Abstract
Imatinib mesylate (Gleevec, STI571), a selective inhibitor of a restricted number of tyrosine kinases, has been effectively used for the treatment of Philadelphia chromosome positive leukemias and gastrointestinal stromal tumors. Imatinib may also directly influence immune cells. Suppressive as well as stimulating effects of this drug on CD4+ and CD8+ T lymphocytes or dendritic cells have been reported. In the current study, we have investigated the influence of imatinib mesylate on CD4+CD25+FoxP3+ regulatory T cells (Treg), a critical population of lymphocytes that contributes to peripheral tolerance. Used at concentrations achieved clinically, imatinib impaired Treg immunosuppressive function and FoxP3 expression but not production of IL-10 and TGF-β in vitro. Imatinib significantly reduced the activation of the transcription factors STAT3 and STAT5 in Treg. Analysis of Treg TCR-induced signaling cascade indicated that imatinib inhibited phosphorylation of ZAP70 and LAT. Substantiating these observations, imatinib treatment of mice decreased Treg frequency and impaired their immunosuppressive function in vivo. Furthermore, imatinib mesylate significantly enhanced anti-tumor immune responses to dendritic cell-based immunization against an imatinib-resistant BCR-ABL negative lymphoma. The clinical applications of imatinib mesylate might thus be expanded with its use as a potent immunomodulatory agent targeting Treg in cancer immunotherapy.
Keywords: Tumor Immunity, T cells, Tolerance/Suppresssion/Anergy, Vaccination, Imatinib Mesylate
Introduction
Imatinib mesylate (Gleevec™) therapy remains the standard of care for patients with chronic myelogenous leukemia (CML). Designed as a selective competitive inhibitor of the ABL tyrosine kinases (BCR-ABL, v-ABL, c-ABL), this drug leads to growth arrest or apoptosis (1, 2). Imatinib also displays strong activity against the platelet-derived growth factor receptor (PDGF-R), c-Kit, ABL-related gene (ARG) and their fusion proteins (1-3) and thus has also been used for the therapy of gastrointestinal stromal tumors (GIST) with mutations in c-Kit (2).
However, the specificity of this molecule may be broader than originally expected, and it is becoming increasingly evident that imatinib also inhibits key tyrosine kinases in immune cells. The exact nature of imatinib effects (activation or suppression) on lymphocytes or dendritic cells remains controversial. Inhibition of CD4+ or CD8+ T cell proliferation and activation by imatinib has been documented (4-8). Some reports have further highlighted the negative effect of imatinib on the TCR-induced ZAP70 signalling pathway identifying the tyrosine kinase Lck as a potential molecular target (4, 9). Similarly, a negative modulation of dendritic cell (DC) development by imatinib and a down-regulation of their antigen presenting function have also been described (10-12). In contrast to these findings, it has been reported that imatinib does not impede the immunogenicity of DC (13) and may enhance their antigen-presenting function (14). Additionally, some reports indicate that imatinib may foster DC-NK reciprocal activation, thereby promoting the anti-tumoral function of NK cells (15).
CD4+CD25+ regulatory T lymphocytes (Treg) critically contribute to the maintenance of self-tolerance and to the prevention of autoimmunity in animals and humans (16, 17). These suppressive cells have also been highlighted as major contributors in the establishment and persistence of cancer-induced immune tolerance (18, 19). Treg expansion detected in the blood, lymph nodes, and spleens of tumor-bearing hosts (20-23) may result from the conversion of CD4+CD25− T cells into CD4+CD25+ (24) or from the proliferation of naturally occurring Treg (25). Tumor-induced Treg compromise the function of anti-tumor effector CD8+ T cells, curtail CD4+ T cell help, impede antigen-presenting cell activity (18, 23, 26) and therefore represent a major obstacle for successful cancer immunotherapy. In support of this concept, studies in humans and in animal models have demonstrated that attempts to disrupt Treg suppressive activity promote anti-tumoral immunity (20, 27, 28). Different strategies have thus been evaluated to deplete/inactivate Treg and include the use of anti-CD25 antibodies, the IL-2/diphtheria toxin fusion protein, the immunotoxin LMB-2, OX-40 antibodies or drugs such as cyclophosphamide (20, 26, 27, 29-31). However, no consensus has been reached regarding the optimal protocol to be used with immunotherapy.
Considering the broad nature of the tyrosine kinases targeted by imatinib mesylate and the prominent role of such proteins in Treg function, we reasoned that imatinib may be endowed with the potential to modulate Treg activity. We have therefore investigated the influence of imatinib on Treg in vitro and in vivo. Our results demonstrate that, in vitro, this tyrosine kinase inhibitor curtailed the immunosupressive activity of Treg and restrained FoxP3 expression. Imatinib mesylate interfered with Treg TCR-signalling pathway as it reduced the phosphorylation of ZAP70 and LAT. The activation of the downstream transcription factors STAT3 and STAT5 was impeded by the drug. The physiologic consequences of imatinib treatment on antitumoral immune responses in vivo was also examined. Imatinib reduced the number and impaired the immunosuppressive function of Treg in vivo and acted synergistically with dendritic cell-based immunization against imatinib-resistant lymphoma. Taken together these findings support the use of this agent as an immunomodulator in cancer immunotherapy.
Material and Methods
Mice
Mice were housed under specific pathogen-free conditions and cared for according to the guidelines of the University of Arizona Institutional Animal Care and Use Committee. Six to 8 -week old female BALB/c (H2d), C57BL6 (H2b) and SCID/NCr mice were obtained from the National Cancer Institute (Bethesda, MD) and used for the experiments.
Cell lines
The murine leukemia and lymphoma cell lines 12B1 and A20 were cultured at 37°C and in 5% CO2 in RPMI medium (Hyclone, Logan, UT) supplemented with 10% heat-inactivated fetal bovine serum (Hyclone). The 12B1 cell line was obtained from Dr. Wei Chen (Cleveland Clinic, Cleveland, OH). Cells were tested routinely and found to be free of Mycoplasma contamination.
Imatinib mesylate
Commercially available 100 mg imatinib capsule content (Gleevec/Glivec, Norvartis Pharmaceutical, Basel, Switzerland) was dissolved in sterile distilled water (Sigma, St Louis, MO) at desired concentrations, aliquoted and stored at −20°C for further in vitro and in vivo experiments. The biologic activity of the drug was tested every week on imatinib-sensitive 12B1 leukemia (BCR/ABL+) or imatinib-resistant A20 lymphoma (BCR/ABL−) using Thiazolyl Blue Tetrazolium Bromide (MTT) assays (Sigma).
Magnetic cell sorting and cultures
Spleens and lymph nodes isolated from BALB/c mice (naïve tumor-free or A20 tumor-bearing) were dissociated. CD4+CD25+ and CD4+CD25− T lymphocytes were purified by magnetic cell sorting using mouse CD4+CD25+ T regulatory cell isolation kits and an autoMACS™ separator according to the manufacturers' instructions (Miltenyi Biotec, Auburn, CA). Both CD4+ T lymphocyte populations (1 × 105 cells per well) were separately activated for 24 hrs in round-bottom 96-well plates coated with anti-CD3ε Ab (5 μg/ml, clone 145-2C11, BD Biosciences Pharmingen, San Diego, CA) and 5 μg/ml soluble anti-CD28 Ab (clone 37.51, BD Biosciences Pharmingen) in the presence of IL-2 (100 U/ml, R & D, Minneapolis, MN).
Flow cytometry analysis and antibodies
Cells (∼106) were washed in PBS containing 3% heat-inactivated fetal bovine serum and 0.09% sodium azide (Sigma Chemical) and were first incubated with an Fc receptor-blocking Ab (BD Biosciences Pharmingen) for 5 min, then with saturating amounts of the appropriate combination of fluorochrome-conjugated Ab for 40 min. Cells were then washed and analysed using a FACS calibur (Becton Dickinson Immunocytometry Systems, San Jose, CA). A minimum of 10,000 events was collected for each sample, and data analysis was performed with the CellQwest software (Becton Dickinson Immunocytometry Systems). For FoxP3 detection, CD4+CD25+ or CD4+CD25− T cells purified by magnetic cell sorting were fixed, permeabilized, stained using an Allophycocyanin (APC) anti-mouse FoxP3 staining set following the provider's instructions (Clone FJK-16, eBioscience, San Diego, CA), and analyzed by flow cytometry. For the monitoring of CD4+CD25+ Treg, cells were first stained with fluorescein isothiocyanate (FITC)-conjugated anti-CD4 (rat IgG2b; BD Biosciences Pharmingen) and phycoerythrin (PE)-conjugated anti-CD25 (rat IgG1; BD Biosciences Pharmingen) antibodies. Then, cells were stained using eBioscience FoxP3 staining set as described above. Isotype control antibodies were purchased from BD Biosciences Pharmingen (PE-conjugated rat IgG1, FITC-conjugated rat IgG2a) or eBioscience (APC-conjugated rat IgG1).
