.
Table 1.
Mean (SD) Characteristics of Participants Stratified by Diabetes Statusa
3MS=modified Mini-Mental State Examination, DSST=Digit Symbol Substitution Test, CES-D=Center for Epidemiologic Studies Depression Scale, LE=lower extremity, AAI=ankle arm index, BMI=body mass index.
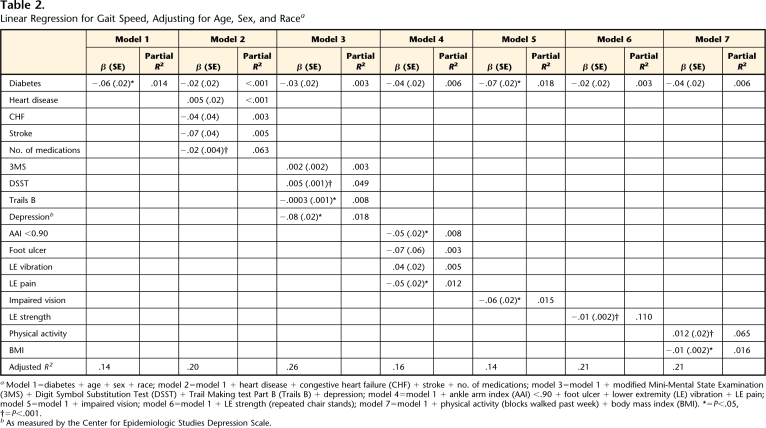
Table 2 provides the results for the series of linear regressions examining the association between diabetes and gait speed. Diabetes status was related to gait speed (P=.006) after adjusting for age, sex, and race. However, this association was primarily explained by measures of health (model 2) or lower-extremity strength (model 6), each which reduced the β by 66%. Measures of cognition and mood attenuated the association between diabetes and gait speed by 50% (model 3), and measures of lower-extremity circulation and sensation (model 4) and physical activity and BMI (model 7) each attenuated the association between diabetes and gait speed by 33%. Although visual impairment was related to gait speed (β=−.06, P=.006), it did not explain the association between diabetes and speed.
Table 2.
Linear Regression for Gait Speed, Adjusting for Age, Sex, and Racea
Model 1=diabetes + age + sex + race; model 2=model 1 + heart disease + congestive heart failure (CHF) + stroke + no. of medications; model 3=model 1 + modified Mini-Mental State Examination (3MS) + Digit Symbol Substitution Test (DSST) + Trail Making test Part B (Trails B) + depression; model 4=model 1 + ankle arm index (AAI) <.90 + foot ulcer + lower extremity (LE) vibration + LE pain; model 5=model 1 + impaired vision; model 6=model 1 + LE strength (repeated chair stands); model 7=model 1 + physical activity (blocks walked past week) + body mass index (BMI). *=P<.05, †=P<.001.
bAs measured by the Center for Epidemiologic Studies Depression Scale.
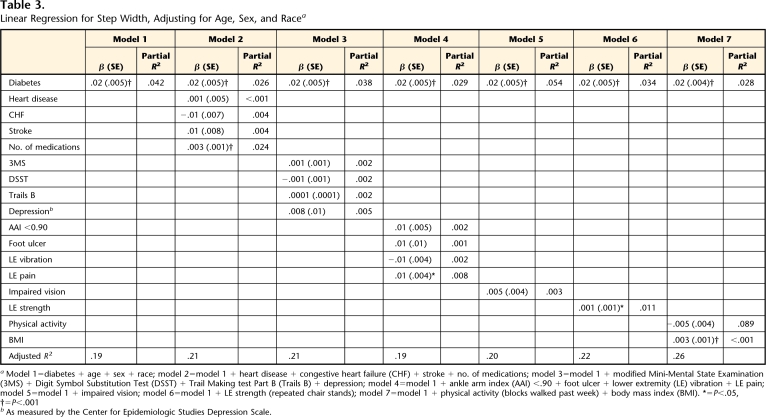
Table 3 provides the results for the series of linear regressions examining the association between diabetes and step width. Diabetes status was related to step width after adjusting for age, sex, and race (model 1). The association between diabetes and step width could not be explained by health status (model 2), cognition and mood (model 3), lower-extremity circulation and sensation (model 4), visual impairment (model 5), lower-extremity strength (model 6), or physical activity and BMI (model 7).
