Abstract
The intensity and prevalence of parasitism by Ascogregarina barretti (Vavra) in Aedes triseriatus (Say) did not differ between tires and tree holes in field samples taken in September 1996. There was significant variation in the intensity of parasitism among containers that was not significantly correlated with the pH, conductivity, or temperature of the container water. In an experiment manipulating habitat drying, treatments had a significant effect on A. barretti infection of Ae. triseriatus, only during midsummer in one of two years. Containers maintained at maximal volume had the lowest prevalence of parasitism, and containers that dried out had the greatest prevalence. In this experiment, there was also a season-dependent difference in the intensity and prevalence of infection between tree holes and tires. The first larvae to reach the fourth instar in tires in the early summer had lower intensity and prevalence of infection than did larvae in tree holes during the same period. The seasonal difference in intensity of parasitism between tires and tree holes was not related to differences in pH, conductivity, and temperature.
Keywords: Aedes triseriatus, Ascogregarina barretti, habitat drying, prevalence and intensity of infection
Environmental factors may affect the dynamics of host/parasite relationships (Lively et al. 1995). For example, Chen and Yang (1996) found that Ascogregarina taiwanensis (Lein & Levine) could complete its life cycle in its host, Aedes albopictus (Skuse), only within a limited range of temperatures. At high temperatures the host reached adulthood too quickly for the development of the parasite and at low temperatures the host developed too slowly. Siegel et al. (1992) found that infection by A. barretti in Ae. triseriatus had a stronger negative effect on A. triseriatus wing length in the more sunlit area of a tire dump, but did not report statistics on either the intensity or prevalence of infection; therefore, it was impossible to determine whether the stronger negative effect of parasitism in sunlit areas may be explained by differences in either measure of infection. Most studies of environmental effects on host/parasite dynamics have focused on the stage of the parasite’s life cycle that occurs in the host (Washburn et al. 1991, Lively et al. 1995, Polak 1996). However, many parasites must survive outside their host for some period. Changes in the environment may affect the survival of the parasite while it is outside its host, thereby altering the intensity (number of parasites per individual) or prevalence (proportion of parasitized individuals in a population) of infection in the host population.
The container dwelling mosquito, Aedes triseriatus (Say), is parasitized by Ascogregarina barretti (Varva), a protozoan intestinal parasite (Varva 1969). Mosquito larvae become parasitized when first instars ingest oocysts. The oocysts rupture to release sporozoites that penetrate epithelial cells of the midgut where they undergo cellular development as trophonts. Late in the fourth stadium the trophonts transform into elongate gamonts and begin migration to the Malpighian tubules. In the Malpighian tubules of pupae the gamonts pair and fuse to become gametocysts. Each gametocyst produces several hundred oocysts, which are released when an adult defecates or dies in a water-filled container (Beier and Craig 1985).
Many studies have shown that A. barretti has significant detrimental effects on Ae. triseriatus development and survival (Beier and Harris 1983, Walker et al. 1987, Siegel et al. 1992; unpublished data). Parasitism by A. barretti increased time to pupation for males and decreased pupal size for females, but had no effect on larval survivorship, emergence, or survival of the adult (Beier and Harris 1983). Walker et al. (1987) found increased development time and decreased size at pupation for highly parasitized, food stressed females, but no effect on larval survival. They estimated a 32% reduction in egg production by parasitized females because of reduced adult body size. Comiskey et al. (1999) found that low food and parasitism by Ascogregarina taiwanensis increased larval mortality of Aedes albopictus Skuse by 35%. Parasitized adults were on average 5% smaller and females produced 23% fewer eggs than did unparasitized females. Aedes triseriatus is the primary vector of LaCrosse virus (Grimstad et al. 1977). Therefore, it is important to understand fully conditions that alter the prevalence or intensity of infection to assess the impact of this parasite on its host population and virus transmission.
