Abstract
We previously described an unidentified lipid purified from calf small intestine that inhibits the in vitro adhesion of Cryptosporidium parvum sporozoites to host cells [Johnson JK, Schmidt J, Gelberg HB, Kuhlenschmidt MS. Microbial adhesion of Cryptosporidium parvum sporozoites: purification of an inhibitory lipid from bovine mucosa. J Parasitol 2004;90:980–90]. Intestinal mucosa from some calves, however, failed to yield this bioactive lipid. Accordingly, we examined other potential sources, especially dietary sources, of the inhibitory lipid and discovered it was principally derived from bovine colostrum. Interestingly, fresh colostrum yielded little or no inhibitory lipid, however, the lipid was found in relatively large quantities following incubation of colostrum with the aqueous fraction of calf intestinal contents. Using FAB-MS and NMR analysis, the sporozoite inhibitory lipid (SIL) was identified as oleic acid, a monounsaturated fatty acid likely released from colostrum triglycerides and phospholipids by digestion in the lumen of the calf small intestine. Oleic acid dose-dependently inhibited in vitro sporozoite–host cell adhesion with an inhibitory constant (IC50) of approximately 5 μM. Comparison of oleic acid with other C-18 fatty acids revealed linolenic, but not stearic acid, also displayed potent inhibitory activity. Neither linolenic nor oleic acid, however, affect either sporozoite or host cell viability at concentrations that inhibit sporozoite adhesion. These results suggest certain colostrum-derived long-chain fatty acids may serve as natural inhibitors of the early steps in C. parvum sporozoite–host cell interactions.
Keywords: Cryptosporidium parvum, Sporozoites, Invasion, Adhesion, Colostrum, Fatty acid, Oleic acid
1. Introduction
Cryptosporidium parvum, a ubiquitous enteric pathogen of the phylum Apicomplexa, is a common parasite of dairy calves and other vertebrate species. Although cryptosporidiosis is self-limiting in the healthy human adult, the infection may be life threatening for those unable to mount an adequate immune response. Recent improvements in C. parvum detection on farms, in medical practices, and in drinking and recreational water supplies have revealed this parasite is a common threat to the health of both humans and livestock [1–10]. Although detection methods have improved greatly in sensitivity and specificity over the last decade, advances in the treatment of cryptosporidiosis have not been as rapid. Treatments currently available for cryptosporidiosis, with the exception of Nitazoxanide, are mostly supportive, accompanied by undesirable side effects, or unacceptable for use in food animals. Although, Nitazoxanide has shown clinical efficacy for the treatment of cryptosporidiosis in both immunocompetent and immunocompromised children and adults, it is not effective in achieving parasitological cure in hospitalized children with HIV [11].
Hyperimmune bovine colostrum (HBC) has previously been used with limited success to treat cryptosporidiosis. HBC has been effective in reducing clinical symptoms and oocyst shedding in numerous animal species [12–18]. Furthermore, antibodies from HBC rendered sporozoites non-infective in vitro and offered some protection to SCID mice in vivo [19]. Interestingly, non-immune bovine colostrum also protected immunosuppressed mice against infection [20]. In humans, both HBC and non-immune colostrum has shown efficacy in the treatment of AIDS patients with cryptosporidiosis [21–25].
The lack of therapeutic options for AIDS patients is especially serious in light of the potentially fatal outcome of cryptosporidiosis in that group, and thus, bovine colostrum appears worthy of further investigation as a treatment for the infection. The bulk of the literature addressing the therapeutic value of colostrum has focused on the antibody component. There is some indication; however, that factors other than antibodies in colostrum may be protective against infection with this parasite [20,26].
A previously unidentified lipid that was shown to inhibit host cell adhesion by C. parvum sporozoites in vitro was originally isolated and purified in our laboratory from mucosal scrapings of neonatal calf small intestine. In subsequent studies, we observed marked variability in the amounts of this sporozoite adhesion inhibitory lipid (SIL) isolated from different calves. This observation led us to question whether the intestinal mucosa was the natural source of SIL. In the work reported here, we identified SIL isolated from either bovine mucosa or colostrum as oleic acid, a monounsaturated fatty acid commonly present in colostrum in the esterified form of di- and triglycerides. We also demonstrate SIL is produced during digestion of dietary colostrum most likely by the action of intestinal lipase activity. A comparison of commercial oleic acid and oleic acid purified from colostrum digests demonstrates oleic acid effects a dose-dependent inhibition of host cell adhesion in vitro, with an IC50 = 5 μM. These results suggest oleic acid, and possibly other long-chain unsaturated fatty acids derived from bovine colostrum, is a natural, non-immune, anti-cryptosporidial component and may have utility not only as a reagent useful for investigating the mechanism of fatty acid mediated-inhibition of sporozoite–host cell adhesion but also as a possible dietary treatment of human and veterinary cryptosporidiosis.
2. Materials and methods
2.1. Preparation of C. parvum oocysts and sporozoites
Oocysts and sporozoites were propagated, collected and purified as previously described [27]. Briefly, oocysts were purified from homogenized, sieved feces by Sheather’s sugar flotation and subsequent discontinuous cesium chloride gradient centrifugation [28]. Sporozoites released following oocyst excystation were chilled, washed in cold minimal essential medium (MEM), enumerated using a hemocytometer and placed on ice until use. Sporozoites were used in cell adhesion assays within 2 h of excystation. All procedures involving animals were part of protocols approved by the University of Illinois Institutional Animal Care and Use Committee (IACUC).
2.2. Organic solvent extraction and fractionation of whole bovine colostrum
Aliquots (500 μl) of first-day bovine colostrum were extracted with chloroform:methanol (10 ml, 30:1) by gentle mixing and pulse sonicated using a Heat Systems-Ultrasonics Inc. probe sonicator at 50% power for three 1-min intervals. Extracts were centrifuged at 1500 × g for 10 min; the organic layers were pooled, dried in a rotary evaporator and lyophilized overnight. Lyophilized material was reconstituted in 40 ml chloroform:methanol (2:1), dried by vacuum centrifugation (Savant, SpeedVac®), and stored at −20 °C. The extract was reconstituted in chloroform:methanol and fractionated by high pressure liquid chromatography (HPLC), high performance thin layer chromatography (HPTLC), and preparative thin layer chromatography (PTLC) as previously described [27].