Table 3.
Linear Regression for Step Width, Adjusting for Age, Sex, and Racea
Model 1=diabetes + age + sex + race; model 2=model 1 + heart disease + congestive heart failure (CHF) + stroke + no. of medications; model 3=model 1 + modified Mini-Mental State Examination (3MS) + Digit Symbol Substitution Test (DSST) + Trail Making test Part B (Trails B) + depression; model 4=model 1 + ankle arm index (AAI) <.90 + foot ulcer + lower extremity (LE) vibration + LE pain; model 5=model 1 + impaired vision; model 6=model 1 + LE strength (repeated chair stands); model 7=model 1 + physical activity (blocks walked past week) + body mass index (BMI). *=P<.05, †=P<.001
bAs measured by the Center for Epidemiologic Studies Depression Scale.
Discussion and Conclusions
Compared with individuals without diabetes, individuals with diabetes walked more slowly, took shorter steps with a greater step width, and spent more time in stance and double support. Our findings in this large sample of older adults support the findings of previous studies on diabetes and gait in adults primarily less than 65 years of age.1,2,4
In this well-characterized cohort of older adults, we were uniquely able to examine potential explanatory factors (health status; cognition and mood; lower-extremity circulation, sensation, and pain; visual impairment; lower-extremity strength; and physical activity and BMI) of the association between diabetes and gait while controlling for age, sex, and race. The association between diabetes and gait speed was partially explained by these factors, with the measures of health and lower-extremity strength explaining the greatest proportion of the association. These data suggest that the comorbidities associated with diabetes may contribute to slower gait.
The association between diabetes and wider step width, however, could not be explained in our analyses. Step width is believed to be related to balance control27 and an increase in step width is thought to improve stability.28 Many of the factors that contribute to balance, such as vision, peripheral sensation, and lower-extremity strength, did not explain the association between diabetes and step width. Petrofsky et al2 have suggested that the changes that occur in gait of people with diabetes may be the result of more central damage to the vestibular, somatic, and autonomic systems as a result of microcirculation changes associated with poor glycemic control and not the result of more peripheral insults. Our peripheral measures did not explain the association of diabetes and wider step width, which supports their hypothesis that the greater step width in individuals with diabetes may be more of a central motor control issue.
The wider step width may be related to changes in the motor circuit of the basal ganglia. In our previous unpublished work, we showed that a wider step width is related to decreased brain volume in the pallidum and the parietal lobules. The basal ganglia are a highly metabolic area that requires good circulation and energy to function properly29,30; therefore, a disease process such as diabetes, which is known to affect circulation and blood glucose levels, could have a detrimental effect on the basal ganglia. Additional work is needed to determine whether these specific brain regions (pallidum and parietal lobules) explain the association between diabetes and step width.
The adjusted R2 values in this study were modest, suggesting that only a small portion (<30%) of gait speed and step width were explained by the factors that were included in the regression models (Tabs. 2 and 3). The goals of this study were to examine the cross-sectional association between diabetes and gait and to examine potential explanatory factors of this association, rather than to describe all factors that might contribute to gait speed and step width. Therefore, it is not surprising that only a small portion of gait was explained because all factors that could potentially contribute to gait were not included in the regression models. In fact, in this type of analysis, it is more important to focus on how the beta coefficient of the independent variable of interest changes when potential contributing factors are added to the model than to focus on the magnitude of the adjusted R2 and partial R2 values.
By examining each set of potential contributors in separate models and examining how the beta coefficient of diabetes status changes when the potential contributors are added to the model, we can begin to understand which factors explain the association between diabetes and gait and how much of the association is explained. By examining the partial R2 values for the potential contributors, we can determine which contributor explains the greatest amount of the association. For example, the association between diabetes and gait speed was primarily explained by measures of health (Tab. 2, model 2), which reduced the β by 66%. Of the measures of health examined, we can determine that the number of medications is the strongest explanatory variable (P<.001), explaining approximately 6% of gait speed (partial R2 for number of medications=.063).
