Abstract
Objective: To examine modulations caused by cyclooxygenase-2 (COX-2) inhibitors on altered microenvironments and overbalanced neurotransmitters in pilocarpine-induced epileptic status rats and to investigate possible mechanisms. Methods: Celecoxib (a COX-2 inhibitor) was administered 45 min prior to pilocarpine administration. The effects of COX-2 inhibitors on mIPSCs (miniature GABAergic inhibitory postsynaptic currents) of CA3 pyramidal cells in the hippocampus were recorded. Expressions of COX-2, c-Fos, newly generated neurons, and activated microgliosis were analyzed by immunohistochemistry, and expressions of α-subunit of γ-amino butyric acid (GABAA) receptors and mitogen-activated protein kinase/extracellular signal-regulated protein kinase (MAPK/ERK) activity were detected by Western blotting. Results: Pretreatment with celecoxib showed protection against pilocarpine-induced seizures. Celecoxib prevented microglia activation in the hilus and inhibited the abnormal neurogenesis and astrogliosis in the hippocampus by inhibiting MAPK/ERK activity and c-Fos transcription. Celecoxib also up-regulated the expression of GABAA receptors. NS-398 (N-2-cyclohexyloxy-4-nitrophenyl-methanesulfonamide), another COX-2 inhibitor, enhanced the frequency and decay time of mIPSCs. Conclusion: The COX-2 inhibitor celecoxib decreased neuronal excitability and prevented epileptogenesis in pilocarpine-induced status epilepticus rats. Celecoxib regulates synaptic reorganization by inhibiting astrogliosis and ectopic neurogenesis by attenuating MAPK/ERK signal activity, mediated by a GABAergic mechanism.
Keywords: Epileptogenesis, Cyclooxygenase-2 (COX-2), Neurogenesis, Microglia, γ-amino butyric acid (GABA), c-Fos, Mitogen-activated protein kinase/extracellular signal-regulated protein kinase (MAPK/ERK), Spontaneous recurrent seizure
INTRODUCTION
The acute insult resulting from status epilepticus (SE) is characterized by functional or morphological changes in the hippocampus over several days to weeks before the onset of spontaneous recurrent seizures (SRS) (Motte et al., 1998). During seizures, the release of glutamate initiates the reorganization of neuronal connections favoring a permanent hyperexcitable state, often associated with plasticity (Esclapez et al., 1999; Morimoto et al., 2004). Recurrent and spontaneous seizures result from long-lasting plastic changes in the brain including synaptic and extracellular matrix remodeling, inhibitory interneuronal loss, reactive astrogliosis and a marked increase in neurogenesis (Mikkonen et al., 1998; Sankar et al., 2000; Jin et al., 2006). Gene expression plays a role in SE-induced alterations in hippocampal physiology. Cellular signaling and inducible transcription events are activated by synchronous synaptic discharges. As the generation of new neurons or glia within the hippocampus is initiated by local signaling (Brock et al., 1998), alterations in the microenvironment such as microglial inflammation and the release of proinflammatory cytokines may affect normal cell proliferation and differentiation, which could cause ectopic neurogenesis, astrogliosis and ectopic synaptic reorganization (Jin et al., 2006). Cyclooxgenase-2 (COX-2), as a proinflammatory mediator encoded in an early-response gene, is induced by synaptic activity (Lukiw et al., 2005); therefore, COX-2 activity could contribute to epileptic neuronal injury.
COX-2 is the predominant isoform of cyclooxygenase in the brain. COX-2 activity produces oxidative stress and results in the production of prostaglandins that have many injurious effects. Cyclooxygenase [COX, or prostaglandin H synthase (PGHS)] catalyzes prostaglandin H2 (PGH2) synthesis from arachidonic acid (AA), the immediate precursor of prostaglandins. COX comprises two isoforms, COX-1 (constitutive) and COX-2 (inducible). COX-2 participates in the inflammatory response (Seibert et al., 1994), cancer growth (Subbaramaiah et al., 1997), neuronal death (Kawaguchi et al., 2005), and neuronal hyperexcitability (Willingale et al., 1997). Recent evidence that COX-2, which is expressed in postsynaptic dendritic spines, regulates prostaglandin E2 (PGE2) signaling in activity-dependent long-term synaptic plasticity at hippocampal perforant path-dentate granule cell synapses (Chen et al., 2002), suggests that COX-2-generated PGE2 has an important role in synaptic signaling.
Specific COX-2 inhibitor protects against experimentally induced epileptogenesis (Shafiq et al., 2003; Dhir et al., 2006), but the molecular mechanisms underlying its chemopreventive activities remain largely unresolved. Little is known how the mediators of COX-2 play a role regulating neuronal signaling in epileptogenesis. The aims of this study were to determine whether seizures activate expression of COX-2 protein in rat hippocampal cells and to investigate the possible signaling pathway(s) involved. Synthesis of protein is thought to form the basis for sustained seizure induced in synaptic reorganization and apoptotic/necrotic neuronal death. We were focused on the role of the extracellular signal-regulated kinase (ERK) signaling pathway. COX-2 products have gained special interest as promoters of cell growth (aside from their role in inflammation) and many reports have demonstrated that mitogen-activated protein kinase/extracellular signal-regulated protein kinase (MAPK/ERK) signaling is implicated in neurogenesis (Lopez-Toledano and Shelanski, 2004; Altar et al., 2004). Therefore, we postulate that seizure-induced accumulation of COX-2, as a bioactive lipid messenger and proinflammatory cytokine, helps drive these changes in synaptic restructuring and cell proliferation and differentiation through the activation of kinase signaling, and consequently, modulation of protein synthesis and gene transcription.
MATERIALS AND METHODS
All animal experimental protocols were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee at Shandong University, Jinan, China.
Lithium-pilocarpine-induced SE model
Immature male Sprague-Dawley (SD) rats (14 d, weighing 30~50 g, genomics) were used for epileptic rats (n=90) and normal controls (n=10). Epileptic rats were divided into an epilepsy-only group and an epilepsy-celecoxib group. Epileptic rats were generated by lithium-pilocarpine-induced SE, as described elsewhere (Motte et al., 1998; Sankar et al., 2000). Lithium chloride (127 mg/kg, i.p.; Sigma, USA) was injected 24 h prior to the administration of pilocarpine. Rats were pretreated with methylscopolamine-bromide (1 mg/kg; Sigma) 30 min prior to pilocarpine, and then a single dose of pilocarpine (30 mg/kg, i.p.; Sigma) was administered. Seizures were scored by Racine’s scale (McIntyre et al., 2002). The beginning of SE was defined as the onset of continuous generalized seizure activity without regaining normal behavior between the seizures. For the rats developing SE, we controlled the duration of SE by injection of diazepam (10 mg/kg, i.p.; Samjin, USA). Normal control rats were treated with lithium-methylscopolamine saline instead of pilocarpine, and were given only a single diazepam injection 2 h after saline injection.