T cell proliferation and suppression assays
CD4+CD25− T cells (1 × 105) from naïve BALB/c mice (H2d) were co-cultured for 60 hrs in round-bottom 96-well plates with 1 × 105 mitomycin C-treated APC (CD90-depleted splenocytes from C57BL/6 mice, H2b) (with or without activated CD4+CD25+ T cells (1 × 105) from BALB/c mice. [3H]-Thymidine (ICN Pharmaceuticals, Costa Mesa, CA) was then added (1 μCi per well) for an additional 12 hrs. The cells were then harvested using a 96-well cell harvester and the radioactivity measured on a Packard beta counter (Packard Biosciences, Meriden, CT). Cultures were set up in triplicate.
Detection of cytokine production by ELISA
The concentrations of IFN-γ, IL-10, or TGF-β1 in cell culture supernatants (1 × 105 cells in 200 μl/well for 48 hrs) were determined using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's procedures (eBiosciences).
Detection of STAT3 and STAT5 phosphorylation by western blotting
CD4+CD25− or CD4+CD25+ T cells purified as described above were pre-incubated for 2 hrs with or without imatinib and were then activated for 6 hrs with soluble anti-CD3ε (5 μg/ml), anti-CD28 Ab (5 μg/ml) and IL-2 (100U/ml). These conditions have been set up to allow the detection of STAT3 and STAT5 phosphorylation, an event that occurs within few hours following cell stimulation. Cells were lysed in lysis buffer (1% Nonidet P40, 50 mM Tris HCl, pH 7.4, 2 mM EDTA, 100mM NaCl, 0.2 mg/mL Aprotinin, 0.2 mg/mL Leupeptin, 1 mM PMSF, 10 mM NaF, 30 mM NaPPi, 10 mM Na3VO4) and western blot analysis was then performed as described (23), using anti-phospho STAT3 (pTyr705), anti-STAT3, anti-phospho STAT5 (pTyr694) or anti-STAT5 antibodies (Cell Signalling, Beverly, MA). In other experiments, STAT3 activation was detected in Treg by DNA-binding transcription factor ELISA assay. Treg nuclear extracts were performed using a Nuclear Extract kit (Active Motif, Carlsbad, CA). Then, STAT3 DNA-binding activity was measured with 10 μg nuclear extract using a STAT3 Trans-AM™ kit according to the manufacturer's recommendations (Active Motif).
Analysis of ZAP-70 and LAT phosphorylation
ZAP-70 and LAT phosphorylation are early events in the transduction of TCR and CD28 signals in T cells. Therefore, purified CD4+CD25− or CD4+CD25+ T cells were pre-incubated for 2 hrs with or without imatinib and were immediately activated for 5 min with soluble anti-CD3ε (5 μg/ml) and anti-CD28 Ab (5 μg/ml). Cells were then lysed and analyzed by western blot as described above. Antibodies used were as follows: anti-phospho ZAP70 (pTyr705), anti-ZAP70, anti-phospho LAT (pTyr694) or anti-LAT antibodies (Cell Signalling, Beverly, MA).
Bone marrow-derived DC
DC were generated from BALB/c bone marrow cells. Cells were harvested from femurs and tibias and filtered through a Falcon 100-μm nylon cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ). Red blood cells were lysed in a hypotonic buffer (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA), and the marrow was cultured at a density of 5 × 105 cells/ml in complete RPMI medium (Gibco/BRL) containing 10% fetal bovine serum (Hyclone). Murine granulocyte-macrophage colony-stimulating factor (GM-CSF; Peprotech, Rocky Hill, NJ) and Interleukine-4 (IL-4; Peprotech) were added at a concentration of 10 ng/ml each. Complete RPMI medium containing GM-CSF and IL-4 was added on day 3. On day 5 medium was replaced and total A20 tumor cell lysate was added. On day 6 cells were activated for 24 hrs with LPS (0.1 μg/ml), and on day 7 cells were recovered, washed in PBS and used as pulsed DC vaccines in chemoimmunotherapy experiments. A20 tumor cell lysate was prepared by six cycles of freezing in liquid nitrogen and thawing in a 37°C water bath, leading to 100% dead cells as assessed by trypan blue. DC cultures were pulsed with 1 equivalent tumor cell/DC.
Immunohistochemical staining
SCID mice were injected s.c. with 106A20 cells or 5 × 103 12B1 cells and were treated with imatinib (gavage, 300 mg/kg daily for 7 days) when tumor became palpable (tumor diameter ∼2-3 mm). Animals were euthanized 2 days after the end of the treatment. Tumors were removed, embedded in Tissue-Tek (Sakura Finetek U.S.A., Torrance, CA) and snap-frozen in liquid nitrogen. Caspase 3 cleavage, a marker of apoptosis, was detected on methanol-fixed 5 μm cryostat sections after inhibition of endogenous peroxidase activity in 3% H2O2and incubation with goat serum (KPL, Gaithersburg, MA) and 1% BSA. Rabbit anti-mouse Ab recognizing the cleaved (active) form of caspase 3 (Cell Signalling) and IgG isotype-matched control (Santa Cruz, CA) were used. Sections were then incubated with biotinylated goat anti-rabbit Ab (KPL), then with streptavidin-peroxidase (KPL) and were stained with 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA). Slide examination of all tumor samples was performed independently by two investigators in a double blind manner.
Chemoimmunotherapy of A20 lymphoma with imatinib mesylate and DC vaccines
Female BALB/c mice (8 mice per group) inoculated at day 0 with A20 cells (5 × 105 cells in 0.1 mL PBS, tail vein injection) were treated at day 3 with imatinib mesylate (gavage, 300 mg/kg, twice daily for 7 days) or control water. DC vaccines (bone marrow-derived DC pulsed with A20 tumor lysate) were injected on days 5 and 9 (s.c., 106 DC per mouse). On day 21 mice were euthanized and the number of liver metastases was determined by two investigators. Spleens and lymph nodes were removed, dissociated and Treg frequency was assessed as described above. Splenocytes (1 × 105) from control, imatinib-treated, DC-vaccinated or imatinib plus DC -treated mice were cultured for 48 hrs in 200 μl of complete medium and IFN-γ secretion in the culture supernatants was detected by ELISA.
Statistical analysis
Unless specified otherwise, all experiments were reproduced 3 times and performed in triplicate. A two-sided student's t test with paired samples was used to determine significant differences (p<0.05) between groups.
Results
Sensitivity of CD4+CD25+FoxP3+ regulatory T cells to imatinib mesylate in vitro
The phenotypic characterization of Treg in the mouse is primarily based on the expression of the transcription factor FoxP3 involved in their lineage commitment, development, and function (32, 33). The majority of CD4+CD25+ T cells isolated by magnetic cell sorting from the lymphoid tissues of BALB/c mice expressed FoxP3 (Fig. 1A). In addition, purified CD4+CD25+ cells inhibited the proliferation of CD4+CD25−cells induced by APC (Fig. 1B), further demonstrating the immunosuppressive nature of these cells.
