Abstract
Background & Aims
The follicle associated epithelium (FAE) plays key roles in antigen uptake and subsequent induction of mucosal immunity. In this study, we examined whether M cell targeting using a protein antigen (Ag) delivery system would induce oral tolerance instead of enhancement of Ag-specific mucosal antibody (Ab) responses.
Methods
Mice were fed different doses of a recombinant protein sigma one of reovirus genetically conjugated to ovalbumin (OVA-pσ1), pσ1 only or PBS prior to oral challenge with OVA plus cholera toxin (CT) as mucosal adjuvant. OVA-specific Ab and CD4-positive (CD4+) T cell responses were determined.
Results
A low dose of OVA-pσ1 reduced anti-OVA Ab and CD4+ T cell responses in both mucosal and systemic lymphoid tissues. OVA / MHC I-Ad tetramer staining revealed that the numbers of OVA-specific CD4+ T cells were significantly reduced in lamina propria of mice fed OVA-pσ1 than of those fed pσ1 only or PBS only. In fact, Foxp3 expressing CD25+, CD4+ T cells were markedly increased in this tissue. Nonetheless, CD25+, CD4+ T cells from the spleen, mesenteric lymph nodes and Peyer’s patches of orally tolerized mice showed increased TGF-β1 and IL-10 production when compared with non-tolerized mice.
Conclusions
These results show that a FAE M cell targeting protein Ag delivery system facilitates oral tolerance induction due to a reduction in Ag-specific CD4+ T cells and increased levels of TGF-β1 and IL-10 producing, CD25+, CD4+ regulatory T cells in both systemic and mucosal lymphoid tissues.
Introduction
Oral administration of a single high dose or repeated low doses of protein has been shown to induce systemic unresponsiveness, presumably in the presence of mucosal IgA Ab responses.1, 2 In earlier studies, this type of immune response was dubbed oral tolerance and the concept was used to refer specifically to immune responses elicited in mucosa-associated as opposed to systemic lymphoid tissues.3 However, our previous studies showed that tolerance induction occurred in the mucosal effector lymphoid tissues.4 This unique response is an important natural physiological mechanism whereby the host presumably avoids development of hypersensitivity reactions to many ingested food proteins and other antigens.5 Thus, tolerance (or systemic unresponsiveness) represents the most common response of the host to the environment. In addition to showing tolerance to several thousand different food proteins, the host tolerates indigenous microflora which colonize the large intestine. Further, the development of mucosal tolerance against pollen and dust Ags could also be essential or the inhibition of allergic reactions, including IgE-mediated hypersensitivity.
It is now generally agreed that oral tolerance is established and maintained at the level of T cells.6–8 Recent studies have identified dendritic cells (DCs) as key players in the direct or indirect (via T cells) induction of oral tolerance.9–12 Though the precise mechanisms by which oral delivery of Ag elicits a state of systemic unresponsiveness are not fully understood, the dosage of Ag has been shown to be an important factor.13–16 On the other hand, repeated delivery of low doses of protein induces cytokine-mediated active immune suppression characterized by the presence of regulatory T cells, which include TGF-β1-producing Th3 cells and IL-10-producing T regulatory one (Tr1) cells or CD4+ CD25+ T regulatory (Treg) cells.17–19 Regulatory-type T cells were first rediscovered as acquired-type Tr1 cells playing a central role in suppressing inflammatory bowel disease development.18 Acquired-type Treg cells, which differentiate from naïve T cells, regulate tolerance to food Ags, bacterial flora and pathogens by producing suppressive cytokines such as TGF-β1 and IL-10.20 In contrast, naturally occurring CD4+ CD25+ T cells or innate-type Treg cells, which are also suppressive, control the proliferation, expansion and differentiation of naïve T cells in a direct cell contact manner21 and migrate preferentially to lymphoid tissues, mainly the spleen.20
We have developed an M cell-targeting Ag delivery system using recombinant reovirus protein sigma one (pσ1).22, 23 Incorporation of pσ1 into liposomes allows the latter to bind to mouse L cells and rat Peyer’s patches,24 and the recombinant pσ1 is also known to bind to NALT M cells.22, 23 In marked contrast to results seen when DNA is given alone, immunization with DNA complexed to poly-L-lysine- conjugated pσ1 leads to elevated S-IgA and plasma IgG Ab responses.23 Accordingly, we have generated recombinant pσ1 of reovirus genetically fused to OVA (OVA-pσ1) and examined whether oral administration of OVA-pσ1 facilitates systemic and mucosal tolerance induction.
Materials and Methods
Mice
BALB/c mice were purchased from the Frederick Cancer Research facility (Frederick, MD). Mice were housed in microisolators, maintained in horizontal laminar flow cabinets, and provided sterile food and water as part of a specific-pathogen-free facility in the University of Alabama at Birmingham (UAB). All mice used in these experiments were free of bacterial and viral pathogens. All of the animal studies were done in accordance with both NIH and UAB institutional guidelines.
