Abstract
Stress-induced hyperalgesia (SIH), a common clinical observation associated with multiple painful diseases including functional urinary disorders, presently has no mechanistic explanation. Using a Footshock treatment, a classical stressor, to magnify physiological responses in a model of urinary bladder pain, we examined one potential group of mediators of SIH, the corticotropin-releasing factor (CRF)-related neuropeptides. Exposure to a Footshock treatment produced bladder hypersensitivity in female Sprague Dawley rats, manifested as significantly more vigorous visceromotor responses (VMRs) to urinary bladder distension (UBD) compared to rats that were exposed to a Non-footshock treatment. This bladder hypersensitivity was significantly attenuated by blocking spinal CRF2 receptors but not CRF1 receptors. Furthermore, spinal administration of urocortin 2, a CRF2 receptor agonist, augmented UBD-evoked VMRs in a way similar to what was observed following exposure to Footshock, an effect significantly attenuated by pretreatment with spinal aSVG30, a CRF2 receptor antagonist. Surprisingly, neither spinal administration of CRF nor the CRF1 receptor antagonist, antalarmin, had an effect on bladder nociceptive responses. The results of the present study not only provide further support for a role of stress in the exacerbation of bladder pain, but also implicate spinal urocortins and their endogenous receptor, the CRF2 receptor, as potential mediators of this effect.
Perspective:
This study presents evidence that spinal urocortins and CRF2 receptors are involved in stress-induced hypersensitivity related to the urinary bladder. This provides a basis for investigating how urocortins mediate SIH, ultimately leading to more effective treatment options for patients suffering from painful bladder syndromes as well as stress-exacerbated chronic pain.
Keywords: Urinary bladder, hypersensitivity, visceral, urocortin, corticotropin-releasing factor
Introduction
A prominent role for stress in the pathophysiology and presentation of multiple clinical pain states has been well-documented 9,11,13,54,64. Acute exposure to numerous stressors can produce an inhibition of cutaneous pain-related responses, a phenomenon referred to as stress-induced analgesia 1,59,63. However, when stress is perceived as uncontrollable, chronic or unpredictable, it may induce long-term pathophysiological changes. Instead of being inhibited, responses to painful stimuli become augmented, a phenomenon known as stress-induced hyperalgesia.
Clinical observations have shown that anxiety and stress may worsen symptoms in functional urinary disorders, such as interstitial cystitis/painful bladder syndrome (IC/PBS) 4,31. This disorder primarily affects the female population and is characterized by pelvic and/or perineal pain, urinary urgency and frequency, and nocturia. A majority of patients report symptom exacerbation by clinical stress, and experimental stress increases bladder pain and urgency in these individuals 24,29. As severity of the disease increases, the relationship between stress and symptom manifestation becomes even more evident 45. Thus, there is clearly a relationship between stress and the exacerbation of bladder pain.
One way in which stress may worsen painful urinary bladder disorders is via mechanisms that involve the corticotropin-releasing factor (CRF) family of peptides. CRF was first characterized for its role in activation of the hypothalamic-pituitary-adrenocortical (HPA) axis, a hallmark of the stress response, and has been subsequently found to modulate autonomic function and behavior 37. The discovery of structurally related peptides in the fish and frog led to the identification of the second CRF-related peptide in mammals, urocortin 1 26,35,57. The CRF family now includes urocortins 1, 2 and 3. CRF binds with high affinity to the CRF1 receptor and with almost no affinity to the CRF2 receptor, despite its name 10,57. Urocortin 1 binds with high affinity to both CRF1 and CRF2 receptors, and urocortins 2 and 3 bind with high affinity to CRF2 receptors with almost no affinity for the CRF1 receptor 27,39,42. The urocortins therefore act as the only identified endogenous agonists for CRF2 receptors at physiological concentrations.
A number of studies indicate that the CRF-related peptides might modulate visceral sensitivity related to stress 12,47,48,49. Systemic administration of CRF1 receptor antagonists has been demonstrated to attenuate visceral perception and anxiety after acute colorectal distension in rats and abolishes restraint stress-induced colonic hypersensitivity 48,49. In humans, peripheral administration of a non-selective CRF receptor antagonist improves gastrointestinal motility, visceral perception and negative mood in response to gut stimulation 47. Intestinal motility, abdominal symptoms and adrenocorticotropic hormone levels are enhanced following systemic administration of CRF; these effects are more exaggerated in individuals with irritable bowel syndrome 12. With regard to the urinary bladder, administration of CRF receptor agonists and antagonists alters cystometric parameters 21,22,38,40, and CRF-related neuropeptides are abundantly expressed in areas involved in the control of micturition (Barrington's nucleus, lumbosacral areas of the spinal cord) 17,25. Following stress or bladder inflammation, increases in CRF receptor expression have been detected in these areas and in the bladder itself 17,25, and nociceptive responses related to the bladder are enhanced 41,44.
These converging lines of evidence implicate CRF-related neuropeptides as potential mediators of stress-induced bladder hyperalgesia. The present studies therefore sought to further investigate the role of CRF receptors in stress-related augmentation of nociceptive reflexes related to the bladder. Based on the pre-existing literature related to the gut, it was expected that CRF1 receptor agonists would be the most likely candidates for involvement. However, this did not prove to be the case.
Materials and Methods
Animal subjects
Female Sprague Dawley rats 11-12 weeks of age (Harlan, Prattville, AL) were used in experiments. Female rats were chosen since disorders of the urinary bladder that are associated with pain are prevalent in and primarily affect the female population. Estrous cycle was not controlled for in these experiments. Food and water were available on an ad libitum basis. A 12:12-h light-dark cycle, where lights were off between 6:00 p.m. and 6:00 a.m., was maintained. There was one week between the time of the animals' arrival and the start of any experimental procedures. Animals were not exposed to any handling or cage habituation prior to the start of experimental procedures. All protocols were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham and adhered to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain.
