Summary
Investigations of competitive interactions emphasize non-detrital resources, even though detritus is a major component of most food webs. Studies of competing species focus usually on single resource types, although consumers in nature are likely to encounter mixtures of resource types that may affect whether competition results in exclusion or coexistence.
The invasive mosquito Aedes albopictus is capable of excluding the native mosquito Ochlerotatus triseriatus in competition for single detritus types in laboratory and field microcosms. In this study, we used nine ratios of two detritus types (animal and leaf) common in natural containers to test whether detritus ratios affect the outcome of competition.
Under intraspecific and interspecific competition, A. albopictus attained higher survival and estimated population growth rate than did O. triseriatus. Unlike past studies, both species had positive growth and high adult survival, with little evidence of competitive effects, under one resource ratio (10:1 ratio of leaf:animal detritus) regardless of mosquito densities, suggesting potential coexistence.
Path analysis showed that densities of larvae had negative effects on population growth for O. triseriatus but not for A. albopictus, indicating competitive superiority of A. albopictus. Population growth of both species was affected strongly by the direct paths from animal (positive) and leaf (negative) detritus, and the indirect effect of leaf detritus via bacterial production (positive).
Field sampling established that detritus entered real tree holes in ratios similar to those in our experiment, suggesting that natural variation in detritus ratios may influence local coexistence of these species. Seasonal variation in ratios of plant and animal detritus indicated that temporal as well as spatial variation in inputs may be important for potential coexistence.
Keywords: aquatic microcosms, bacteria, density effects, mosquito, population growth
Introduction
Resource competition is an indirect negative interaction between species that occurs with the exploitation of shared, limiting resources. Theoretical and empirical work shows that competition of two species for one limiting resource results in competitive exclusion (Tilman 1982; Chase & Leibold 2003). Multiple mechanisms can result in competitive coexistence, including trade-offs between competitive ability and predator avoidance (Chase & Leibold 2003), dispersal ability (Levins & Culver 1971; Tilman 1994) or tolerances to abiotic variation (Chesson 1986; Chesson & Huntly 1997). Another prominent mechanism for coexistence is multiple resources, which can yield coexistence depending on resource ratios and inter-specific differences in use of resource types (Tilman 1982).
Current theory surrounding food web dynamics and species interactions often focuses on direct or indirect manifestations of photosynthetic primary productivity (Moore et al. 2004), even though the majority of energy in food webs passes through detrital pathways (O’Neill & Reichle 1980; Moore et al. 2004). The effect of detritus on community attributes can be large. Detritus inputs can support higher diversity (Hairston & Hairston 1993) or can stabilize otherwise unstable energy fluxes and the dynamics of consumer populations more so than energy derived directly from primary productivity (Moore et al. 2004). Although there appear to be strong effects of detritus in many systems, few studies have investigated whether resource competition theory and predictions about resource ratios are accurate for detritus-based food webs.
Mosquito larvae within detritus-based container systems (e.g. tree holes, bamboo stumps, discarded tyres) have proved to be useful model organisms for tests of hypotheses about processes structuring communities (e.g. predation: Lounibos, Nishimura & Escher 1993; Juliano & Gravel 2002; competition: Livdahl & Willey 1991; Juliano 1998; Teng & Apperson 2000). Natural containers (e.g. tree holes) are home to larvae of a variety of mosquito species, and interspecific competition can be strong (Kitching 2001). Tree holes are cavities in trees that receive inputs of terrestrial detritus, including senescent plant parts (primarily leaves, Kitching 2001), stem flow (water from precipitation flowing along tree trunks and containing organic matter, Carpenter 1982) and terrestrial invertebrate carcasses (Daugherty, Alto & Juliano 2000). Leaf inputs have variable effects on mosquito populations (Lounibos et al. 1993; Léonard & Juliano 1995; Walker et al. 1997; Juliano 1998), but animal-based detritus appears to have a greater effect on container mosquito performance (Yee & Juliano 2006) and can reduce the impact of competitive interactions (Barrera 1996; Daugherty et al. 2000). Mosquito larvae eat microorganisms growing on detritus surfaces (Merritt, Dadd & Walker 1992) and both animal and plant detritus support micro-organism growth (Yee & Juliano 2006). Because tree holes can contain different kinds of detritus resources, and these resources can affect performance of mosquitoes in different ways, we hypothesize that variation in the ratios of these detritus types will affect competitive coexistence of mosquitoes.
We tested for the effect of different detritus ratios on the competitive outcome between two container mosquitoes, Aedes albopictus (Skuse) (Asian tiger mosquito) and Ochlerotatus triseriatus (Say) (eastern tree hole mosquito), (formally in the genus Aedes, Reinert 2000). A. albopictus was introduced into North America in the mid-1980s from Asia, and it now occurs across most of the southern United States (Hawley et al. 1987; Moore 1999). O. triseriatus is typically the dominant consumer in tree holes in the eastern United States (Bradshaw & Holzapfel 1985). Currently, A. albopictus and O. triseriatus overlap considerably in their large-scale distributions within the United States, and co-occur frequently (Lounibos et al. 2001; Aliabadi & Juliano 2002). A. albopictus has negative effects on resident mosquito species and has been shown to be superior to O. triseriatus in competition under field and realistic laboratory conditions (Livdahl & Willey 1991; Edgerly, Willey & Livdahl 1993; Teng & Apperson 2000). These competition experiments have used only leaf detritus as a resource. When raised on animal vs. plant detritus, O. triseriatus larvae grew significantly faster, adults were significantly larger and estimated population growth was significantly higher, suggesting that animal detritus is a higher quality food source (Yee & Juliano 2006). Daugherty et al. (2000) showed that animal and plant detritus could increase the likelihood of coexistence between A. albopictus and another mosquito, A. aegypti, although leaf-only treatments resulted in exclusion of A. aegypti. Whether the outcome of competition between A. albopictus and O. triseriatus is affected by animal detritus or by the ratios of plant and animal detritus is unknown. Livdahl & Willey (1991) suggested that A. albopictus would out-compete O. triseriatus in discarded automobile tyres, whereas coexistence would occur in natural tree holes, and speculated that tyres contained resources that were absent in tree holes and better exploited by A. albopictus, but did not consider that ratios of different kinds of detritus resources may differ between these container types. Variation in micro-organism productivity from different detritus types also could explain patterns of coexistence or exclusion, because mosquito larvae consume microorganisms exploiting detritus. Thus, the mechanism by which A. albopictus excludes or coexists with O. triseriatus remains unknown.
We ask whether variation in the ratios of two detritus types could affect exclusion or coexistence between O. triseriatus and A. albopictus. We used nine ratios of two detritus types (leaves and invertebrate carcasses) in a factorial design with eight different combinations of densities of the two mosquitoes. We also quantified two measures of micro-organism activity (bacterial biomass production and metabolic rates) for each resource ratio, enabling us to quantify the direct food source for larvae. We used path analysis to determine the important direct and indirect casual links among detritus type, micro-organism productivity, mosquito densities and adult mosquito production and estimated population growth to test whether detritus ratios may be important for coexistence of A. albopictus and O. triseriatus. Finally, we measured detritus inputs into natural tree holes to characterize spatial and temporal patterns of these ratios in nature, and to infer whether exclusion or coexistence are likely under natural conditions.