The effects of environmental variation on A. barretti parasitism of Ae. triseriatus in water-filled containers (e.g., tree holes, tires, or cemetery vases) are poorly known. Aedes triseriatus larvae have lower survivorship in tires than in tree holes (Nannini and Juliano 1998), which are two physically different environments. Like their mosquito host, A. barretti may experience reduced survivorship in tires; therefore, the prevalence or intensity of parasitism may be lower in tires than in tree holes. Beier and Harris (1983) studied the level of A. barretti infection in Ae. triseriatus and suggested, without data analysis, that there was no difference between tires and tree holes. These habitats are often subject to drying; and habitat drying and water level fluctuation affects the community structure of these containers (Lounibos 1985, Bradshaw and Holzapfel 1988). The oocyst stage of the A. barretti must survive outside its host. Laboratory studies of Ascogregarina culicis (Ross) showed that viable oocysts could be recovered after 5 mo of drying (McCray et al. 1970). However, these studies provided no quantitative data on the proportion of oocysts that were viable after drying (McCray et al. 1970). Although some viable oocysts remained, drying substantially may have reduced survivorship. The effects of other environmental factors such as pH, conductivity, or temperature on prevalence and intensity parasitism of A. barretti in Ae. triseriatus have not been studied. Garcia et al. (1994) observed infection rates of A. taiwanensis in Ae. albopictus in tires over a 1-yr period and reported a trend for higher prevalence of infection in cold months but provided no statistical analysis. Comiskey et al. (1999) also reported seasonal variation in the prevalence of infection by A. taiwanensis in A. albopictus, with prevalence being lower in the fall and spring. Seasonal studies of infection by A. barretti in Ae. triseriatus have not been performed.
In the current study, we tested the hypothesis that, like their host, A. barretti will have lower survival in tires and predict that the intensity and prevalence of infection will be lower in tires than in tree holes. We also tested the hypothesis that drying will cause mortality of oocysts, resulting in lower intensity and prevalence of infection. Therefore, we predict that containers that dry out completely will have lower intensity and prevalence of infection compared with containers that have fluctuating water volumes but do not dry out, or containers that retain relatively constant water volume. We also tested for seasonal differences in infection and for correlations with various physical characteristics of the container water.
Materials and Methods
Field Sampling
During 1 wk in late September and early October of 1996 fourth instars and pupae of Ae. triseriatus were collected from nine tree holes and 12 experimental tires in a 108-ha tract of riparian and upland woods located 26 km northeast of Normal, IL (89° 3′ W, 40° 54′ N; elevation 291 m). The tires had been placed leaning nearly upright against trees in 1993. Tree holes and tires at this site were sampled continuously from 1995 to 1999, and Ae. triseriatus and Aedes hendersoni Cockerell were the only species of Aedes collected, with Ae. triseriatus comprising >99% of collected individuals. Therefore, we are confident based on host abundance, parasite morphology, and the absence of other Aedes that trophonts in Ae. triseriatus were A. barretti.
Up to 20 larvae were dissected from each container and the number of A. barretti trophonts in each was counted (Beier and Craig 1985). The mean intensity and prevalence of infection for each container were compared between tree holes and tires using multivariate analysis of variance (MANOVA, PROC GLM, SAS Institute 1995). Variation in the intensity of infection among containers was tested using a nested analysis of variance (ANOVA) (PROC GLM, SAS Institute 1995), with container type as a factor and container nested within container type. For the month of September, pH, conductivity, and temperature were recorded weekly for the containers sampled. The mean pH, conductivity, and temperature for a container were tested for correlation with the intensity and prevalence of infection of that container (PROC CORR, SAS Institute 1995).
Experiment
In 1997, an experiment was conducted using 16 tires and 11 tree holes. In 1998, four tree holes were added to attain more equal representation of tree holes and tires. Each container was assigned randomly to one of three treatments: (1) Full and constant: the water level was kept near maximum by refilling two times per week. (2) Fluctuating: the container was allowed to dry to 25% of capacity and then refilled to capacity. (3) Drying: the container was allowed to dry until there was no standing water, left dry for 1 wk, and then refilled to capacity. All treatments were allowed to dry by natural evaporation, so that the manipulation consisted of the timing of water additions. This necessarily resulted in some variation among containers in the timing of refilling. The containers were checked two times per week, and water was added as needed. The tires received deionized water. The tree holes received simulated stem flow created by spraying deionized water to a height of 3–4 m on a tree trunk fitted with a plastic ring that collected the simulated stem flow as it ran down the trunk. Rain was excluded from the tires and tree holes by a covering of plastic sheeting supported by wire mesh frames. For tree holes, additional plastic sheeting was extended to wrap around the trunk of the tree, and stapled, caulked and taped to the tree to exclude natural stem flow. Observations made during rains confirmed that this method was effective in keeping rain water out of the containers.