Drug administration
The rats in the epilepsy-celecoxib group (n=45) were administered celecoxib 45 min prior to an epileptic challenge. They were fed with celecoxib (prescription formulation; Pfizer Incusing 20-mm feeding needles, Popper and Sons, Inc., USA) dissolved in phosphate-buffered saline (PBS) (20 mg/kg) once daily until the day of sacrifice. Epileptic rats fed with vehicle alone served as the epilepsy-only group (n=45), and normal rats fed with the vehicle alone (n=10) served as the normal control group. Also, 5-bromo-2V-deoxyuridine-5V-monophosphate (BrdU, 100 mg/kg, diluted in saline) was injected i.p. once a day for 14 consecutive days from the day of SE. The rats were sacrificed at 14 or 28 d after SE for histological analyses of COX-2, c-Fos, microglial activation, hippocampal cell proliferation, and migration and differentiation of BrdU-positive cells (n=8 for epilepsy-celecoxib, epilepsy-only and normal control groups at each timing). Additional epileptic rats were sacrificed at 1, 4, 7, 14, or 28 d after SE (n=3 for each group at each timing) for Western blotting.
SRS monitoring from Day 28 to Day 42
Since the frequency and duration of SRS in the pilocarpine model are much higher during the light (diurnal) than the dark (nocturnal) period (Motte et al., 1998; Sankar et al., 2000), all recordings for SRS were done during the light period. Epileptic rats (n=9 for the two epilepsy groups) were video-monitored for at least 12 h/d (up to 84 h/week) from Day 28 to Day 42 after SE. Digital video camcorders were used to record 9 rats simultaneously (3 rats per camera). The recordings were analyzed by independent observers for group allocation. The frequency and duration of stage 4 or 5 seizures were recorded, and Racine’s scale was used to rate seizure severity.
Electrophysiology recordings
1. Hippocampal slice preparation
Hippocampal slices were prepared from 2-week-old SD rats (Chen et al., 2002). Briefly, the rats were sacrificed by decapitation and the brains were extracted from the skull and rapidly submerged in cold oxygenated (95% O2-5% CO2) artifical cerebrospinal fluid (aCSF) at 4 °C with the following composition (in mmol/L): NaCl, 126; KCl, 3.5; MgCl2, 1.3; NaHCO3, 25; NaH2PO4, 1.25; CaCl2, 1.0; glucose, 10 (pH=7.3). Slices were cut at 400 µm with a tissue chopper and left in aCSF at room temperature for at least 1 h. Individual slices were fully submerged and superfused with oxygenated aCSF at (32±1) °C, at a flow rate of (3.0±0.2) ml/min. Drugs were applied through the perfusion. Individual pyramidal neurons were viewed with a Zeiss Axioskop microscope, fitted with a 60× (Olympus) water-immersion objective and differential interference contrast (DIC) optics.
2. Whole cell recordings
Experiments were generally performed in the CA3 area of the hippocampus. Whole cell patch-clamp recordings of pyramidal cells were obtained using an Axo clamp-2B patch-clamp amplifier (Axon Instruments, Foster City, CA, USA) in voltage-clamp mode, sampled at 10 kHz (Digidata 1200; Axon Instruments). Recording pipettes (5~7 MΩ for recordings) were pulled from borosilicate glass with a micropipette puller (Sutter Instrument, Washington, USA). The internal pipette solution contained (in mmol/L): CsCl, 140; NaCl, 10; 4-(2-hydroxyethyl)-1-piperarazinee-thanesulfonic acid (HEPES), 10; ethylene glycol tetraacetic acid (EGTA), 1; CaCl2, 0.1; Mg2ATP, 2; Tris2GTP, 0.5. The final solution was adjusted to pH 7.3 and 270~280 mOsm. Isolated GABAergic events were obtained in the presence of NBQX (2,3-dihydroxy-6-nitro-7-sulfamoylbenzoauin-oxaline, 10 μmol/L) and D-APV (2-amino-5-phosphonovaleric acid, 40 μmol/L) to block L-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate and N-methyl-D-aspartate (NMDA) receptor-mediated currents. Miniature GABAergic inhibitory postsynaptic currents (mIPSCs) were recorded in the presence of TTX (tetrodotoxin, 1 μmol/L) and glutamate antagonists. Data were recorded at a holding potential of −70 mV. Drugs were superfused for 30 min as their effects stabilized after 15 min. Occlusion experiments were performed on the same cells. Control (CTR) values were first determined 15 min after applying drug. NS-398 (N-2-cyclohexyloxy-4-nitrophenyl-methanesulfonamide), a COX-2 inhibitor, was then applied for 30 min to the same neurons. The modification induced by the reference drug was compared with the CTR values.
Immunohistochemistry
The method used was one modified from the previous report (Kang et al., 2006). Rats were deeply anesthetized and perfused transcardially first with 100 ml 10 mmol/L PBS (pH 7.4) and then with 100 ml fixative (4% (w/v) paraformaldehyde, 15% (w/v) saturated picric acid, and 10 mmol/L PBS). The brains were removed and postfixed in fixative for 1 d. Coronal blocks (3 mm) were cryoprotected overnight in 30% (w/v) sucrose. We removed the brains and either cryopreserved tissue blocks for cryostat sectioning (30 μm) or embedded them in paraffin for sectioning (7 μm). The coronal sections through the dorsal hippocampus (at the level corresponding to 2.8~4.5 mm posterior to bregma) were selected, and each one-seventh section of the hippocampus (6 sections per animal) was examined for quantitative immunohistochemical analysis. Immunohistochemistry was carried out as described previously (Motte et al., 1998; Sankar et al., 2000). The specimens were fixed in 4% (w/v) paraformaldehyde in PBS (pH 7.5) for 40 min, washed with PBS, incubated for 2 h with blocking buffer (2% (w/v) horse serum/1% (w/v) bovine serum albumin (BSA)/0.1% (w/v) Triton X-100 in PBS, pH 7.5), then incubated with primary antibodies overnight at 4 °C and with secondary antibodies for 1 h at room temperature. Primary antibodies used as markers for microglia were rabbit anti-murine polyclonal antibody to COX-2 (1:300, Caymen Chemicals, Inc., USA); rabbit anti-murine polyclonal antibody to c-Fos (1:500, Abcam, USA); mouse anti-rat GAP-43 antibodies (1:500, Chemicon, USA). Biotinylated goat anti-rabbit IgG (ABC, Sigma, USA) and anti-mouse IgG (1:100, Biodesign, USA) or tetramethylrhodamine isothiocyanate (TRICT)-conjugated anti-mouse antibody were used as the secondary antibodies. Double immunostaining used primary antibodies directed to monoclonal anti-BrdU (1:300, Pharmingen, USA) and TRICT-conjugated secondary anti-mouse antibody to label newly generated cells. Either glial fibrillary acidic protein (GFAP, 1:200, Chemicon) or neuron-specific nuclear protein (NeuN, 1:100, Chemicon) and an anti-mouse fluorescein isothiocyanate (FITC)-conjugated secondary antibody (1:100, Vector Laboratories, USA) were used to label astroglial cells or neurons. The colocalization was analyzed using a laser scanning confocal microscope with a Bio-Rad MRC 1024 (argon and krypton). The fluorescence signals were detected at excitation/emission wavelengths of 550/620 nm (TRICT, red) and 488/522 (FITC, green), respectively. Biotin signals were detected with 3,3′-V-diaminobenzidine (DAB, brown).