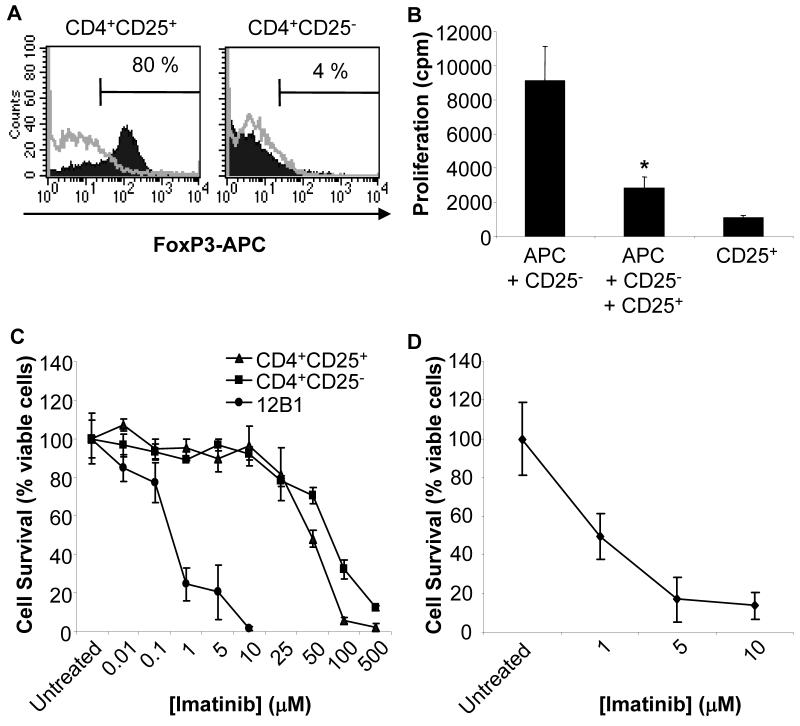
Figure 1. Sensitivity of Treg to imatinib mesylate.
(A) CD4+CD25+ but not CD4+CD25− T cells purified from BALB/c mouse spleen and lymph nodes by magnetic cell sorting express FoxP3. Cells were stained with an anti-FoxP3 antibody (FJK-16S, Ebiosciences) and analyzed by flow cytometry. Representative histograms of 9 independent experiments are presented. FoxP3 was expressed consistently by 80-95% of purified CD4+CD25+ T cells. (B) Inhibition of the proliferation of CD4+CD25− cells by CD4+CD25+ T lymphocytes. Responder BALB/c CD4+CD25− T cells were stimulated with APC (CD90-depleted C57BL6 splenocytes) in the presence or absence of CD4+CD25+ T cells. The data are shown as means ± SD of triplicate wells of 3H-thymidine incorporation. Results are representative of three independent experiments. *, Significant difference when compared to the corresponding control without CD4+CD25+ cells (p<0.01). (C) Purified CD4+CD25+ Treg or CD4+CD25− T cells were pre-activated with anti-CD3, anti-CD28 and IL-2, and were exposed for 48 hrs to the indicated concentrations of imatinib mesylate. The BCR-ABL+ imatinib-sensitive 12B1 leukemia was used as positive control. Cell viability was determined by MTT assays. (D) Purified CD4+CD25+ Treg were activated with anti-CD3, anti-CD28 Ab and IL-2, were then exposed for 72 hrs to the indicated concentration of imatinib mesylate, and cell viability was assessed using MTT. Similar results were obtained with resting Treg cultured for 72 hrs with imatinib mesylate in presence of anti-CD3, anti-CD28 Ab and IL-2.
We first evaluated a possible direct cytotoxic effect of imatinib mesylate against Treg in vitro. Splenic and lymph node CD4+CD25+ and CD4+CD25− T lymphocytes activated and cultured as described in material and methods were exposed to increasing concentrations of imatinib. The mouse leukemia cell line 12B1 expressing the p210 bcr-abl protein and sensitive to imatinib (34, 35) was used as positive control. Only high and continuous concentrations of the drug (> 10 μM) affected the viability of CD4+CD25+ Treg over an incubation period of 48 hours (Fig. 1C). Of note, patients treated with 400 mg of imatinib daily, or mice receiving 200 mg/kg imatinib daily, achieve peak plasma concentrations of only 1-10 μM (36, 37). CD4+CD25+ and CD4+CD25− cells did not exhibit differential sensitivity to the drug at these concentrations (Fig. 1C). Treg exposure to doses of imatinib achieved clinically for more than 48 hours resulted in the reduction of cell viability (Fig. 1D). Concentrations of imatinib of less than 10 μM and exposure time of 48 hours or less were therefore used in further in vitro experiments. Similar results were obtained with Treg isolated from the spleen and draining lymph nodes of mice bearing 12B1 leukemia or A20 lymphoma (data not shown).
Imatinib mesylate minimally affects Treg proliferation in vitro
We next investigated the influence of imatinib on Treg proliferation in vitro. Treg anergic state to TCR-mediated stimulation in vitro can be broken with the combination of anti-CD3/CD28 antibodies and IL-2 (38). CD4+CD25+ and CD4+CD25− cells isolated from mouse lymphoid tissues were stimulated under these conditions in the presence of imatinib mesylate at different concentrations for 48 hours. Consistent with previous studies (4, 5), imatinib at 10 μM slightly but significantly decreased the proliferation of non-regulatory T lymphocytes. However, non-Treg cell proliferation was not significantly affected by the drug used at lower concentrations (Fig. 2). Treg proliferation was not significantly modified by imatinib treatment of the cells (Fig. 2).
Figure 2. Effects of imatinib mesylate on Treg proliferation in vitro.

CD4+CD25+ Treg (CD25+Treg) and CD4+CD25− cells (CD25−) were isolated from BALB/c mouse lymphoid tissues and were cultured for a total of 48 hrs with plate-bound anti-CD3, soluble anti-CD28 and IL-2 with imatinib mesylate at the indicated concentrations. 3[H]-thymidine was added during the last 12 hrs. Results are representative of three independent experiments performed in triplicate. *, Significant difference when compared to the control without imatinib (p<0.05).
Imatinib mesylate inhibits Treg immunosuppressive function and FoxP3 expression
To further document the effects of imatinib mesylate on Treg, imatinib-treated or untreated CD4+CD25+ lymphocytes were tested for their immunosuppressive function. Treg isolated from BALB/c mouse lymphoid tissues were activated with anti-CD3, anti-CD28 antibodies and IL-2. The cells were then exposed to non-toxic concentrations of imatinib mesylate (Fig. 1C) for 48 hours and then washed. Their ability to inhibit the allo-response of BALB/c CD4+CD25− T lymphocytes to mitomycin C-treated C57BL6 APC was then evaluated. The data depicted in Fig. 3A indicate that imatinib mesylate inhibited the capacity of Treg to suppress CD4+CD25− cell proliferation in a dose-dependent manner.
Figure 3. Imatinib mesylate hampers Treg suppressive activity and FoxP3 expression.