Construction of OVA-pσ1 and Oral Immunization
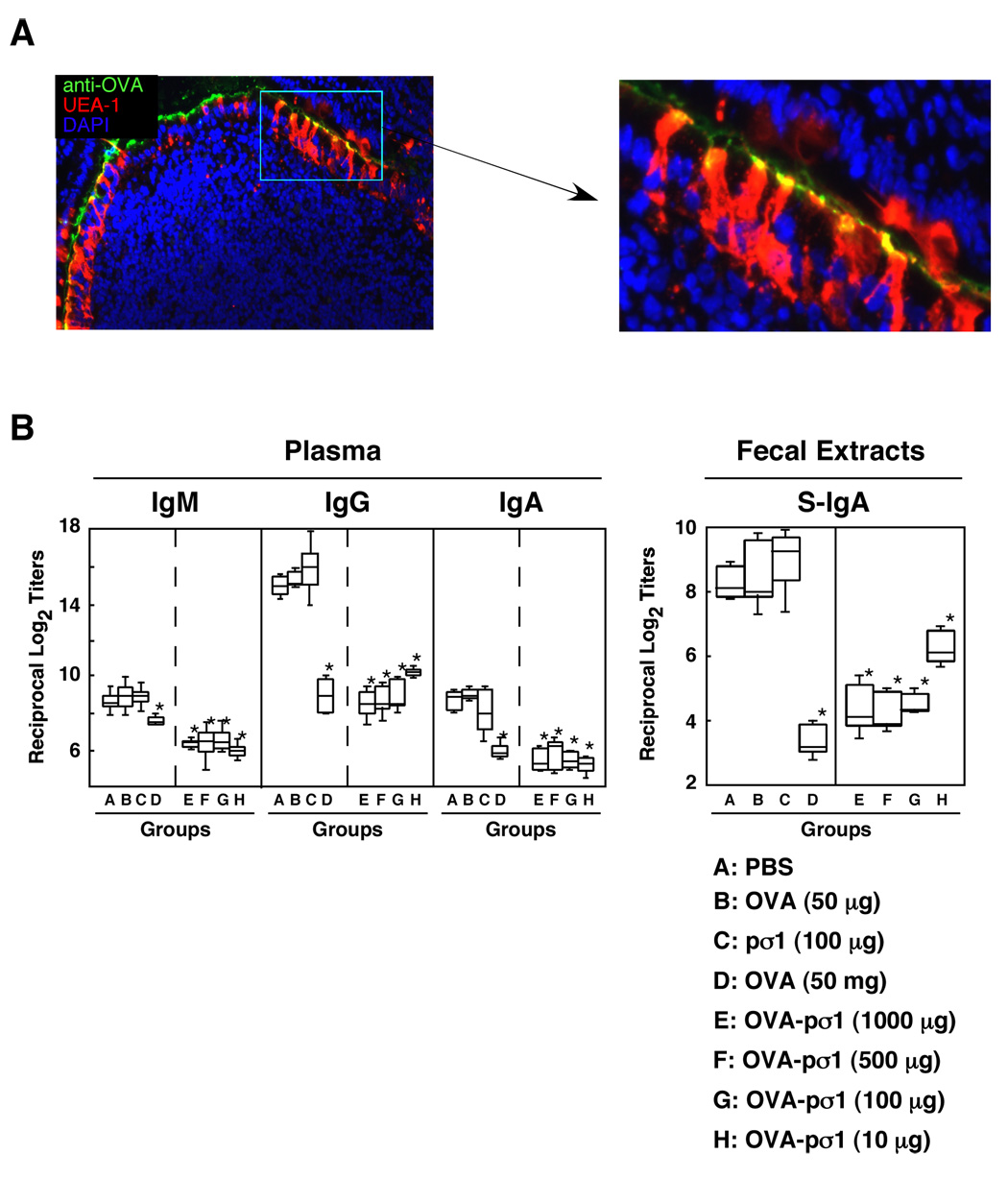
PCR was used to obtain the cloned pσ1 cDNA from reovirus serotype 3 strain Dearing as previously described.22 Ovalbumin (OVA) was genetically fused to pσ1’s N-terminus and is referred to as OVA-pσ1. The OVA-pσ1 was produced using a Pichia pastoris yeast expression system as a his-tag labeled protein and this product specifically bind to M cells (Fig. 1A). Mice were gastrically intubated with different doses of OVA-pσ1 or OVA dissolved in 0.25 ml of PBS. Control mice received PBS or pσ1 only. Seven days later, mice were orally immunized with 1 mg of OVA plus 15 µg of CT three times at weekly intervals.4
Figure 1.
Optimization of OVA-pσ1 for the induction of mucosal tolerance. (A) Frozen section of Peyer’s patches of mice given oral OVA-pσ1 (100 µg) were stained with PE-labeled UEA-1 and biotinylated anti-OVA mAb followed by Alexa488®. The sections were then counterstained with DAPI for histochemical analysis. The picture is a typical example of results of immunofluorescence analysis of over 5 samples. (B) BALB/c mice were fed a single dose of OVA-pσ1 (1000, 500, 100 or 10 µg) prior to oral challenge with OVA plus CT three times at weekly intervals. Other groups of mice were given 50 µg or 50 mg of OVA, 100 µg of pσ1 or PBS orally before oral challenge. Plasma and fecal extract samples were collected 7 days after the last oral challenge and subjected to OVA-specific ELISA. The results represent the median, the upper and lower quartiles and the maximum and minimum values for 12 mice in each experimental group and were taken from three separate experiments. *p < 0.05 when compared with mice fed 50 µg of OVA, pσ1only or PBS alone.
OVA-Specific Antibody Assays
OVA-specific antibody (Ab) levels in plasma and mucosal secretions were determined by an ELISA as previously described.4, 25, 26 The spleen and MLNs were removed aseptically and single-cell suspensions prepared in RPMI 1640 (Cellgro Mediatech, Washington, DC) containing supplemental buffers and antibiotics (incomplete medium) by passage through sterile wire mesh screens.4, 25 Peyer’s patches (PPs) were carefully excised from the small intestinal wall and dissociated using the neutral protease enzyme collagenase type IV (Sigma) in incomplete RPMI 1640 to obtain single-cell preparations.4 Mononuclear cells in the iLP were isolated after removal of PP and intraepithelial lymphocytes from the small intestine using a combination of enzymatic dissociation and discontinuous Percoll gradients (Amersham Biosciences, Pittsburgh, PA).4, 25 Mononuclear cells obtained from mucosal and systemic lymphoid tissues were resuspended in RPMI 1640 containing 10 % FCS (complete RPMI 1640) and subjected to an ELISPOT assay in order to detect numbers of OVA-specific Ab-forming cells (AFCs).4, 25–27 The numbers of OVA-specific AFCs were quantified using an ImmunoSpot® Analyzer (Cellular Technology Ltd., Cleveland, OH).26
Delayed Type Hypersensitivity (DTH) Responses
OVA-specific DTH responses were measured as previously described.4, 28, 29 Briefly, PBS (20 µl) containing 10 µg of OVA was injected into the left ear pinna of mice while the right ear pinna received a PBS control injection. Ear swelling was measured 24 hr later with a dial thickness gauge (Ozaki Manufacturing Co., LTD., Tokyo, JAPAN). The DTH response was expressed as the increase in ear swelling after OVA injection minus the swelling in the PBS-injected control site.