Western blotting analysis
Additional epileptic rats were sacrificed at 1, 4, 7, 14, or 28 d after SE (n=3 for each group at each timing). After the sacrifice, the brain was removed and frozen in liquid nitrogen. The frozen tissues were homogenized in 30 mmol/L Tris-Cl, pH 7.5, 100 nmol/L phenylmethylsulfonyl fluoride (PMSF). Proteins (25 μg) from whole cell lysates were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membrane. The blots were blocked overnight with 5% (w/v) nonfat dry milk in Tris-buffered saline (TBS), followed by incubation for 2 h with rabbit anti-murine polyclonal COX-2 antibody (1:1000, Caymen Chemicals, Inc.) and anti-ERK1/2 antibody (Anaspel, USA) at a dilution of 1:500. After washed with TBS, the blots were incubated with a goat anti-rabbit horseradish peroxidase-conjugated secondary antibody at room temperature. Anti-β-actin antibody (Santa Cruz, USA) was used as a control. The blots were developed via enhanced chemiluminescence (Pierce, Rockford, IL, USA) and were digitally scanned (GS-700, Bio-Rad, Hercules, CA, USA) and analyzed (Molecular Analysts, Bio-Rad).
Statistical analysis
The mIPSCs data from whole cell recordings were analyzed using Clampfit 9.0 (Axon Co., USA) and Origin Professional 6.0 (Microcalsoft, Inc., USA). Frequency and mean amplitudes were determined over a 5-min period; 200~300 consecutive events were selected and averaged to determine 10%~90% rise time and 10%~90% decay-time constants. Results were expressed as mean±standard error of the mean (SEM) of n independent experiments performed on different animals. Paired t-tests were used to determine the significance of differences between mean values. The Kolmogorov-Smirnov test was used to test the normality of distributions. Immunochemical and Western blotting data were processed using the SPSS 13.0 program. Data were analyzed using the unpaired Student’s t-test if they were normally distributed (Kolmogorov-Smirnov test), otherwise the Mann-Whitney U test was used. For repeated measures, the analysis of variance (ANOVA) followed by a post hoc test was used. P values of less than 0.05 were considered to be significant.
RESULTS
Effects of selective COX-2 inhibitors on pilocarpine induced seizures
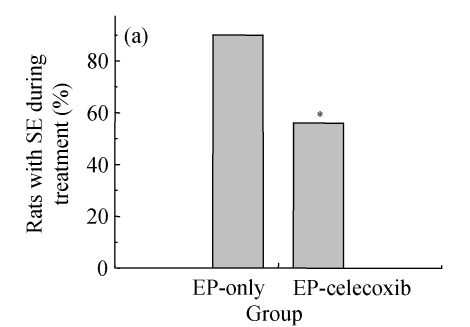
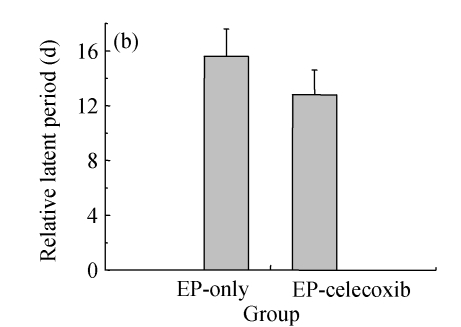
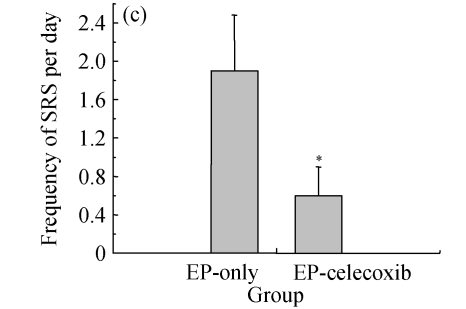
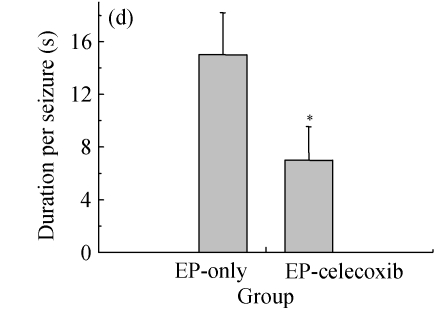
Ninety rats injected with pilocarpine developed SE which was characterized by continuous motor limbic seizures accompanied by intermittent rearing and falling with a mean latency of (10±2) min. The duration of SE was controlled at 60 min. Pretreatment with celecoxib significantly decreased the morbidity and duration of pilocarpine-induced seizures. The morbidity rates during SE were analyzed to provide an external physiologic measure of the effect of celecoxib. The saline-injected animals were not included in this analysis. In celecoxib-treated rats, 56% (25/45) developed SE, indicating the morbidity rate (i.e., rats having at least 1 seizure by about 30 min after pilocarpine administration; Fig.1a) was significantly lower than that in those treated with pilocarpine alone (87%, 35/40; P=0.01). Those animals without motor seizures during treatment in both groups behaved in a similar manner during SE. Rats that had no seizures (n=28) or had five or fewer seizures (n=13) during treatment were also kept for behavioral observation to assess the occurrence of SRS. SRS were observed from 14 to 28 d after SE. The relative latent period was based on an average of 160 h of both direct observation and 24-h video-monitoring. The average time to the first observed SRS was not significantly different between the epilepsy-only [(15.6±2.1) d] and epilepsy-celecoxib [(12.8±1.8) d] groups (P>0.05) (Fig.1b). The frequency of observed SRS was (1.9±0.58) times per day (ranging from 0 to 3.5 times per day) in the epilepsy-only group and (0.6±0.3) times per day (ranging from 0 to 1.6 times per day) in the epilepsy-celecoxib group (Fig.1c). The duration of observed seizures was (15.2+3.3) s in the epilepsy-only group and (7.1±2.53) s in the epilepsy-celecoxib group (Fig.1d). The frequency and duration of SRS in the epilepsy-celecoxib group were significantly reduced compared with the epilepsy-only group (P<0.01).
Fig. 1.
The outcome of pilocarpine-induced SE and SRS (n=45 in each group). (a) Morbidity rate during pilocarpine treatment. Morbidity rate was defined as the number of rats that responded to treatment with at least 1 motor seizure. The morbidity rate was significantly lower if the rats were pretreated with celecoxib (56% vs 87% in the epilepsy (EP)-only; P=0.01, χ 2 test); (b) Relative latent period and seizure rate. The relative latent period was based on an average of 160 h of both direct observation and 24-h video-monitoring. The average time to the first observed SRS was not significantly different between the EP-only [(15.6±2.1) d] and the EP-celecoxib [(12.8±1.8) d] groups (P>0.05, t test); (c) The frequency of observed SRS was (1.9±0.58) times per day (ranging from 0 to 3.5 times per day) in the EP-only group and (0.6±0.3) times per day (ranging from 0 to 1.6 times per day) in the EP-celecoxib group; (d) The duration of observed seizures was (15.2+3.3) s in the EP-only group and (7.1±2.53) s in EP-celecoxib group. The frequency and duration of SRS in the EP-celecoxib group were significantly reduced compared with the EP-only group (P<0.01, t test)
All animals in these groups survived treatment. Data from the rats that did not have any seizures during treatment were not included in any part of this study. These results suggest that celecoxib acted systemically to depress the occurrence of pilocarpine-induced SE in a significant proportion of animals.