Methods
EXPERIMENTAL SET-UP
Experimental microcosms consisted of 100 mL plastic beakers filled with 67 mL de-ionized (DI) water, 30 mL field-collected stem flow and 3 mL of micro-organism inoculum. Stem flow was obtained from 36 stem flow dams attached to trees at ParkLands Merwin Preserve, Lexington, IL (40°39′10″ N, 88°52′21″ W), pooled and mixed thoroughly, and added to all containers to provide a limited amount of nutrients to all treatments. Micro-organism inoculum was collected from 10 tree holes at the Merwin Preserve, pooled and mixed thoroughly, then added to experimental microcosms. Throughout the experiment, DI water was added as needed to maintain water levels. Microcosms were placed in incubators set on a 14:10 h day:night cycle at 27 °C (summer conditions in Illinois; D. A. Yee, personal observation). Two replicates were set up on each of 2 days and housed in three incubators (replicates 1 and 2 in a single incubator, replicates 3 and 4 each in separate incubators). A block term was used in all statistical analyses to account for variation among incubators. Microcosms (10–12) were assigned randomly to 8–16 trays in each incubator. To homogenize variation in environmental conditions within incubators, the vertical positions of trays was shifted within each incubator every other day. Trays were covered loosely to reduce evaporation and light penetration. Microcosms were maintained in incubators undisturbed for 5 days before measurement of micro-organism productivity (see below).
Nine resource ratios of American elm (Ulmus americana L.) leaves (EL) and adult Drosophila melanogaster Meigen (DM) were used. Based on results from experiments on mosquito growth (Yee & Juliano 2006), we used maxima of 0.05 g and 0.50 g of animal and leaf detritus, respectively. Adult mass, survivorship and estimated population growth are statistically equal for O. triseriatus grown on either 0.05 g animal or 0.25 g leaf detritus (Yee & Juliano 2006). Ratios were expressed as relative ratios compared to the situation where leaf and animal amounts were equal (i.e. 1:1 = 0.05 g EL and 0.05 g DM), as follows: (EL:DM) 0:0, 0:0.1, 0:1, 1:0, 10:0, 1:0.1, 1:1, 10:0.1 and 10:1. Individual larvae of O. triseriatus require about 0.05 g of low-quality oak leaves for full development (Kaufman et al. 2001), but fail to develop on 0.025 g (Carpenter 1983). Thus, the low levels of leaf material would probably result in resource competition. Adult fruit flies were obtained from within the Department of Biological Sciences, ISU, and were cold-killed and dried at 50 °C for 48 h before being used. Senescent elm leaves were collected in the autumn from Merwin Preserve and stored dry at room temperature. After removing the petioles, leaves were shredded into pieces similar in size to an adult Drosophila (10–15 mm2).
Mosquito larvae were collected from tree holes at Merwin Preserve (O. triseriatus) and tyres in East St Louis, Illinois (A. albopictus) (38°35′33″ N, 90°08′14″ W). Larvae of both species were raised to adults on bovine liver powder (ICN Biochemicals, Cleveland, OH, USA) and housed in separate cages, where females were blood-fed on anaesthetized laboratory mice. Mosquito eggs (F1) were hatched in a solution of 0.33 g nutrient broth media (Fisher Scientific, Hampton, NH) per 750 mL DI water. After 24 h, hatched larvae were rinsed to remove nutrient solution before being added to microcosms. Eight different density combinations of mosquitoes (O. triseriatus: A. albopictus) were used: intraspecific: 6:0, 0:6, 12:0, 0:12, and interspecific: 6:6, 6:12, 12:6, 12:12. Each detritus ratio (9) and density combination (8) was replicated four times for a total of 288 experimental units. Mosquitoes were added on a single day to all replicates (day 7 after establishment of replicates 1 and 2).
Five days after the initial container set-up, and thus prior to adding mosquitoes, two productivity surrogates were measured: metabolic rates of microorganisms (MR) and production of new bacterial biomass based on measurement of protein synthesis (PS). Productivity was measured on replicates 1 and 3, each on a successive day (72 units per day). PS was quantified by measuring incorporation of tritiated L-leucine (4,5–[3H]) (Kirchman 1993; refined by Kaufman et al. 2001). To a 2-mL fluid sample, [3H]-leucine was added, incubated for 30 min, and then protein was precipitated in trichloracetate. [3H]-Leucine incorporation (nmol ml−1 h−1) was quantified by liquid scintillation (Beckman LS-6500 scintillation counter). Micro-organism community metabolic rates (MR), which measure aerobic respiration from protozoa, bacteria and fungi, were measured using a Gilson Differential Respirometer (Middleton, WI, USA). Values for MR were in μL of O2 consumed h−1. Fluid samples (8 mL) from each microcosm were isolated in a measurement vessel with a CO2 absorbent (Ascarite) and were incubated in the dark. The final MR readings were generated after a 30-min equilibration period. Both productivity measures were obtained under 27 °C.
The experiment ended 30 days after mosquito addition (~2× the time needed for well-fed larvae to complete development, Livdahl & Willey 1991). Each day, newly eclosed adults were collected, sex and species were determined, and then individuals were dried at 50 °C for 48 h. Individual mass was determined to the nearest 0.0001 mg using a Cahn microbalance (Cahn Instruments, Cerritos, CA, USA). For each microcosm, a composite index of mosquito population performance (λ′) was calculated for each species. This measure estimates the finite rate of population increase and is derived from an estimate of the per capita rate of population change (dN/Ndt = r) for each container (Livdahl & Sugihara 1984). Values of λ′ have been used to quantify population level effects of competition for A. albopictus and O. triseriatus (e.g. Juliano 1998; Daugherty et al. 2000; Aliabadi & Juliano 2002). This estimate is:
where N0 is the initial number of females in a cohort (assumed to be 50%), Ax is the number of females eclosing on day x, wx is the mean mass of females eclosing from a container on day x, f(wx) is a function relating fecundity to female mass and D is the days required for a newly eclosed female to mate, to obtain a blood meal and to oviposit [assumed to be 14 days for A. albopictus and 12 days for O. triseriatus (Livdahl & Willey 1991)]. Fecundity was based on female mass using regressions for A. albopictus [f(wx) = 19.5 + 152.7wx; Lounibos et al. 2001] and for O. triseriatus [f(wx) = 0.5exp(4.580 + 0.893 (ln(wx)] − 1; Nannini & Juliano 1998). In addition to λ′, survivor-ship was measured as the proportion of individuals in each microcosm reaching adulthood by day 30.
Larval gut parasites in the genus Ascogregarina may affect competitive interactions of container mosquitoes (Aliabadi & Juliano 2002). Parasite loads were checked by dissecting three to six fourth-instars of each species from a separate set of microcosms treated in the same way as above (three replicates each of 0.05 g DM, 0.5 g EL and 0.05 g DM and 0.5 g EL). All dissected larvae were free of Ascogregarina.
