Summary
Objectives
Methicillin-resistant Staphylococcus aureus (MRSA) infections can be difficult to treat. We evaluated the rate of clinical failure in appropriately-treated patients and determined risk factors for failure.
Methods
We retrospectively studied a cohort of patients with invasive MRSA infections who completed recommended therapy at one hospital over a seven year period.
Results
Two-hundred and fifteen cases were included. Vancomycin monotherapy was given in 73%. Failure rates by infection site were as follows: osteomyelitis 37/81 (46%), epidural abscess 5/18 (28%), surgical wound 4/15 (27%), pneumonia 8/45 (18%), endocarditis 5/32 (16%), bloodstream 5/42 (12%), joint 1/23 (4%), and meningitis 0/1 (0%). In multivariate analysis, only a diagnosis of osteomyelitis was independently associated with relapse (p<0.001).
Conclusions
We found a high rate of treatment failure in an urban population among patients who completed recommended therapy, largely with vancomycin alone. Failure in osteomyelitis was particularly common. High quality comparative studies of antibiotic regimens for MRSA infections, particularly osteomyelitis, are needed.
Keywords: Staphylococcus aureus, methicillin resistance, vancomycin, osteomyelitis
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) infections can be difficult to treat. Clinical failures, even with appropriate treatment, occur regularly. Vancomycin is currently the first-line agent for treating invasive MRSA infections, but this role has been called into question [1], in part because vancomycin has poorer tissue penetration and is less bactericidal than the anti-staphylococcal penicillins [2]. Vancomycin monotherapy has demonstrated inferiority to beta-lactam therapy in the treatment of methicillin sensitive S. aureus osteomyelitis [3] and bacteraemia [4] [5] [6].
Some clinical failures among MRSA infections treated with vancomycin may result from decreased susceptibility to the drug. MRSA strains with intermediate susceptibility to vancomycin (VISA; vancomycin minimum inhibitory concentration [MIC] = 4 – 8 mcg/mL) and strains with frank resistance to vancomycin (VRSA; MIC ≥ 16 mcg/mL) remain uncommon. However, some portion of vancomycin failures may be due to unrecognized heterogeneously resistant S. aureus (hVISA) [2]. Heteroresistance does not correlate well with MIC, and is thus not readily detected by standard clinical laboratory methods [7]. Furthermore, infections due to MRSA which are still considered susceptible to vancomycin but have MICs at the higher end of the susceptible range (MIC = 2) are at higher risk for clinical failure [7].
Apart from the intrinsic pharmacologic properties of vancomycin and emerging resistance, other factors may affect MRSA treatment failure rates. These include the site of infection, characteristics of the patient, antibiotic dosing and serum drug concentrations, and the presence and quality of concomitant surgical therapy where indicated. Such factors, however, are not well studied.
The rate of clinical failure is similarly undefined. Daver and colleagues reported a 35% relapse rate among 37 patients with MRSA osteomyelitis [8]. Gelfand and coworkers reported a case series of five patients with MRSA vertebral osteomyelitis who showed no change in the progression of osteomyelitis after treatment despite receiving prolonged courses of vancomycin monotherapy [9]. These small studies suggest relatively poor outcomes in the treatment of MRSA osteomyelitis, and prior anecdotal experience at our institution supports this. In order to further define rates of treatment failure of MRSA infections and to elucidate factors associated with failure, we studied the epidemiology of clinical failures among patients with MRSA infection who completed appropriate antibiotic therapy at an urban, public hospital.
Methods
Case identification
We retrospectively identified a cohort of patients with culture-proven MRSA infections who completed recommended therapy at San Francisco General Hospital over a seven year period (January 1st, 1998 through December 31st, 2004). Microbiology laboratory records were used to identify the medical records of all patients who had a culture positive for MRSA during the identified time period. All duplicate medical records were examined and only the first incidence of MRSA infection during the time period for each patient was analyzed.
Cases were excluded if the infection was not invasive, if urgent or emergent surgical intervention was the primary therapy indicated (necrotizing fasciitis and abscesses), if therapy was not adequately described, if the patient was under 18 years of age, or if the positive culture was not treated as an infection. Both community-acquired and healthcare-associated cases were included, and we did not attempt to distinguish between the two.
