Abstract
Feeding preferences of larval container-dwelling mosquitoes are not well understood. Primary production is often absent in container systems and external inputs of animal and plant detritus supply the energy base of container food webs by supporting microorganism prey for mosquitoes. We quantified the feeding preferences of Aedes albopictus (Skuse), a non-native invasive mosquito, and Ochlerotatus triseriatus (Say), a native mosquito, when given a choice of animal and plant detritus. We tested for interpopulational (Illinois versus Florida) differences in feeding preferences, and quantified each species’ performance on these two detritus types. When given a choice, both species spent significantly more time feeding at an animal detritus patch. The Illinois populations of both species spent more time feeding at animal detritus patches than did the Florida populations, which spent more time feeding at leaf detritus than did Illinois populations. Both species reached a later instar and had higher survival when reared with animal versus leaf detritus. Ae. albopictus spent more time feeding at animal detritus and had higher survival when reared on either detritus type compared with Oc. triseriatus. Greater preference for and better performance exhibited by Ae. albopictus in high-quality food (animal detritus) may result in preemption of high quality food and may contribute to the superior competitive ability of Ae. albopictus relative to Oc. triseriatus.
Keywords: Aedes albopictus, detritus, feeding behavior, foraging ecology, Ochlerotatus triseriatus
The ecology of mosquitoes inhabiting water-filled artificial and natural containers has received considerable attention (Juliano and Lounibos 2005, and references therein), in part because these mosquitoes often are disease vectors. In containers, allochthonous nutrient inputs are an important influence on community and population dynamics, because primary production is typically absent in these systems (Carpenter 1983). Allochthonous inputs can be classified into plant detritus, including senescent leaves, fruits and seeds, animal detritus, primarily consisting of dead invertebrates, and stem flow, which is organic-rich water that flows down tree surfaces during rain (Yee and Juliano 2006). Stem flow water has been shown to affect ionic concentrations of tree hole water, which can greatly affect the abundance and structure of tree hole insect communities (Paradise and Dunson 1997, Paradise and Dunson 1998). Mosquitoes in these systems feed on bacteria, protozoa, and fungi, which themselves subsist on allochthonous detritus (Walker et al. 1991, Merritt et al. 1992, Sota and Kato 1994). Although the links between plant detritus and mosquito production have been studied (Fish and Carpenter 1982; Léonard and Juliano 1995; Walker et al. 1997; Kaufman et al. 1999, 2002), the role of animal detritus as a source of mosquito nutrition is poorly understood (Daugherty et al. 2000, Wallace and Merritt 2004, Yee and Juliano 2006). Yee and Juliano (2006) showed that when the native North American tree hole mosquito, Ochlerotatus triseriatus (Say), is reared with animal detritus, it attains greater mass and population growth than when they are reared only on plant detritus. Animal detritus is important for the production of mosquitoes in another container system, pitcher plants (Bradshaw and Holzapfel 1986, Sota 1996, Cresswell 2000).
Little is known about the foraging behavior of container-dwelling mosquito larvae or about how they respond to different types of detritus (Merritt et al. 1992). In terrestrial insect systems, it is often easy to observe foraging preferences and then to evaluate how benefits gained from particular resources are related to foraging preferences (Cnaani et al. 2006). In small aquatic systems such as containers, foraging behavior and preferences are harder to observe, and foraging preferences of mosquitoes have seldom been used to assess the impact of different resources on mosquito larvae. Feeding behavior with different resource types may be related to larval success and adult mosquito production, which may be important components of vector-borne disease dynamics (Hawley 1985). In addition, different larval feeding behavior patterns have been suggested to influence competitive ability among medically important mosquito species (Juliano et al. 1993, Grill and Juliano 1996, Yee et al. 2004a).
The Asian tiger mosquito, Aedes albopictus (Skuse), is native to Asia but was introduced into the United States in the mid-1980s (Hawley et al. 1987) and has since invaded most of the southeastern United States (O’Meara et al. 1995). Ae. albopictus is medically important as a vector of arboviruses such as Dengue virus (family Flaviviridae, genus Flavivirus), La Crosse virus (family Bunyaviridae, genus Orthobunyavirus), eastern equine encephalitis virus (family Togaviridae, genus Alphavirus), and West Nile virus (family Flaviviridae, genus Flavivirus) (Mitchell et al. 1992; Ibanez-Bernal et al. 1997; Gerhardt et al. 2001; Turell et al. 2001, 2005). Oc. triseriatus is native to North America and is a vector of La Crosse virus (Eldridge et al. 2000). Both species occupy anthropogenic (tires and cemetery vases) and natural (tree holes) containers. Ae. albopictus and Oc. triseriatus browse surfaces with their mouthparts and are categorized as “collector gatherers” and “shredders” (Merritt et al. 1992). Ae. albopictus is superior to many resident container mosquitoes as a competitor (Daugherty et al. 2000, Aliabadi and Juliano 2002, Yee et al. 2004a). This competitive advantage varies with environmental factors such as habitat drying (Costanzo et al. 2005), container type (Livdahl and Willey 1991), and resource type (Barrera 1996, Daugherty at al. 2000). Although laboratory studies have revealed that Ae. albopictus is a superior competitor to Oc. triseriatus (Teng and Apperson 2000), only one comparison of the foraging behavior of these mosquitoes has been made (Yee et al. 2004b). Preferences of these mosquitoes for animal and plant detritus are not known. Although Ae. albopictus is often the superior resource competitor (Teng and Apperson 2000), the proximate mechanism producing the competitive advantage for Ae. albopictus larvae has not been documented. For example, Barrera (1996) suggest that larvae of Ae. albopictus resist starvation longer when grown on senescent leaf litter because of higher stored energy, but they did not document such a difference in energy storage. This lack of understanding of the mechanism for competitive superiority limits our understanding of ecological forces structuring populations of container mosquitoes.