2.3. Gas chromatography (GC), nuclear magnetic resonance (NMR), and fast atom bombardment mass spectrometry (FAB-MS)
Reconstituted samples in chloroform:methanol were checked for purity by HPTLC, evaporated to dryness, and submitted to the Lipid Analysis Unit, Mylnefield Research Services Ltd., Mylnefield, Invergowrie, Dundee DD2 5DA, Scotland, for GC analysis; the Mass Spectrometry Laboratory, School of Chemical Sciences, University of Illinois for low-resolution fast-atom bombardment mass spectrometry (FAB-MS); and Spectral Data Services, Champaign, IL, for 1H NMR analysis. For GC analysis, the sample was subjected to fatty acid methyl ester derivatization by dissolving in toluene (1 ml) in a test tube fitted with a condenser, and 1% sulfuric acid in methanol (2 ml) was added. The mixture was left overnight in a stoppered tube at 50 °C), water (5 ml) containing sodium chloride (5%) was added, and the resultant esters extracted with hexane (2× 5 ml). The hexane layer was washed with water (4 ml) containing potassium bicarbonate (2%) and dried over anhydrous sodium sulfate. The solution was filtered and the solvent removed under a stream of nitrogen. The recovered esters were subjected to GC using a Hewlett Packard 5890 Series II gas chromatograph. The fused silica column was coated with BPX-70™ (0.25 mm × 50 m, 0.25 μm film; SGE Ltd.). After holding the temperature at 80 °C for 3 min, the column was temperature-programmed at 20 °C/min to 160 °C, then at 4 °C/min to 350 °C, where it was held for 20 min. Helium was the carrier gas. For FAB mass spectroscopy, the samples were reconstituted in Magic Bullet (dithiothreitol:dithioerythritol, 3:1) and analyzed in a ZAB-SE spectrometer using an 8 kV beam of neutral Xenon atoms. Analyses were done at low resolution of 1000 and a scan mass range of 0–1500. Peak profiles of each sample were compared to the profile of the inhibitory lipid previously described [27]. For NMR spectroscopy, purified lipid fractions derived from bovine mucosa or digested colostrum and which matched the FAB-MS profile and HPTLC mobility characteristics of the originally identified inhibitory lipid [27] were lyophilized and submitted for NMR analysis. The 1H NMR spectra were obtained in CDCl3 on a Varian INOVA spectrometer (Varian Assoc., Palo Alto, USA) operating at 400 MHz. The number of data points was 35,000, using a spectral width of 7000 Hz, a 7 μs 90° pulse width and a 5 s relaxation delay time. Peaks were referenced to tetramethylsilane as the internal standard.
2.4. Digestion of bovine colostrum
Fresh bovine colostrum was incubated with the aqueous fraction of calf intestinal contents. The aqueous fraction of calf intestinal contents was produced as follows: a 15.0 ml aliquot of liquid calf intestinal contents was added to an equal volume of chloroform:methanol (30:1) and mixed thoroughly. The mixture was centrifuged at 1500 × g for 10 min and the top aqueous layer was retained. This fraction was placed on ice, brought up to a volume of 15.0 ml with PBS and refrigerated at 4 °C. A 20 μl aliquot was removed and spotted on a HPTLC plate to confirm removal of all lipid components. Aliquots (5 ml) of the aqueous intestinal contents) were either pre-incubated (boiled, 15 min) or added directly to fresh, cold, bovine colostrum (5 ml) and incubated at 37 °C overnight followed by organic solvent extraction, evaporation, and reconstitution as described above. Aliquots from each extract were then analyzed for SIL by HPTLC and FAB-MS. To determine the kinetics of inhibitory lipid formation during in vitro colostrum digestion, aliquots (250 μl) of fresh bovine colostrum were incubated with 250 μl of the aqueous fraction of calf intestinal contents at 37 °C for various times up to 96 h. At each time point, 5 ml chloroform:methanol (30:1) was added and pulse sonication and organic extraction proceeded as described for whole colostrum. Controls included a zero-time, no-enzyme incubation processed immediately and then refrigerated; and a no-enzyme incubation processed at the 96-h time point. All reconstituted extracts were analyzed by HPTLC as previously described.
2.5. Sporozoite adhesion assays
Sporozoite adhesion assays were done essentially as previously described [27]. Briefly, fatty acids were dried under nitrogen and reconstituted in MEM with gentle sonication. C. parvum sporozoites and individualized Madin-Darby Bovine Kidney (MDBK) cells were added at a 10:1 ratio (5 × 106 sporozoites, 5 × 105 MDBK cells). The incubation volume was adjusted to 300 μl with MEM, and a final fatty acid concentration of 5, 50, 100, or 200 μM. Following incubation, 33 μl of 50% glutaraldehyde was immediately added to each tube under gently vortexing. Aliquots were removed and examined by differential interference contrast (DIC) microscopy to quantify sporozoite adhesion. The percentage inhibition of adhesion by fatty acids was determined by comparison of the sporozoite binding to MDBK cells incubated in the absence of fatty acids (control incubations) and is denoted as “% of control”.