Footshock Paradigm
Electrical footshock is an established and readily controlled stressor 18,43 that has been used to produce behavioral and neurochemical changes in a variety of experiments. Factors such as timing, predictability, frequency, intensity and duration of exposure to footshock determine the characteristics of the resultant stress response. We chose to use a chronic intermittent footshock paradigm described by Imaki et al. 16, which produced activation of the HPA axis evidenced by upregulation of CRF mRNA in the brain. Rats that received Footshock Treatments were placed in operant conditioning chambers enclosed in sound-attenuating cubicles and received daily intermittent footshock (15 min/day, 1.0 mA, 1s duration, total of 30 shocks each day) administered via a parallel rod floor under a variable-interval schedule for 7 days. Rats in the Non-footshock Treatment groups were treated in an identical manner except they did not receive any footshocks while in the operant conditioning chambers.
Urinary Bladder Distension(UBD)-Evoked Visceromotor Responses (VMRs)
Under mask isoflurane anesthesia (1-3% isoflurane in oxygen), a 22-gauge polytetrafluoroethylene angiocatheter was placed into the bladder via the urethra and held in place by a tight suture around the distal urethral orifice. Electrodes (silver wire) were inserted into the external oblique musculature immediately superior to the inguinal ligament. Following surgery, isoflurane anesthesia was lowered until flexion reflexes were present in the hind limbs, but spontaneous escape behaviors were absent (1-1.25% isoflurane). Urinary bladder distensions (UBDs; 20 sec) were produced by using compressed air and a previously described distension control device 2, and intravesical pressure was monitored using an in-line, low volume pressure transducer. Visceromotor responses (VMRs; contraction of the abdominal and hindlimb musculature), recorded as electromyographical (EMG) activity, was measured via the electrodes using standard differential amplification and rectification and saved on a computer using Spike-2 software and associated hardware (Micro 1401; CED, Cambridge, United Kingdom). Approximately 15 min after initial anesthesia induction, EMG activity to three presentations of 60 mmHg UBD at 3-minute intervals were recorded (data not shown) to overcome a period of bladder sensitization that occurs before demonstration of vigorous and reliable VMRs 7. Responses to graded stimuli (10-60 mmHg; 1 minute intertrial intervals) were then determined. On a given day, VMRs were obtained from the same number of animals in each treatment condition/group to minimize variability.
General Experimental Protocols
Experiment 1: Effect of Footshock Treatments on UBD-evoked VMRs
Rats were exposed to either the Footshock or Non-footshock treatments. Immediately after the final treatment (day 7), rats were anesthetized with isoflurane, and UBD-evoked VMRs were measured as described above.
Experiment 2: Effect of spinal CRF receptor antagonists on Footshock-induced bladder hypersensitivity
Rats received Footshock Treatments as in Experiment 1, and immediately after the final treatment (day 7) were anesthetized with isoflurane (1-3%) for intrathecal (i.t.) catheter placement and subsequent measure of UBD-evoked VMRs. In brief, while anesthetized, rats were mounted in a stereotaxic instrument, and a midline incision was made to expose the atlanto-occipital membrane. A small slit beginning at the base of the skull was made along the midline of the atlanto-occipital membrane. The catheter, a length of polyethylene tubing (PE10), was advanced 7.8 cm into the i.t. space to the S1 level, and the skin incision was sutured to secure the catheter. An intravesical catheter and EMG electrodes were placed as described in Experiment 1 for recording EMG activity to UBD. Antalarmin (24 μg), a selective CRF1 receptor antagonist, anti-sauvagine-30 (aSVG30; 12 μg), a selective CRF2 receptor antagonist, or appropriate vehicle was administered i.t., and 15 min later VMRs to UBD were measured. Evan's Blue (Sigma) was injected into the spinal catheter at the conclusion of the experiment. Spinal cords were removed and examined for dye to verify proper placement of the catheter.
Experiment 3: Effect of CRF receptor agonists/antagonists on UBD-evoked VMRs
Rats were anesthetized with isoflurane for placement of catheters (i.t. and intravesical) and EMG electrodes, as in Experiment 2. In one group of rats, either CRF (CRF1 receptor agonist; 24 μg), urocortin 2 (CRF2 receptor agonist; 12 μg) or vehicle was administered i.t. In a second group of rats, antalarmin (24 μg) was administered i.t. 10 minutes before i.t. delivery of CRF (24 μg). Similarly, in a third group of rats, aSVG30 (12 μg) was administered i.t. 10 minutes prior to i.t. administration of urocortin 2 (12 μg) or vehicle. A fourth group of rats did not have an i.t. catheter placed and instead received intraperitoneal (i.p.) administration of either CRF (24 μg), urocortin 2 (12 μg) or vehicle. In all groups, VMRs to UBD (10-60 mmHg) were measured 15 minutes after i.t. or i.p. administration of the last drug.
Drugs
Isoflurane was obtained from Minrad, Inc. (Bethleham, PA, USA). Antalarmin, CRF, and urocortin 2 were obtained from Sigma (St. Louis, MO, USA). aSVG30 was obtained from Phoenix Pharmaceuticals, Inc. (Belmont, CA, USA). Urocortin 2, CRF, and aSVG30 were dissolved in sterile water, a solution which served as the vehicle in associated experiments. Antalarmin was dissolved in 9% DMSO/sterile water, a solution which served as the vehicle in its associated experiments. Based on previously published studies of bladder function 21,22,36, 6 μg doses of CRF and urocortin 2 were initially chosen for i.t. administration. In preliminary experiments, neither of these agonists affected UBD-induced VMRs; thus doses were increased to 12 μg for the present experiments. Doses of CRF receptor antagonists antalarmin and aSVG30 used in the present experiments are comparable to i.t. doses employed in other studies of bladder function and visceral perception 21,36.