Geographic variation in behavior is often correlated with variation in environmental conditions experienced by particular populations (De Queiroz et al. 2001). For Argentine ants, environmental cues are important in determining intercolony aggression (Chen and Nonacs 2000) and there is geographic and diet-dependent variation in intercolony aggression (Buczkowski and Silverman 2006). These two examples show the importance of geographical variation in the ecology of a species. Oc. triseriatus and Ae. albopictus are widely distributed in the eastern half of the United States (Darsie and Ward 2005). Reproductive investment, reproductive output, and activity patterns of larval Oc. triseriatus exhibit considerable geographic variation (Juliano and Reminger 1992, Juliano et al. 1993, Hechtel and Juliano 1997, Frankino and Juliano 1999). Information is lacking on geographic variation in behavior of Ae. albopictus and on geographic variation in feeding preferences in both species.
Our objectives in this study were to test whether there are 1) differences in foraging preferences and interpopulation differences in those preferences for animal versus plant detritus between Oc. triseriatus and Ae. albopictus and 2) differences in growth and survival associated with any foraging preferences for those two detritus types.
Materials and Methods
Experiment 1
Interpopulation Foraging Preference
We used Ae. albopictus and Oc. triseriatus eggs from individuals field-collected from Florida and Illinois. The Florida populations of Ae. albopictus were collected from a cemetery (Oak Hill Cemetery, Bartow, FL; 28° 57′ 51 N, 81° 53′ 15 W). The Illinois population of Ae. albopictus was collected from tires in East St. Louis, IL (38° 35′ 33 N, 90° 08′ 14 W), whereas Oc. triseriatus were collected from ParkLands Foundation Preserve, near Lexington (40° 39′ 10 N, 88° 52′ 21 W). The Florida population of Oc. triseriatus was maintained as a laboratory colony to which field-collected adults from tree holes along Indrio Rd., Fort Pierce, FL (27° 31′ 14 N, 80° 23′ 39 W), were added frequently. Aedes albopictus and Oc. triseriatus colonies were blood fed with anesthetized guinea pigs (Cavia porcellus L.) (IACUC protocol 01-2005) to obtain eggs. Ninety, 1-d-old larvae per species per population were isolated in 10-ml vials with 5 ml of deionized (DI) water. Subsequently, larvae were fed 1.0 ml of a liver powder suspension (LPS) (0.3 g of liver power/1,000 ml water) every 2 d until they reached the fourth instar. The LPS was transferred using an Eppendorf pipette while stirring using a stir plate to ensure homogeneous delivery of food (Juliano and Gravel 2002, Kesavaraju and Juliano 2004). When the larvae reached the fourth instar, they were transferred individually into 50-ml cups with 50 ml of DI water and no food to standardize hunger before transferring them to the treatment cups for behavior recording.
Based on a previous study (Yee and Juliano 2006), we used Drosophila melanogaster (Meigen) adults for animal detritus and sugar maple, Acer saccharum (Marsh), leaves for plant detritus (henceforth leaf detritus). Senescent maple leaves were collected in the fall from a woodlot near the Illinois State University campus and stored dry. Oven-dried (50°C for 48 h) D. melanogaster adults and similar-sized maple leaf pieces were soaked separately in 1,000 ml of DI water for 4 d. In addition to DI water, we added 100 ml of tree hole water to each detritus type. Tree hole water was obtained from 10 tree holes at Parklands Preserve, Lexington, IL, and homogenized before being transferred to experimental containers. A line was drawn down the middle of the exterior bottom of each experimental cup (50-ml translucent plastic disposable cups) to delineate two halves. The day of the behavior recording, we randomly assigned one D. melanogaster carcass to one side and a leaf fragment (approximately equivalent in size to the animal detritus) the other side of each cup, which was filled with 50 ml of water. Each treatment cup was prepared 2 min before the larvae were transferred for behavioral recording.