2.6. Cell viability assays
Sporozoite and MDBK cell viability in the presence or absence of added fatty acids was estimated using LIVE/DEAD® Reduced Biohazard Viability/Cytotoxicity Kits (Molecular Probes L-7013). A dye working solution was prepared in Hepes Balanced Salt Solution (HBSS) according to manufacturer’s instructions. Sporozoites (1 × 108) or MDBK cells (1 × 106) were washed once in ice-cold MEM and resuspended in pre-warmed MEM with or without 200 μM α-linolenic or oleic acid in microcentrifuge tubes and incubated as described for sporozoite adhesion assays. Following incubation the tubes were centrifuged 10 min at 1000 × g, the supernatant removed, and 200 μl dye working solution was added. Cells were incubated in the dark at room temperature for 15 min and then centrifuged at top speed in a microcentrifuge 20 s. The supernatants were removed, the pellets resuspended in 50 μl HBSS, and 50 μl 4% glutaraldehyde was added. Tubes were gently mixed, incubated at room temperature in the dark for 15 min, and aliquots examined by fluorescence microscopy. Five fields were counted on each microscope slide; in each field, total, live, and dead sporozoites or MDBK cells were counted using phase-contrast and UV illumination [green, FITC filter cube (Ex = 480/40, Em = 535/50), and red (dead), TRITC filter cube (Ex = 545/30, Em = 620/60)]. The entire experiment was repeated on a different day using fresh reagents and newly harvested MDBK cells and sporozoites. Molecular Probes Inc. kit L-7012 also was used, according to manufacturer’s directions, as an additional dye exclusion assay to measure cell viability and gave the same results as kit L-7013 (see the next section).
3. Results
3.1. Characterization of SIL isolated from bovine mucosa
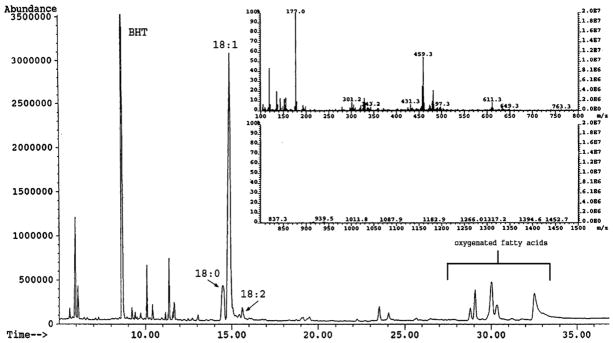
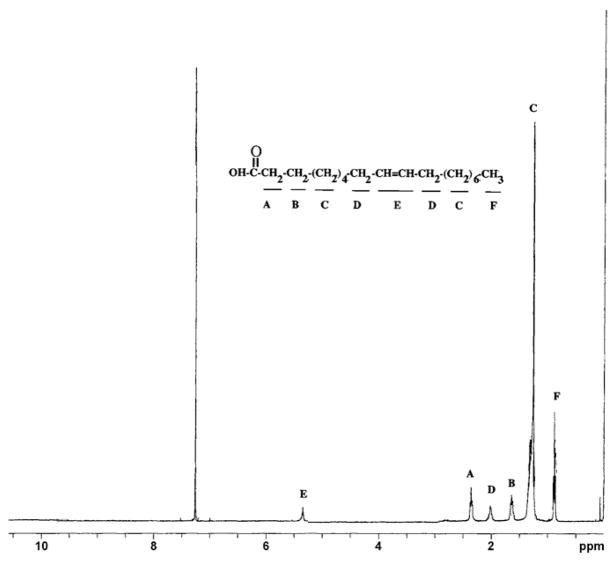
SIL was purified by HPLC and TLC as previously described [27] and analyzed by GC following derivatization of the sample to yield fatty acid methyl esters as described in Section 2. GC analysis demonstrated the majority (~70%) of the sample was composed of methyl oleate with the remainder consisting of small amounts of various oxygenated fatty acid derivatives and trace dimethylacetals derived from aldhehydes (Fig. 1). Analysis by FAB MS revealed a single large molecular ion peak at 459.2 m/z, and a smaller salt peak at 481.1 m/z (Fig. 1, inset). While neither of these values corresponds to oleic acid (282.5 m/z), we subsequently surmised the peak at 459.2 m/z was likely a cluster ion ([29] produced by the additive interaction of oleic acid (282.5 m/z), the Magic Bullet matrix (154 m/z) and a sodium ion (23 m/z). The secondary salt peak (481 m/z) was produced by the addition of a second sodium ion. Although this cluster ion formation initially complicated the identification of SIL, it was ultimately useful as a unique FAB-MS signature profile for identifying SIL in other biological samples such as bovine colostrum (see below). When the magic bullet matrix was replaced with nitrobenzylalcohol (m-NBA) as the matrix, the correct oleic acid molecular ion (282 m/z) was observed (data not shown). Analysis by NMR (Fig. 2) confirmed the identity of SIL as oleic acid, and was consistent with the preliminary characterization as oleic acid based on the results of TLC, GC and mass spectrometry analyses.
Fig. 1.
GC and FAB MS analysis of SIL. SIL was purified from bovine mucosa as previously described [27] and processed for GC and FAB MS analysis as indicated in Section 2. The relative proportions of fatty acid methyl ester peaks corresponding to steric (18:0), oleic (18:1) and linoleic (18:2) acids, and small amounts of oxygenated fatty acids are indicated on the chromatogram. Butylated hydroxytoluene (BHT) was used as the antioxidant. Inset: FAB MS profile of SIL. The molecular ion and its sodium salt peak are seen at 459.2 and 481.1 m/z, respectively. The Magic Bullet matrix and its sodium salt peak are seen at 177 and 199 m/z, respectively.
Fig. 2.
1H NMR analysis of SIL. SIL was purified from bovine mucosa as previously described [27] and processed for NMR analysis as indicated in Section 2. For reference, the assignments for individual signals are annotated (A–F) according to the known chemical shifts for oleic acid.
As mentioned previously, some calf intestinal mucosal extracts failed to yield the characteristic MS profiles, producing instead variable scattered smaller peaks in the 100–500 m/z range (data not shown). These results were surprising, and suggested the possibility that either the production of the inhibitory lipid originally described [27] was variable among calves or that the lipid was principally derived from a source other than bovine intestinal mucosa.
3.2. Fractionation and FAB-MS analysis of laboratory equipment extracts
In order to ensure that SIL was not a laboratory contaminant, organic extracts of all laboratory equipment and supplies containing organic components and used in the fractionation and analysis of mucosa (including squeeze bulbs, pipettes, gloves, etc.) were analyzed by HPTLC. None of these extracts yielded the appropriate HPTLC band or FAB-MS profile characteristic of the inhibitory lipid. The elimination of laboratory contamination as a source of the inhibitory lipid caused us to examine other possible sources, the most likely of which was dietary. Two-day-old calves at the University of Illinois Dairy Farm are fed colostrum; as the strength of the suckle response varies among calves of this age, some will ingest more than others, and this may result in variation in intestinal fat content. Therefore, we extracted and fractionated fresh colostrum in a manner identical to that used for calf intestinal mucosa.
