Abstract
Background
Enterobacter sakazakii (ES) is an emerging pathogen associated with the ingestion of contaminated reconstituted formula that causes necrotizing enterocolitis, sepsis, and meningitis in low-birth-weight preterm neonatal infants. Necrotizing enterocolitis remains the most common gastrointestinal surgical emergency in these infants. In recent years, the International Commission on Microbiological Specifications for Foods has ranked ES a “severe hazard for restricted populations.” Because of its resistance to certain antibiotics, better understanding of ES pathogenesis is needed to aid in the development of new preventive strategies.
Methods
Review of pertinent English-language literature.
Results
Neonatal and older infants appear to be at the highest risk, although adult ES infections have been reported. We discuss the origins of ES, the detection and pathogenesis of the disease, and potential prevention strategies.
Conclusions
The precise pathogenesis of ES remains a mystery. Appropriate measures by parents, infant formula manufacturers, and health care providers, as well as understanding of the pathogenesis, are important in the prevention of ES-related infections.
Enterobacter sakazakii (ES) is a member of the Enterobacteriaceae family that was described as a new species in 1980. Initially, it was noted to be an opportunistic pathogen responsible for neonatal sepsis and meningitis [1–3]. Necrotizing enterocolitis (NEC) is the most common gastrointestinal surgical emergency in neonatal populations, which results in a mortality rate of 40–100% in the most severely affected patients [4].
Koch's postulates are four criteria designed to establish a causal relation between a microorganism and a disease [5]. The organism must be found in individuals suffering from a disease, it must be able to be cultured from those affected, it must be able to induce disease when re-introduced into a host, and it must be able to be isolated from that individual. Of particular note, no other pathogen has been noted to fulfill Koch's postulates with regard to NEC, and ES appears to fulfill these criteria in certain instances.
The presence of bacteria is fundamental to the development of NEC, which cannot develop in a sterile environment [6]. For this reason ES-related NEC is an area of investigation. However, although ES is not isolated from all individuals who develop NEC, it is a member of the Enterobacteriaceae, the group of organisms isolated by far the most often from NEC [6]. In neonatal populations, ES outbreaks have been linked to contaminated infant formula. Most patients who survive ES meningitis suffer severe neurologic sequelae, including hydrocephalus, quadriplegia, and retardation of development [7]. Fecal carriage of ES has been described in patients as old as 18 weeks of age, illustrating the potential for mucosal adherence and long-term colonization of the human intestine by ES [8] and supporting the need for isolation of ES-infected patients. Neonatal enterocolitis is associated with intestinal prematurity, enteral feeding, and microbial colonization. The disease affects 2–5% of all premature infants, and roughly 13% of those with birth weights < 1.5 kg.
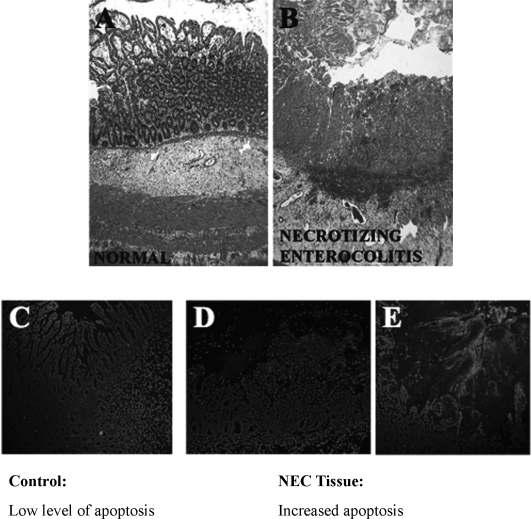
Figure 1 shows an intestinal segment from a child with Enterobacter-associated NEC. Marked loss of villus architecture (Fig. 1B) and epithelial cell apoptosis (Fig. 1D) are clearly visible. In a study of 125 infants with NEC, Enterobacter spp. were the most common bacterial isolates, being recovered from 29% of the patients [9]. Although premature low-birth weight infants are at higher risk, ES infection also has been reported in both normal and immunocompromised adults [10,11]. Currently, the U.S. Centers for Disease Control and Prevention (CDC) estimate that there are approximately six new cases of ES infection diagnosed each year, and approximately 20 deaths in infants and children have been reported to date [12–15]. Although this number may appear small, the implications for those infected are severe, as the mortality rate is between 20–80% [7].