Effects of selective COX-2 inhibitor NS-398 on isolated GABAergic transmission
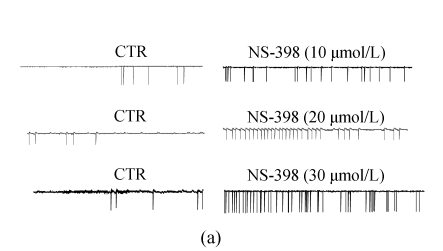
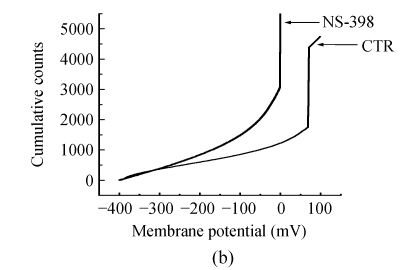
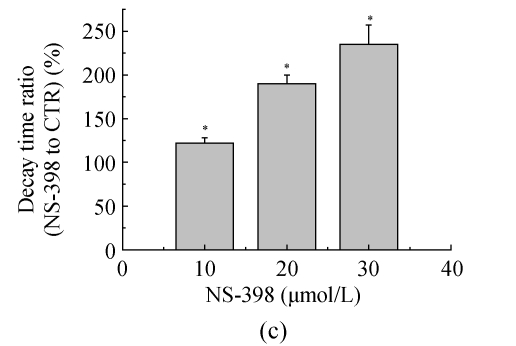
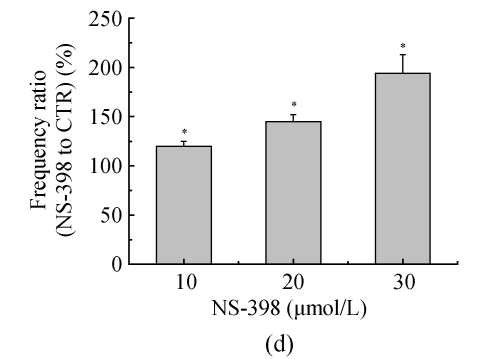
The effects of NS-398 on isolated GABAergic transmission were examined in the hippocampus in the presence of TTX (1 μmol/L; to block action potential-dependent release) and glutamate antagonists (NBQX, 10 μmol/L, and D-APV, 40 μmol/L; to block AMPA/kainite and NMDA receptors, respectively). In CA3 pyramid cells, NS-398 (10 μmol/L) did not modify the amplitude (P=0.4; n=10; Fig.2a and Table 1) of mIPSCs but significantly decreased the mean of interment intervals (Fig.2b), indicating an increase in the frequency of mIPSCs [(120±5)% of CTR; P=0.008; Table 1]. Examination of mIPSC kinetics indicated that NS-398 also markedly increased the mIPSC decay-time constant [(122±6)%; P=0.002; Fig.2c and Table 1] but not the rise time (P=0.7; Table 1). Bicuculline (10 μmol/L) completely blocked mIPSCs (not shown), indicating that these synaptic events were mediated by α-subunit of γ-amino butyric acid (GABAA) receptors. These effects were concentration dependent (Table 1), because NS-398 (10~30 μmol/L) gradually increased duration (Fig.2c) and frequency of mIPSCs (Fig.2c) without modification of their amplitude and rise time. These results clearly indicate that NS-398 enhances GABAergic transmission in the hippocampus.
Fig. 2.
COX-2 inhibitor NS-398 enhances mIPSCs in the hippocampus. (a) Representative sweeps of mIPSCs in the absence or presence of NS-398. mIPSCs were recorded in hippocampal CA3 pyramid neurons. The membrane potential was held at −70 mV. D-APV (400 μmol/L), NBQX (10 μmol/L), and TTX (0.5 μmol/L) were included in the external solution. The synaptic events were analyzed with Clampfit 9; (b) Cumulative probability of mIPSC amplitude in the absence or presence of NS-398. Mean percentage changes (normalized to the baseline) in the presence of NS-398; (c) The decay-time constant of mIPSC (Table 1). The decay time increased from (122±6)% (P=0.002) with 10 μmol/L NS-398 to (235±22)% (P=0.0001) with 30 μmol/L NS-398; (d) The frequency of mIPSC. The frequency of mIPSC (Table 1) increased from (120±5)% (P=0.008) with 10 μmol/L NS-398 to (194±19)% (P=0.0001) with 30 μmol/L NS-398. *A significant change between mean±SEM
Table 1.
Effect of NS-398 on mIPSC characteristics
| Group | n | Frequency (Hz) | Amplitude (×10−12 A) | Rise time (ms) | Decay time (ms) |
| CTR | 10 | 1.0±0.1 | 34.3±1.5 | 1.2±0.1 | 14.4±0.7 |
| NS-398, 10 μmol/L | 10 | 1.2±0.2a | 34.9±2.2 | 1.2±0.1 | 17.6±0.9a |
| CTR | 11 | 0.9±0.1 | 31.6±2.0 | 1.2±0.1 | 13.5±1.1 |
| NS-398, 20 μmol/L | 11 | 1.5±0.3a | 30.6±2.6 | 1.3±0.1 | 25.7±1.6a |
| CTR | 8 | 1.1±0.2 | 35.5±2.8 | 1.3±0.1 | 13.4±1.2 |
| NS-398, 30 μmol/L | 8 | 2.1±0.5a | 34.5±3.9 | 1.3±0.2 | 31.5±3.1a |
mIPSCs were recorded before (CTR) and 30 min after perfusion of NS-398 to the same CA3 pyramidal neurons. Frequency, amplitude, rise time and decay-time constants corresponded to the mean values determined in n independent experiments. For each NS-398 concentration, paired t-tests were performed. Note that amplitude and rise time were not affected by different concentrations of NS-398;
P<0.05, compared with CTR
Inhibition of hippocampal cell proliferation after SE by celecoxib
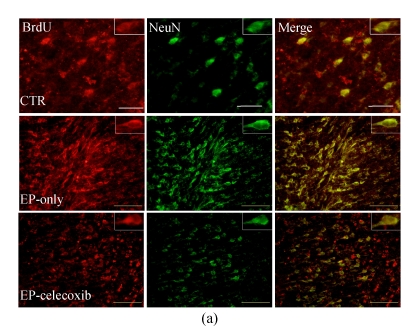
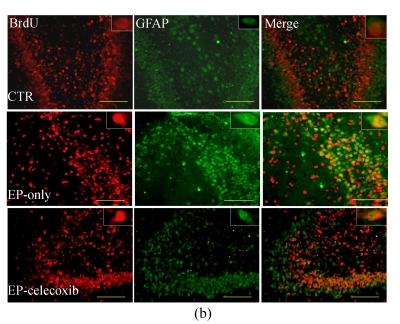
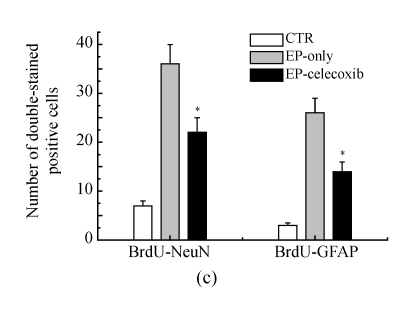
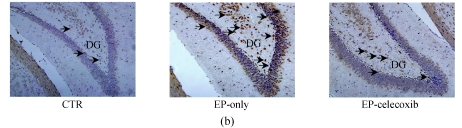
We investigated the effect of COX-2 inhibition on hippocampal cell proliferation by using BrdU fluorescent immunohistochemistry at 14 d after SE. In the normal control group, BrdU-NeuN double stained cells were unevenly distributed in the dentate gyrus (DG). In the epilepsy-only group, the doubled stained cells were up-regulated with subsequent expansion of the DG. Celecoxib treatment for 14 d suppressed neurogenesis (Fig.3a). Also, the BrdU-GFAP doubled stained cells were up-regulated in the epilepsy-only group, indicating that celecoxib attenuated the gliogenesis (Fig.3b). From the quantitative analysis, SE increased the number of BrdU-NeuN double stained positive cells in the hilus by 5-fold compared with the normal control group, while celecoxib treatment caused a 40% decrease in the BrdU-NeuN double stained positive cells within the epilepsy-only group. The BrdU-GFAP double stained cells in dentate gyrus also increased 7.5-fold after SE, and celecoxib treatment caused a 45% decrease of the BrdU-GFAP double stained cells (Fig.3c). Celecoxib suppressed cell proliferation.