Ag-Specific T Cell Responses
CD4+ T cells from spleen, MLNs, and PPs were purified by use of an automated magnetic activated cell sorter (AutoMACS) system (Miltenyi Biotec, Auburn, CA), as described previously.26 The purified CD4+ T cell fraction (> 97 % pure, > 99 % viable) was then suspended in complete RPMI 1640 (4 × 106 cells/ml) and cultured with or without one mg/ml OVA in the presence of T cell-depleted, irradiated (3000 rad) splenic antigen-presenting cells (APCs) taken from non-immunized mice for five days. In some experiments, CD4+ T cells were isolated from DO11.10 mice and cultured with CD4+ CD25+ T cells with or without OVA. To assess OVA-specific T cell proliferative responses, an aliquot of 0.5 µCi of tritiated [3H]-TdR (Amersham Biosciences, Arlington Heights, IL) was added during the final 18 hr of incubation, and the amount of [3H]-TdR incorporation was determined by scintillation counting. The supernatants of identically treated T cell cultures not incubated with [3H]-TdR were then subjected to a cytokine-specific ELISA as described below.
Cytokine-Specific ELISA
Levels of cytokines in culture supernatants were measured by an ELISA. The details of the ELISA for IFN-γ, IL-2, IL-4, IL-5, IL-6 and IL-10 have been described previously.4, 25, 26, 28, 30 A mouse TGF-β1 immunoassay kit, Quantikine™ M (R & D systems, Minneapolis, MN), was used to detect TGF-β1 in the culture supernatants. The levels of Ag-specific cytokine production were calculated by subtracting the results of control cultures (e.g., without Ag stimulation) from those of Ag-stimulated cultures.
Flow Cytometry Sorting and Analysis
In order to determine the frequencies of OVA- specific CD4+ T cells, mononuclear cells from spleen, MLNs, PPs and iLP were stained with FITC-conjugated anti-CD4 (GK1.5), biotinylated anti-CD25 (7D4) mAb and PE-labeled OVA/MHC I-Ad tetramer followed by PerCP-Cy5.5-streptavidin before being subjected to flow cytometric analysis. For intracellular IL-10 analysis, cells were incubated with ionomycin (1 µg/ml, SIGMA, St. Louis, MO) and phorbol 12-myristate 13-acetate (PMA, 25 ng/ml, SIGMA) for 6 hr and then stained with PE-labeled anti-CD4, biotinylated anti-CD25 mAbs followed by PerCP-Cy5.5-streptavidin. These samples were further stained intracellularly with Alexa Fluor® 488 labeled anti-IL-10 mAb (JES5-16E3). In some experiments, cells were stained with FITC-labeled anti-CD4 and biotinylated anti-CD25 mAb followed by PE-streptavidin. CD4+ CD25+ T cells were purified by flow cytometry and their TGF-β1 production was determined as described above. Aliquots of cells were cultured with OVA-specific splenic CD4+ T cells from DO11.10 mice.
Statistics
The significance of the difference (e.g., p values) among groups was evaluated by the Mann Whitney U test using a Statview II program designed for Macintosh computers.
Results
Optimization of Oral Doses of OVA-pσ1
Since it has been shown that pσ1 can bind to mucosal M cells,23 we hypothesized that oral tolerance could also be effectively achieved using OVA-pσ1. OVA-specific plasma IgG and IgA as well as fecal S-IgA Ab titers were not reduced in mice given 50 µg of OVA, 100 µg of pσ1 or PBS (Fig. 1). On the other hand, they were significantly more reduced in all other single OVA-pσ1 treatment groups (10 µg, 100 µg, 500 µg or 1000 µg) as well as in the 50 mg OVA group than in mice fed pσ1 or PBS only (Fig. 1). Further, OVA-specific plasma IgA and mucosal S-IgA Ab responses in mouse groups receiving one feeding of OVA-pσ1 were markedly lower than in the positive control groups (Fig. 1). These results show that a single oral dose of OVA-pσ1 effectively induces both systemic and mucosal unresponsiveness to OVA. Based upon these results, we next employed a single oral dose of 100 µg of OVA-pσ1 for further experiments.
Oral OVA-pσ1 Facilitates Both Systemic and Mucosal Unresponsiveness
To further confirm these findings at the cellular level, we next examined the numbers of OVA-specific Ab-forming cells (AFCs) in various lymphoid tissues of mice given oral OVA-pσ1, pσ1 or PBS. Numbers of OVA-specific IgG and IgA AFCs in spleen and MLNs were reduced significantly (p <0.05) in the oral OVA-pσ1-group when compared with the oral PBS-group (Fig. 2), showing that oral tolerance is indeed induced by feeding 100 µg of OVA-pσ1. In order to assess induction of unresponsiveness in mucosal effector sites, the numbers of OVA-specific AFCs in iLP were compared in groups fed OVA-pσ1, pσ1 only or PBS alone. The numbers of anti-OVA IgA AFCs was reduced in the OVA-pσ1-group when compared with the oral PBS-group (Fig. 2). The oral pσ1-group showed essentially the same anti-OVA AFC responses as the oral PBS-group (data not shown). These results suggest that M cell targeting by OVA-pσ1 effectively induces mucosal tolerance and may contribute to the maintenance of mucosal homeostasis.
Figure 2.
Numbers of OVA-specific AFCs in various lymphoid tissues. BALB/c mice were fed 100 µg of OVA-pσ1 prior to oral challenge with OVA plus CT three times at weekly intervals. As controls, mice were fed PBS prior to oral challenge with OVA plus CT. Mononuclear cells from spleen, MLNs and iLP were isolated 7 days after the last oral challenge and subjected to OVA-specific ELISPOT assays. The results represent the median, the upper and lower quartiles and the maximum and minimum values for 12 mice in each experimental group and are taken from three separate experiments. *p < 0.05 when compared with mice fed PBS.