From March to November 1997 and 1998, censuses of the prevalence and intensity of infection for each experimental tire and tree hole were taken every 6 wk, using the census techniques for larvae described by Lounibos et al. (1997). At each census up to 20 A. triseriatus fourth instars were dissected from each container (Beier and Craig 1985) to assess the number of A. barretti trophonts in each larva. Data on parasite prevalence and intensity were compared among drying regimes and type of container (tree hole or tire) using a two-way MANOVA (PROC GLM, SAS Institute 1995). Mean monthly pH, conductivity, and temperature were compared between tires and tree holes from March to June during 1997 and 1998 using a MANOVA (PROC GLM, SAS Institute 1995). The data for intensity of infection in early summer in 1997 were log10 transformed to meet the assumptions of MANOVA, and back transformed for description of means. All other intensity data were not transformed. For all MANOVAs, we used Pillai’s trace statistic, because it is the most robust to violations of assumptions (Scheiner 1993). Standardized canonical coefficients were used to determine which of the dependent variables explained most of the variation between groups (Scheiner 1993). These coefficients described a function, in units of standard deviations, that synthesized information from the individual dependent variables into a single variable (=canonical variate), which maximized the differences among groups (treatment-habitat combinations in this case). Because the dependent variables’ contributions to the canonical variate were scaled in standard deviation units, the magnitudes of the standardized canonical coefficients indicated the relative contributions of each dependent variable to the multivariate differences (Scheiner 1993). All prevalence data were transformed by arcsine of the square root of the proportion (Zar 1996, p. 282) to meet the assumptions of normality and homogeneity of variances. All pH data for 1997 and 1998 were square root transformed. All conductivity data for 1997 and 1998 were log10 transformed.
Results
FieldSampling
For September and October of 1996 there was no difference in intensity or prevalence of A. barretti infection between tree holes and tires (Pillai’s trace = 0.073; df = 2, 18; P = 0.839). The mean ± SE intensity of infection was 14.39 ± 4.18 and 18.24 ± 11.41 trophonts per larva for tree holes and tires, respectively. The mean prevalence of infection was 83 ± 0.06% and 71 ± 0.12% for tree holes and tires, respectively. There was significant variation among containers in the intensity of infection (F = 3.567; df = 19, 124; P = 0.0001). Intensity and prevalence of infection within containers were not significantly correlated with mean pH, conductivity, or temperature for those containers (Table 1).
Table 1.
Spearman rank correlations between the level of infection by A. barretti in A. triseriatus and physical parameters of container water for September/October 1996 (n = 21 containers)
| Intensity
|
Prevalence
|
|||
|---|---|---|---|---|