Given the cross-sectional design of the study, we could not determine the direction of the association or determine cause and effect. It is reasonable to argue that the diabetes disease process has influenced many of the potential explanatory factors that would then lead to changes in gait characteristics (ie, someone with diabetes develops peripheral neuropathy and lower-extremity weakness that results in slowed gait). The reverse could be true as well; someone with diabetes could start to walk more slowly, which results in lower-extremity weakness.
This was a study of older, community-dwelling adults; therefore, the findings may not be generalizable to younger samples or to individuals with clinically diagnosed diabetes. Several of the individuals classified as having diabetes in this study were unaware that they had diabetes (of the 119 individuals classified as having diabetes, only 67 reported ever being told by a physician that they had diabetes). In addition, this sample may represent recently diagnosed cases of diabetes or cases with minimal complications, because only 6% reported having a sore or ulcer on their foot when the lifetime risk of diabetic foot ulcers is approximately 15%.31
Our findings regarding the discrepancy between diagnosed and undiagnosed diabetes are similar to the findings from the National Health and Nutrition Examination Survey. Based on data from 1999–2002, the crude prevalence of diabetes in the United States was 9.3%, of which 6.5% were diagnosed and 2.8% were undiagnosed cases.32 This discrepancy between diagnosed and undiagnosed cases of diabetes may have important implications for physical therapists. Many therapists are likely treating patients with undiagnosed diabetes, which can greatly influence how an individual responds to exercise (for more on this issue, see the articles by Cade,25 Turcotte and Fisher,33 and Gulve34 in this issue).
Even though our sample may represent those individuals with newly diagnosed diabetes or those in the early stages of the disease, it is interesting to note that they still differed from those individuals without diabetes on a number of key factors such as gait, physical activity, BMI, cognition, and mood. Our findings are similar to those from the Third National Health and Nutrition Examination,35 which examined physical disability among older adults in the United States. Older individuals with diabetes were twice as likely to be unable to walk a quarter of a mile, climb stairs, or do housework compared with older individuals without diabetes.35 Likewise, our finding on physical activity is similar to that of the Medical Expenditure Panel Survey, which found that 39% of adults with diabetes were physically active compared with 58% of adults without diabetes.36 Many of these factors—physical disability, physical activity, BMI, and mood—have been shown to benefit from an exercise program that could be the focus of physical therapy.37–40 In addition, because many older persons with diabetes have gait abnormalities and are less physically active, future research could examine whether the gait abnormalities are contributing to the physical inactivity and whether interventions to improve gait would also increase physical activity.
Several of our measures of potential explanatory variables were not ideal. For example, lower-extremity muscle strength was not formally assessed by tests on individual muscles, but instead was assessed using a surrogate measure of lower-extremity strength: the chair stand.26 Furthermore, we did not have a measure of protective sensation such as Semmes-Weinstein monofilament testing. However, sensory impairment detected by Semmes-Weinstein monofilament testing occurs late in the disease process and may have been a less sensitive measure in our community sample.41 We did measure vibratory sense, which is believed to be an earlier indicator of sensory involvement than discriminant touch42 or peripheral motor nerve function, which may be related to motor performance. In addition, our measures of vision, physical activity level, and lower-extremity pain were all based on self-report and may not accurately reflect these constructs.
Our findings show that diabetes is associated with alterations in gait of older adults. The alterations in gait speed appear to be secondary to the peripheral effect of the disease on other body systems. However, the effect of diabetes on step width was not explained in our analyses and may be related to peripheral motor nerve function or central influences of the disease, which we were unable to assess. Future studies should examine these additional peripheral and central influences underlying the relationship between diabetes and step width.
Dr Brach, Ms Talkowski, and Dr Strotmeyer provided concept/idea/research design. All authors provided writing and consultation (including review of manuscript before submission). Dr Brach provided data collection. Dr Brach and Ms Talkowski provided data analysis. Dr Newman provided fund procurement, subjects, and facilities/ equipment.