We recorded the behavior of larvae using a video-camera attached to a computer with a Winfast XP PCI card (Yee et al. 2004a, b). A single video clip contained six treatment cups (three replicates of each species) and 15 min of video. We played back the video clips and recorded the time spent browsing on leaf or animal detritus patches by each larva. Times spent browsing was converted to proportions of totals. Apart from browsing on the leaf and animal detritus patches, larvae spent time on other behaviors. We analyzed data using multiple analysis of variance (MANOVA) with proportion of time browsing on leaf or animal patches as dependent variables, and species, geographic origin, and interaction as the independent variables (SAS Institute 2004). We used the standardized canonical coefficients (SCC) to interpret the contributions of each variable to any significant effects (Scheiner 2001).
Experiment 2
Performance with Different Detritus Types
We measured survival and growth of Ae. albopictus and Oc. triseriatus with low and high amounts of animal and leaf detritus. Treatments were all combinations of detritus type (animal versus leaf), detritus amount (0.005 or 0.025 g), and species (Ae. albopictus and Oc. triseriatus) for a total of eight treatment combinations. We replicated each treatment combination 10 times for a total of 80 experimental units, each held within a 100-ml plastic beaker with 99 ml of DI water and 1 ml of tree hole water as a microorganism inoculum (described above). Containers remained undisturbed for 5 d before we added larvae. Into each replicate, we added five 1-d-old larvae of one species. Experimental containers were housed in an environmental chamber with a photo-period of 14:10 (L:D) h cycle at 24°C. We checked the cups every other day and recorded the number of larvae that pupated. We transferred pupae into individual glass vials and recorded the day of eclosion, species, and sex of the adults. The duration of the experiment was 31 d, and at the end we counted the number of surviving individuals in each replicate and recorded their developmental stage (larva: 1, 2, 3, and 4, corresponding to instar; pupa: 5; and adult: 6). We analyzed the proportion of individuals that survived per cup and the mean instar for each cup by using MANOVA.
Results
Experiment 1
Interpopulation Foraging Preference
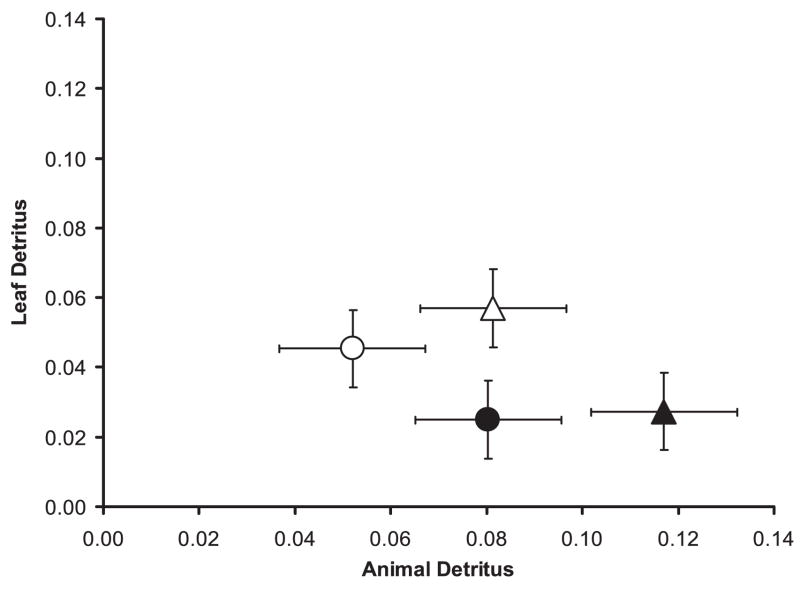
Species and population were significant, but the interaction between them was not (Table 1). As indicated by the SCC interpretation, the species effect, interspecific differences in time foraging on animal detritus patches contributed more than did interspecific differences in time foraging on leaf detritus patches (Table 1; Fig. 1). The SCC for population indicated that both animal detritus and leaf detritus contributed to the significant effect (Table 1). Larvae from Illinois spent more time foraging on animal detritus patches (mean = 0.098 [proportion], SE = 0.117) than did larvae from Florida (mean = 0.066 [Proportion], SE = 0.009), but larvae from Florida spent more time foraging on leaf detritus patches (mean = 0.051 [proportion], SE = 0.009) than did the Illinois populations (mean = 0.026 [proportion], SE = 0.006). Between species, Ae. albopictus spent more time browsing on animal detritus patches (mean = 0.099 [proportion], SE = 0.011) and on leaf detritus patches (mean = 0.042 [proportion], SE = 0.008] than did Oc. triseriatus (animal: mean = 0.066 [proportion], SE = 0.010; leaf: mean = 0.035 [proportion], SE = 0.008). Both species and both populations spent more time foraging on animal detritus patches than on leaf detritus patches (Fig. 1).
Table 1.
Results of MANOVA for effects of species (Oc. triseriatus and Ae. albopictus) and population (Illinois and Florida) on proportions of time foraging on different types detritus patches (animal and leaf)
| Variable | Numerator df | Denominator df | Pillai’s trace | P | Standardized canonical coefficients
|
|
|---|---|---|---|---|---|---|
| Animal | Leaf | |||||
| Species | 2 | 115 | 0.0513* | 0.0484* | 1.0308* | 0.5263* |
| Population | 2 | 115 | 0.0602* | 0.0280* | 0.6074* | −0.6692* |
| Species × Population | 2 | 115 | 0.0016 | 0.9090 | −0.3232 | 0.8774 |
Values followed by an asterisk indicate a significant effect.