FIG. 1.
Human intestine resected from neonatal infant with Enterobacter-positive blood cultures. (Original magnification ∝40). (A) Hematoxylin and eosin-stained segment from resection margin. (B) Resected ileal segment from same patient showing neonatal enterocolitis. Inflammatory cell infiltrate, villus disruption, and hemorrhage are seen clearly. (C) Normal intestine. (D, E) Neonatal enterocolitis tissue stained with nuclear stain (DAPI) and label (APOTAG Red seen as bright cells in these images) to detect apoptosis. In panels D and E, red-stained tissue shows clear increase in apoptotic cells.
Sentinel Case
The first two known cases of neonatal meningitis caused by ES, classified initially as Enterobacter cloacae, were reported in 1961 [16]. The first reported case of ES infection linked definitively to formula was in a male infant born at 33.5 weeks of gestation [17] who was admitted to the neonatal intensive care unit (NICU) because of prematurity and respiratory distress. Subsequently, the patient was noted to be febrile and tachycardic and to exhibit systemic shock. At 11 days of life, he developed seizure activity, and a culture of his cerebrospinal fluid grew ES. Intravenous antibiotics were administered, but the patient died on day of life 20. Close surveillance of the 49 patients in the NICU at that institution revealed an additional nine cases of ES infection. All nine positive cultures were obtained from non-sterile sites (such as tracheal aspirates, stool, and urine), and therefore could not be confirmed as signs of infection. Putative risk factors, including age, birth weight, medications, incubators used, and need for mechanical ventilation were investigated. A significant association was found between a specific powdered infant formula and the patients with ES-positive cultures. Cultures of the formula found a single batch to be contaminated with ES. Of note, cultures from the NICU environment and water supplies were negative. The manufacturer recalled the batch of formula voluntarily.
Enterobacter sakazakii as an Emerging Pathogen and Food Contaminant
Enterobacter sakazakii was formerly referred to as “yellow-pigmented” E. cloacae, and was characterized as a unique species almost 30 years ago [18]. The organism is ubiquitous in food products, being found in milk powder, rice, vegetables, cheese, sausage meat, teas, and various spices [19–24]. Moreover, ES has been identified in the hospital environment in association with infant bottle brushes and food preparation equipment such as blenders [25]. However, most of the attention to ES-related contamination of food products has focused on powdered infant formula. In 2002, the U.S. Food and Drug Administration (FDA) published a warning regarding the presence of ES in baby formula [26]. In addition, there have been several recalls of formula in Asia, Europe, and North and South America as a result of ES contamination. Powdered infant formula is not manufactured as a sterile preparation, and in a study of 141 formulas, 14% were found to contain ES [27]. In total, ES has been isolated from infant formula in 13 countries, yet remarkably, ES has not been found in environmental soil, wood, water samples, avian droppings, or domestic livestock [27]. Enterobacter sakazakii may exhibit long-term persistence in dried infant formula and has been reported to be the only organism isolated after a 2.5-year period of storage [28].
Most of the attention regarding ES infection has focused on infant populations; however, a number of cases have been reported in adults. Enterobacter sakazakii has been isolated on the floors of geriatric wards [29] and from the mouths of stroke victims with pneumonia [30]. In adults, ES infection typically affects patients of age > 55 years having underlying disease such as malignancy, and there is a high fatality rate from sepsis and pneumonia (50–67%) [7,14]. There are, however, rare case reports of ES infection in young, healthy patients, such as a 22-year-old man with postoperative osteomyelitis of the femur [10]. Enterobacter sakazakii has been cultured from a wide range of infected human fluids and tissues, including blood, cerebrospinal fluid, bone marrow, sputum, urine, intestinal tissue, eye, ear, wounds, and fecal samples [8,18,31]. However, the origin of ES remains mysterious. The presence of ES in infant formula and powdered dairy produce has led manufacturers, hospitals, and the FDA to mandate more stringent microbial standards and hygienic practices [32,33]. Given the ubiquity of ES, efforts have been made to develop precise and efficient detection strategies [34]. A variety of commercially available methods, including proteomics and polymerase chain reaction, aid in the detection of ES [35,36].