FIELD DETRITUS COLLECTION
Natural detritus inputs were sampled in 13 tree holes at Merwin Preserve during four 24-h periods: 25 April, 28 April, 20 June and 23 June, 2005. Before sampling, tree hole contents were removed and each hole was lined with 0.5 mm mesh before being refilled with water. Detritus was sorted into leaf litter or animal detritus, dried at 60 °C for 48 h, and weighed to the nearest 0.0001 mg using a Cahn microbalance.
STATISTICAL ANALYSES
Micro-organism productivity
Multiple analysis of variance (MANOVA) (PROC GLM; SAS Institute, Inc. 2004) was used to determine whether the ratio of leaf:animal detritus (independent variable) affected productivity of microorganisms (MR and PS, dependent variables). Standardized canonical coefficients (Scheiner 2001) were used to quantify the contributions of individual dependent variables to significant multivariate effects. Differences among means were identified using multivariate pairwise comparisons (Scheiner 2001). Although transformations did meet assumptions of Manova, statistical outcomes using raw or transformed data were consistent, so we present the raw data for ease of interpretation.
Competition
Differences in survival and estimated finite rate of increase (λ′) for each species were assessed using two-way analysis of variance (ANOVA, PROC GLM; SAS Institute, Inc. 2004) with detritus ratios and density combinations as independent variables. The 0:0, 10:0 and 0:0.1 detritus ratios yielded no adults, so these treatments were omitted from all analyses. Survival for both species {log[(arcsine √x) + 1]} and λ′ for O. triseriatus (x + 1)2 and A. albopictus (x + 1)4 were transformed to meet assumptions of normality and homoscedasticity. For all analyses, Tukey’s honestly significant difference (HSD) tests (Sokal & Rohlf 1995) were used to resolve pairwise differences among means.
Linking productivity to mosquito performance
Path analysis (PROC CALIS; SAS Institute, Inc. 2004) was used to test for effects of multiple independent variables on one or more dependent variables (Wright 1921). Unlike multiple regression, in path analysis a variable may be both dependent (i.e. affected by other variables) and independent (i.e. affecting other variables). Path coefficients, which are similar to standardized regression coefficients, quantify direct effects on a dependent variable caused by variation in an independent variable, independently of direct effects of other independent variables (Hatcher & Stepanski 1994; Mitchell 2001). The importance of a path is tested by testing the fit of reduced models in which paths are removed, relative to a full model (Hatcher & Stepanski 1994; Mitchell 2001). Reduced models were compared to the full model using goodness-of-fit χ2 tests (PROC CALIS; Hatcher 1994; SAS Institute, Inc. 2004).
The first path analysis was used to test for the importance of links between detritus type and amount on measures of MR and PS. This model had direct links from each detritus type to MR and PS, as well as a link from PS to MR. Links were removed, one at a time, from each detritus type to MR and PS. If removing a link did not decrease significantly the fit of the model, then it was assumed that the link was unimportant in explaining variation in the dependent variable.
The subsequent model was constructed to test whether detritus type or amount, micro-organism productivity or mosquito densities were important in explaining variation in λ′ for each species. This model would allow for a test of the mechanistic hypotheses about competition (e.g. that density combinations influence λ′ indirectly through micro-organism productivity, implicating resource competition). The relationship between detritus type and amount and micro-organism productivity for each species was assumed to be the same as that from the analysis of productivity. The following reduced models were tested for each species: removal of direct link from detritus types to λ′, removal of direct links from MR or PS to λ′ and removal of direct links from the density of each species to λ′. If removing a link did not decrease the fit of the model, then it was assumed that the link was unimportant in explaining variation in the dependent variable.
Results
MICRO-ORGANISM PRODUCTIVITY
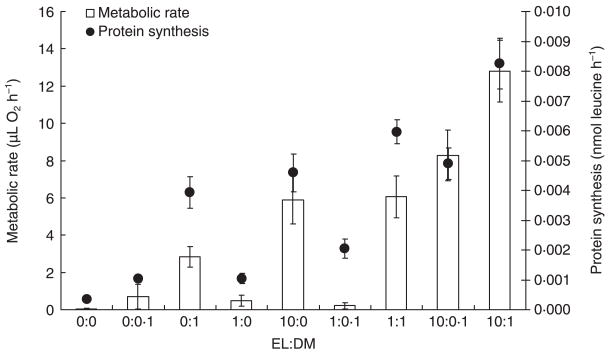
MR values were below detection limits (i.e. ≤ distilled water blanks) for 14 of 16 replicates with no detritus (0:0), 11–12 of 16 replicates with low amounts of single detritus types (0.05 g EL, 0.005 DM) and their combination, and 0–5 of 16 replicates for all other detritus ratios. These values were treated as zeros in all analyses. There were significant differences in micro-organism productivity among the resource ratios (Pillai’s trace = 0.823, F16,270 = 11.80, P < 0.001). Standardized canonical coefficients (SSC) for MR (SSC = 0.874) and PS (SSC = 1.272) were similar, indicating that both contributed to the significant MANOVA effect. No detritus (EL:DM, 0:0) or low total amounts (0:0.1, 1:0, 1:0.1) of either detritus type had the lowest MR and PS values, whereas a 10:1 mixture of leaf and animal detritus had significantly greater productivity (Fig. 1). Other ratios (0:1, 1:1, 10:0, 10:0.1) were intermediate in their values for productivity. MR and PS were correlated strongly and positively (r = 0.591, P < 0.001).
Fig. 1.
Means (± 1 SE) for two measures of micro-organism productivity (MR: metabolic rates, μ O2 h−1; PS: production of new bacteria via measurement of protein synthesis, nmol leucine h−1) in different detritus ratios (elm leaves: Drosophila melanogaster) before the addition of mosquito larvae.