DTH and CD4+ T Cell Proliferative Responses
We next determined whether tolerance was induced at the T cell level after a single oral dose of OVA-pσ1. We assessed OVA-specific delayed-type hypersensitivity (DTH) responses. OVA-specific DTH responses were much more pronounced in the OVA-pσ1-group than in the pσ1- and PBS-group (Fig. 3A), showing that OVA-specific T cell responses were tolerized by a single low dose of OVA-pσ1. We next examined CD4+ T cell proliferative responses in both mucosal (MLNs and PPs) and systemic (spleen) compartments of mice given oral OVA-pσ1. Purified CD4+ T cell cells from spleen, PPs, and MLNs were cultured with or without one mg/ml of OVA in the presence of T cell-depleted, irradiated splenic APCs taken from non-immunized, normal mice. Significant reductions in T cell proliferative responses were seen in the spleen, MLNs and PPs of the OVA-pσ1- when compared with the pσ1- and PBS-group (Fig. 3B). These results suggest that T cell unresponsiveness was initiated in mucosal inductive tissues such as the PPs, by M cell targeting of OVA-pσ1.
Figure 3.
OVA-specific DTH responses and OVA-specific CD4+ T cell proliferative responses. (A) Six days after the last oral challenge, OVA-pσ1-, pσ1- and PBS-fed groups of mice were injected with 10 µg of OVA (20 µl ) into the right ear pinna. PBS (20 µl) was administered to the left ear pinna as a control. The DTH response (24 hr) was expressed as the increase in ear swelling after challenge with Ag after subtraction of swelling in the control site. (B) Immediately after the DTH responses were examined, CD4+ T cells were purified from OVA-pσ1-, pσ1- and PBS-fed mice. Purified CD4+ T cell fractions were cultured with or without one mg/ml of OVA in the presence of APCs for 5 days. An aliquot of 0.5 µCi of tritiated [3H]-thymidine was added during the final 18 hr of incubation, and the amount of [3H]-thymidine incorporation was determined by scintillation counting. The stimulation index was determined as cpm of wells with Ag / cpm of wells without Ag (controls). The levels of [3H] TdR incorporated in each control well ranged from 500 to 1,000 cpm. The results represent the median, the upper and lower quartiles and the maximum and minimum values from three separate experiments (triplicate wells / experiment). *p < 0.05 when compared with mice fed pσ1only or PBS alone.
Cytokine Production by OVA-Stimulated CD4+ T Cells
Since T cell unresponsiveness was induced in both systemic and mucosal lymphoid tissues by a single oral dose of OVA-pσ1, we next examined Th1- and Th2-type cytokine production by OVA-stimulated CD4+ T cells. When culture supernatants were harvested and examined by cytokine-specific ELISA, OVA-pσ1-fed mice showed reduced CD4+ Th1 (IFN-γ and IL-2) and Th2 (IL-4, IL-5, IL-6 and IL-10) cytokine responses, while mice fed oral PBS showed high levels of Th2-type cytokines, especially IL-4 and IL-10 (Table 1). A virtually identical profile of up-regulation of Th1- and Th2-type cytokine synthesis was seen in the spleen and PPs of mice following oral administration of pσ1 only (data not shown). On the other hand, a hyporesponsive Th1- and Th2-type cytokine profile was noted in both PPs and spleen of mice fed OVA-pσ1 before being orally challenged with OVA plus CT (Table 1). Taken together, these results indicate that CD4+ T cell unresponsiveness was induced in both spleen and PPs by a single oral dose of OVA-pσ1.
Table 1.
CD4+ Th1 and Th2 Cytokine Synthesis by OVA-Specific CD4+ T Cellsa.
| Lymphoid Tissue | Orally Immunized With | Th1 typeb | Th2 typeb | ||||
|---|---|---|---|---|---|---|---|
| IFN-γ (ng/ml) | IL-2 (ng/ml) | IL-4 pg/ml) | IL-5 (ng/ml) | IL-6 ng/ml) | IL-10 (ng/ml) | ||
| Spleen | PBS | 5.9 ± 1.3c | 0.9 ± 0.18 | 477 ± 2.6 | 4.54 ± 0.2 | 1.28 ± 0.05 | 44.5 ± 2.9 |
| OVA-pσ1 | 0.3 ± 0.02d | 0.19 ± 0.02e | 30 ± 0.8d | 0.18 ± 0.02d | 0.07 ± 0.02d | 1.8 ± 0.3d | |
| Peyer’s patches | PBS | 4.2 ± 1.7 | 1.6 ± 0.05 | 420 ± 3.0 | 3.1 ± 0.2 | 0.8 ± 0.08 | 40.8 ± 1.1 |
| OVA-pσ1 | 0.3 ± 0.03d | 0.15 ± 0.01d | 110 ± 1.1e | 0.27 ± 0.02e | 0.12 ± 0.01e | 2.2 ± 0.2d | |
Splenic CD4+ T cells (2 × 106 / ml) from each group of mice were cultured with 1 mg / ml of OVA in the presence of T cell-depleted and irradiated splenic feeder cells (4 × 106 / ml).
Culture supernatants were harvested after 5 days (2 days for IL-2) of incubation and analyzed by the cytokine-specific ELISA.
The results represent the mean ± SEM for 15 mice in each experimental group and are taken from three separate experiments.
p < 0.01
p < 0.05 compared with the PBS group.
Mucosal and Systemic Unresponsiveness Is Due to a Reduction in OVA-Specific CD4+ T Cells
In order to examine the role of OVA-specific CD4+ T cells in oral tolerance, mononuclear cells from spleen, PPs, MLNs and iLP were isolated one week after the last immunization and stained with FITC-conjugated anti-CD4, biotin-conjugated anti-CD25 mAbs and PE-labeled OVA/I-Ad tetramer followed by PerCP-Cy5.5-streptavidin. This analysis revealed a lower number of tetramer+ OVA-specific CD4+ T cells in iLPs of mice given OVA-pσ1 prior to oral challenge with OVA plus CT than in mice given oral PBS (Fig. 4). Numbers of OVA-specific CD4+ T cells were also reduced in PPs, MLNs and spleen of mice given oral OVA-pσ1 (Fig. 4). On the other hand, mice fed pσ1 only resulted in no reductions in OVA-specific CD4+ T cells when compared with those of the PBS-fed group (data not shown). When we examined CD25 expression on CD4+ T cells, the actual cell count of CD4+ CD25+ T cells was found to be significantly increased in iLP of orally tolerized mice (Table 2). On the other hand, the numbers of CD4+ CD25+ T cells in PPs, MLNs and spleen of the tolerized group were slightly increased or essentially the same as those in the non-tolerized group. When Foxp3 expression by CD4+ CD25+ T cells was examined, approximately ten-fold increased Foxp3 expressing CD4+ CD25+ T cells were noted in iLP of orally tolerized mice (Table 2). Elevated numbers of Foxp3+ CD4+ CD25+ T cells were seen in MLNs of tolerized mice; however, this cell population in PPs and spleen was essentially unchanged (Table 2). These results suggest that mucosal and systemic unresponsiveness to orally delivered Ag is most likely due to reduced numbers of OVA-specific CD4+ T cells. In the case of mucosal unresponsiveness, increased numbers of Foxp3+ CD4+ CD25+ T cells in iLP and MLNs may play an additional role to down regulate OVA-specific immune responses, although Foxp3 is not always a marker for acquired-type Treg cells.