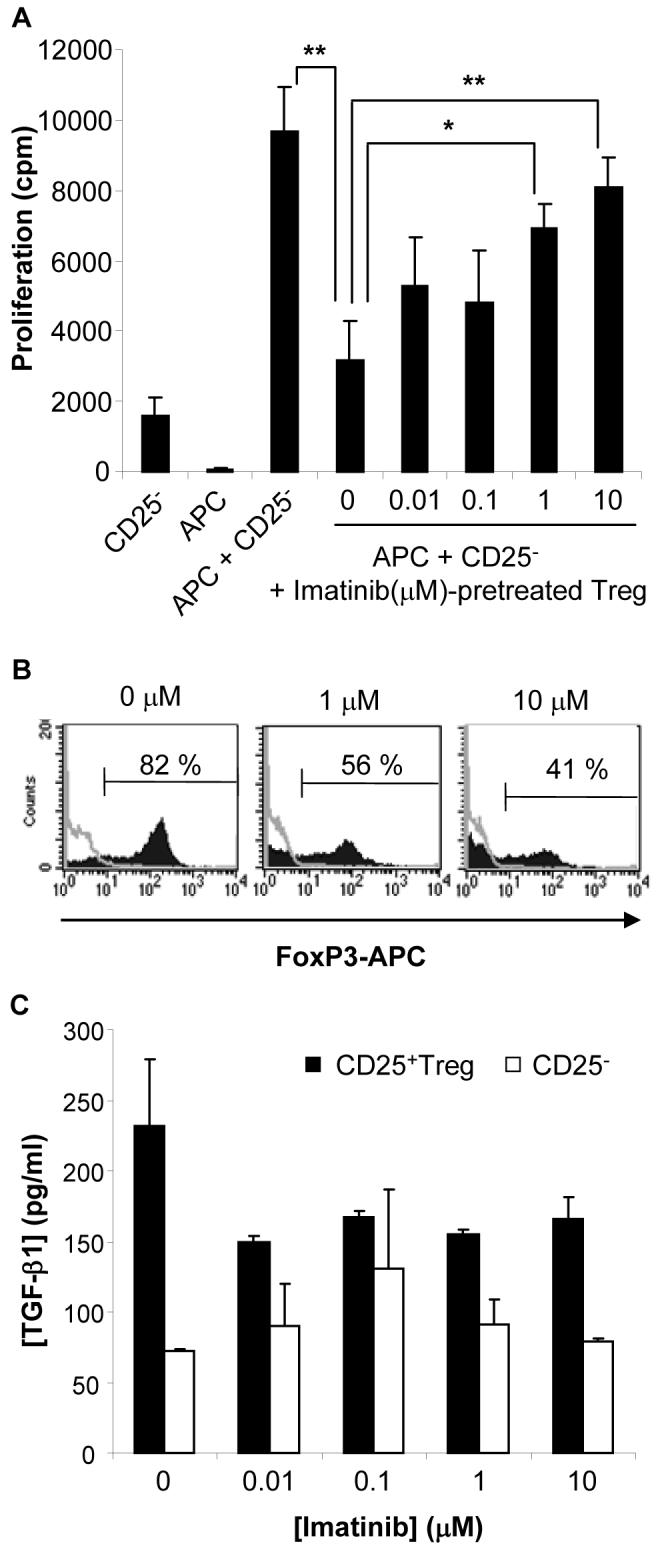
CD4+CD25+ Treg from lymphoid tissues of BALB/c mice were exposed to increasing but non-toxic concentrations of imatinib mesylate for 48 hrs, recovered and washed 3 times in complete medium to eliminate residual imatinib mesylate. (A) The effect of treated or untreated Treg on the proliferation of responder CD4+CD25− T lymphocytes (CD25−) stimulated with mitomycin C-treated allogeneic APC was determined by [3H]-thymidine incorporation assays. *, p<0.02; **, p<0.005. (B) Expression of FoxP3 in CD4+CD25+ Treg treated for 48 hrs with the indicated concentrations of imatinib. Representative results of three independent experiments. (C) TGF-β1 concentration was assessed by ELISA in imatinib mesylate-treated (48 hrs) CD4+CD25+ Treg (CD25+Treg) or CD4+CD25− T cell (CD25−) cultures. No significant difference was found between groups. Representative results of two independent experiments.
The transcription factor FoxP3 is required for the induction of Treg suppressive function, and its expression in non-regulatory cells converts them into immunosuppressive cells (32). Therefore, based on the results presented in Fig. 3A, we reasoned that imatinib may negatively modulate FoxP3 expression in Treg. To test this hypothesis, CD4+CD25+T cells purified from lymphoid tissues and activated as previously described, were treated with imatinib mesylate. Flow cytometry analysis demonstrated a dose-dependent reduction in the proportion of FoxP3-expressing CD4+CD25+ cells (Fig. 3 B).
The influence of imatinib on the production of the immunosuppressive cytokines TGF-β and IL-10 reported to be involved in Treg activity was then examined. Treg or CD4+CD25− cells were isolated and activated as previously mentioned and were exposed to imatinib mesylate for 48 hours. The concentration of TGF-β1 in Treg culture supernatants was low and not significantly impaired by imatinib (Fig. 3C). Similarly imatinib did not significantly affect IL-10 secretion by Treg (data not shown). This result suggests that imatinib mesylate may reduce Treg activity independently of its effect on TGF-β or IL-10.
Imatinib mesylate down-regulates STAT3 and STAT5 phosphorylation in Treg
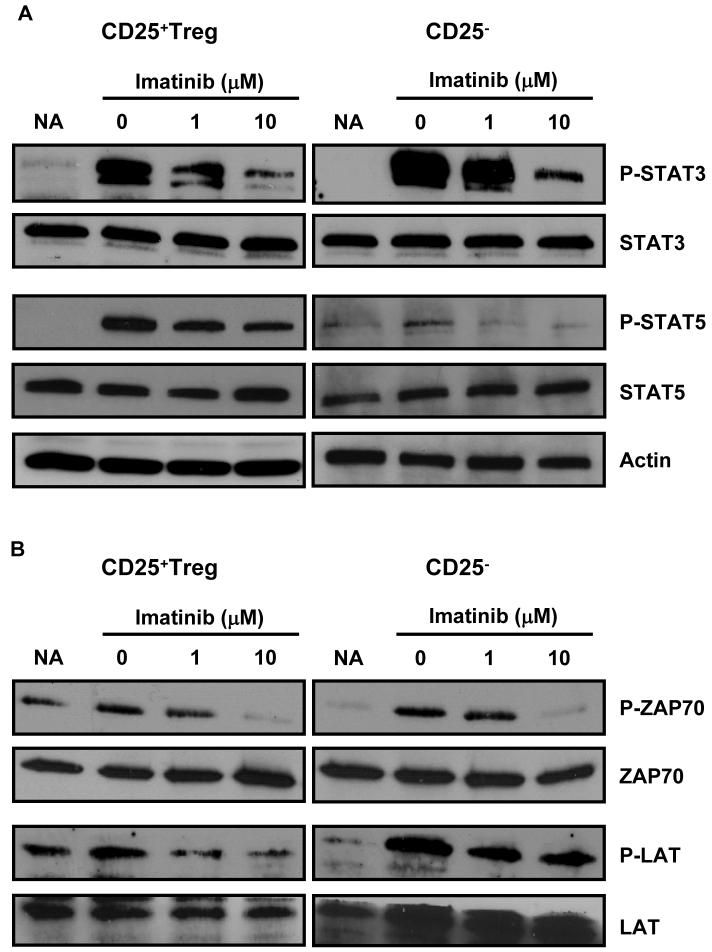
We have previously mentioned that the induction and persistence of Treg immunosuppressive function is intimately related to FoxP3 expression. The activation of the transcription factor STAT3 following TCR/CD28 costimulation has recently been highlighted as a major molecular event required for FoxP3 expression (39). Indeed, activated STAT3 may be necessary for the maintenance of FoxP3 expression in naturally occurring Treg and may also play a critical role in the conversion of CD4+CD25− cells into CD4+CD25+FoxP3+ Treg (39). In addition, it has been reported that FoxP3 upregulation in Treg may involve the binding of the STAT3 and STAT5 proteins to a highly conserved STAT-binding site located in the first intron of the FOXP3 gene (40). Based on this information and on the results presented in Figure 3, we hypothesized that treatment of Treg with this tyrosine kinase inhibitor may result in a negative regulation of these two key transcription factors. Purified CD4+CD25+ or CD4+CD25− pre-exposed to imatinib for 2 hours were stimulated with anti-CD3 and anti-CD28 antibodies for 6 hours. The phosphorylation of STAT3 and STAT5 was then determined by Western blot. Consistent with previous studies, STAT3 was constitutively expressed in both Treg and CD4+CD25− T cells (Fig. 4A). STAT3 Tyr705 phosphorylation induced in Treg by TCR/CD28 signals was impeded by imatinib treatment (Fig. 4A). A downregulation of STAT3 phosphorylation by the tyrosine kinase inhibitor was also observed in CD4+CD25− cells (Fig. 4A). Consistent with these results, ELISA transcription factor assays demonstrated that imatinib inhibited Treg STAT3 activation (not shown). STAT5 Tyr694 phosphorylation was also impaired by imatinib in Treg. In CD4+CD25− T lymphocytes the level of STAT5 phosphorylation was low and further reduced by imatinib treatment (Fig. 4A).
Figure 4. Imatinib mesylate reduces phosphorylation of key cell signaling molecules in Treg.
CD4+CD25− or CD4+CD25+ T cells isolated from BALB/c mouse lymphoid tissues were pre-incubated for 2 hrs with or without the indicated concentration of imatinib. (A) For phospho-STAT3 and Phospho-STAT5 detection, cells were then activated for 6 hrs with anti-CD3 and anti-CD28 Ab. Equivalent amounts of protein from cell lysates were separated by SDS-PAGE and western blot analysis was then performed using anti-phospho STAT3, anti-STAT3, anti-phospho STAT5 or anti-STAT5 Abs. (B) For the analysis of imatinib interference with early TCR signaling events, cells were activated for 5 min with anti-CD3 and anti-CD28 Abs and blots were probed with anti-phospho ZAP70, anti-ZAP70, anti-phospho LAT or anti-LAT antibodies. Untreated, non-activated cells were used as controls (NA).