Statistical analysis
Similar to our previous studies 44, EMG activity was quantified as a response (change) score which represents a signal-to-noise ratio. In this case, baseline mean rectified myoelectrical activity measured prior to the presentation of UBD was treated a “noise” (in mV), and the evoked response (the rectified myoelectrical activity during UBD that exceeded the ongoing activity level immediately prior to UBD) represents the “signal” (in mV). Creating a ratio (signal divided by noise) yields a quantified measure of the vigor of the UBD-evoked physiological response that is independent of other measures. The vigor of the response is thereby represented by a signal-to-noise ratio. Low responders (signal to noise ratio <1) were treated statistically as previously described 44 and were equally distributed between groups. All data are presented as group mean ± SEM. Data were analyzed by repeated measures analysis of variance (ANOVA). Post-hoc tests were performed using Fisher's LSD.
Results
Experiment 1: Effect of Footshock Treatment on bladder nociception
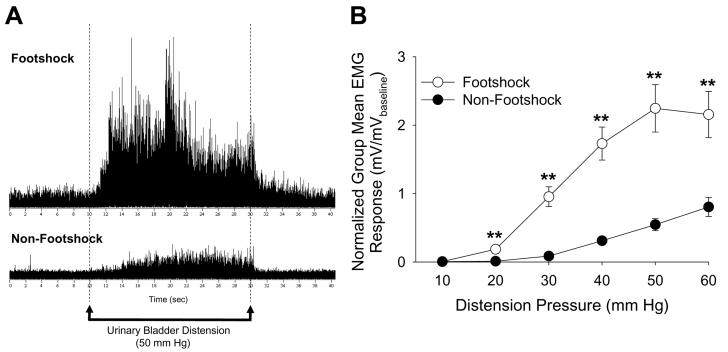
The Footshock Treatment produced bladder hypersensitivity in female Sprague Dawley rats, manifested as significantly more vigorous VMRs to UBD compared to those rats in the Non-footshock Treatment group (F=26.40, p<0.01; ANOVA). Figure 1A shows typical examples of EMG responses to a 50 mmHg distension of the urinary bladder. The upper tracing is from a rat previously exposed to 7 days of Footshock Treatment, while the lower tracing is from a rat exposed to Non-footshock Treatment for 7 days. Post hoc tests revealed significant differences at UBD pressures of 20, 30, 40, 50, and 60 mmHg (Figure 1B).
Figure 1.
A: Typical examples of EMG activity in response to a 50 mmHg urinary bladder distension in a rat exposed to 7 days of the Footshock Treatment (upper) or the Non-Footshock Treatment (lower) for 7 consecutive days. B: Mean data demonstrated that Footshock Treatment rats exhibited significantly greater VMRs to UBD relative to their Non-Footshock Treatment counterparts. ** indicates p<0.01. N=10-11/group.
Experiment 2: Effect of CRF receptor antagonists on Footshock-induced bladder hypersensitivity
Following exposure to 7 days of Footshock Treatment, VMRs of rats treated with i.t. aSVG30, a CRF2 receptor antagonist were significantly lower than VMRs of rats that were treated with i.t. vehicle (Fig. 2A; F=12.43, p=0.002; repeated measures ANOVA). Post hoc tests indicated significant differences at 20, 30, 40, 50, and 60 mmHg. This effect was not observed after i.t. administration of antalarmin, a CRF1 receptor antagonist (Fig. 2B; F=0.191, p=0.34). These data demonstrate that Footshock-induced bladder hypersensitivity can be significantly attenuated by blocking spinal CRF2, but not CRF1, receptors.
Figure 2.
A: Footshock-induced bladder hypersensitivity is significantly attenuated by i.t. administration of a CRF2 receptor antagonist, aSVG30. Following 7 day exposure to the Footshock Treatment, VMRs of rats treated with i.t. aSVG30 were significantly less than VMRs of rats that were given i.t. vehicle. B: This effect was not observed after i.t. administration of antalarmin, a CRF1 receptor antagonist. * indicates p<0.05; ** indicates p<0.01. N=6-8/group.
Experiment 3: Effect of CRF receptor agonists/antagonists on UBD-evoked VMRs
Naïve rats (no Footshock treatments) receiving i.t. administration of the selective CRF2 receptor agonist, urocortin 2, exhibited greater UBD-evoked VMRs compared to those given i.t. CRF (selective CRF1 receptor agonist) or vehicle (Figure 3A; F=9.16, p=0.006 repeated measures ANOVA), and post hoc tests indicated that UBD-evoked VMRs of urocortin 2-treated rats were significantly enhanced at distension pressures of 30, 40, 50, and 60 mmHg. I.p. administration of the same CRF receptor agonists at the same doses given spinally did not significantly affect UBD-evoked VMRs (Fig. 3B; F=0.94, p=0.21) indicating a spinal site of action. Pretreatment with i.t. administration of the CRF2 receptor antagonist, aSVG30, significantly attenuated the augmentation of bladder nociceptive responses induced by i.t. urocortin 2 (Fig. 4A; F=35.43, p<0.01) indicating receptor specificity of the urocortin 2 action. In contrast, i.t. administration of CRF (Fig. 3A; F=0.61, p=0.23) with or without the CRF1 receptor antagonist, antalarmin (Fig. 4B; F=0.24, p=0.32), had any effect on UBD-evoked VMRs.