Figure 4.
Detection of frequency of OVA-specific CD4+ T cells. Mononuclear cells from the spleen, MLNs, PPs and iLPs of mice fed OVA-pσ1 or PBS were stained with FITC-conjugated anti-CD4 mAb and PE-labeled OVA/I-Ad tetramer. Samples were subjected to flow cytometric analysis using FACSCalibur™. The actual percentages of OVA-specific CD4+ T cells were indicated in the upper right region. The results represent the mean values ± SEM from 12 mice in each experimental group and are taken from three separate experiments. The profiles represent typical results and are taken from one of three separate experiments.
Table 2.
The frequency of OVA-specific CD4+ T cells in various lymphoid tissues a.
| Tissue Assessed | Fed | CD4+ (× 103) | CD4+ CD25+ (× 103) | CD4+ CD25+ Foxp3+ |
|---|---|---|---|---|
| Intestinal lamina propria | PBS | 347 ± 11 | 28 ± 1 | 7 ± 0.6 |
| OVA-pσ1 | 1,109 ± 33 b | 271 ± 8 b | 74 ± 2 b | |
| Peyer’s patches | PBS | 2,370 ± 82 | 449 ± 17 | 337 ± 15 |
| OVA-pσ1 | 3,243 ± 59 | 507 ± 5 | 301 ± 2 | |
| MLNs | PBS | 16,633 ± 300 | 1,692 ± 84 | 632 ± 12 |
| OVA-pσ1 | 15,900 ± 193 | 2,042 ± 81 | 1,586 ± 58 b | |
| Spleen | PBS | 14,987 ± 456 | 2,197 ± 64 | 1,335 ± 41 |
| OVA-pσ1 | 18,325 ± 287 | 2,015 ± 40 | 1,600 ± 30 | |
Mononuclear cells (1 ×106) from various lymphoid tissues of mice fed OVA-pσ1 or PBS were stained with FITC-conjugated anti-CD4 (GK1.5) and biotinylated anti-CD25 (7D4) mAbs as well as PE-labeled OVA/I-Ad tetramer followed by PerCP-Cy5.5-streptavidin. In some experiments, samples were stained interacellularly with PE-labeled anti-Foxp3 mAb. Samples were then subjected to flow cytometry analysis using a FACSCalibur™. The actual cell numbers of the respective cell population are calculated using the average of total cell numbers in various lymphoid tissues from one mouse. The results represent the mean values ± SEM from 12 mice in each experimental group and are taken from three separate experiments.
p < 0.05 compared with PBS-group.
TGF-β1- and IL-10 Producing CD4+ CD25+ T Cells Are Induced by Oral OVA-pσ1
The increased numbers of CD4+ CD25+ T cells suggested the possibility that a regulatory type of CD4+ cells was induced in tolerized mice. To test this possibility, we examined the production of IL-10 and TGF-β1 by CD4+ CD25+ T cells. Flow cytometry-purified CD4+ CD25+ T cells from PPs, MLNs and spleen of mice fed OVA-pσ1, pσ1 only or PBS were stimulated with OVA for 5 days. The culture supernatants of CD4+ CD25+ T cells from orally tolerized mice contained higher levels of TGF-β1 than did those from PBS-fed mice (Fig. 5A). Levels of TGF-β1 production by CD4+ CD25+ T cells in mice fed pσ1 only were essentially the same as those in PBS-fed mice (data not shown). Intracellular IL-10 analysis revealed higher numbers of actual IL-10-producing CD4+ CD25+ T cells in mice fed OVA-pσ1 than in mice fed PBS (Fig. 5B). Of interest, the spleens of orally tolerized mice contained approximately three times more IL-10-producing CD4+ CD25+ T cells when compared with those of non-tolerized mice. CD25+ CD4+ T cells were isolated from PPs of mice given oral OVA-pσ1, pσ1 or PBS in order to test their function. Purified CD25+ CD4+ T cells were cultured with splenic CD4+ T cells from DO11.10 mice in the presence of OVA. The cultures without OVA served as negative controls. The cultures containing CD25+ CD4+ T cells from mice fed OVA-pσ1 resulted in lower proliferative responses than the cultures containing naïve CD4+ T cells or CD25+ CD4+ T cells from mice give oral pσ1 or PBS (Fig. 5C). Taken together, these results demonstrate that both TGF-β1- and IL-10 producing CD4+ CD25+ T cells were induced in the PPs, MLNs and spleen of mice fed OVA-pσ1.
Figure 5.