3.3. Fractionation and FAB-MS analysis of bovine colostrum
HPTLC analysis of the organic extract of fresh colostrum produced a very faint band (and occasionally no band at all), which migrated as expected for SIL (Fig. 3A, inset 2). Samples yielding a faint band were pooled and the band was purified by HPTLC and submitted for analysis by FAB-MS; this material yielded only a very small peak at 459.2 m/z, but several other larger mass peaks were also observed (data not shown).
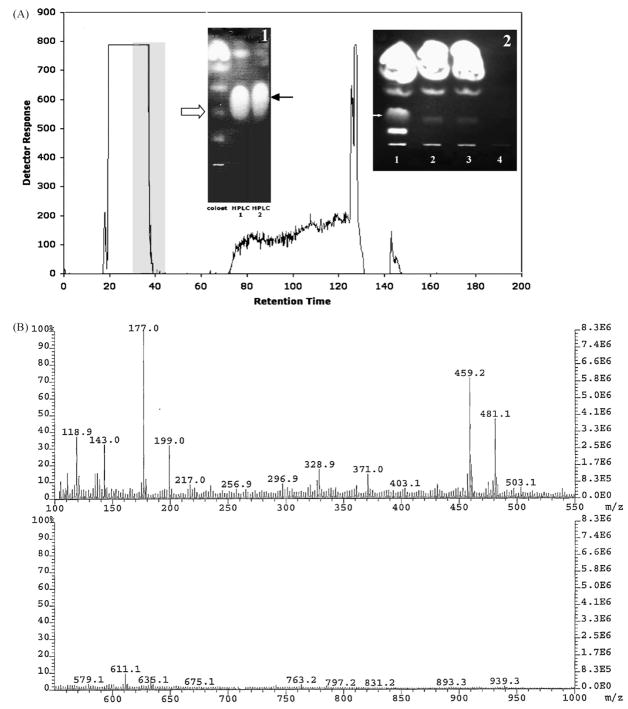
Fig. 3.
Characterization of SIL purified form digested colostrum. SIL was purified from bovine colostrum digested with an aqueous extract of bovine intestinal contents as described in Section 2. (A) HPLC profile of colostrum digested for 72 h. Fractions 30–45 (gray shaded area), were pooled and further purified by two HPTLC runs (inset 1); the black arrow points to the purified SIL bands and the white arrow indicates the position of SIL in the pooled HPLC fraction prior to HPTLC purification. Inset 2: HPTLC showing the relative amounts of SIL in crude colostrum (lane 3), the aqueous extract of bovine intestinal contents (lane 4) and colostrum digested with either native (lane 1) or heat-inactivated bovine intestinal contents (lane 2). (B) Fab-MS characterization of the molecular weight of SIL purified from digested colostrum. Note the presence of the molecular ion peaks at 459.2 and 481.1 m/z.
3.4. Colostrum digestion and SIL purification
Although organic solvent extraction of fresh bovine colostrum failed to yield significant quantities of SIL, we reasoned that dietary lipids, such as those contained in colostrum and isolated from the small intestine, are subject to processing or digestion by pre-gastric and pancreatic lipase. Therefore, in an effort to determine if SIL is produced following colostrum digestion in the gut, we designed an experiment to mimic digestion in vitro using fresh colostrum mixed with the aqueous fraction of calf small intestinal contents. HPLC fractionation of bovine colostrum ‘digested’ in vitro by overnight exposure at 37 °C to the aqueous fraction of bovine intestinal contents yielded a consistent fractionation profile (Fig. 3). HPTLC analysis (Fig. 3A, inset 1) showed a single lipid band was obtained from HPLC fractions eluting at 30–45 min. This band displayed a mobility on HPTLC identical to that originally described for the inhibitory lipid [27]. This band was further purified by HPTLC and submitted for analysis by FAB-MS and NMR. The purified lipid produced a large peak at 459.2 m/z and a smaller salt peak at 481.1 m/z (Fig. 3B), both of which were identical to the peaks previously described for the inhibitory lipid (Fig. 1) [27].
A HPTLC comparison of the lipid fraction from plain colostrum, colostrum incubated with aqueous intestinal contents, and colostrum incubated with pre-boiled aqueous intestinal contents is shown in Fig. 3A (inset 2). Colostrum incubated with untreated aqueous intestinal contents produced a band of much greater intensity in the expected region for SIL as compared to the non-digested colostrum or colostrum incubated with inactivated (boiled) aqueous intestinal content. When the regions corresponding to the SIL band were harvested from the TLC plates of the various control incubations (aqueous extracts of calf intestinal contents, colostrum incubated with pre-boiled aqueous extract of calf intestinal contents, or colostrum incubated without additives) were analyzed by FAB-MS, little or no peaks at either 459.2 m/z or 481.1 m/z were observed (data not shown). These fractions also showed no sporozoite adhesion inhibitory activity.
Digestion of colostrum for longer incubation periods increased the concentration of the SIL in the digested samples, measured as a percentage of the total lipid content of each sample (data not shown). A very faint band was observed at 0 min; from 0 min to 4 h, the band appeared and slowly increased in intensity. From 6 to 72 h, SIL continued to increase in amount, reaching its maximum concentration at 72 h (no increase or decrease was noted between 72 and 96 h). Densitometry analysis showed that concentrations of SIL as a percentage of total lipids increased from less than 1% at 0 min to over 10% at 72 h (data not shown).