Data collection
Data on the following characteristics were collected: age, race, housing status (homeless, incarcerated, or housed/marginally housed), comorbid illnesses, type and route of substance use, sites of infection, dates of positive cultures, type and duration of antibiotics used, dates of treatment, vancomycin trough levels, locations of treatment [inpatient ward, intensive care unit (ICU), skilled nursing facility, outpatient], and treating service.
Definition of site of infection
Bloodstream infections were those cases in which MRSA was cultured from the blood, but in which the patient did not have evidence of endocarditis, joint infection, or osteomyelitis. Surgical wound infections were those that appeared to be superficial infections and were not known to be associated with underlying joint infection or osteomyelitis.
Definition of appropriate treatment
Appropriate treatment was defined as intravenous treatment with an antibiotic to which the infecting bacterial strain was susceptible for at least one week in pneumonia, two weeks in bloodstream infections, four weeks in endocarditis, epidural abscess, and joint infection; and six weeks in osteomyelitis [10]. In cases of bloodstream infection, removal of intravascular catheters was considered a necessary part of appropriate treatment and was therefore required for inclusion. Achievement of a designated vancomycin trough level was not included in our definition of appropriate therapy since the measurement of these levels was not standardized.
In order to judge whether the patient completed therapy, we reviewed the visit history of the patient in the health system computer records. Patients at San Francisco General Hospital who require prolonged courses of anti-staphylococcal antibiotics almost all complete treatment either as an inpatient in the hospital, in a skilled nursing facility within the hospital, in an affiliated skilled nursing facility outside of the hospital that shares our medical records visit history system, or as an outpatient in the infusion clinic. The dates of treatment at all of the above facilities can be reviewed in the computer records. When available, we reviewed notes from subsequent in- and outpatient visits to confirm that adequate therapy was completed. Patients in whom treatment was interrupted for less than 48 hours during vancomycin therapy were included in the analysis. Cases in which the patient died during the inpatient course of treatment were excluded.
Definition of outcomes
Outcomes were coded as ‘failure’, ‘non-failure’, or ‘uncertain’. Failures were patients who had culture, radiographic, or clinical evidence of infection within 60 days of the completion of antibiotic therapy as well as those patients who were treated again for an MRSA infection at the same site as the original infection (except in endocarditis as described below). Non-failures included definitive cures, patients with persistently positive cultures from non-sterile sites who were not treated for a relapsed infection (such as persistently positive tracheal aspirate and sputum cultures). Patients who did not return for follow-up after documented completion of therapy were also considered non-failures in order to avoid overestimation of the failure rate. Patients with a repeat diagnosis of endocarditis in the setting of ongoing injection drug use were coded as ‘uncertain’, since we could not distinguish between relapse and re-infection in these cases. For the multivariate analyses, ‘uncertain’ cases were included in the ‘non-failure’ group.
Statistical analysis
Data were analyzed using STATA version 9.0. The outcome measure was analyzed as a dichotomous variable (failure or non-failure). The ‘uncertain’ cases were included as ‘non-failures’ for the analyses. Independent variables were analyzed as categorical variables (ages were grouped into quartiles and antibiotic therapy was coded as monotherapy or combination therapy), with the exception of vancomycin trough level, which was analyzed as a continuous variable. The vancomycin trough level was a simple average of the measured levels, excluding those >25 µg/mL. Bivariate analysis with Pearson’s chi-square test was used to compare each independent variable with the outcome measure, except for vancomycin trough levels, which were compared with simple linear regression. All variables found to be significantly associated with treatment failure in the bivariate analysis were then included in the multivariate model. Logistic regression was used to identify which factors were independently associated with clinical failures. All variables that were significantly associated with the outcome in bivariate analysis were included in the initial model. Those variables for which the p-value of the beta coefficient was >0.05 were then dropped from the model and a Wald test was performed to confirm whether those variables could be permanently dropped from the model.