TGF-β1 and IL-10 production by CD4+ CD25+ T cells. (A) Mice were fed 100 µg of OVA-pσ1 or PBS before being orally immunized weekly for three weeks with 1 mg of OVA plus 10 µg of CT. CD4+ CD25+ T cells were purified from PPs, MLNs and spleen by flow cytometry and cultured with 1 mg/ml of OVA in the presence of irradiated APCs. The levels of TGF-β1 in the culture supernatants were determined by a TGF-β1-specific ELISA. (B) Interleukin-10 production by CD4+ CD25+ T cell subsets in MLNs and spleen were determined by intracellular analysis. Mononuclear cells were incubated with ionomycin and PMA for 6 hr and then stained with PE-anti-CD4, biotin-CD25 mAbs followed by PerCP-Cy5.5- streptavidin. These samples were further stained intracellularly with Alexa Fluor® 488-labeled anti-IL-10 mAb (JES5-16E3). The actual cell numbers of IL-10-producing CD4+ CD25+ T cells are calculated using the average of total cell numbers in various lymphoid tissues from one mouse. (C) CD4+ CD25+ T cells (2 × 105 cells/ml) from PP of mice fed OVA-pσ1, pσ1 or PBS were purified by flow cytometry and cultured with the same numbers of naïve CD4+ T cells isolated from non-immunized, DO11.10 OVA TCR transgenic mice for three days. The wells without OVA served as negative controls. An aliquot of 0.5 mCi of tritiated [3H]TdR was added during the final 18 h of incubation. The results represent the median, the upper and lower quartiles and the maximum and minimum values for 12 mice in each experimental group and from three separate experiments. *p < 0.05 when compared with mice fed pσ1only or PBS alone.
Discussion
The current study shows that the OVA-pσ1 M cell-targeting delivery system facilitates the induction of oral tolerance. Mucosal and systemic unresponsiveness was induced with a single oral dose of 100 µg of OVA-pσ1 instead of the repeated low doses of oral OVA that would otherwise be required. OVA-specific mucosal S-IgA and plasma IgG Ab responses as well as DTH and T cell proliferative responses were all reduced significantly in OVA-pσ1-fed group when compared with pσ1- and PBS-fed mice. Further, OVA-stimulated CD4+ T cells from spleen and PPs of orally tolerized mice showed marked reductions in the levels of both Th1- and Th2-type cytokine production than did those fed pσ1 or PBS. The use of OVA/MHC I-Ad tetramer staining revealed reduced numbers of OVA-specific CD4+ T cells in mice fed OVA-pσ1. Of significance, marked reductions in OVA-specific CD4+ T cells were noted in iLP of tolerized mice. Further, CD4+ CD25+ T cells in the PPs, MLNs and spleen of orally tolerized mice produced higher levels of TGF-β1 than did the control groups. The numbers of IL-10-producing CD4+ CD25+ T cells were significantly increased in spleens of orally tolerized mice. In addition, CD4+ CD25+ T cells from mice fed OVA-pσ1 exhibited the functional property of reducing CD4+ T cell proliferation. These results show that the M cell-targeted Ag delivery by OVA-pσ1 feeding effectively induces mucosal and systemic unresponsiveness which was mediated by reductions in Ag-specific effector CD4+ T cells along with increased numbers of TGF-β1 or IL-10 producing CD4+ CD25+ T cells.
The M cells are known to take up and transport lumenal Ags, including proteins, viruses, bacteria, small parasites, and microspheres.31–34 M cells have been shown to deliver the intact Ag into underlying lymphoid tissue of the GALT.33, 34 M cells are also thought to be involved in Ag processing and presentation since the GALT M cells express MHC class II molecules and acidic endosomal-lysosomal compartments.35 In addition to serving as a means of transport for lumenal Ags, the M cells also provide an entryway for pathogens including Salmonella typhimurium.36 Based upon these findings, M cell-targeted Ag delivery could be assumed to be the normal pathway for induction of Ag-specific immune responses. Indeed, NALT M cell targeting a DNA vaccine constructed with pσ1 elicited Ag-specific IgG and S-IgA Ab responses.23 However, our current study has now shown that oral administration of OVA-pσ1 facilitates unresponsiveness to OVA in both systemic and mucosal lymphoid tissues instead of inducing OVA-specific immunity. Since we showed that pσ1 alone did not induce tolerance or immunity, pσ1 facilitates Ag targeting to M cells rather than stimulating immune responses. Thus, these opposite outcomes can be partially explained by the nature of the Ag. OVA is only weakly immunogenic and always requires an adjuvant for induction of immune responses. Our recent nasal tolerance studies strongly support this notion37. In contrast, cytomegalovirus plasmid DNA (pCMV), a known ligand for toll-like receptor 9, is recognized by IFN-γ– producing cells and dendritic cells38 and most likely induces innate and acquired immunity. Indeed, although M cells are able to transport lumenal Ags, noninvasive strains of S. typhimurium cannot penetrate M cells and are avirulent.36 An antigen’s immunogenicity and pathogenicity in the GI tract could be one of the most critical factors in determining whether mucosal immunity or tolerance is induced. Mucosal tolerance may be the most common immune response because it is necessary to maintain homeostasis. The normal host must readily establish unresponsiveness to commensal bacteria, food Ag and allergens. Taken together, we conclude that our OVA-pσ1 system, M cell targeting of a non-pathogenic protein Ag is an efficient strategy for the establishment of mucosal tolerance in addition to the systemic unresponsiveness. In this regard, an oral tolerance regimen may have potential advantages to treat inflammatory or allergic diseases occurred in both mucosal and systemic compartments. Since the oral route is most likely safe and easy way to administer Ags, vaccines or drugs, our finding showing that as little as 10 µg of total protein by M cell targeting protein Ag delivery system inducing both mucosal and systemic unresponsiveness, would provide major advantages for the development of therapeutic approaches to treat diseases.
A reduction in Ag-specific T cells occurred in mice given repeated low doses of cytochrome c protein.39 Similarly, our results showed that a reduced frequency of OVA-specific CD4+ T cells occurred in all lymphoid tissues of orally tolerized mice when compared with PBS-fed mice challenged with oral OVA plus CT. Interestingly, in iLP resulted in more significant reduction than those in PPs, spleen and MLNs of orally tolerized mice. In addition, iLP of orally tolerized mice exhibited increased numbers of Foxp3+ CD4+ CD25+ T cells. These findings suggest that mucosal unresponsiveness may be induced by two major mechanisms, i.e., clonal deletion and active suppression by Treg cells in iLP of the small intestine. Although Foxp3 is most likely expressed by natural Treg cells, recent studies showed that this intracellular protein was also expressed by acquired-type Treg cells in orally tolerized mice.40 Since a previous study reported that PP-derived Treg clones produce high levels of TGF-β1 and suppressed Ag-specific Ab responses in spleen41, we next examined TGF-β1 and IL-10 production by OVA-specific CD4+ T cells from mice fed OVA-pσ1 prior to oral challenge with OVA plus CT. Our results clearly showed that CD4+ CD25+ T cells in PPs, MLNs and spleen from orally tolerized mice produce higher levels of TGF-β1 after OVA stimulation than do those from mice fed PBS. Furthermore, intracellular IL-10 production by CD4+ CD25+ T cells from mice fed OVA-pσ1 was significantly increased in the spleens from the orally tolerized group. Taken together with the observation that acquired-type CD4+ Treg cells are Ag-specific and produce inhibitory cytokines including TGF-β1 and IL-10,20 our results indicate that acquired-type CD4+ Treg cells are induced by oral administration of OVA-pσ1. Based upon these findings, it appears likely that mechanisms for the induction of mucosal and systemic unresponsiveness is clearly associated with clonal deletion of OVA-specific effector CD4+ T cells and is achieved by active suppression of acquired-type of Treg cells.