Enterobacter sakazakii is Resistant to Routine Sterilization Methods
Enterobacter sakazakii is fairly resistant to osmotic, heat, and dry stresses, which may explain, in part, its presence and survival in desiccated infant powder and similarly prepared products [35,37]. In fact, Nazarowec-White and Farber found that ES was the most thermotolerant among the Enterobacte-riaceae [35]. Although the optimum temperature for ES growth is 39°C, it is reported to grow at less than 4°C, suggesting that this species would be able to replicate even during refrigeration [38]. Furthermore, ES may form biofilms and thereby resist disinfectants [39,40]. Enterobacter sakazakii is able to survive in powder for at least 12 months. The actual amount of ES contamination usually is low, ranging from 0.36 to 66 colony-forming units (CFU)/100 g [27]. However, in dry environmental samples from infant formula factories, a 40% prevalence rate has been reported. As a result of the widespread prevalence of ES in food products and the risks associated with it, reference detection methods, including selective identification techniques and enrichment procedures, have been established to quantify ES in food products [41,42]. Improper storage and temperature regulation may lead to an increase in bacterial load, thus facilitating outbreaks of infection.
Although ES infection may arise secondary to poor storage and reheating of formula, it has not been proved definitively that hospital staff themselves are not a vector for ES. Unfortunately, hospital personnel may ignore appropriate hygiene practices, and thus may contribute to the spread of infection [43]. Therefore, great care should be taken in adhering to strict hand washing and contact isolation of affected and susceptible infants.
Pathogenesis of Enterobacter sakazakii Infection is Relatively Unknown
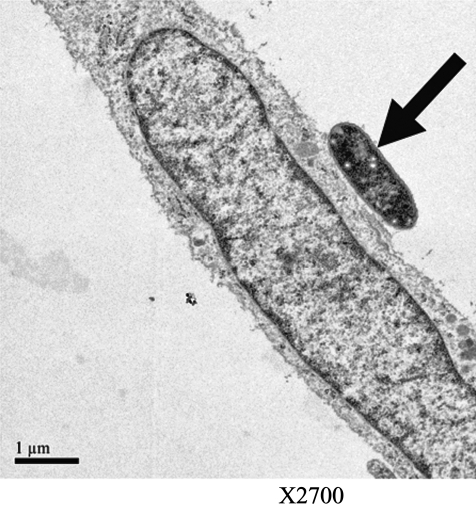
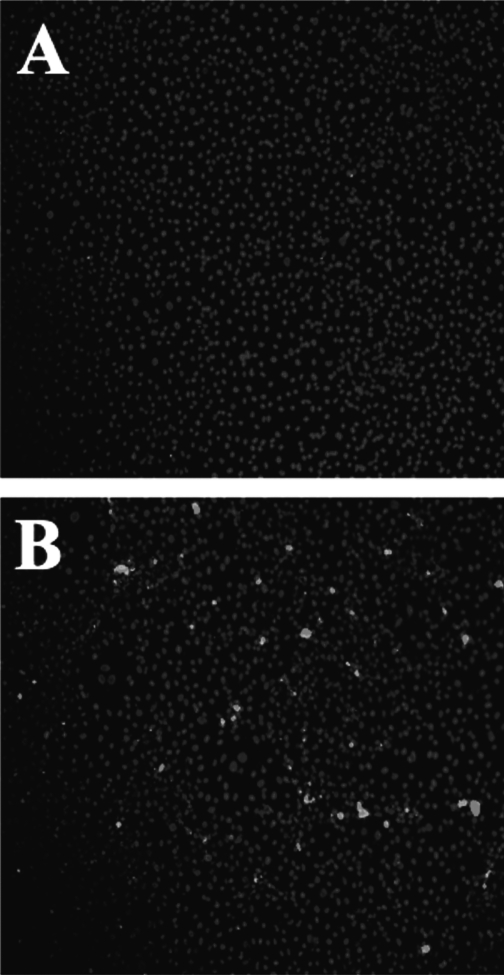
Until recently, little was known about the mechanisms involved in ES pathogenesis. In a review of 17 cases, the outcome of ES meningitis was found to be poorer than in neonatal meningitis caused by more common gram-negative organisms [44]. As with other gram-negative species, ES carries endotoxin on its surface; however, other virulence factors also may be crucial to pathogenicity [45]. In order for a pathogen to cause an infection in a host, it must be able to adhere to and colonize host surfaces. Specific adhesion to host cells is considered to be an essential virulence factor for most bacterial pathogens [46]. In prior studies, strains of opportunistic pathogens have been classified on the basis of their specific adherence pattern to cells in culture, which is described as aggregative or diffuse. Enterobacter sakazakii exhibits clustered adhesion, a pattern that also has been associated with neonatal colitis-causing stains of Klebsiella pneumoniae [47]. Enterobacter sakazakii appears to adhere instantaneously to host surfaces, and then proliferate in a logarithmic manner until an optimal concentration is attained. The adhesion of ES to epithelial cells is mainly non-fimbriaebased, suggesting the role of other virulence factors in binding [47]. Enterobacter sakazakii also produces a viscous capsular material, potentially allowing the organism to form a biofilm on feeding equipment and contact surfaces [38]. This biofilm may provide protection for ES, allowing it to survive osmotic, thermal, and tensile stressors. Mange et al. demonstrated that ES has adhesive capacities to a number of in vitro cell lines, including endothelial and transformed epithelial lines [47]. Similar to many other gram-negative bacteria, ES expresses the outer membrane protein A (ompA) that shows a high degree of homology with ompA genes of other gramnegative bacteria [48]. Singamsetty et al. provided evidence that the ompA protein is crucial in the invasion of brain endothelial cells by ES (unpublished results and personal communication). The organism also induces microtubule condensation at the sites of entry in endothelial cells. However, the mechanism of OmpA-mediated interaction with brain endothelial cells remains to be determined. Similarly, Mohan and Venkitanarayana showed that ES invades moderately into human intestinal epithelial (INT407) cells, for which ompA expression is required [49]. In contrast, our studies revealed that ES does not invade IEC-6 cells efficiently, although the organism binds to the cells in significant numbers. We have demonstrated further, in a rat model of NEC, that ES adheres to the intestinal epithelium during infection [50]. Figure 2 shows the attachment of ES to rat intestinal epithelial cells in vitro. The binding of ES to enterocytes, both in vitro and in the animal model, induces enterocyte apoptosis/necrosis in a dose-dependent manner. Figure 3 illustrates apoptosis of intestinal epithelial cells in vitro after exposure to ES. Studies are under way in our laboratory and others to assess the role of ES in meningitis. Further studies are in progress in our laboratory to evaluate the mechanisms involved in the pathogenesis of NEC by ES, and to develop novel therapeutic strategies.
FIG. 2.
Transmission electron micrograph of rat intestinal epithelial cell (IEC-6) with adherent Enterobacter sakazakii (arrow).
FIG. 3.
Effect of Enterobacter sakazakii (ES) on intestinal epithelial cells in vitro. Confluent rat intestinal epithelial cells stained with nuclear stain (DAPI) and with stain for apoptotic cells (APOTAG red). (A) Control culture not subjected to ES exposure. There are few apoptotic cells. (B) Marked increase in apoptotic cells 3 h after infection with ES. (Seen as bright white stain in these images.)
Treatment of Enterobacter sakazakii Infection
Most infants infected with ES present with sepsis or meningitis, with seizure activity reported in nearly 30% of those affected. After initial resuscitation and prompt administration of antibiotics, a head computed tomography scan should be considered strongly. We believe that these recommendations should be extended to adult patients, especially those who are immunocompromised, who have ES-positive cultures. Intracranial findings that have been described in association with ES, although not exclusive to patients with ES meningitis, include abscess formation, ventricle dilation, infarcts, and hydrocephalus [51].