Results
Study population
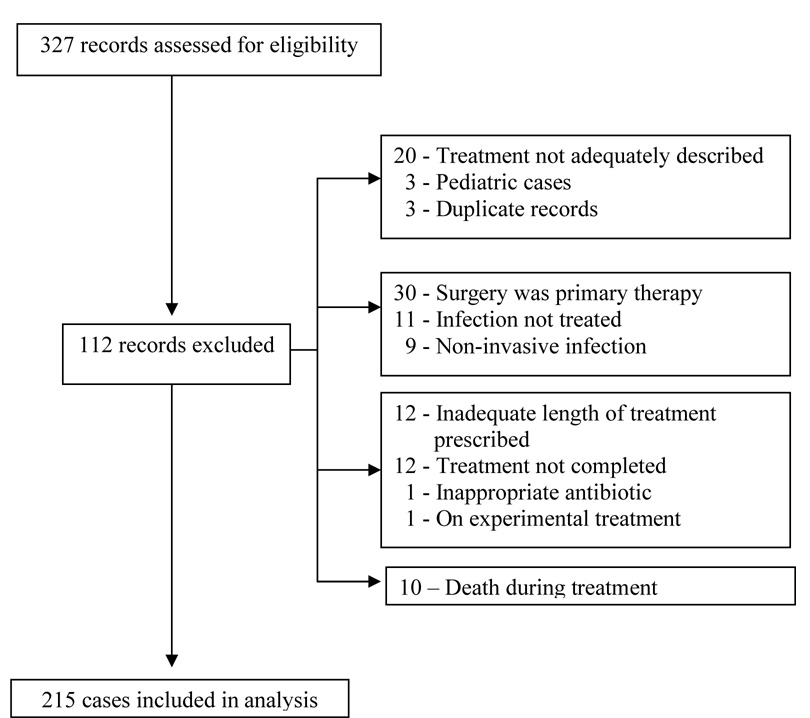
Three hundred and twenty-seven records were identified from the laboratory database, and 215 cases were included in the final analysis. One case was excluded post-hoc because the patient was enrolled in a study of a novel antibiotic. The figure shows the flow of case selection.
Figure 1.
Flow Diagram of Case Selection
Patient and treatment characteristics are summarized in Table 1. The cohort was primarily male (72%). The most common comorbid illnesses were diabetes mellitus (19%) and HIV infection (14%). Approximately one-third (34%) of the cohort was homeless, and 46% were identified as injection drug users. The majority of patients (77%) were not in the intensive care unit at the time of antibiotic initiation.
Table 1.
Patient and treatment characteristics
| Variable | Patients (n=215) |
|---|---|
| Male | 154 (72) |
| Mean age (years) (SD) | 45 (13) |
| Race | |
| White | 93 (43) |
| Black | 78 (36) |
| Hispanic | 26 (12) |
| Asian/Pacific Islander | 14 (6) |
| Native American | 4 (2) |
| Comorbidities | |
| Diabetes mellitus | 40 (19) |
| HIV infection | 30 (14) |
| COPD | 16 ( 7) |
| Renal Failure | 8 ( 4) |
| Cancer | 9 ( 4) |
| Homeless | 72 (34) |
| Substance abuse | 154 (75) |
| Injection drugs | 99 (46) |
| Alcohol | 97 (45) |
| Tobacco | 55 (26) |
| Non-injected cocaine | 34 (16) |
| Intensive care unita | 48 (23) |
| Service at initial therapy | |
| Surgery | 114 (53) |
| Medicine | 94 (44) |
| Other | 6 ( 3) |
NOTE. Data are no. (%) of patients or mean (standard deviation). COPD, chronic obstructive pulmonary disease
At the time of antibiotic initiation
One-quarter of patients had infections at more than one site. The distribution of infections by site was as follows: 81 osteomyelitis (38%), 45 pneumonia (21%), 42 bloodstream without endocarditis (20%), 32 endocarditis (15%), 23 joint (11%), 18 epidural abscesses (8%), 15 surgical wound infections (7%), and 1 meningitis (0.5%).
Treatment
Vancomycin monotherapy was the most common antibiotic regimen used (73%). In the remaining cases, vancomycin was used in combination with another antibiotic, most commonly rifampin (13%) or gentamicin (8%). One hundred and seventy six patients (82%) had at least one vancomycin trough level measured, with an average level of 11.6 µg/mL (SD 4.0 µg/mL). Among osteomyelitis cases, 75 patients (93%) had trough levels measured, and the average level was also 11.6 µg/mL (SD 3.7 µg/mL).
Thirty osteomyelitis cases (38%) were hardware-associated, and in 27 (90%) of those, the hardware was removed. Among those without hardware, 35 (70%) underwent debridement.
Failure rates
The overall failure rate (by patient) was 25%. Eleven cases (5%) were coded as ‘uncertain’ outcome. Nine of these were cases with recurrent endocarditis in the setting of ongoing drug use (as described in the Methods section). The others comprised a patient who died shortly after completion of treatment for MRSA pneumonia of uncertain cause and a patient with recurrent knee pain after completion of treatment for septic arthritis who declined arthrocentesis at the time of pain recurrence.