Imatinib mesylate impairs TCR-induced ZAP70 and LAT phosphorylation in Treg
Imatinib mesylate has been designed as a tyrosine kinase inhibitor, selective to a restricted number of proteins including the ABL tyrosine kinases. However, it has also been reported that in conventional non-regulatory T lymphocytes imatinib may impede TCR-dependent T cell activation by inhibiting the src-family tyrosine kinase Lck involved in the recruitment and activation of the protein ZAP70 during the transduction of the TCR-CD3 signals (4). Lck phosphorylates the immunoreceptor tyrosine-based activation motifs (ITAMs) on the TCR subunits leading to the recruitment and activation of ZAP70. In turn, activated ZAP70 catalyzes the phosphorylation of the adaptor protein linker of activated T cells (LAT), which then initiates a series of downstream intracellular signal transduction events. Treg thymic differentiation or peripheral conversion depends on signals relayed by the TCR and CD28 (17, 41), and it has been reported that Lck activation is required for STAT3 and FoxP3 induction in Treg (39, 42). To further document the molecular mechanisms underlying Treg inhibition by imatinib, we sought to determine whether it may interfere at this level of the cell signaling cascade in Treg. Purified Treg or CD4+CD25− T cells were preincubated for 2 hours with imatinib mesylate, were then stimulated for 5 min with anti-CD3 and anti-CD28 to trigger TCR/CD28 signaling and the phosphorylation status of ZAP70 and LAT was examined as described (4). As depicted in Fig. 4B, decreased ZAP-70 phosphorylation was observed in Treg cells exposed to imatinib. Consistent with this data, phopho-LAT levels were also reduced by imatinib (Fig. 4B). The phosphorylation of these two signaling molecules was also impaired by the drug in CD4+CD25− T lymphocytes, confirming published data (4).
Overall, these results indicate that as in conventional T cells, imatinib negatively interferes with the proximal TCR/CD28 signaling events which may account for the down-regulation of Treg key transcriptions factors STAT3, STAT5, and FoxP3 and the observed inhibition of the suppressive function of these cells.
Imatinib mesylate treatment reduces Treg frequency and immunosuppressive function in vivo
We next sought to evaluate whether imatinib may compromise immunosuppressive Treg in vivo. Experiments were designed to first determine the influence of this drug on the relative frequency of Treg in naïve animals. BALB/c mice were treated for 7 days with imatinib mesylate (gavage, 300 mg/kg daily). We have documented that this dose is well-tolerated in mice with no observed toxicity (34). In other murine models of CML, comparable doses were reported to be well tolerated for as long as 118 days with peak plasma concentrations of 6.5 μM (37, 43). These levels are below the toxic concentrations for Treg that we have observed in vitro (Figure 1). Spleens and draining lymph nodes were removed two days following the end of imatinib mesylate treatment and the proportion of CD4+CD25+FoxP3+ cells was assessed by flow cytometry. Our results indicate that the percentage of CD4+CD25+ and FoxP3+CD4+ T cells within the total CD4+ cell population was significantly reduced in mice treated with imatinib. The number of CD25+ cells co-expressing FoxP3 was also negatively affected by imatinib treatment (Fig. 5A).
Figure 5. Imatinib mesylate restraints Treg in vivo.

(A) Imatinib mesylate decreases the frequency of Treg in vivo. Spleens and lymph nodes from naïve BALB/c mice treated with imatinib (Imat., gavage, 300 mg/kg, twice daily for 7 days) or with control water (CT), were stained with anti-CD4, anti-CD25 and anti-FoxP3 Abs, and then analyzed by flow cytometry. Characteristic dot plots are depicted. The indicated cell ratios (Mean +/− SD) were then determined. A significant reduction of CD4+CD25+/CD4+, FoxP3+CD4+/CD4+ and FoxP3+CD4+/CD25+ ratios was detected (p<0.005, p<0.002, and p<0.002, respectively). Representative results of two independent experiments, n=8 mice per group. (B) Imatinib mesylate treatment of naïve BALB/c mice reduces Treg immunosuppressive activity. CD4+CD25+ Treg were isolated from lymphoid tissues of BALB/c mice treated with imatinib (Imat., gavage, 300 mg/kg, twice daily for 7 days) or with control vehicle only (CT). Treg ability to suppress the proliferation of CD4+CD25− T cells from naïve tumor-free mice was then evaluated as previously described. Representative results of two independent experiments. *, p<0.01.
To further define whether imatinib may modulate the suppressive activity of Treg in vivo, naïve tumor-free BALB/c mice were treated with the tyrosine kinase inhibitor (gavage, 300 mg/kg daily), and CD4+CD25+ cells were isolated from the lymphoid tissues by magnetic cell sorting. The cells were tested for their ability to suppress proliferation of non-regulatory CD4+CD25− T lymphocytes purified from naïve tumor-free mice. Our results indicate that the immunosuppressive activity of Treg from imatinib-treated mice was compromised when compared to Treg from mice treated with control vehicle (Fig. 5B). Together, these results indicate that a short course of imatinib given to mice orally for 7 days not only reduced the relative number of Treg in vivo, but also inhibited their immunosuppressive activity on a per cell basis.
Imatinib mesylate can be efficiently combined with pulsed dendritic cell vaccine to treat established BCR-ABLnegative A20 lymphoma
The therapeutic depletion of Treg improves responses to cancer immunotherapy (20, 27). Since imatinib impaired Treg activity in vitro and in vivo, we next assessed whether this drug may augment the efficiency of cancer vaccination using a mouse model of imatinib-resistant tumors. A20 leukemia/lymphoma cells (BCR-ABL−) are not sensitive in vitro to concentrations of imatinib that can be achieved in vivo (Fig. 6A). Confirming these results imatinib treatment of SCID mice bearing A20 with the tyrosine kinase inhibitor (gavage, 300 mg/kg daily for 7 days) did not affect tumor growth compared to untreated animals (Fig. 6B). Very few dying cells were detected within A20 tumor nodules in mice treated with imatinib, further demonstrating that imatinib does not exert direct killing against A20 cells in vivo (Fig. 6C).
Figure 6. The A20 lymphoma is insensitive to the direct cytotoxic effects of imatinib mesylate.
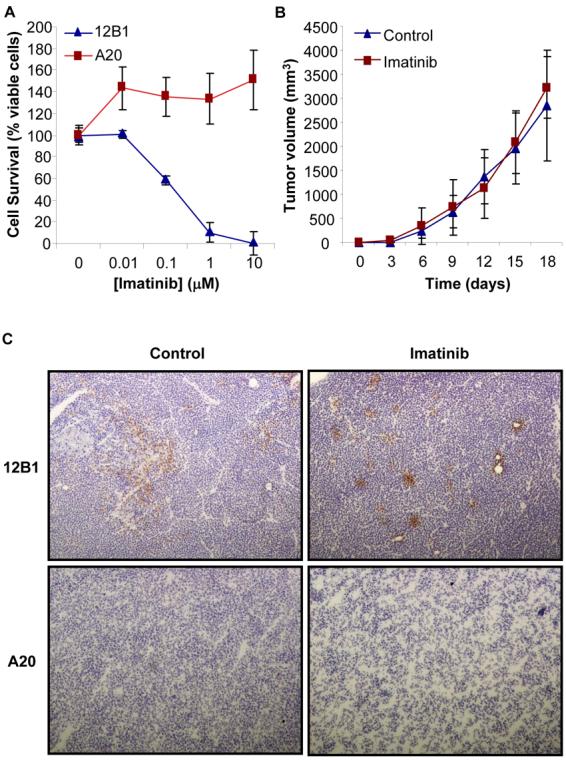
(A) A20 tumor cells (105) were cultured in 96-well plate in complete medium with the indicated concentrations of imatinib for 48 hrs. Cell viability was then evaluated using MTT assays. The BCR-ABL+ leukemia cell line 12B1 was used as positive control. Representative results of three experiments are shown. (B) A20 tumor cells (3 × 106) were injected (s.c) in the right groin of SCID mice. When tumors became palpable, mice were treated with imatinib (gavage, 300 mg/kg, twice daily for 7 days) and tumor volume was measured every three days. (C) Detection of dead cells in A20 tumor nodules from mice treated or not with imatinib. Tumors were removed two days after the end of imatinib treatment and tumor sections from untreated (Control) or imatinib-treated (Imatinib) mice were stained using Abs against the cleaved isoform of caspase 3. Dead cells (positive for cleaved caspase 3) were visualized under microscopy. A representative field is shown (× 100 magnification). 12B1 tumors from imatinib treated or untreated SCID mice were used as controls.