3.5. Comparison of the sporozoite adhesion inhibitory activity of SIL and commercial oleic, linolenic, and stearic acids
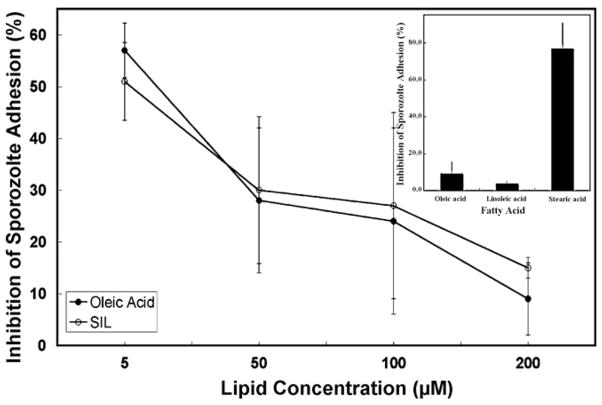
As anticipated, commercial oleic acid and the purified SIL were identical in their ability to dose-dependently inhibit host cell adhesion by C. parvum sporozoites (Fig. 4). Differences between the two fatty acids at each dosage were not statistically significant. Fifty percent inhibition was observed at a fatty acid concentration of 5 μM, with more than 80% inhibition at 200 μM. In initial experiments to determine if there was fatty acid specificity for inhibition of sporozoite adhesion, we observed linolenic acid, a long-chain polyunsaturated fatty acid (L-PUFA), was an even more effective inhibitor than oleic acid (Fig. 4, inset). Linolenate (200 μM) inhibited sporozoite adhesion by greater than 90%; however, stearic acid (200 μM), a saturated long-chain fatty acid, showed only marginal inhibitory activity.
Fig. 4.
Comparison of inhibitory activity of commercial oleic acid, linolenic acid, and stearic acid with SIL purified from digested colostrum. The sporozoite adhesion inhibitory activity of colostrum-derived SIL and commercial oleic acid, as well as linolenic and stearic acids were coompared in parallel parasite-host cell adhesion assays as described in Section 2. (●) commercial oleic acid; (○) SIL. The inset compares the relative sporozoite inhibitory activity of 200 3M commercial oleic, linolenic, and stearic acids. Error bars represent the standard deviation from mean values of triplicate experiments.
3.6. Effect of oleic and linolenic acids on sporozoite and MDBK cell viability
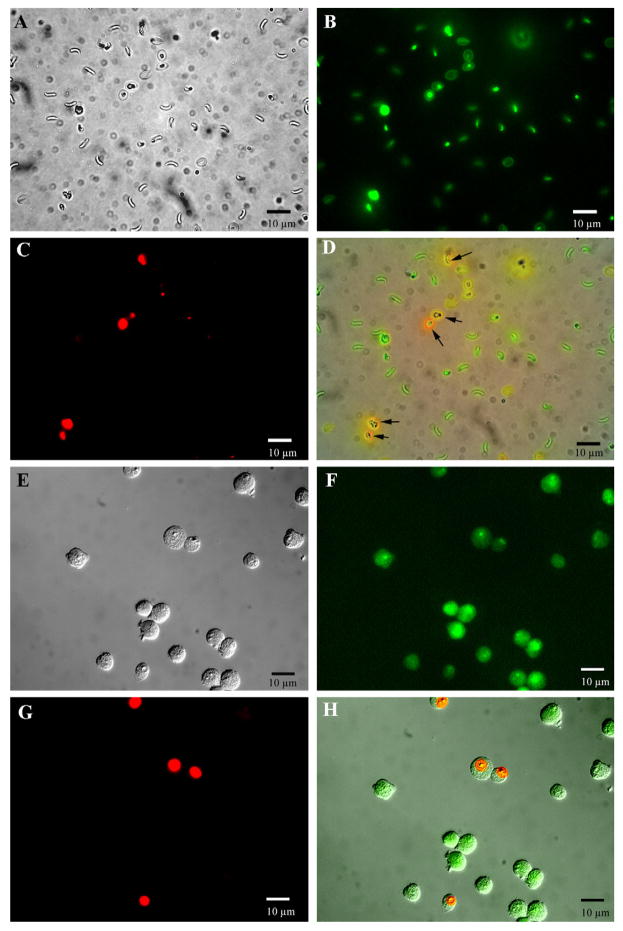
The effect of oleic and linolenic acids on sporozoite and host MDBK cell viability was measured using a dye exclusion assay as described in Section 2. Incubation of either MDBK cells or sporozoites with 200 μM linolenate did not result in measurable loss in cell viability as compared to control incubations performed in the absence of added fatty acids (Fig. 5). Sporozoites and MDBK cells maintained greater than 95% viability when incubated with or without linolenate under the incubation conditions used for sporozoite adhesion assays. Analysis of 20 microscopic fields (total of 3458 sporozoites and 668 MDBK cells) revealed 18/3145 (0.7%) sporozoites and 20/809 (2.5%) MDBK cells were dead when incubated with 200 μM linolenic acid as compared to 21/3458 (0.6%) and 8/668 (1.2%), respectively, for control (absence of fatty acids) incubations. The same results as shown for linolenic acid were also obtained using 200 μM oleic acid (data not shown).
Fig. 5.
Analysis of the effect of oleic and linolenic acids on sporozoite and MDBK cell viability. The effect to SIL on both sporozoite and host cell viability was measured by a two-component fluorescent dye exclusion assay as described in Section 2. The left panel shows representative fluorescent photomicrographs of both sporozoites and host MDBK cells following incubation with 200 μM linolenate, for 25 min at 37 °C. The green fluorescent dye stains all cells (weak stain for dead and vivid green for live cells) and the red dye stains only dead cells. (A) Phase contrast image of sporozoites; (B) green fluorescent image of sporozoites; (C) red fluorescent image of dead sporozoites and empty oocysts; (D) merged image (A, B and C frames). Note the red staining dead or empty oocysts (black arrows). (E) Phase contrast image of MDBK cells; (F) green fluorescent image of MDBK cells; (G) red fluorescent image of dead MDBK cells; (H) merged image (E, F, and G frames). The same results as shown here for linolenic acid were also obtained using 200 μM oleic acid.