Failure rates by infection site were as follows (Table 2): osteomyelitis 37/81 (46%), epidural abscess 5/18 (28%), surgical wound 4/15 (27%), pneumonia 8/45 (18%), endocarditis 5/32 (16%), bloodstream 5/42 (12%), joint 1/23 (4%), and meningitis 0/1 (0%). Five cases had both epidural abscess and osteomyelitis, which were each counted in both categories for the analysis by infection site.
Table 2.
Treatment and Outcome by Site of Infection
| Site | Total | Failure (%) | Treatment Duration (days) | Monotherapy (%) |
|---|---|---|---|---|
| Osteomyelitis | 81 | 37 (46) | 42.9 (SD 5.1) | 55 (68) |
| Bloodstream (without endocarditis) | 42 | 5 (12) | 25.8 (14.5) | 32 (76) |
| Pneumonia | 45 | 8 (18) | 24.3 (14.8) | 36 (80) |
| Endocarditis | 32 | 5 (16) | 37.4 (8.4) | 19 (59) |
| Joint | 23 | 1 (4) | 39 (5.7) | 19 (83) |
| Epidural abscess | 18 | 5 (28) | 40.1 (3.6) | 12 (67) |
| Surgical site | 15 | 4 (27) | 34.6 (9.2) | 13 (87) |
| Meningitis | 1 | 0 (0) | 42 (0) | 1 (100) |
| Overall (by patient)a | 215 | 53 (23) | see note | 157 (73) |
NOTE. Data not included since treatment duration was based primarily on site of infection
25% of patients had infections at more than one site.
Failure risk factors
The following factors were associated with an increased risk of failure at a p-value of <0.05 in bivariate analysis: diabetes [OR for failure (95%CI): 2.9 (1.4–5.9)], admission to the orthopedics service [3.7 (1.8–7.4)], osteomyelitis [6.2 (3.1–12.3)], and hospitalization in the intensive care unit at the time of antibiotic initiation [2.9 (1.2–6.9)]. Pneumonia [0.3 (0.1–0.9)] and endocarditis [0.8 (0.01–0.6)] were associated with decreased risk of failure.
In multivariate analysis, only a diagnosis of osteomyelitis was independently associatedwith failure (p<0.001). Among osteomyelitis cases, the presence of hardware was not associated with failure [OR (95%CI): 1.0 (0.4–2.4)], nor was surgical debridement [OR (95%CI): 0.9 (0.5–1.7)]. Vancomycin monotherapy, treatment duration, and vancomycin trough levels were not associated with failure.
Discussion
This study documents a high rate of treatment failure among patients with invasive MRSA infections at an urban hospital who completed recommended therapy, largely with vancomycin alone. Failure in cases of osteomyelitis was particularly common (46%).
The high rate of treatment failure in osteomyelitis that we report is likely due in part to the intrinsic difficulty of treating osteomyelitis. Tice and coworkers reported a failure rate of 30.6% among 454 patients with osteomyelitis due to various bacterial species who completed outpatient intravenous antimicrobial therapy [3]. Daver and colleagues recently reported a failure rate of 35% in patients with MRSA osteomyelitis treated with either ≥4 weeks of parenteral antibiotics or <4 weeks of parenteral therapy, followed by oral therapy, with no difference in the relapse rates between the two groups [8]. The failure rate we report is comparable to that reported in the latter study. The removal of surgical hardware in cases of hardware-associated osteomyelitis is an established cornerstone of therapy. The lack of association between hardware removal and clinical failure in our study may reflect a limitation of the model.
The substantial failure rate in cases of MRSA osteomyelitis that we found may also indicate poor performance of vancomycin in bone. Vancomycin has higher penetration into infected bone than non-infected bone, but it may function more poorly in the more anaerobic environment of osteomyelitic bone [11]. We were not able to analyze vancomycin in comparison to other antibiotics since all of the patients in our study received vancomycin and only a minority received combination therapy with a heterogeneous group of drugs.
The average vancomycin trough level among cases of osteomyelitis in our study (11.6 µg/mL) is lower than the range of 15–20 µg/mL that is currently the standard target (but was not during the period of our study). Recent guidelines from the American Thoracic Society and the Infectious Diseases Society of America recommend targeting vancomycin trough levels of 15 to 20 ug/ml in the treatment of MRSA pneumonia [12]. Although there are no definitive data to support the practice, many clinicians now dose vancomycin to obtain a trough level of 15 to 20 in osteomyelitis and other invasive MRSA infections.