Fig. 1.
Mean proportion (±1 SE) of time spent feeding on animal and leaf detritus patches by Ae. albopictus and Oc. triseriatus from Florida and Illinois. Triangles are Ae. albopictus and circles are Oc. triseriatus. Closed symbols represent Illinois populations and open symbols represent Florida populations.
Experiment 2
Performance in Different Detritus
Species, detritus amount, and detritus type were significant, but the interaction among them was not (Table 2). SCC’s for detritus type indicated that differences in mean instar of mosquito individuals contributed more to the significant effect than did survival. SCCs for detritus amount and for species indicated that survival contributed more to the significant effect than did mean instar (Table 2).
Table 2.
Results of MANOVA for the effects of different types (animal and leaf) and amounts (0.005 and 0.025 g) of detritus, and species (Oc. triseriatus and Ae. albopictus) on mean instar and survival after 31 d
| Variable | Numerator df | Denominator df | Pillai’s trace | P | Standardized canonical coefficients
|
|
|---|---|---|---|---|---|---|
| Instar | Survival | |||||
| Detritus(D) | 2 | 31 | 0.6993* | <0.0001* | 1.8285* | 0.3686* |
| Amount(A) | 2 | 31 | 0.7501* | <0.0001* | 0.6997* | 1.6108* |
| Species(S) | 2 | 31 | 0.2173* | 0.0224* | 0.1901* | 1.9241* |
| D × A | 2 | 31 | 0.0431 | 0.5050 | 1.2635 | 1.1163 |
| D × S | 2 | 31 | 0.0299 | 0.6237 | 0.2142 | 1.9118 |
| A × S | 2 | 31 | 0.1429 | 0.0916 | 1.4206 | 0.9419 |
| D × A × S | 2 | 31 | 0.0342 | 0.5830 | −0.8206 | 2.2254 |
Values followed by an asterisk indicate a significant effect.
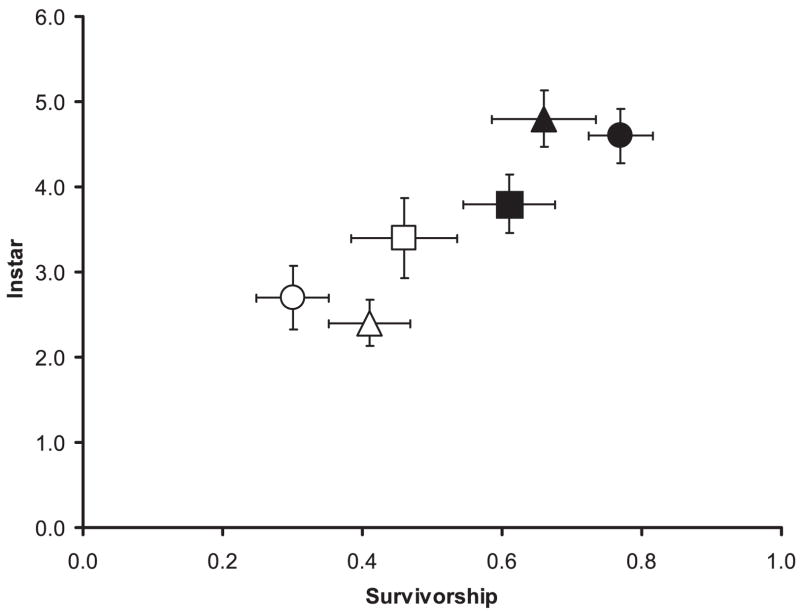
Survival was greater on animal detritus (mean = 0.66 [proportion], SE = 0.074) than on leaf detritus (mean = 0.41 [proportion], SE = 0.058) and greater in high-detritus containers (Mean = 0.77 (Proportion), SE = 0.047) than in low-detritus containers (mean = 0.30 [proportion], SE = 0.051). Regardless of detritus type or amount, Ae. albopictus had significantly greater survival (mean = 0.61 [proportion], SE = 0.066) than did Oc. triseriatus (mean = 0.46 [proportion], SE = 0.075) (Fig. 2).
Fig. 2.
Mean proportion of survival and instar (performance) for larvae in type of detritus (animal and leaf), amount of detritus (high and low) and species (Ae. albopictus and Oc. triseriatus). Closed triangles represent animal detritus and open triangle represent leaf detritus. Closed circles represent high detritus and open circles represent low detritus. Closed squares represent Ae. albopictus and open squares represent Oc. triseriatus.
For the detritus effect, it was apparent that mosquitoes reached a greater mean instar when grown on animal (mean = 4.89 [instar], SE = 0.331) versus leaf detritus (mean = 2.46 [instar], SE = 0.270), and they reached a greater instar with high detritus (mean = 4.64 [instar], SE = 0.317) than with low detritus (mean = 2.71 [instar], SE = 0.372). Regardless of detritus type or amount, Ae. albopictus attained slightly greater mean instar (mean = 3.89 [instar], SE = 0.339) than did Oc. triseriatus (mean = 3.46 [instar], SE = 0.467) (Fig. 2).