Enterobacter sakazakii infection typically has been treated with ampicillin and gentamicin. However, many Enterobacter species are resistant to narrow-spectrum penicillins that traditionally have good activity against Enterobacteriaceae such as E. coli (i.e., ampicillin and amoxicillin). As pointed out by Lai et al., increasing resistance of ES to antibiotics should prompt physicians to consider carbapenems (i.e., imipenem-cilastatin, meropenem, or ertapenem) or the newer cephalosporins (e.g. cefepime) in concert with a second agent such as an aminoglycoside [7]. However, as per standard recommendations, minimizing the use of broad-spectrum antibiotics and selecting antimicrobials on the basis of culture and sensitivity results are of paramount importance.
Because most of the data regarding antibiotic susceptibility and resistance profiles of Enterobacter were determined on established species such as E. cloacae, Stock and Wiede-mann studied the specific antibiotic profiles of various ES strains. Interestingly, no natural resistance to cephalosporins was detected in wild-type populations of ES, and these strains appear to lack beta-lactamases. Therefore, ES is the Enterobacter species most susceptible to beta-lactams. Enterobacter sakazakii, typical of most Enterobacteriaceae, has natural resistance to lincosamides, glycopeptides, strep-togramins, and fusidic acids.
Prevention Strategies
A variety of strategies have been suggested to minimize the risk of ES contamination of infant formula, including the use of gamma radiation and ES-targeted bacteriophage therapy to reduce bacterial growth [52,53]. However, simple strategies may be just as effective, including the promotion of breast milk feeding, inclusion of warnings on packages of powdered infant formula that they may be contaminated with ES, and discouraging re-warming of reconstituted formula. In adults, despite a lack of definitive source identification, it would be prudent to limit the use of reconstituted dairy products in immunosuppressed populations. In hospitals, further encouragement and incentives for health care providers to use universal precautions should be mandated, and the “hang time” of enteral feeding solutions should be minimized.
Conclusions
Because of the ubiquitous nature of ES and the mystery surrounding its pathogenesis, preventive measures by parents, infant formula manufacturers, and health care providers will be important in the prevention of ES-related infections. We recommend a focus on simple preventative strategies such as the promotion of breast milk feeding, inclusion of warnings on powdered infant formula packages that they may be contaminated with ES, and abstinence from the practice of re-warming of reconstituted formula. In adults, we believe that reconstituted dairy products should be avoided in immunosuppressed populations. Appropriate barrier precautions should be observed in ICU settings (both adult and neonatal), where spread of infection may be more prevalent. Given increasing reports of Enterobacter antibiotic resistance, we currently recommend the use of carbapenems in suspected ES infection pending final sensitivity reports. Better understanding of the progression and pathogenesis ES-related diseases will help in the development of new modes of prevention for this emerging pathogen.
References
- 1.Simmons BP. Gelfand MS. Haas M, et al. Enterobacter sakazakii infections in neonates associated with intrinsic contamination of a powdered infant formula. Infect Control Hosp Epidemiol. 1989;10:398–401. doi: 10.1086/646060. [DOI] [PubMed] [Google Scholar]
- 2.Biering G. Karlsson S. Clark NC, et al. Three cases of neonatal meningitis caused by Enterobacter sakazakii in powdered milk. J Clin Microbiol. 1989;27:2054–2056. doi: 10.1128/jcm.27.9.2054-2056.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark NC. Hill BC. O'Hara CM, et al. Epidemiologic typing of Enterobacter sakazakii in two neonatal nosocomial outbreaks. Diagn Microbiol Infect Dis. 1990;13:467–472. doi: 10.1016/0732-8893(90)90078-a. [DOI] [PubMed] [Google Scholar]