This single-center, retrospective study has several limitations. During the time period of the study, the microbiology laboratory at our hospital, like many others, reported only whether S. aureus was "sensitive" to vancomycin, not the actual MIC. Therefore, it could not be determined whether higher MICs that were still within the susceptible range were associated with treatment failure. Furthermore, The Antimicrobial Susceptibility Testing subcommittee of the Clinical and Laboratory Standards Institute changed the MIC interpretive criteria for breakpoints of vancomycin against S. aureus in January of 2006 [13], after the period of our study. Undetected heteroresistant strains of S. aureus may have been present among the cases we studied. This seems unlikely to be the primary reason behind the failures we observed, however, because the prevalence of h-VISA at San Francisco General Hospital was less then 1.4% in one study of patients on dialysis [14].
Our study did not examine the adequacy of medical and surgical therapy for MRSA infections other than the antibiotic regimen given, removal of catheters in cases of bloodstream infections, and the presence or absence of surgical therapy in bone and joint infections. It is conceivable that other aspects of the medical and surgical treatment could be responsible for a portion of the failure rate. The retrospective design of our study precluded an assessment of the adequacy of surgical therapy in cases of bone and joint infections.
Regimens other than vancomycin monotherapy and/or longer duration of monotherapy may be more appropriate for treatment of MRSA osteomyelitis. In animal models of S. aureus osteomyelitis, vancomycin monotherapy has demonstrated inferiority to moxifloxacin [15], tigecycline [16], and rifampin-vancomycin combination therapy [16]. Linezolid was found to be superior to vancomycin in a study of complicated skin and soft tissue infections [17], and daptomycin was at least as effective as vancomycin in the treatment of MRSA bacteraemia and right-sided endocarditis [18]. In patients with infections caused by MRSA with MIC ≥1.5 who received vancomycin in combination with linezolid, daptomycin and/or rifampin, end of treatment responses were higher than in those who received vancomycin monotherapy [19]. Higher doses of vancomycin (40 mg/kg/day) may also be superior to standard dosing [20].
The recommendations for antibiotic treatment of MRSA osteomyelitis at our hospital were recently updated. Initial recommended therapy is now six weeks of parenteral vancomycin plus rifampin, vancomycin plus clindamycin, or oral linezolid, followed by two to three months of consolidation therapy with rifampin plus levofloxacin, clindamycin, trimethoprimsulfamethoxazole, or doxycycline (or if linezolid was used initially, continued linezolid). There are currently no clinical data to support these recommendations, however, and their impact will be monitored prospectively.
In summary, this study showed a high rate of treatment failure in an urban population among patients who completed recommended therapy, largely with vancomycin alone. Failure in osteomyelitis was particularly common. High quality comparative studies of antibiotic regimens, including varying duration and combination therapy, are needed.
Acknowledgements
Financial support. This work was carried out with assistance from the General Clinical Research Center at San Francisco General Hospital with support from Grant 5-MO1-RR00083 from the Division of Research Resources, National Institutes of Health. The study sponsor had no role in the study design, in the collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflict of interest. None
References
- 1.Stevens DL. The role of vancomycin in the treatment paradigm. Clin Infect Dis. 2006 Jan 1;42 Suppl 1:S51–S57. doi: 10.1086/491714. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Chambers HF. Staphylococcus aureus with heterogeneous resistance to vancomycin: epidemiology, clinical significance, and critical assessment of diagnostic methods. Antimicrobial agents and chemotherapy. 2003 Oct;47(10):3040–3045. doi: 10.1128/AAC.47.10.3040-3045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tice AD, Hoaglund PA, Shoultz DA. Risk factors and treatment outcomes in osteomyelitis. The Journal of antimicrobial chemotherapy. 2003 May;51(5):1261–1268. doi: 10.1093/jac/dkg186. [DOI] [PubMed] [Google Scholar]