Discussion
Our data show that when given a choice, Ae. albopictus and Oc. triseriatus prefer to forage on animal detritus but that the expression of this preference in time allocation varies with geographic origin and species. Ae. albopictus and Oc. triseriatus also realize greater developmental progress and survival when reared on animal detritus as opposed to an equal amount of leaf detritus. Ae. albopictus showed a stronger preference for animal detritus (i.e., allocated relatively more time foraging on patches of animal detritus; Fig. 1) than did Oc. triseriatus, and it also had higher survival overall (Fig. 2). Geographic differences also were evident in our study, with Florida populations of both species allocating relatively more time to leaf detritus than did Illinois populations, suggesting a weaker preference for animal detritus for Florida populations.
In general, animals maximize their foraging efficiency by increasing the time spent foraging on high-quality food (De Haas et al. 2006). Studies of stream systems show that animal detritus is a higher quality resource than is plant detritus for certain aquatic macroinvertebrates (Cloe and Garman 1996). Yee and Juliano (2006) showed that mosquitoes have higher survival and growth rate from ingesting this type of resource versus plant material. Deciduous tree leaves are generally a poor-quality resource for aquatic insects because energy first must be converted to microorganism production before it is consumed (Merritt et al. 1992). Similarly, in our study, mosquitoes that were raised on animal detritus had greater survival and attained a later instar than did those raised on leaf detritus, indicating that animal detritus is a higher quality resource. Aedes albopictus and Oc. triseriatus compete and co-occur in many parts of the United States (Livdahl and Willey 1991, Lounibos et al. 2001). Laboratory and field studies have shown that Ae. albopictus is competitively superior to other co-occurring mosquito species, including Oc. triseriatus (Ho et al. 1989, Livdahl and Willey 1991, Novak et al. 1993, Lounibos et al. 2001, Aliabadi and Juliano 2002) and Ae. aegypti (Barrera 1996, Juliano 1998, Daugherty et al. 2000, Juliano and Lounibos 2005). Greater efficiency in acquiring a limited resource can be an important determinant of competitive advantage. Yee et al. (2004a) showed that Ae. albopictus allocated more foraging effort to browsing on leaves than did a related species, Ae. aegypti, and that Ae. albopictus were able to reach later instar than Ae. aegypti when reared with low amounts of detritus. Yee et al. (2004a) suggested that more efficient allocation of foraging effort to high-resource patches contributed to greater success of Ae. albopictus when resources were scarce. Our current experiments extend these results to choices among different classes of resource patches. The greater preference for foraging in high-quality resource patches (animal detritus) by Ae. albopictus compared with Oc. triseriatus suggests that the foraging pattern of Ae. albopictus may contribute to the well-documented superior competitive ability of Ae. albopictus.
Tree hole or container-breeding mosquitoes meet their nutritional needs primarily by consuming microorganisms from the surfaces of detritus or suspended in the water column (Merritt et al. 1992, Walker et al. 1997). Nucleic acids and nucleotides emanating from any kind of detritus act as phagostimulants for mosquitoes (Merritt et al. 1992, Walker et al. 1997). Larvae may be attracted to high-quality animal detritus because of greater concentrations of phagostimulants or particular kinds of phagostimulants not found in lower quality detritus. In our behavior experiments, we often observed mosquitoes using their mouthparts to carry entire Drosophila carcasses, but not leaf fragments of equal size, from the bottom of the containers to the surface. Gut content analysis of mosquito larvae have shown that individuals reared with Drosophila carcasses do not simply ingest epidetrital microorganisms, but instead consume some parts of this animal detritus directly (Yee 2006). Direct ingestion of high-protein animal detritus may be a mechanism contributing to greater developmental progress and survival of mosquitoes reared with animal versus leaf detritus (Wallace and Merritt 2004, Yee and Juliano 2006).
Geographic differences in foraging time were apparent between Illinois and Florida populations. Local environmental differences combined with biological or geographic barriers can produce local differentiation in phenotypic traits (O’Hara et al. 2005), including behavior. Behavioral characters may diverge due to selection so that individuals from different populations respond differently to an environmental variable such as resource availability (Reznick et al. 2001, Stoks et al. 2003). Container-dwelling mosquitoes have been shown to exhibit geographic variation in behavioral, life history, and population dynamic characters (Black et al. 1989, Juliano and Reminger 1992, Juliano et al. 1993, Frankino and Juliano 1999, Armbruster et al. 2001). Variation in food quality affects the development of aquatic insect larvae (Macchiusi and Baker 1992, Wallace and Merritt 1999) and may be one source of environmental variation affecting behavior patterns in local populations. Container mosquito activity in Illinois is highly seasonal, occurring from April to August (S.A.J., unpublished data). During winter, temperatures often fall below freezing; hence, Ae. albopictus and Oc. triseriatus must overwinter as diapausing eggs. In contrast, conditions in Florida are more favorable for mosquito growth throughout most of the year, although a December to May dry season can inhibit larval development (Juliano et al. 2002). Because of the shorter active season in Illinois, selection may have favored individuals that maximize growth during favorable months. We hypothesize that individuals from Illinois populations of these species exhibit stronger preference for higher quality food (e.g., animal detritus) because that resource will maximize growth and development during the relatively short active season, but further research is needed to assess the degree of variation among populations in these traits.