| rs | P>rs | rs | P>rs | |
| Conductivity | 0.181 | 0.507 | 0.006 | 0.983 |
| pH | 0.233 | 0.384 | −0.266 | 0.319 |
| Temp | −0.052 | 0.846 | −0.068 | 0.802 |
Experiment
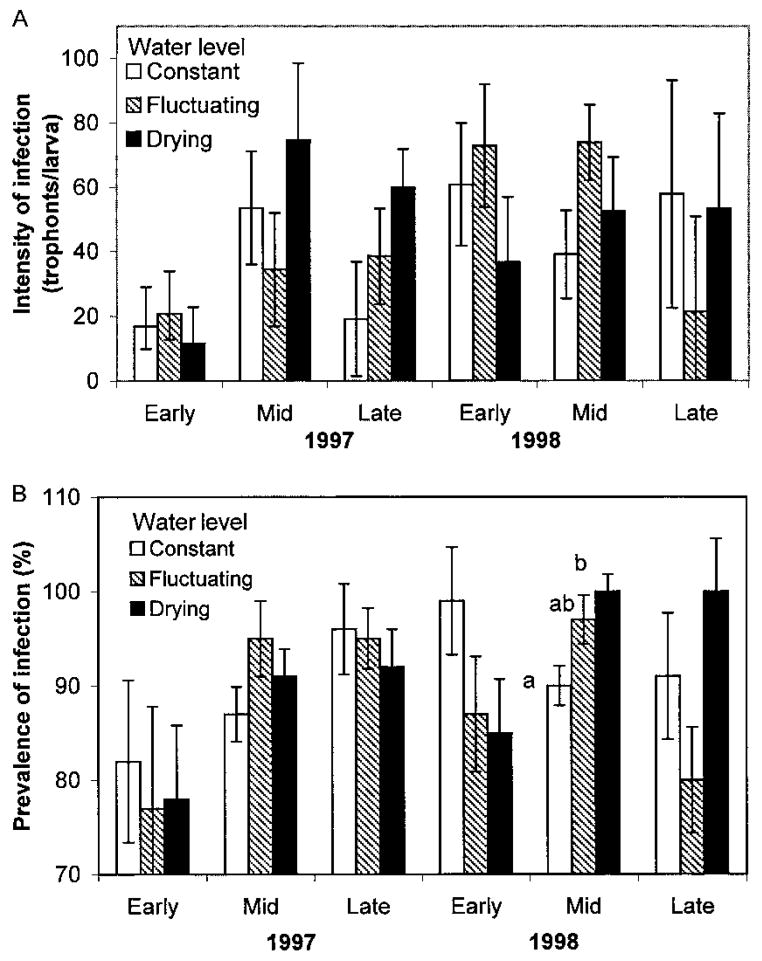
For both years, only three periods yielded sufficient numbers of fourth-instar Ae. triseriatus for analysis: early summer (early June), mid- summer (mid-July), and late summer (late August/early September). Comparing the intensity and prevalence of A. barretti infection for each year across all sample periods yielded no significant effects of drying regime or container type (tree hole or tire) in either year (Table 2). However, when the sample periods were considered separately, there was a significant effect of container type on prevalence and intensity for the early summer sample in both 1997 and 1998 (Tables 3 and 4). The standardized canonical coefficients for container type showed that most of the variation was caused by prevalence of infection in 1997 and intensity of infection in 1998 (Table 5; Fig. 1). Over all, early-season prevalence and intensity of parasitism were lower in tires than in tree holes during both years. Neither the drying regime nor the interaction had an effect on parasitism for the early summer sample (Tables 3 and 4). There was a significant effect of drying regime in the midsummer sample in 1998, but only a marginally significant effect in 1997 (Tables 3 and 4; Fig. 2). For midsummer in both 1997 and 1998 the standardized canonical coefficients showed that prevalence of infection accounted for more of the variation among treatments (standardized canonical coefficients: 1997 –– prevalence = 1.211, intensity = 0.927; 1998 –– prevalence = 1.035, intensity = 0.673. In midsummer 1998, the drying treatment yielded significantly greater prevalence of infections than did the constant treatment (Fig. 2). The late summer samples did not show significant effects of container type or drying regime in either year (Tables 3 and 4).
Table 2.
MANOVA results over all samples for 1997 and 1998 response variables were intensity and prevalence of infection by A. barretti in A. triseratus
| 1997
|
1998
|
|||||
|---|---|---|---|---|---|---|
| Pillai’s trace | df | P | Pillai’s trace | df | P | |
| Drying regime | 0.059 | 4, 44 | 0.853 | 1.129 | 4, 50 | 0.353 |
| Container type | 0.215 | 2, 76 | 0.078 | 0.051 | 2, 24 | 0.531 |
| Regime*type | 0.085 | 4, 44 | 0.743 | 0.163 | 4, 50 | 0.363 |
Table 3.
MANOVA results for 1997 samples by time of year
| Early summer
|
Mid-summer
|
Late summer
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pillai’s trace | df | P | Pillai’s trace | df | P | Pillai’s trace | df | P | |
| Drying regime | 0.184 | 4, 42 | 0.384 | 0.433 | 4, 34 | 0.068 | 0.450 | 4, 28 | 0.160 |
| Container type | 0.433 | 2, 20 | 0.003a | 0.089 | 2, 16 | 0.473 | 0.159 | 2, 13 | 0.324 |
| Regime*Type | 0.380 | 4, 42 | 0.093 | 0.226 | 4, 34 | 0.381 | 0.194 | 4, 28 | 0.564 |
Response variables were intensity and prevalence of infection by A. barretti in A. triseriatus.
Significant P values.
Table 4.