Figure 3.
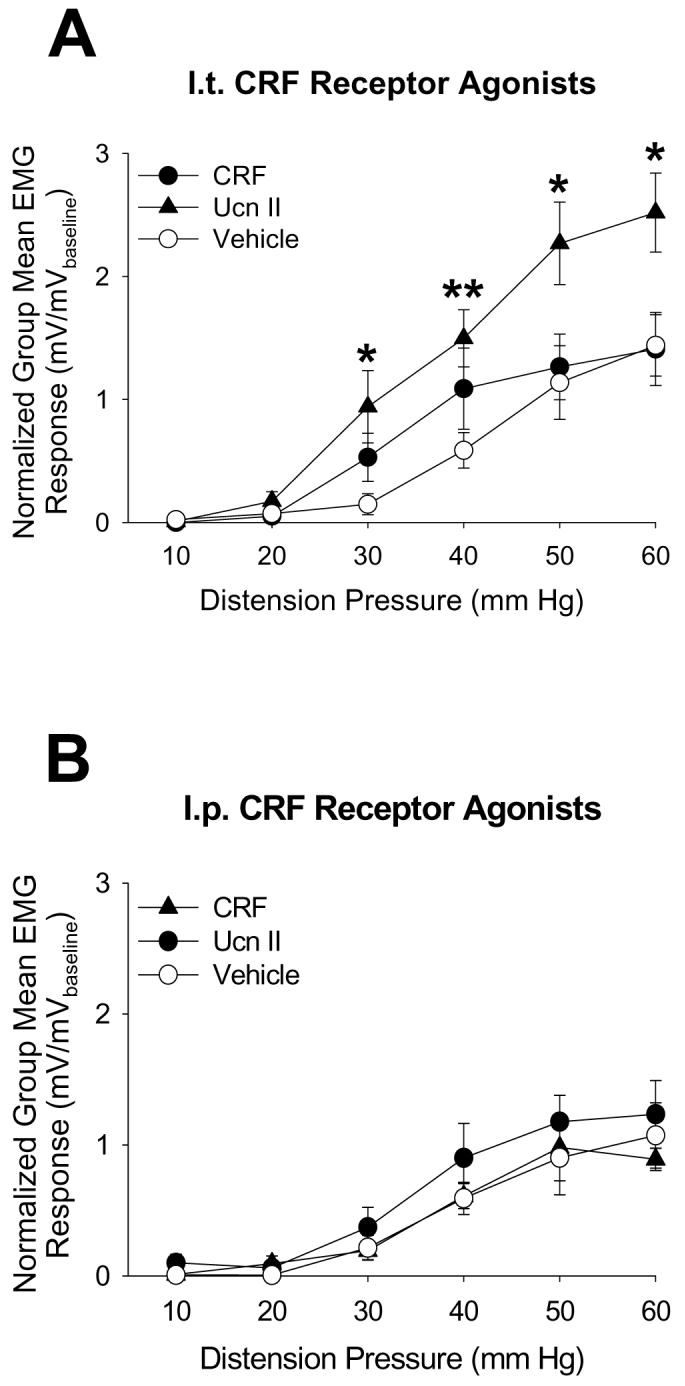
Activation of spinal CRF2 receptors significantly enhances VMRs evoked by UBD. A: I.t. administration of the CRF2 receptor agonist, urocortin 2, but not the CRF1 receptor agonist, CRF, significantly increased bladder nociceptive responses relative to i.t. administration of vehicle (* and ** indicate significantly different from vehicle at p<0.05 and p<0.01, respectively). B: I.p. administration of the same CRF receptor agonists at the same i.t. doses did not affect EMG responses. N=5-8/group
Figure 4.

A: I.t. administration of the CRF2 receptor antagonist, aSVG30, significantly attenuated the augmentation of bladder nociceptive responses induced by urocortin 2 (** indicates significant difference from aSVG30 + urocortin 2 and from aSVG30 + vehicle at p<0.01). B: In contrast, i.t. administration of the CRF1 receptor antagonist, antalarmin, had no effect on UBD-evoked VMRs. N=5-8/group.
Discussion
The present study demonstrates that a 7-day exposure to Footshock Treatments, a well characterized experimental stressor, is capable of producing bladder hypersensitivity as measured by augmented VMRs to UBD. Using this model system, the present study also showed that spinal administration of a selective CRF2 receptor antagonist attenuates this Footshock-induced effect. Furthermore, spinal administration of a CRF2 receptor agonist produces an augmentation of UBD-evoked VMRs, similar to what was observed following Footshock, an effect that was also abolished by pretreatment with a spinal CRF2 receptor antagonist. These converging lines of evidence implicate the CRF-related neuropeptide system as a potential mediator of Footshock-induced hyperalgesia in the urologic system with a specific role for the CRF2 receptor, and its endogenous agonists, the urocortins.