Effects of leaf detritus in container habitats are well known (Carpenter 1983, Léonard and Juliano 1995, Kaufman et al. 2002), but we know little about the effect of animal detritus on mosquito performance (Daugherty et al. 2000, Wallace and Merritt 2004, Yee and Juliano 2006). Our results on the performance of mosquitoes with different detritus types are similar to the finding of Yee and Juliano (2006) who showed that Oc. triseriatus had higher population growth and survival when offered animal versus leaf detritus. Our study is novel in that it shows that mosquitoes also exhibit a foraging preference between detritus types and that these preferences can differ between species and geographic populations. It is clear that container mosquitoes had higher survival and growth rates when reared with animal detritus compared with leaf detritus (also shown by Yee and Juliano 2006). Animal detritus contains higher amounts of nitrogen and phosphorus compared with an equal amount of leaf detritus (Yee and Juliano 2006). This added nutrient content may benefit mosquito larvae directly if they can ingest it, or it may benefit their microorganism food base (Yee and Juliano 2006). Future studies should focus on determining the mechanism by which animal detritus enhances mosquito performance, quantifying the amount of animal detritus that enters containers in nature, and determining the effects of such natural variation in resource inputs on population performance and community composition.
Acknowledgments
We thank Natalka Kinal for helping with the experiments. This project was supported by National Institute of Allergy and Infectious Disease Grants R01-AI-44793 and R15-AI-051374 and a 2005 Mockford summer dissertation fellowship (Phi Sigma Biological Society, Illinois State University) to B.K.
References Cited
- Aliabadi BW, Juliano SA. Escape from gregarine parasites affects the competitive interactions of an invasive mosquito. Biol Invasions. 2002;4:283–297. doi: 10.1023/A:1020933705556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armbruster P, Bradshaw WE, Ruegg K, Holzapfel CM. Geographic variation and the evolution of reproductive allocation in the pitcher-plant mosquito, Wyeomyia smithii. Evolution. 2001;55:439 – 444. doi: 10.1111/j.0014-3820.2001.tb01306.x. [DOI] [PubMed] [Google Scholar]
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol Entomol. 1996;21:112–127. [Google Scholar]
- Black WC, Rai KS, Turco BJ, Arroyo DC. Laboratory study of competition between United States strains of Aedes albopictus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 1989;32:847 – 852. doi: 10.1093/jmedent/26.4.260. [DOI] [PubMed] [Google Scholar]
- Bradshaw WE, Holzapfel CM. Geography of density-dependent selection in pitcher-plant mosquitoes. In: Taylor F, Karban R, editors. The evolution of insect life cycles. Springer; New York: 1986. pp. 44–65. [Google Scholar]
- Buczkowski G, Silverman J. Geographical variation in Argentine ant aggression behaviour mediated by environmentally derived nestmate recognition cues. Anim Behav. 2006;71:327–335. [Google Scholar]
- Carpenter SR. Resource limitation of larval treehole mosquitoes subsisting on beech detritus. Ecology. 1983;64:219 – 223. [Google Scholar]
- Chen JSC, Nonacs P. Nestmate recognition and intraspecific aggression based on environmental cues in Argentine ants (Hymenoptera: Formicidae) Ann Entomol Soc Am. 2000;93:1333–1337. [Google Scholar]
- Cloe WW, Garman GC. The energetic importance of terrestrial arthropod inputs to three warm-water streams. Freshw Biol. 1996;36:105–114. [Google Scholar]
- Cnaani J, Thomson JD, Papaj DR. Flower choice and learning in foraging bumblebees: effects of variation in nectar volume and concentration. Ethology. 2006;112:278 – 285. [Google Scholar]
- Costanzo KS, Kesavaraju B, Juliano SA. Condition-specific competition in container mosquitoes: the role of noncompeting life-history stages. Ecology. 2005;86:3289 – 3295. doi: 10.1890/05-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresswell JE. Resource input and the community structure of larval infaunas of an eastern tropical pitcher plant Nepenthes bicalcarata. Ecol Entomol. 2000;25:362–366. [Google Scholar]
- Darsie RF, Jr, Ward RA. Identification and geographical distribution of the mosquitoes of North America and North of Mexico. University of Florida; Gainesville, FL: 2005. [Google Scholar]
- Daugherty MP, Alto BW, Juliano SA. Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2000;37:364 – 372. doi: 10.1093/jmedent/37.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haas EM, Wagner COEN, Koelmans AA, Kraak MHS, Admiraal WIM. Habitat selection by chironomid larvae: fast growth requires fast food. J Anim Ecol. 2006;75:148 – 155. doi: 10.1111/j.1365-2656.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- De Queiroz A, Henke C, Smith HM. Geographic variation and ontogenetic change in the diet of the Mexican Pacific lowlands garter snake, Thamnophis validus. Copeia. 2001:1034 – 1042. [Google Scholar]