Fig. 3.
Hippocampal cell proliferation after SE. BrdU immunostainings showed the distribution of newly generated cells in the hippocampus at 14 d after SE. (a) BrdU-positive cells (red) in the hippocampus were co-localized with NeuN (green); (b) The BrdU-positive cells in the hilus also were found localized in the nucleus of a GFAP-positive cell (green). The insets are higher magnification of BrdU-immunoreactive neurons or astrocytes; (c) Quantification of BrdU-positive cells in each group demonstrates the significant inhibitory effect of celecoxib in the dentate gyrus at 14 d. In (a) and (b), scale bars=100 μm; In (c), bars represent mean±SD. * P<0.05 compared with the epilepsy-only group (n=8, ANOVA followed by post hoc test)
Suppression of activated microglia at damaged sites by celecoxib
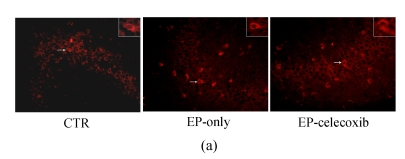
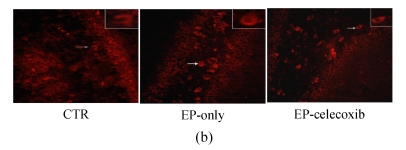
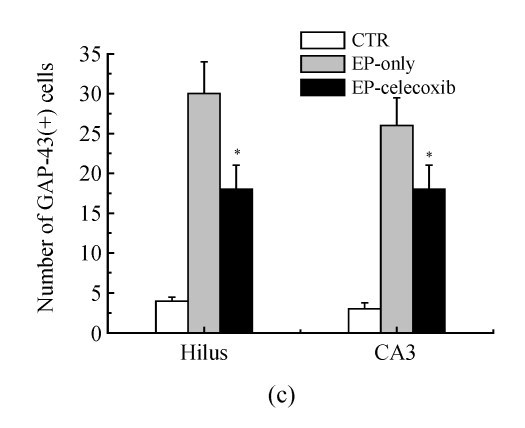
Activated microglia cells immunostained with GAP-43 were observed in the damaged hippocampus areas, especially in the hilus and CA3 14 d after SE (Fig.4a). The number of GAP-43 positive cells significantly increased at 14 d after SE. From the quantitative analysis, celecoxib treatment showed a 40% decrease in the number of activated microglia cells in the hilus and a 35% decrease in CA3, compared with the epilepsy-only group (Fig.4c).
Fig. 4.
Fluorescent immunohistochemical analysis of GAP-43-positive activated microglia (arrows shown). GAP-43-positive activated microglia in the (a) CA3 and (b) hilus at 14 d after SE. The number of GAP-43-positive cells was reduced by celecoxib treatment; (c) Quantitative analysis showed that the numbers of GAP-43-positive cells were reduced by 40% in the hilus and by 35% in the CA3. Bars represent mean±SD; * P<0.05 compared with the epilepsy-only group (n=8, t-test)
Inhibition of ERK activity in the epileptic rat brain by celecoxib
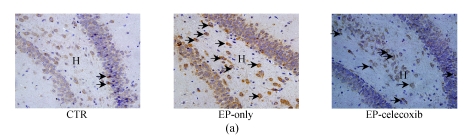
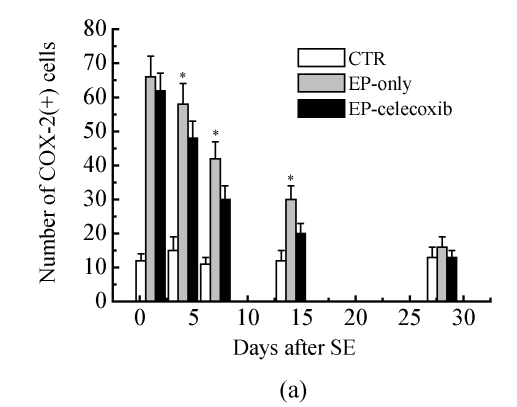
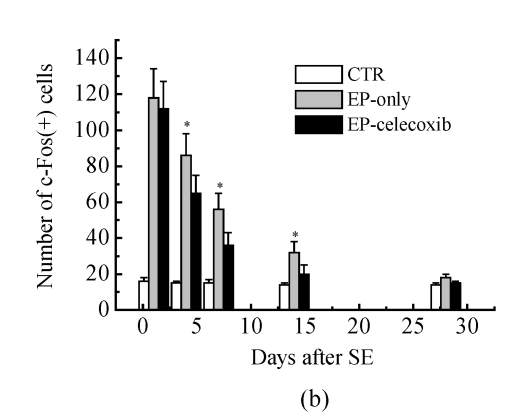
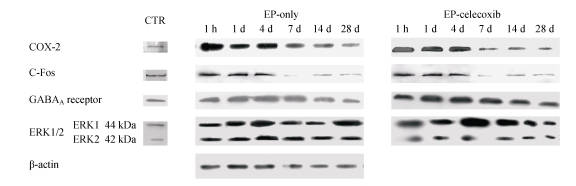
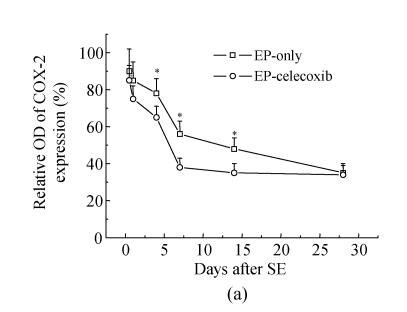
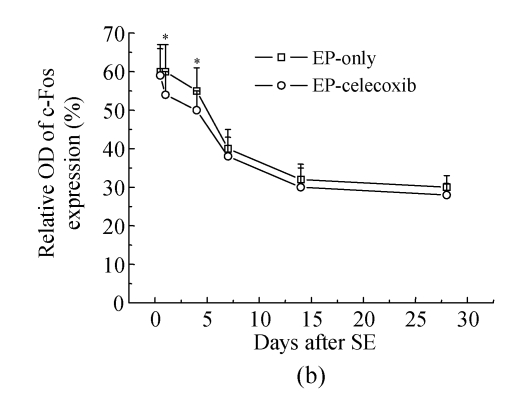
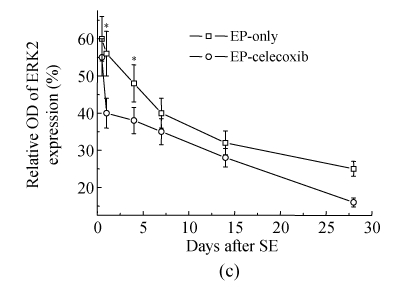
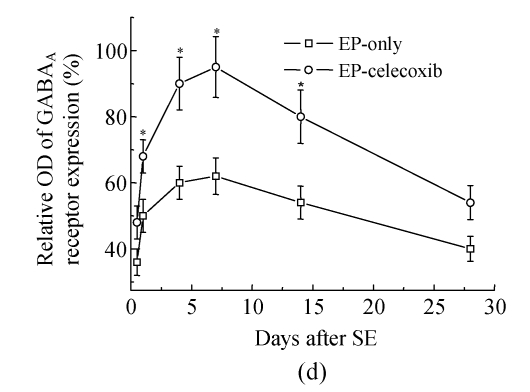
COX-2 immunoreactivity was observed in the perinuclear region of neurons in both the normal control rats and epileptic rats (Fig.5a). C-Fos immunoreactivity was detected in many cells throughout the hippocampus, especially in the DG (Fig.5b). The c-Fos positive cells appeared up-regulated in both the epilepsy-only group and the epilepsy-celecoxib group at 14 d after SE, compared with the normal control group. Also the COX-2 and c-Fos proteins were expressed in a time-dependent manner after SE (Fig.6). SE caused an up-regulation in COX-2 and c-Fos expressions. Both peaked at 1 h after SE and then declined. COX-2 expression of the epilepsy-only group was 1.3 times and 1.5 times higher than that of the epilepsy-celecoxib group at 4 and 14 d after SE, respectively. C-Fos expression of the epilepsy-only group was 1.2 times higher than that of the epilepsy-celecoxib group at both 1 and 4 d after SE. Quantitative analysis of positive cells demonstrated that celecoxib down-regulated the expression of COX-2 and c-Fos. The expression of ERK1/2 in the hippocampus was also detected (Fig.7). The MAPK was activated by pilocarpine-induced SE, and celecoxib attenuated that activation. Phosphospecific ERK expression of the epilepsy-only group was 1.4 and 1.2 times higher than that of epilepsy-celecoxib group at 1 and 4 d after SE, respectively. GABAA receptors mediated the majority of fast inhibitor synaptic transmissions in the brain (Fig.8) (n=5, Mann-Whitney U test).
Fig. 5.
(a) COX-2 immunoreactivity (arrow) was detected in many cells throughout the hippocampus, especially in the dentate gyrus (DG). The COX-2 positive cells appeared unregulated in the epilepsy-only and epilepsy-celecoxib groups at 14 d after SE, compared with the normal control group; (b) C-Fos immunoreactivity (arrow) was detected in many cells throughout the hippocampus, especially in the dentate gyrus (DG). The c-Fos positive cells appeared up-regulated in the epilepsy-only and epilepsy-celecoxib groups at 14 d after SE, compared with the normal control group