COMPETITION
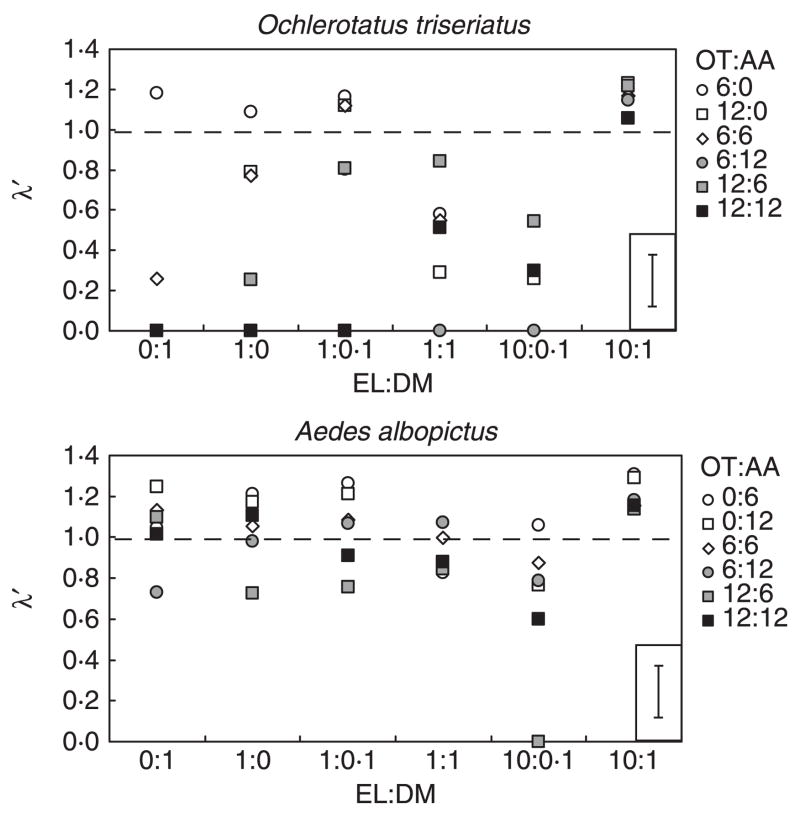
There were significant effects of detritus ratios, mosquito densities and their interaction on λ′ for O. triseriatus (Table 1). O. triseriatus had mean values of λ′ > 1.0 for the low intraspecific densities in four of the six resource ratios (0:1, 1:0, 1:0.1, 10:1), for the low interspecific and high intraspecific densities (1:0.1) and for all densities in the 10:1 ratio (Fig. 2). In these cases, O. triseriatus cohorts would be expected to increase. All other density × detritus ratio combinations had mean values of λ′ < 1.0. For A. albopictus there were significant detritus ratio and density combination effects, but their interaction was not significant (Table 1). Averaged across mosquito density combinations, all detritus ratios except the 10:0.1 EL:DM had mean λ′ ≥ 1.0 (Fig. 2). Averaged across resource ratios, mean λ′ was ≥ 1.0 for all mosquito densities except when there were twice as many O. triseriatus as A. albopictus (Fig. 2). For both species, the 10:1 ratio of leaf to animal detritus always yielded λ′ > 1.0 (Fig. 2).
Table 1.
Results of two-way ANOVA (density and detritus ratio) on transformed values for estimated population growth (λ′) and survival for Ochlerotatus triseriatus and Aedes albopictus in experimental microcosms. Significant effects are shown in bold type
| λ′
|
Survivorship
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
O. triseriatus |
A. albopictus |
O. triseriatus |
A. albopictus |
|||||||
| Source | d.f. | F | P | F | P | d.f. | F | P | F | P |
| Resource (R) | 5 | 23.85 | < 0.001 | 9.79 | < 0.001 | 5 | 63.84 | < 0.001 | 30.27 | < 0.001 |
| Density (D) | 5 | 9.08 | < 0.001 | 4.65 | 0.007 | 5 | 19.09 | < 0.001 | 1.57 | 0.175 |
| R × D | 25 | 2.65 | < 0.001 | 0.99 | 0.484 | 25 | 3.86 | < 0.001 | 1.39 | 0.126 |
Fig. 2.
Means of estimated population growth (λ′) of each mosquito species across resource ratios (elm leaves: Drosophila melanogaster) and mosquito densities (OT = Ochlerotatus triseriatus, AA = Aedes albopictus). The dashed line at λ′ = 1 in each panel represents population growth equal to zero. The standard error bar based on LS means has been inset in the lower right of each panel to improve visualization of the means. Shadings of symbols reflect larval densities (open = 6–12 larvae, grey = 18 larvae, black = 24 larvae).
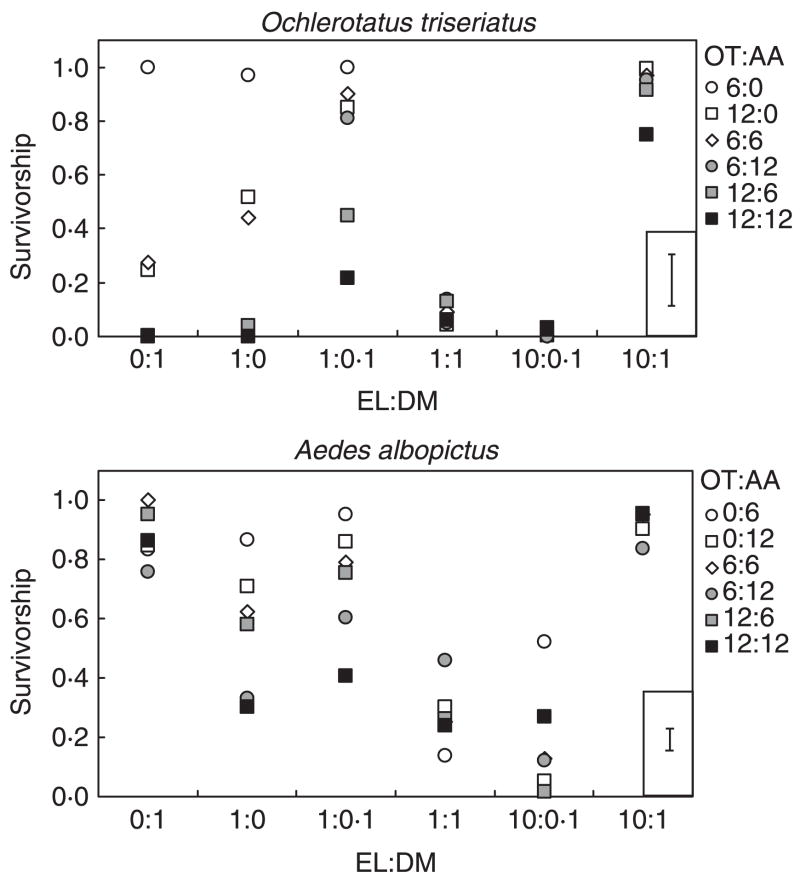
Analyses of survivorship for O. triseriatus yielded a significant detritus ratio × mosquito density interaction (Table 1), with the highest survival in the low intraspecific density across detritus ratios (6:0 OT:AA). A decrease in survival in all other densities was apparent, although these differences were strongest in the 0:1, 1:0 and 1:0.1 ratios (Fig. 3). In the 1:1 and 10:0.1 ratios, survival in all densities was near zero, whereas survival was near 1.0 in all densities in the 10:1 ratio (Fig. 3). In general, survival of A. albopictus was highest when raised alone (Fig. 3), although differences among density combinations in the 1:1 ratio were non-significant and survival was low. There were no significant differences in survivorship among densities in the 10:1 ratio (Fig. 3).
Fig. 3.
Means (± 1 SE) of survivorship of adults of each mosquito species across resource ratios (elm leaves: Drosophila melanogaster) and mosquito densities (OT = Ochlerotatus triseriatus, AA = Aedes albopictus). The standard error bar based on LS means has been inset in the lower right of each panel to improve visualization of the means. Shadings of symbols reflect larval densities (open = 6–12 larvae, grey = 18 larvae, black = 24 larvae).