The research reported in this article was supported by contract numbers N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01-HC-15103, N01-HC-55222, N01-HC-75150, and N01-HC-45133 and grant number U01 HL080295 from the National Heart, Lung, and Blood Institute, with additional contribution from the National Institute of Neurological Disorders and Stroke. A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. At the time of data collection, Dr Brach was supported by the National Institutes of Health Public Health Service grant TG 32AG00181. Currently, Dr Brach is supported by a National Institutes on Aging and American Federation of Aging Research Paul Beeson Career Development Award (1 K23 AG026766-01) and the University of Pittsburgh Older Americans Independence Center grant (1 P30 AG024827-01).
E.Q. Inc, PO Box 16, Chalfont, PA 18914-0016.
Parks Electronics, 19460 SW Shaw, Aloha, OR 97007.
SAS Institute Inc, PO Box 8000, Cary, NC 27511.
References
- 1.Mueller MJ, Minor SD, Sahrmann SA, et al. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys Ther. 1994;74:299–308; discussion 309–313. [DOI] [PubMed] [Google Scholar]
- 2.Petrofsky J, Lee S, Bweir S. Gait characteristics in people with type 2 diabetes mellitus. Eur J Appl Physiol. 2004;93:640–647. [DOI] [PubMed] [Google Scholar]
- 3.Katoulis EC, Ebdon-Parry M, Lanshammar H, et al. Gait abnormalities in diabetic neuropathy. Diabetes Care. 1997;20:1904–1907. [DOI] [PubMed] [Google Scholar]
- 4.Petrofsky J, Lee S, Macnider M, Navarro E. Autonomic, endothelial function and the analysis of gait in patients with type 1 and type 2 diabetes. Acta Diabetol. 2005;42:7–15. [DOI] [PubMed] [Google Scholar]
- 5.Sacco IC, Amadio AC. A study of biomechanical parameters in gait analysis and sensitive cronaxie of diabetic neuropathic patients. Clin Biomech (Bristol, Avon). 2000;15:196–202. [DOI] [PubMed] [Google Scholar]
- 6.Menz HB, Lord SR, St George R, Fitzpatrick RC. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil. 2004;85:245–252. [DOI] [PubMed] [Google Scholar]
- 7.Salsich GB, Mueller MJ. Effect of plantar flexor muscle stiffness on selected gait characteristics. Gait Posture. 2000;11:207–216. [DOI] [PubMed] [Google Scholar]
- 8.Arvanitakis Z, Wilson RS, Schneider JA, et al. Diabetes mellitus and progression of rigidity and gait disturbance in older persons. Neurology. 2004;63:996–1001. [DOI] [PubMed] [Google Scholar]
- 9.Dingwell JB, Cavanagh PR. Increased variability of continuous overground walking in neuropathic patients is only indirectly related to sensory loss. Gait Posture. 2001;14:1–10. [DOI] [PubMed] [Google Scholar]
- 10.Cavanagh PR, Derr JA, Ulbrecht JS, et al. Problems with gait and posture in neuropathic patients with insulin-dependent diabetes mellitus. Diabet Med. 1992;9:469–474. [DOI] [PubMed] [Google Scholar]
- 11.Hilton TN, Tuttle LJ, Bohnert KL, et al. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88:1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mueller MJ, Zou D, Bohnert KL, et al. Plantar stresses on the neuropathic foot during barefoot walking. Phys Ther. 2008;88:1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeMaster JW, Mueller MJ, Reiber GE, et al. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: Feet First randomized controlled trial. Phys Ther. 2008;88:1385–1398. [DOI] [PubMed] [Google Scholar]
- 14.Sinacore DR, Hastings MK, Bohnert KL, et al. Inflammatory osteolysis in diabetic neuropathic (Charcot) arthropathies of the foot. Phys Ther. 2008;88:1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tell GS, Fried LP, Hermanson B, et al. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–366. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright PL, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. [DOI] [PubMed] [Google Scholar]
- 17.Walsh JP. Foot fall measurement technology. In: Craik RL, Oatis CA, eds. Gait Analysis: Theory and Application. St Louis, MO: Mosby; 1995:125–142.
- 18.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 19.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. New York, NY: Psychological Corporation; 1981.