MANOVA results for 1998 samples by time of year
| Early summer
|
Mid-summer
|
Late summer
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pillai’s trace | df | P | Pillai’s trace | df | P | Pillai’s trace | df | P | |
| Drying regime | 0.329 | 4, 44 | 0.088 | 0.466 | 4, 36 | 0.035 | 0.635 | 2, 20 | 0.366 |
| Container type | 0.385 | 2, 21 | 0.006 | 1.123 | 2, 24 | 0.109 | 0.059 | 2, 9 | 0.764 |
| Regime*Type | 0.187 | 4, 44 | 0.353 | 0.669 | 4, 36 | 0.387 | 0.162 | 2, 20 | 0.777 |
Response variables were intensity and prevalence of infection by A. barretti in A. triseriatus.
Significant P values.
Table 5.
Standardized canonical coefficients for the response variables, intensity and prevalence of infection, between container type
| 1997 early summer | 1998 early summer | |
|---|---|---|
| Intensity | −0.061 | 1.368 |
| Prevalence | 1.291 | −0.137 |
Fig. 1.
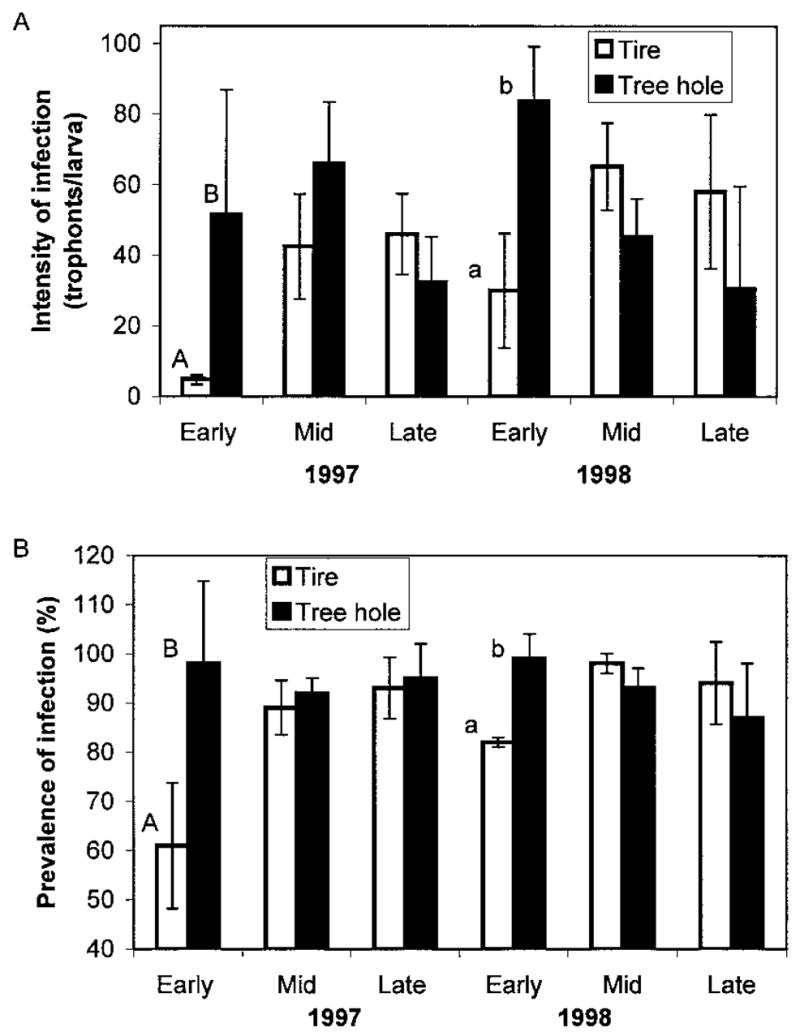
(A) Prevalence of infection expressed as the percentage (±SE) of A. triseriatus larvae parasitized by A. barretti and (B) least square means (±SE) for intensity of infection in tree holes and tires for each sample period of 1997 and 1998. Different letters denote statistically significant pairwise differences within a sampling period (t-tests, Bonferroni experimentwise α = 0.05).
Fig. 2.
(A) Prevalence of infection expressed as the percentage (±SE) of A. triseriatus larvae parasitized by A. barretti and (B) least square means (±SE) for intensity of infection for each drying regime treatment for each sample period of 1997 and 1998. The means for intensity in early 1997 are back transformed.