It should be noted that the present study examined both CRF1 and CRF2 receptor effects after the presentation of an environmental stressor. These receptor-mediated effects are therefore envisioned not as anxiolytic effects but rather as representative of spinal mechanisms related to hypersensitivity. The findings of the present study were surprising because pre-existing literature related to visceral sensation suggested a predominant role for the CRF1 receptor. In contrast to the present findings, it appears that CRF, via the CRF1 receptor, is involved in modulation of stress-related functional gastrointestinal disorders such as irritable bowel syndrome (IBS). Colorectal distention (CRD) enhances abdominal EMG activity, induces noradrenaline release in the rat hippocampus, and a causes a significant rise in plasma adrenocorticotropic hormone 36,48. Administration of CRF1 receptor antagonists significantly suppresses all of these CRD-induced effects 14,36>. Anxiety-like behaviors evoked by CRD and stress-induced increases in VMRs to CRD are also significantly attenuated by selective CRF1 receptor antagonists 49. CRF1 receptor-deficient mice have greater response thresholds to CRD 55. Results of human studies provide further support for a role of the CRF1 receptor in IBS. Compared to normal control subjects, individuals with IBS exhibit robust colonic motility, exaggerated colonic motility indices, and enhanced secretion of adrenocorticotropic hormone following exogenous administration of CRF 12. Thus, results of both animal and human studies support a pronociceptive role for the CRF1 receptor, in the pathophysiology of stress-related digestive diseases. In contrast, activation of CRF2 receptors blunts CRD-induced gastrointestinal pain 34,36. Together with the findings of the present study, this suggests that the function of these receptors may be organ-specific instead of applicable to stress-related pain responses in general. Obviously, great differences in methodological specifics are also present between the present study and the previously noted studies of gastrointestinal sensation which include the spinal versus systemic administration of drugs, the use of anesthetics, differing rat strains, the stressors employed and pre- versus post-stressor drug treatments. As such, a precise comparison of studies is not possible.
Several other lines of evidence, in addition to the present findings, implicate the urocortins in the regulation of stress-related physiology. Urocortins are localized to stress-related neuronal groups involved in the integration of afferent signals from the viscera (PVN, supraoptic nucleus, locus coeruleus), and the expression of urocortins in the PVN, supraoptic nucleus and Edinger-Westphal nucleus is upregulated by different stressors 14,52,58,60. Infusion of urocortins into the lateral septum or amygdala increases anxiogenic-like behavior 16,46, and uncontrollable stressors sensitize serotonergic neurons in the dorsal raphe, an effect that is mediated by activation of CRF2 receptors 32. Urocortins are also expressed in immunological tissues, and urocortin 1 has been detected in various immune cells (lymphocytes, mast cells, macrophages, fibroblasts). Stress induces mast cells to synthesize and release both CRF and urocortin 1, and activation of CRF receptors on mast cells potentiates the secretion of other pro-inflammatory mediators 19,24,50,53. This suggests that CRF-related neuropeptides may mediate stress-related inflammatory conditions via enhanced mast cell activation. Indeed, urocortin 1-like immunoreactivity and mRNA are elevated in the synovium of patients with rheumatoid arthritis and in rats with asthma 56,61,62. Inflammatory conditions of the reproductive tract, such as endometriosis, are also associated with elevated urocortin 1-like immunoreactivity, as well as increased numbers of mast cells 20.
With regard to the bladder, central nervous system areas involved in the control of micturition (i.e., Barrington's nucleus, lumbosacral areas of the spinal cord) express CRF-related neuropeptides and administration of nonselective CRF receptor agonists and antagonists alters cystometric parameters 21,22,38,40. These studies found that that the state of arousal profoundly influences the effect of CRF agonists and antagonists on urodynamic function (inhibition versus facilitation) and clearly demonstrated a role for the CRF-related neuropeptides in micturition. Imaki et al. reported that both acute and chronic footshock stress enhanced CRF mRNA expression in Barrington's nucleus 17. Significant increases in CRF mRNA were also observed in the hypothalamus following chronic exposure to footshock. LaBerge et al. demonstrated increases in CRF and CRF2 receptor expression in the lumbosacral spinal cord and in the bladder itself as a result of cyclophosphamide-induced bladder inflammation 25. These converging lines of evidence implicate the CRF-related neuropeptide family, including urocortins, as potential mediators of stress-induced bladder hyperalgesia, and the present studies provide further evidence for CRF2 receptors in stress-related sensory augmentation in the bladder. Doses of CRF and urocortin 2 used in the present study are at least two-fold higher than doses previously reported to affect urodynamic function 21 suggesting that the doses used in these studies should have been adequate to observe CRF1 receptor-related effects.
There remain a number of unanswered questions related to the precise mechanism by which the urocortins and their endogenous receptors mediate urinary bladder hypersensitivity. Our preliminary data demonstrate that the site of action of urocortins is spinal, however the source of these urocortins, whether they are intrinsic to the spinal cord or released from a supraspinal site, is unknown. Intrinsic urocortin 1 and 2 expression have been reported in the spinal cord 5,42, but given that this has been localized predominantly to lamina IX, release from this area is unlikely to activate CRF2 receptors in the dorsal cord. There are a number of supraspinal sites which have spinal projections, including the lateral septum, hypothalamus, Edinger-Westphal nucleus, dorsal raphe, locus coerelus, and amygdala 23,28,33,42, that express urocortin immunoreactivity and thus may release urocortins that activate spinal CRF2 receptors. It remains to be determined which, if any, of these candidate sites contains urocortin-immunoreactive neurons with axonal extensions to the spinal cord.
In summary, while CRF has been extensively studied as a mediator of endocrine, autonomic and behavioral functions, particularly in terms of the stress response, these data suggest that it is not CRF, but rather urocortin 2 and the CRF2 receptor in the spinal cord that is critical to the bladder hypersensitivity related to stress. There is very little known about urocortin 2 in mammalian systems, and a role for this neuropeptide in nociceptive processing is nonexistent, apart from the present work. Additional studies investigating the precise mechanism(s) by which urocortins mediate stress-induced bladder hypersensitivity would prove beneficial, not only as the first step in devising more effective treatment options for patients suffering specifically from painful bladder syndromes, but potentially for individuals with stress-exacerbated chronic pain, in general.
Acknowledgement
This work was supported by DK51413 and 1K99DK080981-01.