In conclusion, our current study provides the first evidence that M cell targeting of a non-pathogenic Ag OVA-pσ1 can induce mucosal unresponsiveness via two major mechanisms including clonal deletion of Ag-specific CD4+ T cells and the induction of acquired-type Treg cells. This M cell-targeting system allowed us to elucidate the immunoregulatory mechanisms of the PP-mediated oral tolerance pathway from other potential mechanisms. Thus, our findings suggest that regulatory-type CD4+ T cells may be initially induced in the PPs and then migrate into MLNs and spleen. These CD4+ Treg cells contribute to the successful systemic unresponsive state that ensues. Further, our results clearly show that mucosal unresponsiveness to orally administered Ag can be attributed to a lack of Ag-specific CD4+ T helper cells in the iLP. The potential roles of other GALT-mediated oral tolerance induction pathways are currently under investigation by our group.
Acknowledgments
This work is supported by U.S. Public Health Service Grants AI 18958, DE 12242, AG 025873, and DE 13812.
We thank Dr. Kimberly K. McGhee for editorial assistance and Ms. Sheila D. Turner for the final preparation of this manuscript.
Abbreviations used in this paper
- CT
native cholera toxin
- GALT
gut-associated lymphoreticular tissues
- iLP
intestinal lamina propria
- OVA-pσ1
protein sigma one of reovirus genetically fused to ovalbumin
- PPs
Peyer’s patches
- S-IgA
secretory-IgA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest exist.
References
- 1.Challacombe SJ, Tomasi TB., Jr Systemic tolerance and secretory immunity after oral immunization. J. Exp. Med. 1980;152:1459–1472. doi: 10.1084/jem.152.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mestecky J, Blumberg RS, Kiyono H, McGhee JR. The mucosal immune system. In: Paul WE, editor. Fundamental Immunology. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. pp. 965–1020. [Google Scholar]
- 3.Tomasi TB., Jr Oral tolerance. Transplantation. 1980;29:353–356. doi: 10.1097/00007890-198005000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Kato H, Fujihashi K, Kato R, Yuki Y, McGhee JR. Oral tolerance revisited: prior oral tolerization abrogates cholera toxin-induced mucosal IgA responses. J. Immunol. 2001;166:3114–3121. doi: 10.4049/jimmunol.166.5.3114. [DOI] [PubMed] [Google Scholar]
- 5.Garside P, Mowat AM, Khoruts A. Oral tolerance in disease. Gut. 1999;44:137–142. doi: 10.1136/gut.44.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strobel S, Mowat AM. Immune responses to dietary antigens: oral tolerance. Immunol. Today. 1998;19:173–181. doi: 10.1016/s0167-5699(97)01239-5. [DOI] [PubMed] [Google Scholar]
- 7.Strober W, Kelsall B, Marth T. Oral tolerance. J. Clin. Immunol. 1998;18:1–30. doi: 10.1023/a:1023222003039. [DOI] [PubMed] [Google Scholar]
- 8.Wardrop RM, 3rd, Whitacre CC. Oral tolerance in the treatment of inflammatory autoimmune diseases. Inflamm. Res. 1999;48:106–109. doi: 10.1007/s000110050433. [DOI] [PubMed] [Google Scholar]
- 9.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 10.Nagler-Anderson C, Shi HN. Peripheral nonresponsiveness to orally administered soluble protein antigens. Crit. Rev. Immunol. 2001;21:121–131. [PubMed] [Google Scholar]
- 11.Viney JL, Mowat AM, O'Malley JM, Williamson E, Fanger NA. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J. Immunol. 1998;160:5815–5825. [PubMed] [Google Scholar]
- 12.Weiner HL. Induction and mechanism of action of transforming growth factor-beta-secreting Th3 regulatory cells. Immunol. Rev. 2001;182:207–214. doi: 10.1034/j.1600-065x.2001.1820117.x. [DOI] [PubMed] [Google Scholar]
- 13.Friedman A, Weiner HL. Induction of anergy or active suppression following oral tolerance is determined by antigen dosage. Proc. Natl. Acad. Sci. (USA) 1994;91:6688–6692. doi: 10.1073/pnas.91.14.6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melamed D, Friedman A. Direct evidence for anergy in T lymphocytes tolerized by oral administration of ovalbumin. Eur. J. Immunol. 1993;23:935–942. doi: 10.1002/eji.1830230426. [DOI] [PubMed] [Google Scholar]
- 15.Whitacre CC, Gienapp IE, Orosz CG, Bitar DM. Oral tolerance in experimental autoimmune encephalomyelitis. III. Evidence for clonal anergy. J. Immunol. 1991;147:2155–2163. [PubMed] [Google Scholar]
- 16.Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177–180. doi: 10.1038/376177a0. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 18.Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 19.Nagler-Anderson C, Bhan AK, Podolsky DK, Terhorst C. Control freaks: immune regulatory cells. Nat. Immunol. 2004;5:119–122. doi: 10.1038/ni0204-119. [DOI] [PubMed] [Google Scholar]
- 20.Cottrez F, Groux H. Specialization in tolerance: innate CD4+ CD25+ versus acquired Tr1 and Th3 regulatory T cells. Transplantation. 2004;77:S12–S15. doi: 10.1097/01.TP.0000106471.23410.32. [DOI] [PubMed] [Google Scholar]