- Eldridge BF, Scott TW, Day JF, Tabachnick WJ. Arbovirus diseases. In: Eldridge BF, Edman JD, editors. Medical entomology. Kluwer Academic; Dordrecht, The Netherlands: 2000. pp. 415–460. [Google Scholar]
- Fish D, Carpenter SR. Leaf litter and larval mosquito dynamics in tree-hole ecosystems. Ecology. 1982;63:283–288. [Google Scholar]
- Frankino WA, Juliano SA. Costs of reproduction and geographic variation in the reproductive tactics of the mosquito Aedes triseriatus. Oecologia (Berl) 1999;120:59 – 68. doi: 10.1007/s004420050833. [DOI] [PubMed] [Google Scholar]
- Gerhardt RR, Gottfried KL, Apperson CS, Davis BS, Erwin PC, Smith AB, Panella NA, Powell EE, Nasci RS. First isolation of LaCrosse virus from naturally infected Aedes albopictus. Emerg Inf Dis. 2001;37:807 – 811. doi: 10.3201/eid0705.017506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill CP, Juliano SA. Predicting species interactions based on behaviour: predation and competition in container-dwelling mosquitoes. J Anim Ecol. 1996;65:63–76. [Google Scholar]
- Hawley WA. Population dynamics of Aedes sierrensis. In: Lounibos LP, Rey JR, Frank JH, editors. Ecology of Mosquitoes: Proceedings of a Workshop. Florida Medical Entomology Laboratory, University of Florida; Vero Beach, FL: 1985. [Google Scholar]
- Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB., Jr Aedes albopictus in North America: probable introduction in used tires from northern Asia. Science (Wash, DC) 1987;236:1114 – 1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- Hechtel LJ, Juliano SA. Effects of a predator on prey metamorphosis: plastic responses by prey or selective mortality? Ecology. 1997;78:838 – 851. [Google Scholar]
- Ho BC, Ewert A, Chew L. Interspecific competition among Aedes aegypti, Ae. albopictus and Ae triseriatus (Diptera: Culicidae): larval development in mixed cultures. J Med Entomol. 1989;26:615 – 623. doi: 10.1093/jmedent/26.6.615. [DOI] [PubMed] [Google Scholar]
- Ibanez-Bernal BBS, Mutebi JP, Argot E, Rodriguez G. First record in America of Aedes albopictus naturally infected with dengue virus during the 1995 outbreak at Reynosa, Mexico. Med Vet Entomol. 1997;11:305–309. doi: 10.1111/j.1365-2915.1997.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Juliano SA, Gravel ME. Predation and the evolution of prey behavior: an experiment with tree hole mosquitoes. Behav Ecol. 2002;13:301–311. [Google Scholar]
- Juliano SA, Hechtel LJ, Waters JR. Behavior and risk of predation in larval tree hole mosquitoes – effects of hunger and population history of predation. Oikos. 1993;68:229 – 241. [Google Scholar]
- Juliano SA, O’Meara GF, Morrill JR, Cutwa MM. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia (Berl) 2002;130:458 – 469. doi: 10.1007/s004420100811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, Reminger L. The relationship between vulnerability to predation and behavior of larval tree-hole mosquitoes: geographic and ontogenetic differences. Oikos. 1992;63:465 – 467. [Google Scholar]
- Juliano SA. Species Introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558 – 574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MG, Goodfriend W, Kohler-Garrigan A, Walker ED, Klug MJ. Soluble nutrient effects on microbial communities and mosquito production in Ochlerotatus triseriatus habitats. Aquat Microb Ecol. 2002;29:73 – 88. [Google Scholar]
- Kaufman MG, Walker ED, Smith TW, Merritt RW, Klug MJ. Effects of larval mosquitoes (Aedes triseriatus) and stemflow on microbial community dynamics in container habitats. Appl Environ Microbiol. 1999;65:2661–2673. doi: 10.1128/aem.65.6.2661-2673.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesavaraju B, Juliano SA. Differential behavioral responses to water-borne cues to predation in two container-dwelling mosquitoes. Ann Entomol Soc Am. 2004;97:194 – 201. doi: 10.1603/0013-8746(2004)097[0194:dbrtwc]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léonard PM, Juliano SA. Effect of leaf litter and density on fitness and population performance of the hole mosquito Aedes triseriatus. Ecol Entomol. 1995;20:125 – 136. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedestriseriatus. Science (Wash, DC) 1991;253:189 – 191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, O’Meara GF, Escher RL, Nishimura N, Cutwa M, Nelson T, Campos RE, Juliano SA. Testing predictions of displacement of native Aedes by the invasive Asian tiger mosquito Aedes albopictus in Florida, USA. Biol Invasions. 2001;3:151–166. [Google Scholar]