References
- 1.Amit Z, Galina ZH. Stress-induced analgesia: adaptive pain suppression. Physiol Rev. 1986;66:1091–1120. doi: 10.1152/physrev.1986.66.4.1091. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RH, Ness TJ, Gebhart GF. A distension control device used for quantitative studies of hollow organ sensation. Physiol Behav. 1988;41:635–638. doi: 10.1016/0031-9384(87)90322-2. [DOI] [PubMed] [Google Scholar]
- 3.Avishai-Eliner S, Brunson KL, Sandman CA. Stressed out, on in (utero)? Trends Neurosci. 2002;25:578–524. doi: 10.1016/s0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldoni F, Ercolani M, Baldaro B, Trombini G. Stressful events and psychological symptoms in patients with functional urinary disorders. Percept Mot Skills. 1995;80:605–606. doi: 10.2466/pms.1995.80.2.605. [DOI] [PubMed] [Google Scholar]
- 5.Bittencourt JC, Vaughn J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- 6.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289:G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 7.Castroman P, Ness TJ. Vigor of visceromotor responses to urinary bladder distension in rats increases with repeated trials and stimulus intensity. Neurosci Lett. 2001;306:97–100. doi: 10.1016/s0304-3940(01)01886-9. [DOI] [PubMed] [Google Scholar]
- 8.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 mRNA expression. J Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dancey CP, Taghavi M, Fox RJ. The relationship between daily stress and symptoms of irritable bowel. J Psychosom Res. 1998;44:537–545. doi: 10.1016/s0022-3999(97)00255-9. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson CJ, Sutton SW, Perrin MH, Corrigan AZ, Lewis KA, Rivier JE, Vaughan JM, Vale WW. Cloning and characterization of human urocortin. Endocrinol. 1996;137:2167–2170. doi: 10.1210/endo.137.5.8612563. [DOI] [PubMed] [Google Scholar]
- 11.Farber EM, Nickoloff BJ, Recht B, Fraki JE. Stress, symmetry and psoriasis: possible role of neuropeptides. Am J Acad Dermatol. 1986;2:305. doi: 10.1016/s0190-9622(86)70034-0. [DOI] [PubMed] [Google Scholar]
- 12.Fukudo S, Nomura T, Hongo M. Impact of corticotropin releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut. 1998;42:845–849. doi: 10.1136/gut.42.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garrett VD, Brantley PJ, Jones GN, McKnight GT. The relation between daily stress and Crohn's disease. J Behav Med. 1991;14:87–96. doi: 10.1007/BF00844770. [DOI] [PubMed] [Google Scholar]
- 14.Greenwood-van Meerveld B, Johnson AC, Cochrane S, Schulkin J, Myers DA. Corticotropin-releasing factor 1 receptor-mediated mechanisms inhibit colonic hypersensitivity in rats. Neurogastroenterol Motil. 2005;17:415–422. doi: 10.1111/j.1365-2982.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- 15.Hara Y, Ueta Y, Isse T, Kabashima N, Shibuya I, Hattori Y, Yamashita Y. Increase of urocortin-like immunoreactivity in the rat hypothalamoneurohypophysial system after salt loading and hypophysectomy. Neurosci Lett. 1997;227:124–130. doi: 10.1016/s0304-3940(97)00327-3. [DOI] [PubMed] [Google Scholar]
- 16.Henry B, Vale W, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imaki T, Nahan JL, Rivier C. Differential regulation of corticotrophin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kant GJ, Mougey EH, Pennington LL, Meyerhoff JL. Graded footshock stress elevates pituitary cyclic AMP and plasma β –endorphin, β-LPH, corticosterone and prolactin. Life Sci. 1983;33:2657–2663. doi: 10.1016/0024-3205(83)90350-8. [DOI] [PubMed] [Google Scholar]
- 19.Kempuraj D, Papadopoulou NG, Lytinas M, Huang M, Kandere-Grzybowska K, Madhappan B, Boucher W, Christodoulou S, Athanassiou A, Theoharides TC. Corticotropin-releasing hormone and its structurally related urocortin are synthesized and secreted by human mast cells. Endocrinol. 2004;145:43–48. doi: 10.1210/en.2003-0805. [DOI] [PubMed] [Google Scholar]
- 20.Kempuraj D, Papadopoulou NG, Stanford EJ, Christodoulou S, Madhappan B, Sant GR, Solage K, Adams T, Theoharides TC. Increased numbers of activated mast cells in endometriosis lesions positive for corticotropin-releasing hormone and urocortin. Am J Reprod Immunol. 2004;52:267–275. doi: 10.1111/j.1600-0897.2004.00224.x. [DOI] [PubMed] [Google Scholar]
- 21.Kidoo DA, Valentino RJ, Zderic S, Ganesh A, Leiser SC, Hale L, Grigoriadis DE. Impact of state of arousal and stress neuropeptides on urodynamic function in freely moving rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1697–R1706. doi: 10.1152/ajpregu.00742.2005. [DOI] [PubMed] [Google Scholar]
- 22.Klausner AP, Streng T, Na YG, Raju J, Batts TW, Tuttle JB, Andersson KE, Steers WD. The role of corticotrophin releasing factor and its antagonist, astressin, on micturition in the rat. Auton Neurosci. 2005;123:26–35. doi: 10.1016/j.autneu.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Kozicz T, Yanaihara H, Arimura A. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J Comp Neurol. 1998;391:1–10. doi: 10.1002/(sici)1096-9861(19980202)391:1<1::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Koziol JA, Clark DC, Gittes RF, Tan EM. The natural history of interstitial cystitis: a survey of 374 patients. J Urol. 1993;149:465–469. doi: 10.1016/s0022-5347(17)36120-7. [DOI] [PubMed] [Google Scholar]