- 4.Chang FY, Peacock JE, Jr., Musher DM, Triplett P, MacDonald BB, Mylotte JM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine. 2003 Sep;82(5):333–339. doi: 10.1097/01.md.0000091184.93122.09. [DOI] [PubMed] [Google Scholar]
- 5.Siegman-Igra Y, Reich P, Orni-Wasserlauf R, Schwartz D, Giladi M. The role of vancomycin in the persistence or recurrence of Staphylococcus aureus bacteraemia. Scandinavian journal of infectious diseases. 2005;37(8):572–578. doi: 10.1080/00365540510038488. [DOI] [PubMed] [Google Scholar]
- 6.Stryjewski ME, Szczech LA, Benjamin DK, Jr., Inrig JK, Kanafani ZA, Engemann JJ, et al. Use of vancomycin or first-generation cephalosporins for the treatment of hemodialysis-dependent patients with methicillin-susceptible Staphylococcus aureus bacteremia. Clin Infect Dis. 2007 Jan 15;44(2):190–196. doi: 10.1086/510386. [DOI] [PubMed] [Google Scholar]
- 7.Tenover FC, Moellering RC., Jr. The rationale for revising the Clinical and Laboratory Standards Institute vancomycin minimal inhibitory concentration interpretive criteria for Staphylococcus aureus. Clin Infect Dis. 2007 May 1;44(9):1208–1215. doi: 10.1086/513203. [DOI] [PubMed] [Google Scholar]
- 8.Daver NG, Shelburne SA, Atmar RL, Giordano TP, Stager CE, Reitman CA, et al. Oral step-down therapy is comparable to intravenous therapy for Staphylococcus aureus osteomyelitis. The Journal of infection. 2007 Jun;54(6):539–544. doi: 10.1016/j.jinf.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Gelfand MS, Cleveland KO. Vancomycin therapy and the progression of methicillin-resistant Staphylococcus aureus vertebral osteomyelitis. Southern medical journal. 2004 Jun;97(6):593–597. doi: 10.1097/00007611-200406000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert DN, Mollering RC, Eliopoulos GM, Sande MA, editors. The Sanford Guide to Antimicrobial Therapy. Sperryville, VA: Antimicrobial Therapy, Inc.; 2007. [Google Scholar]
- 11.Norden CW, Shaffer M. Treatment of experimental chronic osteomyelitis due to staphylococcus aureus with vancomycin and rifampin. The Journal of infectious diseases. 1983 Feb;147(2):352–357. doi: 10.1093/infdis/147.2.352. [DOI] [PubMed] [Google Scholar]
- 12.Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005 Feb 15;171(4):388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 13.CLSI. Performance standards for antimicrobial susceptibility testing. Wayne, PA: CLSI; 2006. [Google Scholar]
- 14.Eguia JM, Liu C, Moore M, Wrone EM, Pont J, Gerberding JL, et al. Low colonization prevalence of Staphylococcus aureus with reduced vancomycin susceptibility among patients undergoing hemodialysis in the San Francisco Bay area. Clin Infect Dis. 2005 Jun 1;40(11):1617–1624. doi: 10.1086/429906. [DOI] [PubMed] [Google Scholar]
- 15.Kalteis T, Beckmann J, Schroder HJ, Handel M, Grifka J, Lehn N, et al. Moxifloxacin superior to vancomycin for treatment of bone infections--a study in rats. Acta orthopaedica. 2006 Apr;77(2):315–319. doi: 10.1080/17453670610046082. [DOI] [PubMed] [Google Scholar]
- 16.Yin LY, Lazzarini L, Li F, Stevens CM, Calhoun JH. Comparative evaluation of tigecycline and vancomycin, with and without rifampicin, in the treatment of methicillin-resistant Staphylococcus aureus experimental osteomyelitis in a rabbit model. The Journal of antimicrobial chemotherapy. 2005 Jun;55(6):995–1002. doi: 10.1093/jac/dki109. [DOI] [PubMed] [Google Scholar]
- 17.Weigelt J, Itani K, Stevens D, Lau W, Dryden M, Knirsch C. Linezolid versus vancomycin in treatment of complicated skin and soft tissue infections. Antimicrobial agents and chemotherapy. 2005 Jun;49(6):2260–2266. doi: 10.1128/AAC.49.6.2260-2266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowler VG, Jr., Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. The New England journal of medicine. 2006 Aug 17;355(7):653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 19.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Archives of internal medicine. 2006 Oct 23;166(19):2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 20.Boffi El Amari E, Vuagnat A, Stern R, Assal M, Denormandie P, Hoffmeyer P, et al. High versus standard dose vancomycin for osteomyelitis. Scandinavian journal of infectious diseases. 2004;36(10):712–717. doi: 10.1080/00365540410020901. [DOI] [PubMed] [Google Scholar]