- 4.Stoll BJ. Epidemiology of necrotizing enterocolitis. Clin Perinatol. 1994;21:205–218. doi: 10.1016/S0095-5108(18)30341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munch R. Robert Koch. Microbes Infect. 2003;5:69–74. doi: 10.1016/s1286-4579(02)00053-9. [DOI] [PubMed] [Google Scholar]
- 6.Hunter CJ. Upperman JS. Ford HR. Camerini V. Understanding the susceptibility of the premature infant to necrotizing enterocolitis (NEC) Pediatr Res. 2008;63:117–123. doi: 10.1203/PDR.0b013e31815ed64c. [DOI] [PubMed] [Google Scholar]
- 7.Lai KK. Enterobacter sakazakii infections among neonates, infants, children, and adults: Case reports and a review of the literature. Medicine. 2001;80:113–122. doi: 10.1097/00005792-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Block C. Peleg O. Minster N, et al. Cluster of neonatal infections in Jerusalem due to unusual biochemical variant of Enterobacter sakazakii. Eur J Clin Microbiol Infect Dis. 2002;21:613–616. doi: 10.1007/s10096-002-0774-5. [DOI] [PubMed] [Google Scholar]
- 9.Chan KL. Saing H. Yung RW, et al. A study of pre-antibiotic bacteriology in 125 patients with necrotizing enterocolitis. Acta Paediatr Suppl. 1994;396:45–48. doi: 10.1111/j.1651-2227.1994.tb13242.x. [DOI] [PubMed] [Google Scholar]
- 10.Corti G. Panunzi I. Losco M. Buzzi R. Postsurgical osteomyelitis caused by Enterobacter sakazakii in a healthy young man. J Chemother. 2007;19:94–96. doi: 10.1179/joc.2007.19.1.94. [DOI] [PubMed] [Google Scholar]
- 11.Pribyl C. Salzer R. Beskin J, et al. Aztreonam in the treatment of serious orthopedic infections. Am J Med. 1985;78(Suppl 2A):51–56. doi: 10.1016/0002-9343(85)90205-0. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Enterobacter sakazakii infections associated with the use of powdered infant formula—Tennessee, 2001. JAMA. 2002;287:2204–2205. [PubMed] [Google Scholar]
- 13.Drudy D. Mullane NR. Quinn T, et al. Enterobacter sakazakii: An emerging pathogen in powdered infant formula. Clin Infect Dis. 2006;42:996–1002. doi: 10.1086/501019. [DOI] [PubMed] [Google Scholar]
- 14.Gurtler JB. Kornacki JL. Beuchat LR. Enterobacter sakazakii: A coliform of increased concern to infant health. Int J Food Microbiol. 2005;104:1–34. doi: 10.1016/j.ijfoodmicro.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Nazarowec-White M. Farber JM. Enterobacter sakazakii: A review. Int J Food Microbiol. 1997;34:103–113. doi: 10.1016/s0168-1605(96)01172-5. [DOI] [PubMed] [Google Scholar]
- 16.Urmenyi AM. Franklin AW. Neonatal death from pigmented coliform infection. Lancet. 1961;1:313–315. doi: 10.1016/s0140-6736(61)91481-7. [DOI] [PubMed] [Google Scholar]
- 17.Enterobacter sakazakii infections associated with the use of powdered infant formula—Tennessee, 2001. MMWR Morb Mortal Wkly Rep. 2002;51:297–300. [PubMed] [Google Scholar]
- 18.Muytjens HL. Zanen HC. Sonderkamp HJ, et al. Analysis of eight cases of neonatal meningitis and sepsis due to Enterobacter sakazakii. J Clin Microbiol. 1983;18:115–120. doi: 10.1128/jcm.18.1.115-120.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanivets VI. Pishchur IN. [Bacterial microflora on disinfected sugar beets] Mikrobiologiia. 2001;70:370–373. [PubMed] [Google Scholar]
- 20.Osterblad M. Pensala O. Peterzens M, et al. Antimicrobial susceptibility of Enterobacteriaceae isolated from vegetables. J Antimicrob Chemother. 1999;43:503–509. doi: 10.1093/jac/43.4.503. [DOI] [PubMed] [Google Scholar]