Fig. 6.
Quantitative analysis of positive cells demonstrated that celecoxib down-regulates the expressions of (a) COX-2 and (b) c-Fos
Fig. 7.
Western blotting documented a time course of COX-2, c-Fos, phosphorylation of ERKs. SE caused an up-regulation of COX-2 and c-Fos expressions. Both peaked at 1 h after SE and then declined. GABAA receptors mediated the majority of fast inhibitor synaptic transmissions in the brain. Celecoxib up-regulated the expression of GABAA receptors. Time-dependent pilocarpine-induced ERK1 and ERK2 phosphorylation in the hippocampus. Phosphorylations of ERKs were determined by Western blotting analysis using an anti-phosphorylated ERK antibody and activated by pilocarpine-induced SE, and celecoxib attenuated the activation
Fig. 8.
(a) Relative optical density (OD) of COX-2 expression of the epilepsy-only group was 1.3 times and 1.5 times higher than that of the epilepsy-celecoxib group at 4 and 14 d after SE, respectively; (b) Relative optical density of c-Fos expression of the epilepsy-only group was 1.2 times higher than that of the epilepsy-celecoxib group at 1 and 4 d after SE, respectively; (c) Relative optical density of ERK2 expression of the epilepsy-only group was 1.4 times and 1.2 times higher than that of the epilepsy-celecoxib group at 1 and 4 d after SE, respectively. The MAPK was activated by pilocarpine-induced SE and celecoxib attenuated the activation; (d) Relative optical density of GABAA receptors expression of the epilepsy-celecoxib group was 1.5 times higher than that of epilepsy-only group at 4 d after SE. Data are mean±SD of experiments. * P<0.05 compared with the epilepsy-only group (n=5, Mann-Whitney U test)
DISCUSSION
Many previous studies indicated that COX-2 expression was induced after seizures in different animal models of epilepsy and epilepsy patients with hippocampal sclerosis (Okada et al., 2001; Tu and Bazan, 2003; Kawaguchi et al., 2005; Dhir et al., 2006). Furthermore, the concentrations of prostaglandins (PGs) increased in the cerebrospinal fluid of epilepsy, hippocampal sclerosis and febrile seizures patients (Desjardins et al., 2003). Our findings confirmed the previous observations that COX-2 expression in the rat brain is increased markedly following SE. This suggests that the activation of COX-2 has a central role in the genesis of epilepsy, as well in the pathways targeted by new anti-epileptogenic drugs.
The effects of COX-2 inhibitors in epileptic animal models have been contradictory. Some previous studies demonstrated that COX-2 inhibitors such as nimesulide and rofecoxib treatment prior to an epileptic challenge showed anticonvulsant role and reduced hippocampal cell death in bicuculline- and picrotoxin-induced convulsions and kainite-induced epilepsy model in mice (Kunz and Oliw, 2001; Tu and Bazan, 2003; Dhir et al., 2006). Kawaguchi et al. (2005) also reported that SC58125, another COX-2 inhibitor, attenuated the seizure-induced increase of the major COX-2 product, PGE2, and improved neuronal survival. In contrast, NS-398 showed proconvulsant effects by increasing neuronal injury and mortality in mice, resulting in a paradoxical increase in PGE2 (Baik et al., 1999). In our study, pretreatment with celecoxib significantly decreased the morbidity and duration of pilocarpine-induced seizures. Also the frequency and duration of SRS in celecoxib-treated group were significantly reduced compared with the epilepsy-only group, which indicated that celecoxib attenuated seizure and the likelihood of developing SRS. The comparison and interpretation of these studies are complicated by differences in the selectivity of the studied drugs, as well as by differences in the treatment protocols and outcome measures. In the current study, we attempted to treat the epileptic rats with a selective COX-2 inhibitor, celecoxib, throughout the latent period in the pilocarpine-induced SE model, and to investigate various parameters such as hippocampal neurogenesis, astrogliosis, microglia infiltration, which are all closely related to synaptic reorganization in the hippocampus after prolonged-seizures.