LINKING PRODUCTION TO MOSQUITO POPULATION GROWTH
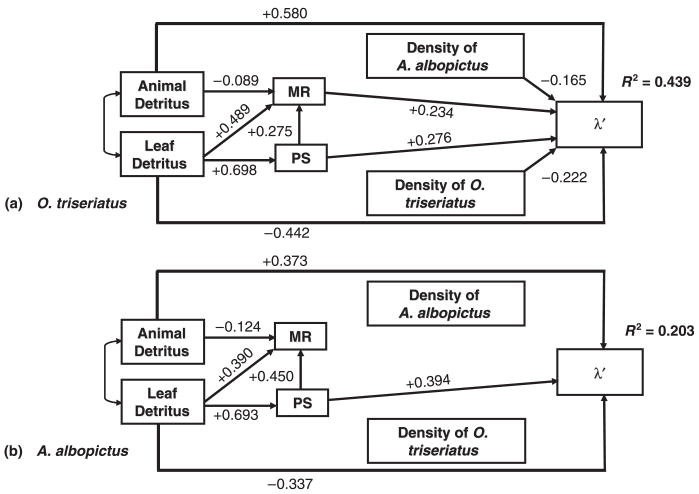
The final path analysis for both species (Fig. 4) indicated that relationships between detritus type and micro-organism productivity were best explained by links from leaf detritus to both MR and PS, and from PS to MR, but links from animal detritus to PS were unimportant. There were small differences in the relative strength of these path coefficients among the variables for each species (compare Fig. 4a and b). For both species, the removal of the direct paths from animal detritus (O. triseriatus , P < 0.01, A. albopictus , P < 0.01) and leaf detritus (O. triseriatus , P < 0.01, A. albopictus , P < 0.05) to λ′ resulted in a significant decrease in fit, indicating that these paths were necessary for explaining variation in λ′. For both species, the path to λ′ was large and positive from animal detritus, but was moderate and negative from leaf detritus (Fig. 4). Removal of the direct link from MR to λ′ resulted in significant decrease in fit for O. triseriatus ( , P < 0.01) but not for A. albopictus ( , P > 0.05). There was a significant decrease in fit when the link from PS to λ′ was removed for both species (O. triseriatus , P < 0.05, A. albopictus , P < 0.05). PS had a moderate positive effect on λ′ for both species. The effect of intra- and interspecific mosquito densities on A. albopictus λ′ could be removed with no significant effect on fit (density of A. albopictus , P > 0.05, density of O. triseriatus , P > 0.05); however, neither of these paths could be removed for O. triseriatus (density of A. albopictus , P < 0.05, density of O. triseriatus , P < 0.01), with both densities having a negative effect on growth. The final models explained 44% and 20% of the variation in λ′ for O. triseriatus (Fig. 4a) and A. albopictus (Fig. 4b), respectively.
Fig. 4.
Final path diagram testing for the relationships among detritus type (animal, leaf), micro-organism productivity (MR: metabolic rates; PS: production of new bacteria via measurement of protein synthesis), and mosquito densities on estimated population growth (λ′) of (a) Ochlerotatus triseriatus and (b) Aedes albopictus in laboratory microcosms.
FIELD DETRITUS COLLECTION
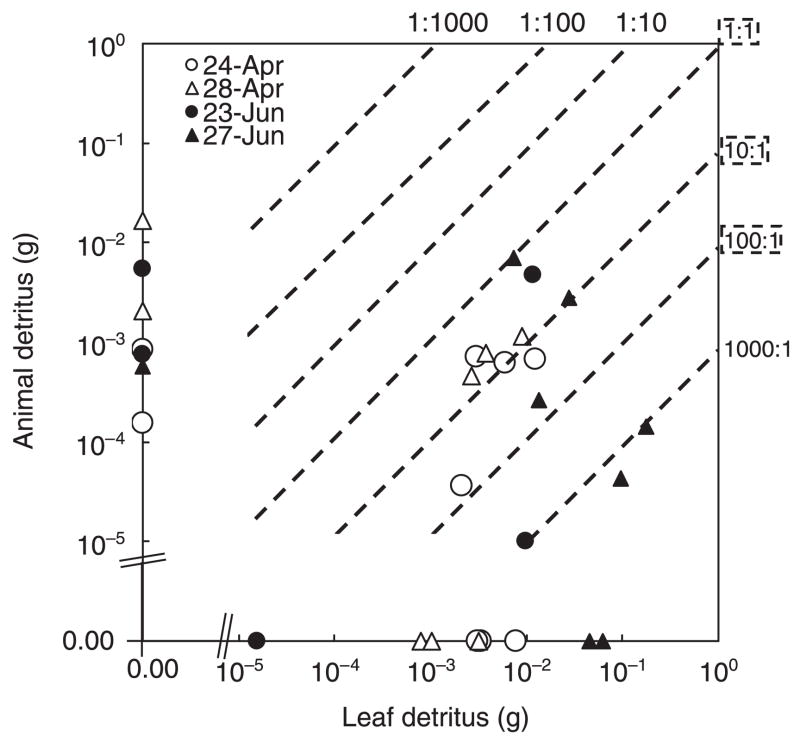
Detritus was observed in 60% of the tree holes across collection dates, with all tree holes receiving input of detritus on at least one date. A diverse set of ratios of leaf to animal detritus was found across the sampling dates (Fig. 5). In April, most tree holes contained about 10 times more leaf than animal detritus, although some tree holes received either exclusively leaf or animal material (Fig. 5). In June, the ratios were variable, ranging from 1:1 to 1000:1 leaf:animal detritus (Fig. 5). Leaf inputs were generally higher in June than in April, although animal inputs were similar in both months.
Fig. 5.
Variation in the ratio of leaf to animal detritus from 13 natural tree holes sampled at two times in April and June 2005. Data are represented on a log-log scale. Each point represents the total amount of animal and leaf detritus within each tree hole on each date. The axes have been broken to facilitate placement of single detritus observations. Dashed lines represent relative ratios of the two detritus types. The dashed rectangles identify the relative detritus ratios used in the laboratory experiment.
Discussion
Our results indicate that both coexistence and exclusion may occur depending on the detritus ratios used, and appear to be consistent with theory concerning effects of resource ratios on coexistence (Tilman 1982). The most striking outcome was that the 10:1 ratio of elm leaves to D. melanogaster resulted in λ′ values greater than 1.0 and high but equal survival for both species, regardless of intra- or interspecific densities (Figs 2 and 3), suggesting that both species should persist and potentially coexist at this ratio. To date, single resource experiments testing for competition between A. albopictus and O. triseriatus have shown that the invasive A. albopictus is uniformly the superior competitor (Edgerly et al. 1993; Teng & Apperson 2000; Aliabadi & Juliano 2002). Ours is the first experiment to suggest that coexistence may be dependent on mixed detritus types. Coexistence between A. albopictus and another mosquito, A. aegypti, has been predicted using leaf and animal detritus, where exclusion would be predicted with only leaves (Daugherty et al. 2000). Daugherty et al. (2000) used a narrower range of ratios of resources compared to the present study, did not examine animal detritus-only situations and did not attempt to link micro-organism productivity to competitive outcomes. Our results on variation in micro-organism productivity with different ratios suggest that different detritus types constitute different resources that may alter the outcome of competition between container mosquitoes.