To determine if water quality during the times larvae became infected was related to the difference between container types in the early summer, the mean pH, conductivity, and temperature were compared for tree holes and tires for the 2 mo before the early- and midsummer samples. If there were differences in water quality between tree holes and tires during the 2 mo before the early summer sample, they may be related to the difference in parasitism between container types. However, if those differences in water quality persisted during the 2 mo before the midsummer sample, when the level of parasitism in each type of container was similar, they would seem to be unrelated to the difference in parasitism between container types in early summer. The mean physical parameters (pH, conductivity, and temperature) for both March and April, and May and June differed significantly between tree holes and tires in both 1997 and 1998 (Table 6). Standardized canonical coefficients for both years and both sample periods showed that most of the variation between container types was the result of differences in conductivity, with much greater conductivity in tree holes at all times (Table 7).
Table 6.
MANOVA results over all samples for 1997 and 1998
| 1997
|
1998
|
|||||
|---|---|---|---|---|---|---|
| Pillai’s trace | df | P | Pillai’s trace | df | P | |
| March and April | 0.524 | 3, 26 | 0.0018 | 0.584 | 3, 27 | 0.0001 |
| May and June | 0.546 | 3, 26 | 0.0001 | 0.682 | 3, 27 | 0.0001 |
Response variables were conductivity, pH, and temperature, compared between tires and tree holes.
Table 7.
Standardized canonical coefficients for the response variables, conductivity, pH, and temperature
| 1997
|
1998
|
|||
|---|---|---|---|---|
| March and April | May and June | March and April | May and June | |
| Conductivity (μmhos/cm) | ||||
| Coefficients | 1.066 | 1.287 | 1.477 | 1.200 |
| LS Mean ± SE | ||||
| Tires | 371.48 ±59.79 | 403.13 ± 90.87 | 208.85 ± 47.77 | 355.81 ± 64.08 |
| Treeholes | 919.36 ± 66.18 | 1,576.19 ± 100.13 | 906.76 ± 51.07 | 1,272.56 ± 65.43 |
| pH | ||||
| Coefficients | −0.030 | −0.617 | 0.076 | 0.655 |
| Mean ± SE | ||||
| Tires | 6.91 ± 0.07 | 7.37 ± 0.06 | 7.11 ± 0.08 | 7.38 ± 0.07 |
| Treeholes | 7.33 ± 0.08 | 7.41 ± 0.06 | 7.34 ± 0.08 | 7.88 ± 0.07 |
| Temp, °C | ||||
| Coefficients | 0.574 | −0.627 | −0.071 | −0.709 |
| Mean ± SE | ||||
| Tires | 7.47 ± 0.53 | 15.09 ± 0.28 | 8.46 ± 0.84 | 18.09 ± 0.40 |
| Treeholes | 8.28 ± 0.53 | 14.31 ± 0.30 | 9.60 ± 0.88 | 17.87 ± 0.38 |
Discussion
Our field results in early fall of 1996 were consistent with the conclusions of Beier and Harris (1983) that there was no significant difference in the intensity or prevalence of infection by A. barretti between Ae. triseriatus larvae collected in tree holes and tires. There was significant variation in the intensity of infection among containers that was not related to pH, conductivity, or temperature of the water in the containers. Therefore, A. barretti, unlike its host, does not appear to have lower survival in tires than tree holes. However, lower survival of A. barretti oocysts in tires may have been masked by a greater rate of replacement with new oocysts. The possibility that oocysts are replaced at a rate greater than they are lost from a container because of mortality is also important in our discussion of habitat drying and season-dependent differences between tires and tree holes (see below).
Previous studies have shown that habitat drying affects the structure of container communities (Lounibos 1985, Bradshaw and Holzapfel 1988). However, it seems that the parasite–host relationship of A. barretti and Ae. triseriatus is only slightly affected by habitat drying. The effect of our experimental drying regimes was significant only in the midsummer sample of 1998 and marginally significant in the midsummer sample of 1997. The direction of the significant effect in 1998 was the opposite of our predictions. The drying treatment had the highest prevalence of infection, followed by the fluctuating and constant water levels. In midsummer 1997 it was also the constant treatments that had the lowest prevalence of infection. Therefore, although there was a significant effect of drying regime on parasitism by A. barretti, this transient effect was not consistent with mortality caused by drying. It may be that one week of dry conditions is insufficient to increase oocyst mortality significantly or that the rate of input of fresh oocysts is high enough to compensate for increased mortality. Prevalence of parasitism in midsummer tended to be lowest in the constant treatment, which had container water volumes maintained close to maximum values. This effect, which was significant in one year and marginally significant in the other, may be a simple result of greater average water volume in the constant treatments, which may have resulted in a lower concentration of oocysts per milliliter, and increased the likelihood of some larvae escaping parasitism.