- 21.Dieckmann D, Bruett CH, Ploettner H, Lutz MB, Schuler G. Human CD4+ CD25+ regulatory, contact-dependent T cells induce interleukin 10-producing, contact-independent type 1-like regulatory T cells [corrected] J. Exp. Med. 2002;196:247–253. doi: 10.1084/jem.20020642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, Boysun MJ, Csencsits KL, Pascual DW. Gene transfer facilitated by a cellular targeting molecule, reovirus protein sigma1. Gene Ther. 2000;7:61–69. doi: 10.1038/sj.gt.3301046. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Wang X, Csencsits KL, Haddad A, Walters N, Pascual DW. M cell-targeted DNA vaccination. Proc. Natl. Acad. Sci. (USA) 2001;98:9318–9323. doi: 10.1073/pnas.161204098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubas W, Banerjea AC, Gallati H, Speiser PP, Joklik WK. Incorporation of the reovirus M cell attachment protein into small unilamellar vesicles: incorporation efficiency and binding capability to L929 cells in vitro. J Microencapsul. 1990;7:385–395. doi: 10.3109/02652049009021848. [DOI] [PubMed] [Google Scholar]
- 25.Fujihashi K, Dohi T, Rennert PD, Yamamoto M, Koga T, Kiyono H, McGhee JR. Peyer's patches are required for oral tolerance to proteins. Proc. Natl. Acad. Sci. (USA) 2001;98:3310–3315. doi: 10.1073/pnas.061412598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kataoka K, McGhee JR, Kobayashi R, Fujihashi K, Shizukuishi S, Fujihashi K. Nasal Flt3 ligand cDNA elicits CD11c+ CD8+ dendritic cells for enhanced mucosal immunity. J. Immunol. 2004;172:3612–6319. doi: 10.4049/jimmunol.172.6.3612. [DOI] [PubMed] [Google Scholar]
- 27.Fujihashi K, McGhee JR, Kweon MN, Cooper MD, Tonegawa S, Takahashi I, Hiroi T, Mestecky J, Kiyono H. γ/δ T cell-deficient mice have impaired mucosal immunoglobulin A responses. J. Exp. Med. 1996;183:1929–1935. doi: 10.1084/jem.183.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato H, Fujihashi K, Kato R, Dohi T, Fujihashi K, Hagiwara Y, Kataoka K, Kobayashi R, McGhee JR. Lack of oral tolerance in aging is due to sequential loss of Peyer's patch cell interactions. Int. Immunol. 2003;15:145–158. doi: 10.1093/intimm/dxg011. [DOI] [PubMed] [Google Scholar]
- 29.Fujihashi K, Kato H, van Ginkel FW, Koga T, Boyaka PN, Jackson RJ, Kato R, Hagiwara Y, Etani Y, Goma I, Kiyono H, McGhee JR. A revisit of mucosal IgA immunity and oral tolerance. Acta. Odontol. Scand. 2001;59:301–308. doi: 10.1080/000163501750541174. [DOI] [PubMed] [Google Scholar]
- 30.Hagiwara Y, McGhee JR, Fujihashi K, Kobayashi R, Yoshino N, Kataoka K, Etani Y, Kweon M-N, Tamura S, Kurata T, Takeda Y, Kiyono H, Fujihashi K. Protective mucosal immunity in aging is associated with functional CD4+ T cells in nasopharyngeal-associated lymphoreticular tissue. J. Immunol. 2003;170:1754–1762. doi: 10.4049/jimmunol.170.4.1754. [DOI] [PubMed] [Google Scholar]
- 31.Ermak TH, Dougherty EP, Bhagat HR, Kabok Z, Pappo J. Uptake and transport of copolymer biodegradable microspheres by rabbit Peyer's patch M cells. Cell Tissue Res. 1995;279:433–436. doi: 10.1007/BF00318501. [DOI] [PubMed] [Google Scholar]
- 32.Neutra MR, Frey A, Kraehenbuhl JP. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 33.Gebert A, Rothkotter HJ, Pabst R. M cells in Peyer's patches of the intestine. Int. Rev. Cytol. 1996;167:91–159. doi: 10.1016/s0074-7696(08)61346-7. [DOI] [PubMed] [Google Scholar]
- 34.Wolf JL, Bye WA. The membranous epithelial (M) cell and the mucosal immune system. Annu. Rev. Med. 1984;35:95–112. doi: 10.1146/annurev.me.35.020184.000523. [DOI] [PubMed] [Google Scholar]
- 35.Allan CH, Mendrick DL, Trier JS. Rat intestinal M cells contain acidic endosomal-lysosomal compartments and express class II major histocompatibility complex determinants. Gastroenterology. 1993;104:698–708. doi: 10.1016/0016-5085(93)91004-2. [DOI] [PubMed] [Google Scholar]
- 36.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rynda A, Maddaloni M, Mierzejewska D, Ochoa-Reparaz J, Maslanka T, Crist K, Riccardi C, Barszczewska B, Fujihashi K, McGhee JR. Low-Dose Tolerance Is Mediated by the Microfold Cell Ligand, Reovirus Protein s1. J. Immunol. 2008;180:5187–5200. doi: 10.4049/jimmunol.180.8.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Gutgemann I, Fahrer AM, Altman JD, Davis MM, Chien YH. Induction of rapid T cell activation and tolerance by systemic presentation of an orally administered antigen. Immunity. 1998;8:667–673. doi: 10.1016/s1074-7613(00)80571-3. [DOI] [PubMed] [Google Scholar]
- 40.Sun JB, Raghavan S, Sjoling A, Lundin S, Holmgren J. Oral tolerance induction with antigen conjugated to cholera toxin B subunit generates both Foxp3+CD25+ and Foxp3- CD25- CD4+ regulatory T cells. J. Immunol. 2006;177:7634–7644. doi: 10.4049/jimmunol.177.11.7634. [DOI] [PubMed] [Google Scholar]
- 41.Tsuji NM, Mizumachi K, Kurisaki J. Antigen-specific, CD4+ CD25+ regulatory T cell clones induced in Peyer's patches. Int. Immunol. 2003;15:525–534. doi: 10.1093/intimm/dxg051. [DOI] [PubMed] [Google Scholar]