Our study indicates that plant and animal detritus are different resources for microorganisms and mosquito consumers. Terrestrial plant detritus has been well studied in aquatic systems (e.g. Cloe & Garman 1996), and plant-derived detritus must be processed typically by decomposers before it is available to consumers. In contrast, detritus from animal sources may be ingested directly and thus available more immediately to consumers (Mason & MacDonald 1982; Garman 1991). Animal detritus decomposes at a greater rate than does plant material (Swift, Heal & Anderson 1979; Yee & Juliano 2006), but mosquito feeding may accelerate further the breakdown of animal detritus or mosquitoes may consume animal detritus directly (Daugherty et al. 2000; Yee & Juliano 2006; Yee et al. 2007). From path analysis it was clear that different detritus types have opposite direct effects on mosquito rate of increase. The positive effect of animal detritus on λ′ and the lack of necessary paths from animal detritus through micro-organism productivity to λ′ suggests that animal detritus benefits mosquito larvae directly through ingestion (Yee & Juliano 2006; Yee et al. 2007).
COMPETITION
A. albopictus was superior in resource competition for multiple detritus ratios and density combinations that led to λ′ < 1 for O. triseriatus and λ′ > 1 for A. albopictus (Fig. 2). Path analysis indicated that larval densities had essentially no effect on A. albopictus λ′ (Fig. 4). These results are consistent with previous studies that have shown highly asymmetrical competitive effects for A. albopictus compared to other container mosquitoes (Livdahl & Willey 1991; Juliano 1998; Aliabadi & Juliano 2002; Costanzo et al. 2005). The mechanism by which A. albopictus has a competitive advantage over other mosquitoes remains elusive, although A. albopictus may be more efficient at resource acquisition (Barrera 1996) through differential allocation of time to foraging vs. non-foraging activities (Yee, Kesavaraju & Juliano 2004a). A. albopictus from Illinois displays a greater preference for foraging on animal vs. leaf material than does O. triseriatus (Kesavaraju, Yee & Juliano 2007), providing further evidence that interspecific behavioural differences may affect the outcome of competition.
We found no evidence that detritus ratios could reverse competitive dominance and lead to the prediction of exclusion of A. albopictus by O. triseriatus. It remains possible that under some ratios such a result could be obtained; however, a more extensive selection of individual and paired detritus types may be required to find such ratios. Because A. albopictus and O. triseriatus exhibit similar feeding patterns (Yee, Kesavaraju & Juliano 2004b), they probably exploit resources in the same way. Competitive reversal, which did not occur in our study, may be found with different detritus types when considering species with different feeding modes.
LINKING PRODUCTIVITY TO MOSQUITO POPULATION GROWTH
The mechanisms underlying the differences in competitive interactions with different detritus ratios are unknown, although it is likely that differences in micro-organism communities or differences in mosquito foraging may be involved. The detritus types we used were important to mosquito success, as containers without detritus produced no adults. We did not collect data on micro-organism species composition or quality, but analysis of micro-organism productivity revealed that detritus quantity alone is an inadequate predictor of productivity. There were no differences in either productivity measure between the 10:0 (0.50 g) and 1:1 ratios (0.10 g), even though there was a fivefold difference in the total detritus amount between these ratios. Further, the 0:1 ratio (0.05 g DM only) produced significantly greater MR and PS values than did an equal amount of leaf detritus (i.e. 1:0, 0.05 g EL only). These results suggest differences in nutritional quality between these sources of detritus.
There is a possibility that coexistence may be possible under the 10:1 ratio because this ratio contained the most detritus (0.55 g). Values for MR and PS were significantly greater in the 10:1 ratio relative to other ratios (Fig. 1). The next lowest amount of total detritus (10:0.1, 0.505 g) had significantly lower MR and PS values, and resulted in significantly lower survival and estimated population growth for both species. However, the difference between the 10:1 and 10:0.1 ratios may be related to the amount of animal detritus, and not the total amount of detritus per se. The 0:1 ratio supported values for λ′ and survival closer to the 10:1 ratio than the 10:0.1 ratio for both species, suggesting a central role of animal detritus in influencing this outcome. It appears that the specific combination of leaf and animal detritus was important in generating our results.
Unexpectedly, we did not obtain any adult females from high leaf-only (10:0) interspecific treatments, although other treatments containing this amount of leaf material (10:1, 10:0.1) did produce females. Micro-organism productivity values for 10:0 containers (Fig. 1) were moderate and similar to values for ratios that produced adults (e.g. 1:1). Containers receiving only 0.50 g leaves developed fungus-rich leaf mats at the water’s surface before the addition of larvae. These mats were probably an artefact of shredding leaves, which would not normally occur in nature. These fungi may have been unsuitable as food for larvae, may have out-competed edible microorganisms or may have prevented access to the surface for air-breathing larvae. The fact that the addition of even a small amount of animal detritus may have altered the micro-organism community sufficiently to inhibit the growth of mat-forming fungi indicates that animal detritus may change micro-organism communities in important ways that affect mosquito populations. This argument is consistent with the observed increase in micro-organism productivity with the addition of only 0.005 or 0.05 g of animal detritus to 0.50 g leaf only ratios (10:0.1, 10:1, Fig. 1). It is also possible that there were interactions between resources that could make it more difficult to interpret our results based on ratios alone. For instance, the effect of adding both animal and leaf detritus could be that adding animal material added nitrogen, which increased leaf material quality indirectly by stimulating fungal decay (Kaufman & Walker 2006). Animal detritus has been shown to contain high amounts of nitrogen and phosphorus (Yee & Juliano 2006), and thus this interaction between different kinds of detritus is possible.
An unexpected result generated by path analysis was the positive effect of bacterial production from leaf detritus on mosquito population growth, even though the direct link from leaf detritus was negative (Fig. 4). This is also consistent with the negative effects of leaf detritus already discussed, but may indicate that bacterial growth supported by leaves is still an important positive factor in mosquito population growth. Within the context of our experiment, the negative effects of leaf material on population growth is explained at least partially by the results from the 10:0 ratio with unusual fungal growth (discussed above). However, leaf detritus must also be considered a direct source of inhibiting compounds (e.g. tannins and phenols). These compounds have been shown to retard larval mosquito growth and affect survival negatively (e.g. Sota 1993; Rey et al. 1999). Because these compounds can also both stimulate and inhibit micro-organism growth (Steinberg et al. 2006), it is not surprising that both MR and PS were still affected positively by leaf material.
It is unclear if leaf-associated inhibitory compounds affected competitive outcomes in our study. Sota (1993) found that A. albopictus was as tolerant of leaf tannins as a native tree hole mosquito in Japan, and A. albopictus larvae showed higher survival rates than the native species at the highest tannin concentrations tested. In the case of A. albopictus invading leaf-dominated tree hole habitats of O. triseriatus, a tolerance to tannins or phenols would be an essential component of success and very probably interacts with nutritional acquisition and assimilation capacities of the two species to explain overall growth and development.