To evaluate the potential synergistic effects of imatinib with tumor immunization, BALB/c mice inoculated (i.v.) with A20 cells were treated with imatinib mesylate and vaccinated twice with dendritic cells pulsed with A20 lysates as described in Figure 8. The number of liver metastases (Fig. 7A and Supplemental Fig. S1) was significantly reduced in mice treated with imatinib plus DC pulsed with tumor lysates compared to control mice or to mice receiving imatinib mesylate or vaccination alone. Splenocytes from control, imatinib-treated, vaccinated or imatinib plus vaccine-treated mice were cultured for 48 hours and IFN-γ concentration was detected by ELISA. Total splenocytes from mice treated with the combination therapy produced significantly higher amounts of IFN-γ compared to the other groups (Fig. 7B). Further analysis indicated that IFN-γ was produced by both CD4+ and CD8+ T lymphocytes (data not shown). Interestingly, analysis of each individual mouse in the group treated with the combination therapy revealed a negative correlation between the levels of IFN-γ and the number of liver metastases (Fig. 7C). Additionnally the frequency of Treg was significantly decreased following treatment of tumor-bearing mice with imatinib plus DC vaccine combination treatment (Fig. 8). These results thus demonstrate that imatinib promotes the anti-tumor response to vaccination against cancers that are not sensitive to the drug's direct cytotoxicity.
Figure 8. Decrease of Treg following imatinib treatment with or without DC vaccination of A20 tumor-bearing mice.

A20 tumor-bearing mice were treated with control vehicle, imatinib, DC, or imatinib plus DC as described in Fig. 7. The number of Treg in spleens and lymph nodes was then determined by flow cytometry. Representative results of 2 independent experiments. * p<0.001.
Figure 7. Imatinib mesylate enhances DC based immunization.
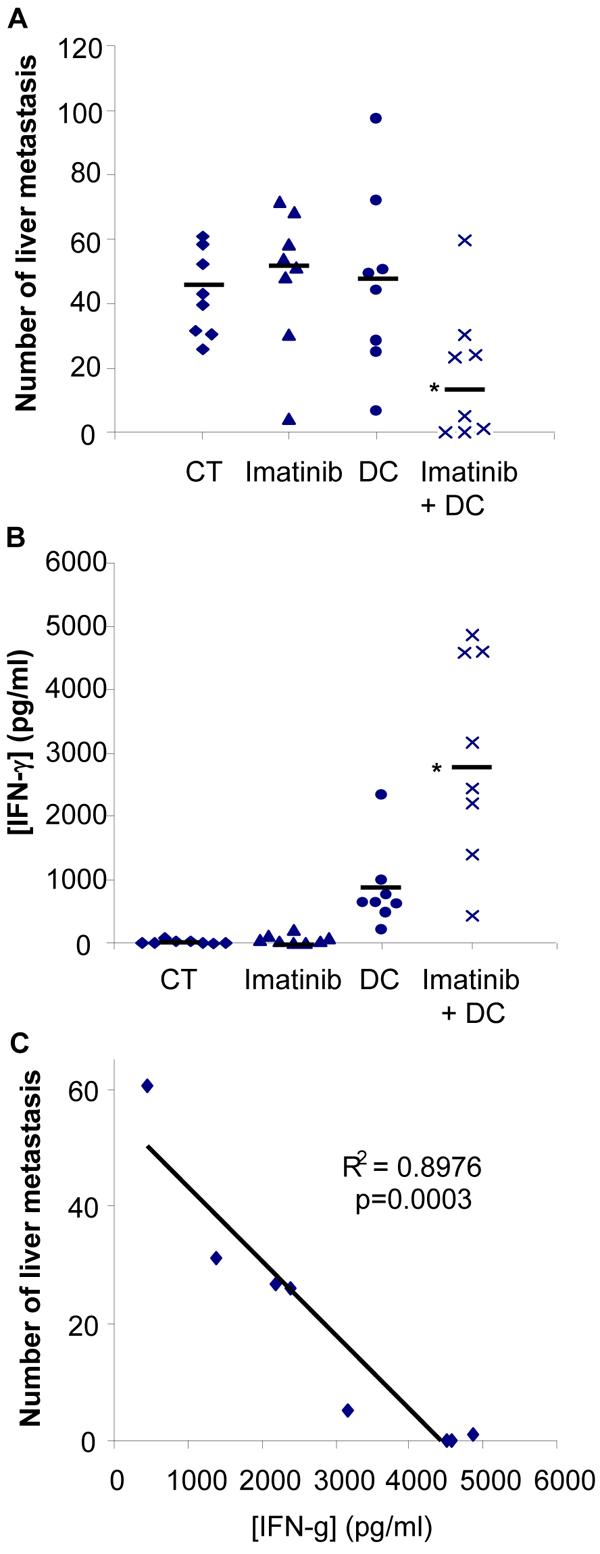
Female BALB/c mice (8 mice per group) inoculated at day 0 with A20 cells (5 × 105 cells, tail vein injection) were treated with imatinib mesylate (gavage, 300 mg/kg, twice daily for 7 days) or control water. DC vaccination (bone marrow-derived DC pulsed with A20 tumor lysate) was given at days 5 and 9 (s.c., 106 DC per mouse). On day 21 mice were euthanized and the number of liver metastases was determined by two investigators (A). *, p<0.05. IFN-γ concentration in the supernatants of cultured spleen cells (1 × 105 cells in 200 μl medium /well) from each individual mouse was detected by ELISA after 48 hrs incubation (B). *, p<0.05. In the group of mice treated with the combination therapy a negative correlation was found between the levels of IFN-γ produced by splenocytes and the number of liver metastases (C).
Discussion
CD4+CD25+FoxP3+ Treg represent major contributors of tumor-induced tolerance. The benefit of integrating Treg neutralization or elimination in cancer immunotherapy strategies has been advocated in several studies (20, 27, 28, 44). We report here that the widely used tyrosine kinase inhibitor imatinib mesylate may represent an additionnal approach to suppress Treg and may therefore be efficiently combined with immunotherapy against imatinib-resistant tumors. Treg immunosuppressive function and FoxP3 expression are impaired by therapeutic concentrations of imatinib but Treg proliferation is not significantly modified by the inhibitor in vitro. In contrast, a recent study reported inhibition of CD4+CD25+ T cell proliferation after treatment for 72 hours with imatinib (45). However, we found that continuous exposure of activated Treg to imatinib in vitro for period of time exceeding 48 hours results in a significant loss of cell viability, which may explain the reduced BrdU incorporation observed in this report.
We attempted to further identify the molecular mechanisms underlying imatinib-mediated inhibition of Treg activity. The survival, homeostasis and immunosuppressive function of regulatory T cells are dependent on external signals, some of which are relayed by the TCR, CD28, the IL-2 receptor, CTLA-4, GITR, or TGF-β receptors and other yet to be identified molecules (17, 19), converging towards the regulation of specific gene expression such as FoxP3. Particularly, activated STAT3 and STAT5 are required for FoxP3 upregulation in Treg (39, 40). In line with the observed inhibition of FoxP3 expression, the phosphorylation of both transcription factors STAT3 and STAT5 is attenuated by imatinib mesylate. Previous studies have reported that imatinib hinders TCR-dependent activation of conventional T lymphocytes, highlighting the src-family tyrosine kinase Lck as a possible molecular target of this drug (4). In Treg, CD28-induced Lck activation is critical for STAT3 induction (39). These data prompted us to examine whether imatinib mesylate may interfere with these TCR/CD28 early cell signaling events in Treg. The tyrosine kinase inhibitor alters the phosphorylation status of ZAP-70 and LAT, located immediately downstream Lck in the signal transduction cascade. These observations thus substantiate and provide a molecular basis for the attenuation of Treg suppressive activity by imatinib. However, if the intracellular events leading to conventional T lymphocyte activation are well-characterized, the signaling pathways and their regulation controlling Treg activity are more elusive, and it is thus possible that imatinib suppresses other unidentified key tyrosine kinases involved in the immunosuppressive function of these cells.