4. Discussion
We previously reported the isolation of an unidentified lipid fraction from calf small intestinal mucosa that inhibited host cell adhesion by C. parvum sporozoites in vitro [27]. Subsequent attempts to isolate SIL from mucosal extracts of other calves met with varied success in yielding a fraction displaying an identical inhibitory specific activity and FAB-MS profile, leading us to suspect the principal source of the lipid may be dietary. Contrary to our expectations, the lipids isolated from fresh colostrum failed to produce the expected dominant FAB-MS peaks at 459.2 m/z and 481.1 m/z. However, as dietary lipids isolated from the small intestine are subject to processing by pre-gastric and pancreatic lipase, we designed an experiment to mimic digestion in vitro using the aqueous fraction of calf small intestinal contents. We found that SIL is generated from colostrum incubated at physiological temperatures with the fraction of intestinal contents expected to include digestive enzymes. Furthermore, the production of SIL under these conditions can be blocked by boiling the aqueous intestinal extract fraction prior to incubation with colostrum, a treatment known to destroy the activity of most enzymes. Fatty acids are typically generated from colostrum in vivo by the interaction of colostrum fats with pre-gastric and/or pancreatic lipase in the small intestine. Our results suggest that processing of colostrum lipids by digestive enzymes present in the small intestine of the calf produces the SIL, which we have now characterized as free, non-esterified, oleic acid.
During colostrum digestion experiments, we also observed very small amounts of SIL (mass spectrum peaks at 459.2 m/z) produced from colostrum stored at 4 °C in the refrigerator for 24 h. The storage of human milk at 4 °C for similar lengths of time results in the release of milk lipids active against enveloped viruses [30] and Giardia lamblia [31]. Others have reported the slow accumulation of free fatty acids in bovine milk over time at 4 °C caused by lipolysis of triglycerides by milk lipoprotein lipase [32] and by the release of other lipases by lactic acid bacteria [33]. These reports closely paralleled our own observations of the gradual appearance of the SIL at 4 °C.
Thin-layer chromatography, HPLC, GC, FAB-MS and NMR analysis of SIL originally isolated from bovine mucosa as well as from digested bovine colostrum clearly identified the inhibitory lipid as oleic acid, a long-chain monounsaturated fatty acid commonly found in a variety of dietary sources. The isolation of oleic acid from bovine colostrum (or from the small intestine of a colostrum-fed calf) in relatively large quantities was not unreasonable in light of the fact that approximately 21% of the fatty acid content in a typical milk sample is composed of monounsaturated fatty acids, the most abundant of which is oleic acid. Only 4% of the fatty acids are polyunsaturated, with the remaining 75% as saturated fatty acids. Also, the vast majority of the fatty acids (97%) in milk are present in the form of triglycerides, with very few free fatty acids present before cold storage or digestion [34].
Commercial oleic acid and oleic acid purified from colostrum (SIL) were equally effective in blocking sporozoite adhesion, with 50% inhibition of adhesion occurring at a concentration of 5 μM oleic acid. Although we have found no previous reports on the effects of fatty acids from milk or colostrum on Cryptosporidium species, some studies have demonstrated milk-mediated protection against a variety of protozoan pathogens and the effects of fatty acids on related parasites of phylum Apicomplexa have also been investigated. One report indicated oleic acid was cytotoxic to sporozoites of Plasmodium species, both free and within infected cells, in vitro within thirty minutes [35]. The release of free fatty acids during digestion of human milk was also found to be cytotoxic to G. lamblia [31,36]. A subsequent study showed that although increasing the concentration of intestinal mucous protected those parasites from fatty acid-mediated cytotoxicity in vitro; an increase in either the fatty acid concentration or the incubation time negated this effect [37]. Together, these results suggest that in addition to the antibody component of bovine colostrum and milk, the lipid fraction may also offer some protection against cryptosporidiosis.
Our research suggest some free fatty acids, particularly long-chain unsaturated fatty acids, inhibit the adhesion of C. parvum sporozoites to host cells in vitro. Preliminary data using two different commercial fluorescent live-dead cell viability assays (Molecular Probes Inc. kits #L7012 and L7013) indicate these fatty acids are not affecting cell membrane integrity or otherwise causing obvious cytotoxic effects since both the host MDBK cells or sporozoites maintain greater than 95% viability in the presence or absence of exogenous fatty acids. Furthermore, the effect appears to be on the sporozoite since pre-incubation of MDBK cells with 200 μM oleic acid for 30 min followed by washing prior to adding sporozoites has no effect on the kinetics or extent of sporozoite adhesion. We are currently investigating the molecular mechanism as well as the fatty acid specificity and structural characteristics responsible for fatty acid-mediated inhibition of sporozoite adhesion. Initial results indicate sporozoites display a marked specificity for their sensitivity to fatty acids. Only certain fatty acids, e.g., linolenic and oleic acid, show inhibitory activity, whereas long-chain saturated fatty acids, such as stearic acid, have little or no effect on sporozoite adhesion. Based on these initial results, we are currently engaged in a series of experiments to comprehensively address the fatty acid structural requirements, such as chain length, degree of saturation, esterification, and double bond configuration, necessary for optimal inhibition of sporozoite adhesion to host cells.
Although, the ability to inhibit sporozoite adhesion in vitro by exogenously supplied fatty acids suggests the possibility of dietary intervention using fatty acid supplementation for treatment or prevention of cryptosporidiosis, we have yet to determine whether fatty acid supplementation can inhibit infectivity in vivo. It is possible, that fatty acids may not be effective on sporozoites while they reside in the oocysts. Also, the process of in vivo excystation, which may position sporozoites in a mucus gel and in direct juxtaposition to the enterocyte surface membrane as they exit the oocysts, may effectively protect them from exogenous or dietary fatty acids. Even if fatty acids prove not to be effective as a direct anti-cryptosporidial agent, oleic or linolenic acid (and perhaps other long-chain unsaturated or polyunsaturated fatty acids) should serve as a very useful reagent for probing the molecular mechanism(s) involved in early sporozoite–host cell invasion. In this regard, we are currently examining the effect of L-PUFAs on sporozoite gliding motility, a process shown to be required for sporozoite invasion of host cells [38,39]. Such studies should likely reveal new parasite targets for the development anti-cryptosporidial and perhaps anti-Apicomplexa therapeutics.