FIELD DETRITUS COLLECTION
Survival may be possible for poor competitors if the environment is sufficiently heterogeneous to provide some patches where favourable conditions exist (Tilman 1982; Chesson 2000). Temporal or spatial heterogeneity may disrupt competitive equilibrium, and in turn increase species richness (Huston 1979). Tree holes show both temporal and spatial variation in detritus inputs (Kitching 2000) and form a mosaic of patches of different detritus ratios, as we observed in our field sampling (Fig. 5). Individual tree holes may function like resource patches within a variable landscape of resources (Abrams 1995). In this scenario, spatial variation in ratios means that each tree hole contains a particular set of resources wherein species may coexist or one may be excluded (Tilman 1982). At any one ratio competitive exclusion may occur, but extinction would be unlikely at the scale of the forest. Besides coexistence stemming from spatial variation, local extinction could be prevented by temporal variation in ratios of resource inputs (Kirk 2002). Different frequencies of temporal variation in resource inputs over time may change competitive outcomes, affect the rate of competitive exclusion or allow the coexistence of competitors (Grover 1997). In fact, the ratio most likely to promote coexistence in our study was found more often early in the season (Fig. 5), when population densities of both species are relatively low (Costanzo et al. 2005; D. A. Yee, unpublished data). Low population densities would probably decrease negative density-dependent effects on O. triseriatus, effects that do not seem to impact A. albopictus (Fig. 4). Based on our analyses, it appears that competitive interactions between A. albopictus and O. triseriatus may vary considerably with the dynamics of detritus inputs.
Our data from the field demonstrate that ratios of leaf and animal detritus that may lead to coexistence do occur in natural tree holes. Future studies will need to explore how populations of these mosquitoes and the microorganisms they consume vary in response to detritus ratios in natural containers, if coexistence of A. albopictus and O. triseriatus is to be understood. Because our experiment could not determine what outcome would be expected if increasing populations of these species in the 10:1 ratio were allowed to grow over an extended period, multigenerational studies are needed to examine the implications for mixed detritus types on long-term competitive outcomes between these species.
Acknowledgments
We thank S. Harrell Yee, H. Les, S. Hohm, C. Villanueva, C. Janiec, J. Rakauskas and B. Kesavaraju for assistance with the laboratory experiment, K. Edwards for providing fruit flies, two anonymous reviewers for providing comments on an earlier draft of this manuscript, D. King and G. Butler of the East Side Health District, East St Louis, Illinois, for aid in collecting mosquitoes and the ParkLands Foundation for access to their property. This work was supported by grants to D. A. Yee from the ISU Department of Biological Sciences and the Phi Sigma Biological Society, and to M. G. Kaufman (R01 AI-21884) and S. A. Juliano and D. A. Yee (R15 AI-051374) from the National Institute of Allergy and Infectious Disease.
References
- Abrams PA. Monotonic or unimodal diversity–productivity gradients: what does competition theory predict? Ecology. 1995;76:2019–2027. [Google Scholar]
- Aliabadi BK, Juliano SA. Escape from gregarine parasites affects the competitive impact of an invasive mosquito. Biological Invasions. 2002;4:283–297. doi: 10.1023/A:1020933705556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecological Entomology. 1996;21:112–127. [Google Scholar]
- Bradshaw WE, Holzapfel CM. The distribution and abundance of tree holes mosquitoes in eastern North America: perspectives from Florida. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of Mosquitoes: Proceedings of a Workshop. Florida Medical Entomology Laboratory; Vero Beach, FL: 1985. pp. 3–23. [Google Scholar]
- Carpenter SR. Stemflow chemistry: effects on population dynamics of detrivorous mosquitoes in tree-hole ecosystems. Oecologia. 1982;53:1–6. doi: 10.1007/BF00377128. [DOI] [PubMed] [Google Scholar]
- Carpenter SR. Resource limitation of larval treehole mosquitoes subsisting on beech detritus. Ecology. 1983;64:219–223. [Google Scholar]
- Chase JM, Leibold MA. Ecological Niches: Linking Classical and Contemporary Approaches. University of Chicago Press; Chicago, IL: 2003. [Google Scholar]
- Chesson P. Mechanisms of maintenance of species diversity. Annual Review of Ecology and Systematics. 2000;31:343–366. [Google Scholar]
- Chesson PL. Environmental variation and the coexistence of species. In: Diamond J, Case TJ, editors. Community Ecology. Harper and Row; New York, NY: 1986. pp. 240–256. [Google Scholar]
- Chesson P, Huntly N. The roles of harsh and fluctuating conditions in the dynamics of ecological communities. American Naturalist. 1997;150:519–553. doi: 10.1086/286080. [DOI] [PubMed] [Google Scholar]
- Cloe WW, III, Garman GC. The energetic importance of terrestrial arthropod inputs to three warm-water streams. Freshwater Biology. 1996;36:105–114. [Google Scholar]
- Costanzo KS, Mormann K, Juliano SA. Asymmetrical competition and patterns of abundance of Aedes albopictus and Culex pipiens (Diptera: Culicidae) Journal of Medical Entomology. 2005;42:559–570. doi: 10.1093/jmedent/42.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty MP, Alto BM, Juliano SA. Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae) Journal of Medical Entomology. 2000;37:364–372. doi: 10.1093/jmedent/37.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerly JS, Willey MS, Livdahl T. Intraguild predation among larval treehole mosquitoes, Aedes albopictus, Ae. aegypti, and Ae. triseriatus (Diptera: Culicidae) in laboratory microcosms. Journal of Medical Entomology. 1993;36:394–399. doi: 10.1093/jmedent/36.3.394. [DOI] [PubMed] [Google Scholar]
- Garman GC. Use of terrestrial arthropod prey by a stream-dwelling cyprinid fish. Environmental Biology of Fishes. 1991;30:325–331. [Google Scholar]
- Grover JP. Resource Competition. Chapman & Hall; New York: 1997. [Google Scholar]
- Hairston NG, Jr, Hairston NG., Jr Cause-Effect Relationships in Energy Flow, Trophic Structure, and Interspecific Interactions. The American Naturalist. 1993;142:379–411. [Google Scholar]
- Hatcher L. A Step-by-Step Approach to Using the SAS System for Factor Analysis and Structural Equation Modeling. SAS Institute Inc.; Cary, NC: 1994. [Google Scholar]
- Hatcher L, Stepanski HE. A Step by Step Approach to Using SAS System for Univariate and Multivariate Analyses. SAS Institute Inc.; Cary, NC: 1994. [Google Scholar]
- Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB., Jr Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- Huston M. A general hypothesis of species diversity. American Naturalist. 1979;113:81–101. [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Gravel ME. Predation and the evolution of prey behavior: an experiment with tree hole mosquitoes. Behavioral Ecology. 2002;13:301–311. [Google Scholar]
- Kaufman MG, Bland SN, Worthen ME, Walker ED, Klug MJ. Bacterial and fungal biomass responses to feeding by larval Aedes triseriatus (Diptera: Culicidae) Journal of Medical Entomology. 2001;38:711–719. doi: 10.1603/0022-2585-38.5.711. [DOI] [PubMed] [Google Scholar]