Prolonged seizure can induce the endogenous cell proliferation in the rodent hippocampus (Scharfman et al., 2000; Dashtipour et al., 2001). Newly generated cells induced by prolonged seizure migrated and differentiated to granule cell-like neurons (Scharfman et al., 2000; Dashtipour et al., 2001), which had 3 times more total synapses on their somata than mature granule cells in the granular cell layer (GCL), but they lack the inhibitory synapses on the somata and proximal dendrites (Dashtipour et al., 2001). These ectopic cells axons in this region creating recurrent excitatory circuits, these circuits may contribute to hyperexcitability and seizures (Scharfman et al., 2000). On the other hand, astrogliosis is another prominent feature of the epileptic brain (Rothstein et al., 1996; Tashiro et al., 2002). Animal studies have shown that kindled seizures up-regulated the expression of GFAP (Torre et al., 1993) and caused glial cell hypertrophy and proliferation (Torre et al., 1993). Jung et al.(2004) found that the suppression of new neurons and glia by cytosine-b-D-arabinofuranoside infusion led to the attenuation of SRS frequency, suggesting an epileptogenic role of the altered neurogenesis or astrogliosis. In our study, SE increased the BrdU-NeuN double stained positive cells 5-fold in the hilus and celecoxib treatment suppressed the neurogenesis by 40%. BrdU-GFAP double stained positive cells in dentate gyrus also increased 7.5-fold after SE while celecoxib treatment attenuated the gliogenesis by 45%. The present findings extend theses previous observations to a therapeutic trial to correct the upstream pathway of altered cell generation. Neurogenesis within the hippocampus is controlled by local signaling and alterations in the microenvironment of stem cell niches may cause ectopic neurogenesis. The activation of microglial cells is the hallmark of neuroinflammation. A gradient of microglial cells could induce the migration of precursor cells, and soluble factors from microglia affect the differentiation of precursor cells towards neuronal lineage. In our findings, celecoxib treatment showed a 40% decrease in activated microglia cells in the hilus and a 35% decrease in the CA3, compared with the epilepsy-only group. We speculate that the suppression of ectopic hilar neurogenesis and astrogliosis is partly due to a decreased gradient of microglial inflammation and proinflammatory cytokines. The suppression of ectopic neurons and astrogliosis by celecoxib might result not only from an indirect effect via correction of an inflammatory environment but from a direct effect on progenitor cells. COX-2 is an important modulator inducing proliferation of progenitor cells (Sasaki et al., 2003).
A large body of experimental evidences supports the involvement of γ-amino butyric acid (GABA) in seizures. The GABAA receptor is responsible for most fast inhibitory neurotransmission in the central nervous system. In our study, NS-398, a selective COX-2 inhibitor, increased the frequency and decay time of mIPSCs. These results show clearly that NS-398 enhances GABAergic transmission in the hippocampus. Also the Western blotting analysis results showed that celecoxib up-regulated the expression of GABAA receptors. The previous study also showed that nimesulide and rofecoxib enhanced the effect of subprotective dose of muscimol against picrotoxin-induced convulsions, which suggested that the protective effect of rofecoxib against pentetrazole (PTZ)-induced convulsions may be achieved through GABAergic modulation (Dhir et al., 2006). COX-2 inhibitors might be acting through GABAergic neurons thus increasing the inhibitory neurotransmitter and the expression of GABAA receptor protein. One possibility is that activation of cyclooxygenases causes an increase in free radical production leading to oxidative stress and apoptosis of GABAergic neurons, thus increasing glutamate and causing epileptic discharges.
ERK is important for regulation of neuronal function, particularly playing a role in the regulation of synaptic plasticity and long-term memory formation (Sweatt, 2004; Ivanov et al., 2006). Activation of ERK results in pleiotropic effects such as modulation of the transcription and activation of enzymes involved in signal transduction. C-Fos was the downstream signal molecule. These molecules mediate short-term (acute) signaling functions in neurotransmission and long-term (chronic) signaling functions in cell proliferation, differentiation, and death involved in development and regeneration (Gass et al., 1997). In the current study, we showed that ERK is activated with a short latency (<1 h) in hippocampal neurons by pilocarpine-induced seizures. Activated ERK2 can be attenuated by celecoxib, which is in line with the results of neurogenesis and gliogenesis inhibitions by celecoxib in epileptic rats. These results indicate that the enhancement of COX-2 expression can activate the MAPK/ERK, modulating cell proliferation. We also found a persistent activation of c-Fos in the hippocampus after SE. C-Fos, is rapidly and transiently expressed in cells after activation by seizures. We also found that the altered level of c-Fos expression corresponded with that of ERK. The stimulation of seizures also induces phosphorylation of ERKs and a robust induction of c-Fos transcription in the hippocampus. Celecoxib suppressed ectopic neurogenesis and astrogliosis by modulating the phosphorylation of ERKs and c-Fos transcription.