Acknowledgments
This work was supported by grants from the U.S. National Institutes of Health (AI44967) and USDA (NC-1041-CSREES RRF ILLU-888-382) to MSK and a training fellowship grant (Agricultural Genome Science and Public Policy Training Program) from the USDA (2001-52100-11527) to JS. The mass spectrometer used in these studies was purchased in part with grants from the Division of Research Resources, National Institutes of Health (RR01575), the National Science Foundation (PCM 8121494), and the National Institute of General Medical Sciences (GM 27029). The AUCP-1 isolate of C. parvum used in these studies was originally a gift from Dr. Byron Blagburn, Auburn University. We thank Dr. Theresa Banet Kuhlenschmidt and Mr. William Hanafin for their excellent technical assistance; Spectral Data Services, Champaign, IL, and Professor Eric Oldfield, Department of Chemistry, University of Illinois for their assistance in NMR analyses; the Lipid Analysis Unit, Mylnefield Research Services Ltd., Mylnefield, Invergowrie, Dundee DD2 5DA, Scotland, for GC–MS analysis; and the Mass Spectrometry Laboratory, School of Chemical Sciences, University of Illinois for low-resolution fast-atom bombardment mass spectrometry (FAB-MS).
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited.
In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier’s archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/copyright
References
- 1.Hill VR, Kahler AM, Jothikumar N, Johnson TB, Hahn D, Cromeans TL. Multistate evaluation of an ultrafiltration-based procedure for simultaneous recovery of enteric microbes in 100-liter tap water samples. Appl Environ Microbiol. 2007;73:4218–25. doi: 10.1128/AEM.02713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruecker NJ, Braithwaite SL, Topp E, et al. Tracking host sources of Cryptosporidium spp. in raw water for improved health risk assessment. Appl Environ Microbiol. 2007;73:3945–57. doi: 10.1128/AEM.02788-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molloy SL, Montgomery AE, Huffman DE, Rose JB. Detection of Cryptosporidium parvum oocysts in sediment and biosolids by immunomagnetic separation. Water Environ Res. 2006;78:1013–6. doi: 10.2175/106143005x86619. [DOI] [PubMed] [Google Scholar]
- 4.Ochiai Y, Takada C, Hosaka M. Detection and discrimination of Cryptosporidium parvum and C. hominis in water samples by immunomagnetic separation-PCR. Appl Environ Microbiol. 2005;71:898–903. doi: 10.1128/AEM.71.2.898-903.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schets FM, Engels GB, During M, de Roda Husman AM. Detection of infectious Cryptosporidium oocysts by cell culture immunofluorescence assay: applicability to environmental samples. Appl Environ Microbiol. 2005;71:6793–8. doi: 10.1128/AEM.71.11.6793-6798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang J, Alderisio KA, Singh A, Xiao L. Development of procedures for direct extraction of Cryptosporidium DNA from water concentrates and for relief of PCR inhibitors. Appl Environ Microbiol. 2005;71:1135–41. doi: 10.1128/AEM.71.3.1135-1141.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thurston-Enriquez JA, Gilley JE, Eghball B. Microbial quality of runoff following land application of cattle manure and swine slurry. J Water Health. 2005;3:157–71. [PubMed] [Google Scholar]
- 8.Simmons OD, 3rd, Sobsey MD. A chemiluminescent immunofocus assay (CIFA) for non-microscopic enumeration of Cryptosporidium parvum infectivity in cell culture. Water Sci Technol. 2003;47:137–42. [PubMed] [Google Scholar]
- 9.Coulliette AD, Huffman DE, Slifko TR, Rose JB. Cryptosporidium parvum: treatment effects and the rate of decline in oocyst infectivity. J Parasitol. 2006;92:58–62. doi: 10.1645/GE-534R.1. [DOI] [PubMed] [Google Scholar]
- 10.Hunter PR, Hadfield SJ, Wilkinson D, Lake IR, Harrison FC, Chalmers RM. Subtypes of Cryptosporidium parvum in humans and disease risk. Emerg Infect Dis. 2007;13:82–8. doi: 10.3201/eid1301.060481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson VR, Curran MP. Nitazoxanide: a review of its use in the treatment of gastrointestinal infections. Drugs. 2007;67:1947–67. doi: 10.2165/00003495-200767130-00015. [DOI] [PubMed] [Google Scholar]
- 12.Graczyk TK, Cranfield MR, Bostwick EF. Successful hyperimmune bovine colostrum treatment of Savanna monitors (Varanus exanthematicus) infected with Cryptosporidium sp. J Parasitol. 2000;86:631–2. doi: 10.1645/0022-3395(2000)086[0631:SHBCTO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Graczyk TK, Cranfield MR, Bostwick EF. Hyperimmune bovine colostrum treatment of moribund Leopard geckos (Eublepharis macularius) infected with Cryptosporidium sp. Vet Res. 1999;30:377–82. [PubMed] [Google Scholar]
- 14.Graczyk TK, Cranfield MR, Helmer P, Fayer R, Bostwick EF. Therapeutic efficacy of hyperimmune bovine colostrum treatment against clinical and subclinical Cryptosporidium serpentis infections in captive snakes. Vet Parasitol. 1998;74:123–32. doi: 10.1016/s0304-4017(97)00153-2. [DOI] [PubMed] [Google Scholar]
- 15.Fayer R, Guidry A, Blagburn BL. Immunotherapeutic efficacy of bovine colostral immunoglobulins from a hyperimmunized cow against cryptosporidiosis in neonatal mice. Infect Immun. 1990;58:2962–5. doi: 10.1128/iai.58.9.2962-2965.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fayer R, Andrews C, Ungar BL, Blagburn B. Efficacy of hyperimmune bovine colostrum for prophylaxis of cryptosporidiosis in neonatal calves. J Parasitol. 1989;75:393–7. [PubMed] [Google Scholar]