Modulation of Treg activity by imatinib mesylate in vivo had not been studied. In line with our in vitro observations, treatment of animals for seven days with imatinib reduced Treg number and dampened their suppressive function. The potential benefit of combining imatinib with immunotherapy to treat CML patients has been highlighted in different clinical trials (46, 47). We have previously documented that imatinib mesylate can be successfully combined with specific anti-tumor vaccination to treat established BCR-ABL+ leukemia in mouse (48). However, since imatinib directly induces BCR-ABL+ leukemia cell death, the observed synergistic effects with tumor vaccination could be related to the extensive release of tumor antigens as well as ‘danger’, proinflammatory signals associated with the liberation of the intracellular contents of the cells. The distinction between the killing effects of imatinib and its immunomodulatory function was therefore difficult to ascertain. Thus, although imatinib has been successfully combined with tumor vaccines to treat cancers that are sensitive to the cytotoxic effects of this drug, the integration of imatinib in chemoimmunotherapeutic strategies to treat malignancies that are resistant to direct killing by this molecule had not been previously examined. We therefore sought to further investigate the physiologic relevance of Treg inhibition by imatinib using a tumor model, A20 lymphoma, that is resistant to the cytotoxic activity of the drug. Such an approach allowed for the evaluation of the synergistic effects of imatinib with immunotherapy separately from its direct tumoricidal properties. The dose of imatinib given to animals lead to plasma levels comparable to the concentrations used to treat cells in vitro (37, 43). Our data demonstrate that imatinib significantly enhances the efficacy of DC-based immunization, resulting in a reduction of the number of liver metastases that correlates with higher IFN-γ production by the splenocytes of treated animals. These results thus provide the proof of principle that imatinib can be successfully incorporated in immunotherapy protocols against imatinib resistant tumors. Although humans differ from mice in many different aspects (genetic heterogeneity, personal medical history, previous immunosuppressive therapies etc.), our data further support previous sudies indicating that imatinib may improve responses to tumor-specific vaccination (46, 47, 49, 50).
Our current findings uncover Treg as novel targets for imatinib mesylate and further advocate for the combination of this tyrosine kinase inhibitor with tumor vaccination strategies against malignancies that are typically not affected by imatinib alone. The therapeutic applications of this well-tolerated drug may thus be broadened with its use as an immunomodulator in cancer chemoimmunotherapy protocols.
Supplementary Material
Acknowledgments
Grant support: This work was supported in part by the NIH grant R01 CA104926, the Leukemia and Lymphoma Society Fellow Award 5188-07 (NL), the Tee Up for Tots, and Raise a Racquet for Kids Funds.
Abbreviations
- BCR-ABL
Breakpoint Cluster Region-Abelson
- CML
Chronic Myelogenous Leukemia
- FoxP3
Forkhead box P3
- GIST
gastrointestinal stromal tumors
- LAT
Linker of Activation of T cells
- Lck
Leukocyte-specific protein tyrosine kinase
- PDGF-R
platelet-derived growth factor receptor
References
- 1.Druker BJ, Lydon NB. Lessons learned from the development of an abl tyrosine kinase inhibitor for chronic myelogenous leukemia. J. Clin. Invest. 2000;105:3–7. doi: 10.1172/JCI9083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savage DG, Antman KH. Imatinib mesylate--a new oral targeted therapy. N. Engl. J. Med. 2002;346:683–693. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- 3.Carroll M, Ohno-Jones S, Tamura S, Buchdunger E, Zimmermann J, Lydon NB, Gilliland DG, Druker BJ. CGP 57148, a tyrosine kinase inhibitor, inhibits the growth of cells expressing BCR-ABL, TEL-ABL, and TEL-PDGFR fusion proteins. Blood. 1997;90:4947–4952. [PubMed] [Google Scholar]
- 4.Seggewiss R, Lore K, Greiner E, Magnusson MK, Price DA, Douek DC, Dunbar CE, Wiestner A. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105:2473–2479. doi: 10.1182/blood-2004-07-2527. [DOI] [PubMed] [Google Scholar]
- 5.Dietz AB, Souan L, Knutson GJ, Bulur PA, Litzow MR, Vuk-Pavlovic S. Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood. 2004;104:1094–1099. doi: 10.1182/blood-2003-12-4266. [DOI] [PubMed] [Google Scholar]
- 6.Mumprecht S, Matter M, Pavelic V, Ochsenbein AF. Imatinib mesylate selectively impairs expansion of memory cytotoxic T cells without affecting the control of primary viral infections. Blood. 2006;108:3406–3413. doi: 10.1182/blood-2006-04-018705. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Schmitt A, Chen B, Rojewski M, Ringhoffer M, von Harsdorf S, Greiner J, Guillaume P, Dohner H, Bunjes D, Schmitt M. Imatinib impairs CD8+ T lymphocytes specifically directed against the leukemia-associated antigen RHAMM/CD168 in vitro. Cancer Immunol. Immunother. 2007;56:849–861. doi: 10.1007/s00262-006-0232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boissel N, Rousselot P, Raffoux E, Cayuela JM, Soulier J, Mooney N, Charron D, Dombret H, Toubert A, Rea D. Imatinib mesylate minimally affects bcr-abl+ and normal monocyte-derived dendritic cells but strongly inhibits T cell expansion despite reciprocal dendritic cell-T cell activation. J. Leukoc. Biol. 2006;79:747–756. doi: 10.1189/jlb.0705419. [DOI] [PubMed] [Google Scholar]
- 9.Gao H, Lee BN, Talpaz M, Donato NJ, Cortes JE, Kantarjian HM, Reuben JM. Imatinib mesylate suppresses cytokine synthesis by activated CD4 T cells of patients with chronic myelogenous leukemia. Leukemia. 2005;19:1905–1911. doi: 10.1038/sj.leu.2403933. [DOI] [PubMed] [Google Scholar]
- 10.Appel S, Rupf A, Weck MM, Schoor O, Brummendorf TH, Weinschenk T, Grunebach F, Brossart P. Effects of imatinib on monocyte-derived dendritic cells are mediated by inhibition of nuclear factor-kappaB and Akt signaling pathways. Clin. Cancer Res. 2005;11:1928–1940. doi: 10.1158/1078-0432.CCR-04-1713. [DOI] [PubMed] [Google Scholar]
- 11.Appel S, Boehmler AM, Grunebach F, Muller MR, Rupf A, Weck MM, Hartmann U, Reichardt VL, Kanz L, Brummendorf TH, Brossart P. Imatinib mesylate affects the development and function of dendritic cells generated from CD34+ peripheral blood progenitor cells. Blood. 2004;103:538–544. doi: 10.1182/blood-2003-03-0975. [DOI] [PubMed] [Google Scholar]
- 12.Taieb J, Maruyama K, Borg C, Terme M, Zitvogel L. Imatinib mesylate impairs Flt3L-mediated dendritic cell expansion and antitumor effects in vivo. Blood. 2004;103:1966–1967. doi: 10.1182/blood-2003-10-3475. author reply 1967. [DOI] [PubMed] [Google Scholar]
- 13.Wehner R, Wendisch M, Schakel K, Bornhauser M, Platzbecker U, Mohr B, Temme A, Bachmann M, Rieber EP, Schmitz M. Imatinib mesylate does not impair the immunogenicity of human myeloid blood dendritic cells. Leukemia. 2006;20:1629–1632. doi: 10.1038/sj.leu.2404315. [DOI] [PubMed] [Google Scholar]
- 14.Wang H, Cheng F, Cuenca A, Horna P, Zheng Z, Bhalla K, Sotomayor EM. Imatinib mesylate (STI-571) enhances antigen-presenting cell function and overcomes tumor-induced CD4+ T-cell tolerance. Blood. 2005;105:1135–1143. doi: 10.1182/blood-2004-01-0027. [DOI] [PubMed] [Google Scholar]