- 21.Farmer JJ., 3rd Davis BR. Hickman-Brenner FW, et al. Biochemical identification of new species and biogroups of Enterobacteriaceae isolated from clinical specimens. J Clin Microbiol. 1985;21:46–76. doi: 10.1128/jcm.21.1.46-76.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedemann M. Enterobacter sakazakii in food and beverages (other than infant formula and milk powder) Int J Food Microbiol. 2007;116:1–10. doi: 10.1016/j.ijfoodmicro.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 23.Richards GM. Gurtler JB. Beuchat LR. Survival and growth of Enterobacter sakazakii in infant rice cereal reconstituted with water, milk, liquid infant formula, or apple juice. J Appl Microbiol. 2005;99:844–850. doi: 10.1111/j.1365-2672.2005.02656.x. [DOI] [PubMed] [Google Scholar]
- 24.Kandhai MC. Reij MW. Gorris LG, et al. Occurrence of Enterobacter sakazakii in food production environments and households. Lancet. 2004;363:39–40. doi: 10.1016/S0140-6736(03)15169-0. [DOI] [PubMed] [Google Scholar]
- 25.Noriega FR. Kotloff KL. Martin MA. Schwalbe RS. Nosocomial bacteremia caused by Enterobacter sakazakiki and Leuconostoc mesenteroides resulting from extrinsic contamination of infant formula. Pediatr Infect Dis J. 1990;9:447–449. [PubMed] [Google Scholar]
- 26.Administration USFaD. Isolation and enumeration of Enterobacter sakazakii from dehydrated infant formula. www.cfsanfdagov/∼comm/mmesakaz.html www.cfsanfdagov/∼comm/mmesakaz.html
- 27.Muytjens HL. Roelofs-Willemse H. Jaspar GH. Quality of powdered substitutes for breast milk with regard to members of the family Enterobacteriaceae. J Clin Microbiol. 1988;26:743–746. doi: 10.1128/jcm.26.4.743-746.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riedel K. Lehner A. Identification of proteins involved in osmotic stress response in Enterobacter sakazakii by proteomics. Proteomics. 2007;7:1217–1231. doi: 10.1002/pmic.200600536. [DOI] [PubMed] [Google Scholar]
- 29.Masaki H. Asoh N. Tao M, et al. [Detection of gram-negative bacteria in patients and hospital environment at a room in geriatric wards under the infection control against MRSA] Kansenshogaku Zasshi. 2001;75:144–150. doi: 10.11150/kansenshogakuzasshi1970.75.144. [DOI] [PubMed] [Google Scholar]
- 30.Gosney MA. Martin MV. Wright AE. Gallagher M. Enterobacter sakazakii in the mouths of stroke patients and its association with aspiration pneumonia. Eur J Intern Med. 2006;17:185–188. doi: 10.1016/j.ejim.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 31.van Acker J. de Smet F. Muyldermans G, et al. Outbreak of necrotizing enterocolitis associated with Enterobacter sakazakii in powdered milk formula. J Clin Microbiol. 2001;39:293–297. doi: 10.1128/JCM.39.1.293-297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Commission E. Commission regulation (EC) number 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Official J Eur Union. 2005:1–26. [Google Scholar]
- 33.Report of the Thirty-fifth Session of the Codex Committee on Food Hygiene. Orlando, Florida: Alinorm 03/13A; 2003. [Google Scholar]
- 34.Kim K. Jang SS. Kim SK, et al. Prevalence and genetic diversity of Enterobacter sakazakii in ingredients of infant foods. Int J Food Microbiol. 2008;122:196–203. doi: 10.1016/j.ijfoodmicro.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 35.Nazarowec-White M. Farber JM. Thermal resistance of Enterobacter sakazakii in reconstituted dried-infant formula. Lett Appl Microbiol. 1997;24:9–13. doi: 10.1046/j.1472-765x.1997.00328.x. [DOI] [PubMed] [Google Scholar]
- 36.Kang SE. Nam YS. Hong KW. Rapid detection of Enterobacter sakazakii using TaqMan real-time PCR assay. J Microbiol Biotechnol. 2007;17:516–519. [PubMed] [Google Scholar]
- 37.Breeuwer P. Lardeau A. Peterz M. Joosten HM. Desiccation and heat tolerance of Enterobacter sakazakii. J Appl Microbiol. 2003;95:967–973. doi: 10.1046/j.1365-2672.2003.02067.x. [DOI] [PubMed] [Google Scholar]
- 38.Iversen C. Lane M. Forsythe SJ. The growth profile, thermotolerance and biofilm formation of Enterobacter sakazakii grown in infant formula milk. Lett Appl Microbiol. 2004;38:378–382. doi: 10.1111/j.1472-765X.2004.01507.x. [DOI] [PubMed] [Google Scholar]