- 17.Perryman LE, Kapil SJ, Jones ML, Hunt EL. Protection of calves against cryptosporidiosis with immune bovine colostrum induced by a Cryptosporidium parvum recombinant protein. Vaccine. 1999;17:2142–9. doi: 10.1016/s0264-410x(98)00477-0. [DOI] [PubMed] [Google Scholar]
- 18.Naciri M, Mancassola R, Reperant JM, Canivez O, Quinque B, Yvore P. Treatment of experimental ovine cryptosporidiosis with ovine or bovine hyperimmune colostrum. Vet Parasitol. 1994;53:173–90. doi: 10.1016/0304-4017(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 19.Riggs MW, Cama VA, Leary HL, Jr, Sterling CR. Bovine antibody against Cryptosporidium parvum elicits a circumsporozoite precipitate-like reaction and has immunotherapeutic effect against persistent cryptosporidiosis in SCID mice. Infect Immun. 1994;62:1927–39. doi: 10.1128/iai.62.5.1927-1939.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watzl B, Huang DS, Alak J, Darban H, Jenkins EM, Watson RR. Enhancement of resistance to Cryptosporidium parvum by pooled bovine colostrum during murine retroviral infection. Am J Trop Med Hyg. 1993;48:519–23. doi: 10.4269/ajtmh.1993.48.519. [DOI] [PubMed] [Google Scholar]
- 21.Nord J, Ma P, DiJohn D, Tzipori S, Tacket CO. Treatment with bovine hyperimmune colostrum of cryptosporidial diarrhea in AIDS patients. Aids. 1990;4:581–4. doi: 10.1097/00002030-199006000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Shield J, Melville C, Novelli V, et al. Bovine colostrum immunoglobulin concentrate for cryptosporidiosis in AIDS. Arch Dis Child. 1993;69:451–3. doi: 10.1136/adc.69.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenberg PD, Cello JP. Treatment of severe diarrhea caused by Cryptosporidium parvum with oral bovine immunoglobulin concentrate in patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:348–54. doi: 10.1097/00042560-199612010-00008. [DOI] [PubMed] [Google Scholar]
- 24.Ungar BL, Ward DJ, Fayer R, Quinn CA. Cessation of Cryptosporidium-associated diarrhea in an acquired immunodeficiency syndrome patient after treatment with hyperimmune bovine colostrum. Gastroenterology. 1990;98:486–9. doi: 10.1016/0016-5085(90)90842-o. [DOI] [PubMed] [Google Scholar]
- 25.Floren CH, Chinenye S, Elfstrand L, Hagman C, Ihse I. ColoPlus, a new product based on bovine colostrum, alleviates HIV-associated diarrhoea. Scand J Gastroenterol. 2006;41:682–6. doi: 10.1080/00365520500380817. [DOI] [PubMed] [Google Scholar]
- 26.Flanigan T, Marshall R, Redman D, Kaetzel C, Ungar B. In vitro screening of therapeutic agents against Cryptosporidium: hyperimmune cow colostrum is highly inhibitory. J Protozool. 1991;38:225S–7S. [PubMed] [Google Scholar]
- 27.Johnson JK, Schmidt J, Gelberg HB, Kuhlenschmidt MS. Microbial adhesion of Cryptosporidium parvum sporozoites: purification of an inhibitory lipid from bovine mucosa. J Parasitol. 2004;90:980–90. doi: 10.1645/GE-231R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Current WL. Techniques and laboratory maintenance of Cryptosporidium. In: Dubey JP, Speer CA, Fayer R, editors. Cryptosporidiosis of man and animals. Boca Raton, FL: CRC Press; 1990. pp. 31–49. [Google Scholar]
- 29.Fenselau C, Cotter RJ. Chemical aspects of fast atom bombardment. Chem Rev. 1987;87:501–12. [Google Scholar]
- 30.Thormar H, Isaacs CE, Brown HR, Barshatzky MR, Pessolano T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1987;31:27–31. doi: 10.1128/aac.31.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hernell O, Ward H, Blackberg L, Pereira ME. Killing of Giardia lamblia by human milk lipases: an effect mediated by lipolysis of milk lipids. J Infect Dis. 1986;153:715–20. doi: 10.1093/infdis/153.4.715. [DOI] [PubMed] [Google Scholar]
- 32.Clegg RA. Activation of milk lipase by serum proteins: possible role in the occurrence of lipolysis in raw bovine milk. J Dairy Res. 1980;47:61–70. doi: 10.1017/s0022029900020884. [DOI] [PubMed] [Google Scholar]
- 33.Coskun H, Ondul E. Free fatty acid accumulation by mesophilic lactic acid bacteria in cold-stored milk. J Microbiol. 2004;42:133–8. [PubMed] [Google Scholar]
- 34.Mansbridge RJ, Blake JS. Nutritional factors affecting the fatty acid composition of bovine milk. Br J Nutr. 1997;78(Suppl 1):S37–47. doi: 10.1079/bjn19970133. [DOI] [PubMed] [Google Scholar]
- 35.Krugliak M, Deharo E, Shalmiev G, Sauvain M, Moretti C, Ginsburg H. Anti-malarial effects of C18 fatty acids on Plasmodium falciparum in culture and on Plasmodium vinckei petteri and Plasmodium yoelii nigeriensis in vivo. Exp Parasitol. 1995;81:97–105. doi: 10.1006/expr.1995.1097. [DOI] [PubMed] [Google Scholar]
- 36.Reiner DS, Wang CS, Gillin FD. Human milk kills Giardia lamblia by generating toxic lipolytic products. J Infect Dis. 1986;154:825–32. doi: 10.1093/infdis/154.5.825. [DOI] [PubMed] [Google Scholar]
- 37.Zenian AJ, Gillin FD. Intestinal mucus protects Giardia lamblia from killing by human milk. J Protozool. 1987;34:22–6. doi: 10.1111/j.1550-7408.1987.tb03124.x. [DOI] [PubMed] [Google Scholar]
- 38.Wetzel DM, Schmidt J, Kuhlenschmidt MS, Dubey JP, Sibley LD. Gliding motility leads to active cellular invasion by Cryptosporidium parvum sporozoites. Infect Immun. 2005;73:5379–87. doi: 10.1128/IAI.73.9.5379-5387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sibley LD. Intracellular parasite invasion strategies. Science. 2004;304:248–53. doi: 10.1126/science.1094717. [DOI] [PubMed] [Google Scholar]