In our experiment, there was a significant difference in parasitism between container types (tree holes and tires), but this difference depended on the time of year. Larvae sampled in early summer were the first generation of the year and appeared to suffer much lower parasitism in tires than in tree holes. This difference disappeared by midsummer. Our unpublished data and Walker et al. (1987) have shown that A. triseriatus females infected with A. barretti may experience a 32–50% reduction in fecundity. Therefore, females emerging from tires early in the year with reduced parasitism may realize higher fecundity, on average, than will females emerging from tree holes. This gives a possible advantage to females from tires early in the season.
There are several hypotheses that may explain this phenomenon. First, oocysts may suffer greater mortality over winter in tires than in tree holes. Tree holes may be better insulated and oocysts may experience higher mean temperatures than in tires. We know that the water in our tires became completely frozen for a period of weeks in both winters, and such solid freezing may have increased oocyst mortality. However, we do not know whether tree holes also froze, because the form of most rot holes precludes determination of whether the water has frozen to the bottom of the hole.
Second, oocysts may not experience greater overwinter mortality, but rather may be less likely to infect their host under conditions in the tires early in the spring when the first hatch of A. triseriatus occurs. Our comparison of pH, conductivity, and temperature of container water between tree holes and tires in early spring (when larvae collected in early summer became infected) indicated that these containers differed mainly in conductivity, with relatively little difference in pH or temperature. Although it is possible that this difference in conductivity in some way contributed to the difference in parasitism between tree holes and tires in early summer, we think that this difference is unlikely to explain the differences in prevalence of infection. Water quality in tires and tree holes over May and June (months during which larvae sampled in midsummer would have been subject to infection) had similar differences in conductivity, yet there was no difference in the level of parasitism between container types for the midsummer sample. If the difference in conductivity was responsible for differences in parasitism early in the summer, then the same situation would be expected in the midsummer sample.
A third hypothesis is that there is a difference in the traits of Ae. triseriatus larvae from the first hatch of the year between tree holes and tires. This implies that early larvae in tires are in some way intrinsically more resistant to parasitism. These larvae would be produced from overwintering (diapause) eggs laid late in the season of the previous year. We know of no evidence for or against this hypothesis.
Whatever the explanation, it is clear from our study that parasitism of Ae. triseriatus by A. barretti does not show strong, consistent relationships to habitat type or to some major environmental variables. Differences between habitats, such as they are, are season-dependent. Effects of habitat drying are subtle and variable, and most importantly, do not seem to result in the reduction in parasitism that we predicted. We suggest that effects of freezing and the genetic composition of larval Ae. triseriatus on parastism by A. barretti would be fruitful areas for further investigation of natural variation in this host-parasite relationship.
Acknowledgments
We thank David L. Williams, Kathleen W. Miller, Marc Gravel, Scott Hegrenes, Matt Daugherty, Barry Alto, John Styrsky, and Phil Lounibos for their comments and suggestions. We thank the Illinois State University Department of Biological Sciences, its faculty and staff for support throughout this research. This research was funded by grants from the Beta Lambda chapter of Phi Sigma (to S.L.V.) and from the National Institutes of Health (R15#AI39700-01) and the Illinois State University Office of Research (to S.A.J.).