- Kaufman MG, Walker ED. Indirect effects of soluble nitrogen on growth of Ochlerotatus triseriatus larvae in container habitats. Journal of Medical Entomology. 2006;43:677–688. doi: 10.1603/0022-2585(2006)43[677:ieosno]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kesavaraju B, Yee DA, Juliano SA. Interspecific and intraspecific differences in foraging preferences of container-dwelling mosquitoes. Journal of Medical Entomology. 2007;44:215–221. doi: 10.1603/0022-2585(2007)44[215:iaidif]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman DL. Leucine incorporation as a measure of biomass production by heterotrophic bacteria. In: Kemp PF, Sherr BF, Sherr EB, Cole JJ, editors. Handbook of Methods in Aquatic Microbiology. Lewis Publishing; Boca Raton FL: 1993. pp. 509–512. [Google Scholar]
- Kirk KL. Competition in variable environments: experiments with planktonic rotifers. Freshwater Biology. 2002;47:1089–1096. [Google Scholar]
- Kitching RL. Food Webs and Container Habitats. The Natural History and Ecology of Phytotelmata. Cambridge University Press; Cambridge: 2000. [Google Scholar]
- Kitching RL. Food webs in phytotelmata: ‘bottom-up’ and ‘top-down’ explanations for community structure. Annual Review of Entomology. 2001;46:729–760. doi: 10.1146/annurev.ento.46.1.729. [DOI] [PubMed] [Google Scholar]
- Léonard PM, Juliano SA. Effect of leaf litter and density on fitness and populations performance of the hole mosquito Aedes triseriatus. Ecological Entomology. 1995;20:125–136. [Google Scholar]
- Levins R, Culver D. Regional coexistence of species and competition between rare species. Proceedings of the National Academy of Sciences USA. 1971;68:1246–1248. doi: 10.1073/pnas.68.6.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livdahl TP, Sugihara G. Non-linear interactions of populations and the importance of estimating per capita rates of change. Journal of Animal Ecology. 1984;53:573–580. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Nishimura N, Escher RL. Fitness of a treehole mosquito: influences of food type and predation. Oikos. 1993;66:114–118. [Google Scholar]
- Lounibos LP, O’Meara GF, Escher RL, Nishimura N, Cutwa M, Nelson T, Campos RE, Juliano SA. Testing predicted competitive displacement of native Aedes by the invasive Asian tiger mosquito Aedes albopictus in Florida, USA. Biological Invasions. 2001;3:151–166. [Google Scholar]
- Mason CF, MacDonald SM. The input of terrestrial invertebrates from tree canopies to a stream. Freshwater Biology. 1982;12:305–311. [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annual Review of Entomology. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ. Path analysis: pollination. In: Schiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. Oxford University Press; Oxford: 1993. pp. 211–223. [Google Scholar]
- Moore CG. Aedes albopictus in the United States: current status and prospects for further spread. Journal of the American Mosquito Control Association. 1999;15:221–227. [PubMed] [Google Scholar]
- Moore JC, Berlow EL, Coleman DC, Ruiter PC, Dong Q, Hastings A, Johnson NC, McCann KS, Melville K, Morin PJ, Nadelhoffer K, Rosemond AD, Post DM, Sabo JL, Scow KM, Vanni MJ, Wall DH. Detritus, trophic dynamics and biodiversity. Ecology Letters. 2004;7:584–600. [Google Scholar]
- Nannini MA, Juliano SA. Effects of the facultative predator Anopheles barberi on population performance of Aedes triseriatus. Annals of the Entomological Society of America. 1998;91:33–42. [Google Scholar]
- O’Neil RV, Reichle DA. Dimensions of ecosystem theory. In: Waring RH, editor. Forests: Fresh Perspectives from Ecosystem Analysis. Oregon State University Press; Corvallis, OR: 1980. pp. 11–26. [Google Scholar]
- Reinert JF. New classification for the composite genus Aedes (Diptera, Culicidae, Aedini), elevation of subgenus Ochlerotatus to generic rank, reclassification of the other subgenera, and notes on certain subgenera and species. Journal of the American Mosquito Control Association. 2000;16:175–188. [PubMed] [Google Scholar]
- Rey D, Cuany A, Patou MP, Meyran JC. Differential sensitivity of mosquito taxa to vegetable tannins. Journal of Chemical Ecology. 1999;25:537–548. [Google Scholar]
- SAS Institute Inc. SAS/STAT 9.1 User’s Guide. SAS Institute Inc.; Cary, NC.: 2004. [Google Scholar]
- Scheiner SM. MANOVA. Multiple response variables and multi species interactions. In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. 2. Oxford University Press; Oxford: 2001. pp. 99–133. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry, the Principles and Practices of Statistics in Biological Research. W.H. Freeman; New York: 1995. [Google Scholar]
- Sota T. Performance of Aedes albopictus and A. riversi larvae (Diptera: Culicidae) in waters that contain tannic acid and decaying leaves: is the treehole species better adapted to treehole water? Annals of the Entomological Society of America. 1993;86:450–457. [Google Scholar]
- Steinberg CEW, Kamara S, Prokhotskaya VY, Manusadzianas L, Karasyova TA, Timofeyev MA, Zhang J, Paul A, Meinelt T, Farjalla VF, Matsuo AYO, Burnison BK, Menzel R. Dissolved humic substances: ecological driving forces from the individual to the ecosystem level? Freshwater Biology. 2006;51:1189–1210. [Google Scholar]
- Swift MJ, Heal OW, Anderson TM. Decomposition in Terrestrial Ecosystems. Blackwell; Oxford: 1979. [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: effects of density, food, and competition on response to temperature. Journal of Medical Entomology. 2000;37:40–52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Tilman D. Resource Use and Community Structure. Princeton University Press; Princeton, NJ: 1982. [Google Scholar]
- Tilman D. Competition and biodiversity in spatially structured habitats. Ecology. 1994;75:2–16. [Google Scholar]
- Walker ED, Kaufman MG, Ayres MP, Riedel MH, Merritt RW. Effects of variation in quality of leaf detritus on growth of the eastern tree-hole mosquito, Aedes triseriatus (Diptera: Culicidae) Canadian Journal of Zoology. 1997;75:706–718. [Google Scholar]
- Wright S. Correlation and causation. Journal of Agricultural Research. 1921;20:557–585. [Google Scholar]
- Yee DA, Juliano SA. Consequences of detritus type in an aquatic microsystem: effects on water quality, micro-organisms, and performance of the dominant consumer. Freshwater Biology. 2006;51:448–459. doi: 10.1111/j.1365-2427.2005.01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kesavaraju B, Juliano SA. Interspecific differences in feeding behavior and survival under food-limited conditions for larval Aedes albopictus and Aedes aegypti (Diptera: Culicidae) Annals of the Entomological Society of America. 2004a;97:720–728. doi: 10.1603/0013-8746(2004)097[0720:IDIFBA]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kesavaraju B, Juliano SA. Larval feeding behavior of three co-occurring species of container mosquitoes. Journal of Vector Ecology. 2004b;29:315–322. [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Keseravaju B, Juliano SA. Direct and indirect effects of animal detritus on growth, survival, and mass of the invasive container mosquito Aedes albopictus (Diptera: Culicidae) Journal of Medical Entomology. 2007;44:580–588. doi: 10.1603/0022-2585(2007)44[580:daieoa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]