- 20.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tuscon, AZ: Neuropsychological Press; 1985.
- 21.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Applied Psychological Measures. 1977;1:385–401. [Google Scholar]
- 22.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale). Am J Prev Med. 1994;10:77–84. [PubMed] [Google Scholar]
- 23.Lipsitz LA. Physiological complexity, aging, and the path to frailty. Sci Aging Knowl Environ. 2004(16):pe16. [DOI] [PubMed]
- 24.Grabiner PC, Biswas T, Grabiner MD. Age-related changes in spatial and temporal gait variables. Arch Phys Med Rehabil. 2001;82:31–35. [DOI] [PubMed] [Google Scholar]
- 25.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csuka M, McCarthy DJ. Simple method of measurement of lower extremity muscle strength. Am J Med. 1985;78:77–81. [DOI] [PubMed] [Google Scholar]
- 27.Gabell A, Nayak USL. The effect of age and variability in gait. J Gerontol. 1984;39:662–666. [DOI] [PubMed] [Google Scholar]
- 28.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear? J Am Geriatr Soc. 1997;45:313–320. [DOI] [PubMed] [Google Scholar]
- 29.Tucker DM, Penland JG, Sandstead HH, et al. Nutrition status and brain function in aging. Am J Clin Nutr. 1990;52:93–102. [DOI] [PubMed] [Google Scholar]
- 30.Parent M, Parent A. Relationship between axonal collateralization and neuronal degeneration in basal ganglia. J Neural Transm Suppl. 2006;70:85–88. [DOI] [PubMed] [Google Scholar]
- 31.Reiber GE, Boyko E, Smith DG. Lower extremity ulcers and amputations in individuals with diabetes. In: Harris MI, ed. Diabetes in America. Bethesda, MD: National Institutes of Health; 1995:409–427.
- 32.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health and Nutrition Examination Survey 1999–2002. Diabetes Care. 2006;29:1263–1268. [DOI] [PubMed] [Google Scholar]
- 33.Turcotte LP, Fisher JS. Skeletal muscle insulin resistance: roles of fatty acid metabolism and exercise. Phys Ther. 2008;88:1279–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gulve EA. Exercise and glycemic control in diabetes: benefits, challenges, and adjustments to pharmacotherapy. Phys Ther. 2008;88:1297–1321. [DOI] [PubMed] [Google Scholar]
- 35.Gregg EW, Beckles GLA, Williamson DF, et al. Diabetes and physical disability among older U.S. adults. Diabetes Care. 2000;23:1272–1277. [DOI] [PubMed] [Google Scholar]
- 36.Morrato EH, Hill JO, Wyatt HR, et al. Physical activity in U.S. adults with diabetes and at risk for developing diabetes, 2003. Diabetes Care. 2007;30:203–209. [DOI] [PubMed] [Google Scholar]
- 37.Diabetes Prevention Program Research Group; prepared by Crandall J, Schade D, Ma Y, et al. The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci. 2006;61:1075–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The LIFE Study Investigators; Pahor M, Blair SN, Espeland M, et al. Effects of a physical activity intervention on measures of physical performance; results of the Lifestyle Interventions and Independence in Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. [DOI] [PubMed] [Google Scholar]
- 39.Singh NA, Clements KM, Singh MA. The efficacy of exercise as a long-term antidepressant in elderly subjects: a randomized, controlled trial. J Gerontol A Biol Sci Med Sci 2001;56:M497–M504. [DOI] [PubMed] [Google Scholar]
- 40.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol A Biol Sci Med Sci. 1997;52:M27–M35. [DOI] [PubMed] [Google Scholar]
- 41.Sosenko JM, Sparling YH, Hu D, W et al. Use of Semmes-Weinstein monofilament in the Strong Heart Study: risk factors for clinical neuropathy. Diabetes Care. 1999;22:1715–1721. [DOI] [PubMed] [Google Scholar]
- 42.Mackinnon S, Dellon AL. Diagnosis of Nerve Injury: Surgery of the Peripheral Nerve. New York, NY: Theme Medical Publishers; 1988:65–87.