References
- 1.Altar CA, Laeng P, Jurata LW, Brockman JA, Lemire A, Bullard J, Bukhman YV, Young TA, Charles V, Palfreyman MG. Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. J Neurosci. 2004;24(11):2667–2677. doi: 10.1523/JNEUROSCI.5377-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baik EJ, Kim EJ, Lee SH, Moon CH. Cyclooxygenase-2 selective inhibitors aggravate kainic acid induced seizure and neuronal cell death in the hippocampus. Brain Res. 1999;843(1-2):118–129. doi: 10.1016/S0006-8993(99)01797-7. [DOI] [PubMed] [Google Scholar]
- 3.Brock SC, Bonsall J, Luskin MB. The neuronal progenitor cells of the forebrain subventricular zone: intrinsic properties in vitro and following transplantation. Methods. 1998;16(3):268–281. doi: 10.1006/meth.1998.0684. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002;87(12):2851–2857. doi: 10.1152/jn.2002.87.6.2851. [DOI] [PubMed] [Google Scholar]
- 5.Dashtipour K, Tran PH, Okazaki MM, Nadler JV, Ribak CE. Ultrastructural features and synaptic connections of hilar ectopic granule cells in the rat dentate gyrus are different from those of granule cells in the granule cell layer. Brain Res. 2001;890(2):261–271. doi: 10.1016/S0006-8993(00)03119-X. [DOI] [PubMed] [Google Scholar]
- 6.Desjardins P, Sauvageau A, Bouthillier A, Navarro D, Hazell AS, Rose C, Butterworth RF. Induction of astrocytic cyclooxygenase-2 in epileptic patients with hippocampal sclerosis. Neurochem Int. 2003;42(4):299–303. doi: 10.1016/S0197-0186(02)00101-8. [DOI] [PubMed] [Google Scholar]
- 7.Dhir A, Naidu PS, Kulkarni SK. Effect of cyclooxygenase-2 (COX-2) inhibitors in various animal models (bicuculline, picrotoxin, maximal electroshock-induced convulsions) of epilepsy with possible mechanism of action. Indian J Exp Biol. 2006;44(2):286–291. [PubMed] [Google Scholar]
- 8.Esclapez M, Hirsch JC, Ben-Ari Y, Bernard C. Newly formed excitatory pathways provide a substrate for hyperexcitability in experimental temporal lobe epilepsy. J Comp Neurol. 1999;408(4):449–460. doi: 10.1002/(SICI)1096-9861(19990614)408:4<449::AID-CNE1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 9.Gass P, Bruehl C, Herdegen T, Kiessling M, Lutzenburg M, Witte OW. Induction of FOS and JUN proteins during focal epilepsy: congruences with and differences to [14C] deoxyglucose metabolism. Brain Res Mol Brain Res. 1997;46(1-2):177–184. doi: 10.1016/S0169-328X(96)00300-2. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov A, Pellegrino C, Rama S, Dumalska I, Salyha Y, Ben-Ari Y, Medina I. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572(6):789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin X, Prince DA, Huguenard JR. Enhanced excitatory synaptic connectivity in layer v pyramidal neurons of chronically injured epileptogenic neocortex in rats. J Neurosci. 2006;26(18):4891–4900. doi: 10.1523/JNEUROSCI.4361-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung KH, Chu K, Kim M, Jeong SW, Song YM, Lee ST, Kim JY, Lee SK, Roh JK. Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur J Neurosci. 2004;19(12):3219–3226. doi: 10.1111/j.0953-816X.2004.03412.x. [DOI] [PubMed] [Google Scholar]
- 13.Kang TC, Kim DS, Kwak SE, Kim JE, Won MH, Kim DW, Choi SY, Kwon OS. Epileptogenic roles of astroglial death and regeneration in the dentate gyrus of experimental temporal lobe epilepsy. Glia. 2006;54(4):258–271. doi: 10.1002/glia.20380. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi K, Hickey RW, Rose ME, Zhu L, Chen J, Graham SH. Cyclooxygenase-2 expression is induced in rat brain after kainate-induced seizures and promotes neuronal death in CA3 hippocampus. Brain Res. 2005;1050(1-2):130–137. doi: 10.1016/j.brainres.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 15.Kunz T, Oliw EH. The selective cyclooxygenase-2 inhibitor rofecoxib reduces kainate-induced cell death in the rat hippocampus. Eur J Neurosci. 2001;13(3):569–575. doi: 10.1046/j.1460-9568.2001.01420.x. [DOI] [PubMed] [Google Scholar]
- 16.Lopez-Toledano MA, Shelanski ML. Neurogenic effect of beta-amyloid peptide in the development of neural stem cells. J Neurosci. 2004;24(23):5439–5444. doi: 10.1523/JNEUROSCI.0974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukiw WJ, Cui JG, Musto AE, Musto BC, Bazan NG. Epileptogenesis in diacylglycerol kinase epsilon deficiency up-regulates COX-2 and tyrosine hydroxylase in hippocampus. Biochem Biophys Res Commun. 2005;338(1):77–81. doi: 10.1016/j.bbrc.2005.08.109. [DOI] [PubMed] [Google Scholar]
- 18.McIntyre DC, Poulter MO, Gilby K. Kindling: some old and some new. Epilepsy Res. 2002;50(1-2):79–92. doi: 10.1016/S0920-1211(02)00071-2. [DOI] [PubMed] [Google Scholar]
- 19.Mikkonen M, Soininen H, Kalvianen R, Tapiola T, Ylinen A, Vapalahti M, Paljarvi L, Pitkanen A. Remodeling of neuronal circuitries in human temporal lobe epilepsy: increased expression of highly polysialylated neural cell adhesion molecule in the hippocampus and the entorhinal cortex. Ann Neurol. 1998;44(6):923–934. doi: 10.1002/ana.410440611. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol. 2004;73(1):1–60. doi: 10.1016/j.pneurobio.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Motte J, Fernandes MJ, Baram TZ, Nehlig A. Spatial and temporal evolution of neuronal activation, stress and injury in lithium-pilocarpine seizures in adult rats. Brain Res. 1998;793(1-2):61–72. doi: 10.1016/S0006-8993(98)00135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada K, Yuhi T, Tsuji S, Yamashita U. Cyclooxygenase-2 expression in the hippocampus of genetically epilepsy susceptible El mice was increased after seizure. Brain Res. 2001;894(2):332–335. doi: 10.1016/S0006-8993(01)02019-4. [DOI] [PubMed] [Google Scholar]
- 23.Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16(3):675–686. doi: 10.1016/S0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- 24.Sankar R, Shin D, Liu H, Katsumori H, Wasterlain CG. Granule cell neurogenesis after status epilepticus in the immature rat brain. Epilepsia. 2000;41(s6):S53–S56. doi: 10.1111/j.1528-1157.2000.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki T, Kitagawa K, Sugiura S, Omura-Matsuoka E, Tanaka S, Yagita Y, Okano H, Matsumoto M, Hori M. Implication of cyclooxygenase-2 on enhanced proliferation of neural progenitor cells in the adult mouse hippocampus after ischemia. J Neurosci Res. 2003;72(4):461–471. doi: 10.1002/jnr.10595. [DOI] [PubMed] [Google Scholar]
- 26.Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20(9):6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seibert K, Zhang Y, Leahy K, Hauser S, Masferrer J, Perkins W, Lee L, Isakson P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci USA. 1994;91(25):12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shafiq N, Malhotra S, Pandhi P. Anticonvulsant action of celecoxib (alone and in combination with sub-threshold dose of phenytoin) in electroshock induced convulsion. Methods Find Exp Clin Pharmacol. 2003;25(2):87–90. doi: 10.1358/mf.2003.25.2.723681. [DOI] [PubMed] [Google Scholar]
- 29.Subbaramaiah K, Zakim D, Weksler BB, Dannenberg A. Inhibition of cyclooxygenase: a novel approach to cancer prevention. Proc Soc Exp Biol Med. 1997;216(2):201–210. doi: 10.3181/00379727-216-44170. [DOI] [PubMed] [Google Scholar]
- 30.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14(3):311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Tashiro A, Goldberg J, Yuste R. Calcium oscillations in neocortical astrocytes under epileptiform conditions. J Neurobiol. 2002;50(1):45–55. doi: 10.1002/neu.10019. [DOI] [PubMed] [Google Scholar]
- 32.Torre ER, Lothman E, Steward O. Glial response to neuronal activity: GFAP-mRNA and protein levels are transiently increased in the hippocampus after seizures. Brain Res. 1993;631(2):256–264. doi: 10.1016/0006-8993(93)91543-2. [DOI] [PubMed] [Google Scholar]
- 33.Tu B, Bazan NG. Hippocampal kindling epileptogenesis upregulates neuronal cyclooxygenase-2 expression in neocortex. Exp Neurol. 2003;179(2):167–175. doi: 10.1016/S0014-4886(02)00019-5. [DOI] [PubMed] [Google Scholar]
- 34.Willingale HL, Gardiner NJ, McLymont N, Giblett S, Grubb BD. Prostanoids synthesized by cyclo-oxygenase isoforms in rat spinal cord and their contribution to the development of neuronal hyperexcitability. Br J Pharmacol. 1997;122(8):1593–1604. doi: 10.1038/sj.bjp.0701548. [DOI] [PMC free article] [PubMed] [Google Scholar]