- 25.LaBerge J, Malley SE, Zvarova K, Vizzard MA. Expression of corticotrophin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R692–R703. doi: 10.1152/ajpregu.00086.2006. [DOI] [PubMed] [Google Scholar]
- 26.Ledaris K, Letter A, McMaster D, Moore G, Schlesinger D. Complete amino acid sequence of urotensin I, a hypotensive and corticotrophin-releasing neuropeptide from Catostomus. Science. 1982;218:162–164. doi: 10.1126/science.6981844. [DOI] [PubMed] [Google Scholar]
- 27.Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, Bilezikjian L, Rivier J, Sawchenko PE, Vale WW. Identification of urocortin III, an additional member of the corticotrophin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci USA. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotropin-releasing factor receptor expression. J Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lutgendorf SK, Kreder KJ, Rothrock NE, Ratliff TL, Zimmerman B. Stress and symptomatology in patients with interstitial cystitis: a laboratory stress model. J Urol. 2000;164:1265–1269. [PubMed] [Google Scholar]
- 30.Lytinas M, Kempuraj D, Huang M, Boucher W, Esposito P, Theoharides TC. Acute stress results in skin corticotropin-releasing hormone secretion, mast cell activation and vascular permeability, an effect mimicked by intradermal corticotropin-releasing hormone and inhibited by histamine-1 receptor antagonists. Int Arch Allergy Immunol. 2003;130:224–231. doi: 10.1159/000069516. [DOI] [PubMed] [Google Scholar]
- 31.Macaulay AJ, Stern RS, Holmes DM, Santon SL. Micturition and the mind: psychological factors in the etiology and treatment of urinary symptoms in women. Br Med J. 1987;294:540–543. doi: 10.1136/bmj.294.6571.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Mano-Otagiri A, Shibasaki T. Distribution of urocortin 2 and urocortin 3 in rat brain. J Nippon Med Sch. 2004;71:358–359. doi: 10.1272/jnms.71.358. [DOI] [PubMed] [Google Scholar]
- 34.Million M, Wang L, Stenzel-Poore MP, Coste SC, Yaun PQ, Lamy C, Rivier J, Buffington T, Taché Y. Enhanced pelvic responses to stressors in female CRF-overexpressing mice. Am J Physiol Regul Integr Comp Physiol. 2006;292:R1429–R1438. doi: 10.1152/ajpregu.00626.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montecucchi PC, Henschen A. Amino acid composition and sequence analysis of sauvagine, a new active peptide from the skin of Phyllomedusa sauvagei. Int J Peptide Protein Res. 1981;18:113–120. doi: 10.1111/j.1399-3011.1981.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 36.Nijsen M, Ongenae N, Meukemans A, Coulie B. Divergent role for CRF1 and CRF2 receptors in the modulation of visceral pain. Neurogastroenterol Motil. 2005;17:423–432. doi: 10.1111/j.1365-2982.2005.00644.x. [DOI] [PubMed] [Google Scholar]
- 37.Owens MJ, Nereroff CB. Physiology and pharmacology of corticotrophin-releasing factor. Pharmacol Rev. 1991;43:425–474. [PubMed] [Google Scholar]
- 38.Pavcovich LA, Valentino RJ. Central regulation of micturition in the rat by the corticotrophin-releasing hormone from Barrington's nucleus. Neurosci Lett. 1995;196:185–188. doi: 10.1016/0304-3940(95)11873-u. [DOI] [PubMed] [Google Scholar]
- 39.Perrin MH, Vale WW. Corticotropin releasing factor receptors and their ligand family. Ann NY Acad Sci. 1999;885:312–328. doi: 10.1111/j.1749-6632.1999.tb08687.x. [DOI] [PubMed] [Google Scholar]
- 40.Puder BA, Papka RE. Distribution and origin of corticotrophin-releasing factor-immunoreactive axons in the female rat lumbosacral spinal cord. J Neurosci Res. 2001;66:1217–1225. doi: 10.1002/jnr.10033. [DOI] [PubMed] [Google Scholar]
- 41.Randich A, Uzzell T, Cannon R, Ness TJ. Inflammation and enhanced nociceptive responses to bladder distension produced by intravesical zymosan in the rat. BMC Urol. 2006;6:2–8. doi: 10.1186/1471-2490-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reyes TM, Lewis K, Perrin MH, Kunitake K, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Sawchenko PE, Vale WW. Urocortin II: a member of the corticotrophin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 receptors. Proc Natl Acad Sci USA. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivier C, Vale W. Diminished responsiveness of the hypothalamic-pituitary-adrenal axis of the rat during exposure to prolonged stress: a pituitary-mediated mechanism. Endocrinol. 1987;121:1320–1328. doi: 10.1210/endo-121-4-1320. [DOI] [PubMed] [Google Scholar]
- 44.Robbins MT, DeBerry J, Ness TJ. Chronic psychological stress enhances nociceptive processing in the urinary bladder in high-anxiety rats. Physiol Behav. 2007;91:544–550. doi: 10.1016/j.physbeh.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rothrock NE, Lutgendorf SK, Kreder KJ, Ratliff TL, Zimmerman B. Daily stress and symptom exacerbation in interstitial cystitis patients. Urology. 2001;57:422–427. doi: 10.1016/s0090-4295(01)01075-5. [DOI] [PubMed] [Google Scholar]