- 39.Lehner A. Riedel K. Eberl L, et al. Biofilm formation, extracellular polysaccharide production, and cell-to-cell signaling in various Enterobacter sakazakii strains: Aspects promoting environmental persistence. J Food Prot. 2005;68:2287–2294. doi: 10.4315/0362-028x-68.11.2287. [DOI] [PubMed] [Google Scholar]
- 40.Kim H. Ryu JH. Beuchat LR. Effectiveness of disinfectants in killing Enterobacter sakazakii in suspension, dried on the surface of stainless steel, and in a biofilm. Appl Environ Microbiol. 2007;73:1256–1265. doi: 10.1128/AEM.01766-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guillaume-Gentil O. Sonnard V. Kandhai MC, et al. A simple and rapid cultural method for detection of Enterobacter sakazakii in environmental samples. J Food Prot. 2005;68:64–69. doi: 10.4315/0362-028x-68.1.64. [DOI] [PubMed] [Google Scholar]
- 42.Iversen C. Waddington M. Farmer JJ., 3rd Forsythe SJ. The biochemical differentiation of Enterobacter sakazakii genotypes. BMC Microbiol. 2006;6:94. doi: 10.1186/1471-2180-6-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gould DJ. Chudleigh JH. Moralejo D. Drey N. Interventions to improve hand hygiene compliance in patient care. Cochrane Database Syst Rev. 2007;2 doi: 10.1002/14651858.CD005186.pub2. CD005186. [DOI] [PubMed] [Google Scholar]
- 44.Willis J. Robinson JE. Enterobacter sakazakii meningitis in neonates. Pediatr Infect Dis J. 1988;7:196–199. doi: 10.1097/00006454-198803000-00012. [DOI] [PubMed] [Google Scholar]
- 45.Townsend S. Caubilla Barron J. Loc-Carrillo C. Forsythe S. The presence of endotoxin in powdered infant formula milk and the influence of endotoxin and Enterobacter sakazakii on bacterial translocation in the infant rat. Food Microbiol. 2007;24:67–74. doi: 10.1016/j.fm.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Klemm P. Schembri MA. Bacterial adhesins: Function and structure. Int J Med Microbiol. 2000;290:27–35. doi: 10.1016/S1438-4221(00)80102-2. [DOI] [PubMed] [Google Scholar]
- 47.Mange JP. Stephan R. Borel N, et al. Adhesive properties of Enterobacter sakazakii to human epithelial and brain microvascular endothelial cells. BMC Microbiol. 2006;6:58. doi: 10.1186/1471-2180-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohan Nair MK. Venkitanarayanan KS. Cloning and sequencing of the ompA gene of Enterobacter sakazakii and development of an ompA-targeted PCR for rapid detection of Enterobacter sakazakii in infant formula. Appl Environ Microbiol. 2006;72:2539–2546. doi: 10.1128/AEM.72.4.2539-2546.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mohan Nair MK. Venkitanarayanan K. Role of bacterial OmpA and host cytoskeleton in the invasion of human intestinal epithelial cells by Enterobacter sakazakii. Pediatr Res. 2007;62:664–966. doi: 10.1203/PDR.0b013e3181587864. [DOI] [PubMed] [Google Scholar]
- 50.Hunter CJ. Singamsetty VK. Chokshi NK, et al. Enterobacter sakazakii enhances epithelial cell injury by inducing apoptosis in a rat model of necrotizing enterocolitis. J Infect Dis. 2008;198:1–9. doi: 10.1086/590186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burdette JH. Santos C. Enterobacter sakazakii brain abscess in the neonate: The importance of neuroradiologic imaging. Pediatr Radiol. 2000;30:33–34. doi: 10.1007/s002470050009. [DOI] [PubMed] [Google Scholar]
- 52.Lee JW. Oh SH. Kim JH, et al. Gamma radiation sensitivity of Enterobacter sakazakii in dehydrated powdered infant formula. J Food Prot. 2006;69:1434–1437. doi: 10.4315/0362-028x-69.6.1434. [DOI] [PubMed] [Google Scholar]
- 53.Kim KP. Klumpp J. Loessner MJ. Enterobacter sakazakii bacteriophages can prevent bacterial growth in reconstituted infant formula. Int J Food Microbiol. 2007;115:195–203. doi: 10.1016/j.ijfoodmicro.2006.10.029. [DOI] [PubMed] [Google Scholar]