References Cited
- Beier JC, Craig GB., Jr . Gregarine parasites of mosquitoes. In: Laird M, Miles JW, editors. Integrated mosquito control methodologies. Vol. 2. Academic; London: 1985. pp. 167–184. [Google Scholar]
- Beier JC, Harris C. Ascogregarina barretti (Sporozoa: Diplocytidae) infections in natural populations of Aedes triseriatus (Diptera: Culicidae) J Parasitol. 1983;69:430–431. [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Drought and the organization of tree-hole mosquito communities. Oecologia. 1988;74:507–514. doi: 10.1007/BF00380047. [DOI] [PubMed] [Google Scholar]
- Chen W, Yang C. Developmental synchrony of Ascogregarina taiwanensis (Apicomplexa: Lecudinidae) within Aedes albopictus (Diptera: Culicidae) J Med Entomol. 1996;33:212–215. doi: 10.1093/jmedent/33.2.212. [DOI] [PubMed] [Google Scholar]
- Comiskey NM, Lowrie RC, Jr, Wesson DM. Role of habitat components on the dynamics of Aedes albopictus (Diptera: Culicidae) from New Orleans. J Med Entomol. 1999;36:313–320. doi: 10.1093/jmedent/36.3.313. [DOI] [PubMed] [Google Scholar]
- Garcia JJ, Fukuda T, Becnel JJ. Seasonality, prevalence and pathogenicity of the gregarine Ascogregarina taiwanensis (Apicomplexa: Lecudinidae) in mosquitoes from Florida. J Am Mosq Control Assoc. 1994;10:413–418. [PubMed] [Google Scholar]
- Grimstad PR, Craig GB, Jr, Ross QE, Yuill TM. Aedes triseriatus and LA crosse virus: geographic variation in vector susceptibility and ability to transmit. Am J Trop Med Hyg. 1977;26:990–996. doi: 10.4269/ajtmh.1977.26.990. [DOI] [PubMed] [Google Scholar]
- Lively CM, Johnson SG, Delph LF. Thinning reduces the effect of rust infection on jewelweed (Impatiens capensis) Ecology. 1995;76:1859–62. [Google Scholar]
- Lounibos LP. Interactions influencing production of treehole mosquitoes in south Florida. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of Mosquitoes: Proceedings of a Workshop. Florida Medical Entomology Laboratory; Vero Beach, FL: 1985. pp. 65–78. [Google Scholar]
- Lounibos LP, Escher RL, Nishimura N, Juliano SA. Long-term dynamics of a predator used for biological control and decoupling from mosquito prey in a subtropical treehole ecosystem. Oecologia. 1997;111:189–200. doi: 10.1007/s004420050225. [DOI] [PubMed] [Google Scholar]
- McCray EM, Jr, Fay RW, Schoof HF. The bionomics of Lankesteria culicis and Aedes aegypti. J Invertebr Pathol. 1970;16:42–53. doi: 10.1016/0022-2011(70)90203-x. [DOI] [PubMed] [Google Scholar]
- Nannini MA, Juliano SA. Effects of the facultative predator Anopheles barberi on population performance of its prey Aedes triseriatus (Diptera Culicidae) Ann Entomol Soc Am. 1998;91:33–42. [Google Scholar]
- Polak M. Ectoparasitic effects on host survival and reproduction: the Drosophila-Macrocheles association. Ecology. 1996;77:1379–1389. [Google Scholar]
- SAS Institute. SAS/STAT user’s guide, version 6. 4. 1 and 2. SAS Institute; Cary, NC: 1995. [Google Scholar]
- Scheiner SM. MANOVA: Mutliple response variables and multispecies interactions. In: Sheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. Chapman & Hall; New York: 1993. pp. 94–112. [Google Scholar]
- Siegel JPR, Novak J, Maddox JP. Effects of Ascogregarina barretti (Eugregarinida: Lecudinidae) infection on Aedes triseriatus (Diptera: Culicidae) in Illinois. J Med Entomol. 1992;29:968–973. doi: 10.1093/jmedent/29.6.968. [DOI] [PubMed] [Google Scholar]
- Walker ED, Poirier SJ, Veldman WT. Effects of Ascogregarina barretti (Eugregarinida: Lecudinidae) infection on emergence success, development time, and size of Aedes triseriatus (Diptera: Culicidae) in microcosms and tires. J Med Entomol. 1987;24:303–309. doi: 10.1093/jmedent/24.3.303. [DOI] [PubMed] [Google Scholar]
- Washburn JO, Mercer DR, Anderson JR. Regulatory role of parasites: impact on host population shifts with resource availability. Science. 1991;253:185–188. doi: 10.1126/science.1906637. [DOI] [PubMed] [Google Scholar]
- Varva J. Lankesteria barretti n. sp. (Eugregarinida, Diplocystidae), a parasite of the mosquito Aedes triseriatus (Say) and a review of the genus Lankesteria Mingassini. J Protozool. 1969;16:546–570. doi: 10.1111/j.1550-7408.1969.tb02314.x. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. 3. PrenticeHall; London: 1996. [Google Scholar]