- 15.Borg C, Terme M, Taieb J, Menard C, Flament C, Robert C, Maruyama K, Wakasugi H, Angevin E, Thielemans K, Le Cesne A, Chung-Scott V, Lazar V, Tchou I, Crepineau F, Lemoine F, Bernard J, Fletcher JA, Turhan A, Blay JY, Spatz A, Emile JF, Heinrich MC, Mecheri S, Tursz T, Zitvogel L. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J. Clin. Invest. 2004;114:379–388. doi: 10.1172/JCI21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 17.Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389–400. doi: 10.1038/nri821. [DOI] [PubMed] [Google Scholar]
- 18.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J. Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 20.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 21.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J. Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 22.Viguier M, Lemaitre F, Verola O, Cho MS, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J. Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 23.Larmonier N, Marron M, Zeng Y, Cantrell J, Romanoski A, Sepassi M, Thompson S, Chen X, Andreansky S, Katsanis E. Tumor-derived CD4(+)CD25 (+) regulatory T cell suppression of dendritic cell function involves TGF-beta and IL-10. Cancer Immunol. Immunother. 2007;56:48–59. doi: 10.1007/s00262-006-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valzasina B, Piconese S, Guiducci C, Colombo MP. Tumor-induced expansion of regulatory T cells by conversion of CD4+CD25-lymphocytes is thymus and proliferation independent. Cancer Res. 2006;66:4488–4495. doi: 10.1158/0008-5472.CAN-05-4217. [DOI] [PubMed] [Google Scholar]
- 25.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J. Exp. Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larmonier N, Cantrell J, Lacasse C, Li G, Janikashvili N, Situ E, Sepassi M, Andreansky S, Katsanis E. Chaperone-rich tumor cell lysate-mediated activation of antigen-presenting cells resists regulatory T cell suppression. J. Leukoc. Biol. 2008;83:1049–1059. doi: 10.1189/jlb.0907635. [DOI] [PubMed] [Google Scholar]
- 27.Prasad SJ, Farrand KJ, Matthews SA, Chang JH, McHugh RS, Ronchese F. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+CD25+ regulatory T cells. J. Immunol. 2005;174:90–98. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 28.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Attia P, Powell DJ, Jr., Maker AV, Kreitman RJ, Pastan I, Rosenberg SA. Selective elimination of human regulatory T lymphocytes in vitro with the recombinant immunotoxin LMB-2. J. Immunother. 2006;29:208–214. doi: 10.1097/01.cji.0000187959.45803.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105:2845–2851. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 31.Comes A, Rosso O, Orengo AM, Di Carlo E, Sorrentino C, Meazza R, Piazza T, Valzasina B, Nanni P, Colombo MP, Ferrini S. CD25+ regulatory T cell depletion augments immunotherapy of micrometastases by an IL-21-secreting cellular vaccine. J. Immunol. 2006;176:1750–1758. doi: 10.4049/jimmunol.176.3.1750. [DOI] [PubMed] [Google Scholar]
- 32.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 33.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 34.Zeng Y, Graner MW, Feng H, Li G, Katsanis E. Imatinib mesylate effectively combines with chaperone-rich cell lysate-loaded dendritic cells to treat bcr-abl+ murine leukemia. Int. J. Cancer. 2004;110:251–259. doi: 10.1002/ijc.20115. [DOI] [PubMed] [Google Scholar]
- 35.Zeng Y, Chen X, Larmonier N, Larmonier C, Li G, Sepassi M, Marron M, Andreansky S, Katsanis E. Natural killer cells play a key role in the antitumor immunity generated by chaperone-rich cell lysate vaccination. Int. J. Cancer. 2006;119:2624–2631. doi: 10.1002/ijc.22150. [DOI] [PubMed] [Google Scholar]
- 36.Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R, Ohno-Jones S, Sawyers CL. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 37.Wolff NC, Randle DE, Egorin MJ, Minna JD, Ilaria RL., Jr. Imatinib mesylate efficiently achieves therapeutic intratumor concentrations in vivo but has limited activity in a xenograft model of small cell lung cancer. Clin Cancer Res. 2004;10:3528–3534. doi: 10.1158/1078-0432.CCR-0957-03. [DOI] [PubMed] [Google Scholar]
- 38.Ring S, Thome M, Pretsch L, Enk AH, Mahnke K. Expanded murine regulatory T cells: analysis of phenotype and function in contact hypersensitivity reactions. J. Immunol. Methods. 2007;326:10–21. doi: 10.1016/j.jim.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Pallandre JR, Brillard E, Crehange G, Radlovic A, Remy-Martin JP, Saas P, Rohrlich PS, Pivot X, Ling X, Tiberghien P, Borg C. Role of STAT3 in CD4+CD25+FOXP3+ regulatory lymphocyte generation: implications in graft-versus-host disease and antitumor immunity. J. Immunol. 2007;179:7593–7604. doi: 10.4049/jimmunol.179.11.7593. [DOI] [PubMed] [Google Scholar]
- 40.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, Bellucci R, Raderschall E, Canning C, Soiffer RJ, Frank DA, Ritz J. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006 doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thornton AM, Piccirillo CA, Shevach EM. Activation requirements for the induction of CD4+CD25+ T cell suppressor function. Eur. J. Immunol. 2004;34:366–376. doi: 10.1002/eji.200324455. [DOI] [PubMed] [Google Scholar]
- 42.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat. Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 43.Wolff NC, Richardson JA, Egorin M, Ilaria RL., Jr. The CNS is a sanctuary for leukemic cells in mice receiving imatinib mesylate for Bcr/Abl-induced leukemia. Blood. 2003;101:5010–5013. doi: 10.1182/blood-2002-10-3059. [DOI] [PubMed] [Google Scholar]
- 44.Knutson KL, Dang Y, Lu H, Lukas J, Almand B, Gad E, Azeke E, Disis ML. IL-2 immunotoxin therapy modulates tumor-associated regulatory T cells and leads to lasting immune-mediated rejection of breast cancers in neutransgenic mice. J. Immunol. 2006;177:84–91. doi: 10.4049/jimmunol.177.1.84. [DOI] [PubMed] [Google Scholar]
- 45.Chen J, Schmitt A, Giannopoulos K, Chen B, Rojewski M, Dohner H, Bunjes D, Schmitt M. Imatinib impairs the proliferation and function of CD4+CD25+ regulatory T cells in a dose-dependent manner. Int. J. Oncol. 2007;31:1133–1139. [PubMed] [Google Scholar]
- 46.Bocchia M, Gentili S, Abruzzese E, Fanelli A, Iuliano F, Tabilio A, Amabile M, Forconi F, Gozzetti A, Raspadori D, Amadori S, Lauria F. Effect of a p210 multipeptide vaccine associated with imatinib or interferon in patients with chronic myeloid leukaemia and persistent residual disease: a multicentre observational trial. Lancet. 2005;365:657–662. doi: 10.1016/S0140-6736(05)17945-8. [DOI] [PubMed] [Google Scholar]
- 47.Li Z, Qiao Y, Liu B, Laska EJ, Chakravarthi P, Kulko JM, Bona RD, Fang M, Hegde U, Moyo V, Tannenbaum SH, Menoret A, Gaffney J, Glynn L, Runowicz CD, Srivastava PK. Combination of imatinib mesylate with autologous leukocyte-derived heat shock protein and chronic myelogenous leukemia. Clin. Cancer Res. 2005;11:4460–4468. doi: 10.1158/1078-0432.CCR-05-0250. [DOI] [PubMed] [Google Scholar]
- 48.Zeng Y, Feng H, Graner MW, Katsanis E. Tumor-derived, chaperone-rich cell lysate activates dendritic cells and elicits potent antitumor immunity. Blood. 2003;101:4485–4491. doi: 10.1182/blood-2002-10-3108. [DOI] [PubMed] [Google Scholar]
- 49.Bocchia M, Abruzzese E, Forconi F, Ippoliti M, Trawinska MM, Pirrotta MT, Raspadori D, Tozzi M, Gozzetti A, Lauria F. Imatinib does not impair specific antitumor T-cell immunity in patients with chronic myeloid leukemia. Leukemia. 2006;20:142–143. doi: 10.1038/sj.leu.2404029. [DOI] [PubMed] [Google Scholar]
- 50.Chen CI, Maecker HT, Lee PP. Development and dynamics of robust T-cell responses to CML under imatinib treatment. Blood. 2008;111:5342–5349. doi: 10.1182/blood-2007-12-128397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.