- 46.Sajdyk TJ, Schober DA, Gehlert DR, Shekhar A. Role of corticotropin-releasing factor and urocortin within the basolateral amygdala of rats in anxiety and panic responses. Behav Brain Res. 1999;100:207–215. doi: 10.1016/s0166-4328(98)00132-6. [DOI] [PubMed] [Google Scholar]
- 47.Sagami Y, Shimada Y, Tayama J, Nomura T, Satake M, Endo Y, Shoji T, Karahashi K, Hongo M, Fukudo S. Effect of a corticotropin-releasing hormone receptor antagonist on colonic sensory and motor function in patients with irritable bowel syndrome. Gut. 2004;53:958–964. doi: 10.1136/gut.2003.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saito K, Kasai T, Nagura Y, Ito H, Kanazawa M, Fukudo S. Corticotropin-releasing hormone receptor-1 antagonist blocks brain-gut activation induced by colonic distension in rats. Gastroenterol. 2005;129:1533–1543. doi: 10.1053/j.gastro.2005.07.053. [DOI] [PubMed] [Google Scholar]
- 49.Schwetz I, Bradesi S, McRoberts JA, Sablad M, Miller JC, Zhou H, Ohning G, Mayer EA. Delayed stress-induced colonic hypersensitivity in male Wistar rats: role of neurokinin-1 and corticotropin-releasing factor-1 receptors. Am J Physiol Gastrointest Liver Physiol. 2004;286:G683–G691. doi: 10.1152/ajpgi.00358.2003. [DOI] [PubMed] [Google Scholar]
- 50.Singh LK, Boucher W, Pang X, Letourneau R, Seretakis D, Green M, Theoharides TC. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of corticotropin-releasing hormone receptors. J Pharmacol Exp Ther. 1999;288:1349–1356. [PubMed] [Google Scholar]
- 51.Taché Y, Martinez V, Million M, Rivier J. Corticotropin-releasing factor and the brain-gut motor response to stress. Can J Gastroenterol. 1999;13:18A–25A. doi: 10.1155/1999/375916. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka Y, Makino S, Noguchi T, Tamura K, Kaneda T, Hashimoto K. Effects of stress and adrenalectomy on urocortin II mRNA expression in the hypothalamic paraventricular nucleus of the rat. Neuroendocrinology. 2003;78:1–11. doi: 10.1159/000071700. [DOI] [PubMed] [Google Scholar]
- 53.Theoharides TC, Donelan JM, Papadopoulou N, Cao J, Kempuraj D, Conti P. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563–568. doi: 10.1016/j.tips.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Thomason BT, Brantley PJ, Jones GN, Dyer HR, Morris JL. The relation between stress and disease activity in rheumatoid arthritis. J Behav Med. 1992;15:215–220. doi: 10.1007/BF00848326. [DOI] [PubMed] [Google Scholar]
- 55.Trimble N, Johnson AC, Foster A, Greenwood van-Meerveld B. Corticotropin-releasing factor receptor 1-deficient mice show decreased anxiety and colonic sensitivity. Neurogastrentrol Motil. 2007;19:754–760. doi: 10.1111/j.1365-2982.2007.00951.x. [DOI] [PubMed] [Google Scholar]
- 56.Uzuki M, Sasano H, Muramatsu Y, Totsune K, Takahashi K, Oki Y, Iino K, Sawai T. Urocortin in the synovial tissue of patients with rheumatoid arthritis. Clin Sci. 2001;100:577–589. [PubMed] [Google Scholar]
- 57.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, Rivier J, Sawchenko PE, Vale WW. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotrophin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 58.Venihaki M, Sakihara S, Subraminian S, Dikkes P, Weninger SC, Liapakis G, Graf T, Majzoub JA. Urocortin III, a brain neuropeptide of the corticotropin-releasing hormone family: modulation by stress and attenuation of some anxiety-like behaviors. J Neuroendocrinol. 2004;16:411–422. doi: 10.1111/j.1365-2826.2004.01170.x. [DOI] [PubMed] [Google Scholar]
- 59.Watkins LR, Cobelli DA, Mayer DJ. Opiate vs. non-opiate footshock induced analgesia (FSIA): descending and intraspinal components. Brain Res. 1982;245:97–106. doi: 10.1016/0006-8993(82)90342-0. [DOI] [PubMed] [Google Scholar]
- 60.Weninger SC, Peters LL, Majzoub JA. Urocortin expression in the Edinger-Westphal nucleus is up-regulated by stress and corticotropin-releasing hormone deficiency. Endocrinol. 2000;141:256–263. doi: 10.1210/endo.141.1.7277. [DOI] [PubMed] [Google Scholar]
- 61.Wu Y, Xu Y, Zhou H, Tao J, Li S. Expression of urocortin in rat lung and its effect on pulmonary vascular permeability. J Endocrinol. 2006;189:167–178. doi: 10.1677/joe.1.06607. [DOI] [PubMed] [Google Scholar]
- 62.Wu Y, Zhou H, Xu Y, Li S. Enhanced expression of urocortin in lung tissues of rats with allergic asthma. Biochem Biophys Res Commun. 2006;341:532–540. doi: 10.1016/j.bbrc.2005.12.214. [DOI] [PubMed] [Google Scholar]
- 63.Young E, Akil H. Changes in releasibility of ACTH and beta-endorphin with chronic stress. Neuropep. 1985;5:545–548. doi: 10.1016/0143-4179(85)90075-7. [DOI] [PubMed] [Google Scholar]
- 64.Zautra AJ, Hoffman J, Potter P, Matt KS, Yocum D, Castro L. Examination of changes in interpersonal stress as a factor in disease exacerbations among women with rheumatoid arthritis. Ann Behav Med. 1997;19:279–286. doi: 10.1007/BF02892292. [DOI] [PubMed] [Google Scholar]