- Macchiusi F, Baker RL. Effects of predators and food availability on activity and growth of Chironomus tentans (Chironomidae, Diptera) Freshw Biol. 1992;28:207–216. [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 1992;37:349 – 376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Mitchell DJ, Niebylski ML, Karabatsos N, Martin D, Mutebi JP. Isolation of eastern equine encephalitis from Aedes albopictus in Florida. Science (Wash, DC) 1992;257:526 – 527. doi: 10.1126/science.1321985. [DOI] [PubMed] [Google Scholar]
- Novak MG, Higley LG, Christianssen CA, Rowley WA. Evaluating larval competition between Aedes albopictus and A. triseriatus (Diptera: Culicidae) through replacement series experiments. Environ Entomol. 1993;22:311–318. [Google Scholar]
- O’Hara PD, Fernandez G, Becerril F, de la Cueva H, Lank DB. Life history varies with migratory distance in western sandpipers Calidris mauri. J Avian Biol. 2005;36:191–202. [Google Scholar]
- O’Meara GF, Evans LF, Getman AD, Cuda JP. Spread of Aedes albopictus and decline of Aedes aegypti (Diptera, Culicidae) in Florida. J Med Entomol. 1995;32:554 – 562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Paradise CJ, Dunson WA. Effects of water cations on treehole insect communities. Ann Entomol Soc Am. 1997;90:798 – 805. [Google Scholar]
- Paradise CJ, Dunson WA. Relationship of atmospheric deposition to the water chemistry and biota of treehole habitats. Environ Toxicol Chem. 1998;17:362–368. [Google Scholar]
- Reznick D, Butler MJ, Rodd H. Life-history evolution in guppies. VII The comparative ecology of high- and low-predation environments. Am Nat. 2001;157:126 – 140. doi: 10.1086/318627. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT® 9.1 user’s guide. SAS Institute; Cary, NC: 2004. [Google Scholar]
- Scheiner SM. MANOVA: multiple response variables and multispecies interaction. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. 2. Oxford University Press; Oxford, United Kingdom: 2001. pp. 99–115. [Google Scholar]
- Sota T. Effects of capacity on resource input and the aquatic metazoan community structure in phytotelmata. Res Popul Ecol. 1996;38:65–73. [Google Scholar]
- Sota T, Kato K. Bacteria as diet for the mosquito larvae Aedes (Stegomyia) (Diptera, Culicidae) – preliminary experiments with Pseudomonas fluorescens. Appl Entomol Zool. 1994;29:598 – 3600. [Google Scholar]
- Stoks R, MAMcPeek M, Mitchell JL. Evolution of prey behavior in response to changes in predation regime: damselflies in fish and dragonfly lakes. Evolution. 2003;57:574 – 585. doi: 10.1554/0014-3820(2003)057[0574:EOPBIR]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: effects of density, food, and competition on response to temperature. J Med Entomol. 2000;37:40 – 52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130 – 134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, O’Guinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57 – 62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Walker ED, Lawson DL, Merritt RW, Morgan WT, Klug MJ. Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems and microcosms. Ecology. 1991;72:1529 – 1546. [Google Scholar]
- Walker ED, Kaufman MG, Ayres MP, Riedel MH, Merritt RW. Effects of variation in quality of leaf detritus on growth of the eastern tree-hole mosquito, Aedes triseriatus (Diptera, Culicidae) Can J Zool. 1997;75:706 – 718. [Google Scholar]
- Wallace JR, Merritt RW. Influence of microclimate, food, and predation on Anopheles quadrimaculatus(Diptera: Culicidae) growth and development rates, survivorship, and adult size in a Michigan pond. Environ Entomol. 1999;28:233–239. [Google Scholar]
- Wallace JR, Merritt RW. Diel feeding periodicity of larval anopheline mosquitoes on microorganisms and microinvertebrates: a spatial and temporal comparison of Anopheles quadrimaculatus (Diptera: Culicidae) diets in a Michigan pond. J Med Entomol. 2004;41:853–860. doi: 10.1603/0022-2585-41.5.853. [DOI] [PubMed] [Google Scholar]
- Yee DA. PhD dissertation. Illinois State University; Normal, IL: 2006. Effects of species interactions and productivity on aquatic macroinvertebrate diversity and community composition in tree holes: patterns and mechanisms. [Google Scholar]
- Yee DA, Juliano SA. Consequences of detritus type in an aquatic microsystem: effects on water quality, microorganisms and performance of the dominant consumer. Freshw Biol. 2006;51:448 – 459. doi: 10.1111/j.1365-2427.2005.01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kesavaraju B, Juliano SA. Inter-specific differences in feeding behavior and survival under food-limited conditions for larval Aedes albopictus and Aedes aegypti (Diptera: Culicidae) Ann Entomol Soc Am. 2004a;97:720 – 728. doi: 10.1603/0013-8746(2004)097[0720:IDIFBA]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kesavaraju B, Juliano SA. Larval feeding behavior of three co-occuring species of container mosquitoes. J Vector Ecol. 2004b;29:315–322. [PMC free article] [PubMed] [Google Scholar]