Abstract
Background:
Fatigue is highly prevalent and has a negative impact on quality of life and performance in a variety of disorders. The 9-item Fatigue Severity Scale (FSS) is one of the most commonly used self-report questionnaires to measure fatigue, but has only been validated in small sample-sized studies and in single disorders.
Objective:
To validate the FSS in healthy subjects and different disorders known to be commonly associated with fatigue.
Material and Methods:
The FSS was administered to 454 healthy subjects, 188 patients with multiple sclerosis (MS), 235 patients with recent ischemic stroke, and 429 patients with sleep-wake disorders including narcolepsy with cataplexy (n = 22), restless legs syndrome (RLS) (n = 79), sleep apnea (n = 108), insomnia (n = 62), parasomnia (n = 25), excessive daytime sleepiness/hypersomnia of other origin (n = 84), and other sleep-wake disorders (n = 49).
Results:
FSS scores were 4.66 ± 1.64 (mean ± SD) in patients with MS, 3.90 ± 1.85 in patients after ischemic stroke, and 4.34 ± 1.64 in patients with sleep-wake disorders. Compared to patients, values were significantly lower in healthy subjects (3.00 ± 1.08, P < 0.01). Scores did not correlate with gender, age, or education. Item analysis showed an excellent internal consistency and reliability (Cronbach α = 0.93). Test-retest variability was assessed in 104 healthy subjects, showing stable values over time (2.94 ± 0.90 vs. 2.90 ± 0.74; P = 0.27).
Conclusions:
This first validation of a fatigue scale in a large sample size demonstrates that the FSS is a simple and reliable instrument to assess and quantify fatigue for clinical and research purposes.
Citation:
Valko PO; Bassetti CL; Bloch KE; Held U; Baumann CR. Validation of the fatigue severity scale in a swiss cohort. SLEEP 2008;31(11):1601–1607.
Keywords: Fatigue, sleep, multiple sclerosis, validity, reliability
FATIGUE CAN BE DEFINED AS A SUBJECTIVE EXPERIENCE, AND INCLUDES SUCH SYMPTOMS AS RAPID INANITION, PERSISTING LACK OF ENERGY, EXHAUSTION, physical and mental tiredness, and apathy.1 It can be a consequence of many sleep-wake disorders, but also of a large variety of other disorders including multiple sclerosis (MS) (present in as many as 76% to 92%; experienced as worst symptom by 50% to 60% of patients), and stroke (poststroke fatigue, up to 68%).2–10 Fatigue represents one of the most frequent complaints of primary care patients (6% to 45%).6
The large number of publications on fatigue in the last decade reflects the increasing awareness and the substantial role it has gained in clinical practice and research. In spite of this increasing interest in fatigue, as well as its prevalence and clinical significance, fatigue is underrecognized, possibly because of the lack of sufficiently validated and widespread instruments to quantify fatigue. Numerous fatigue scales have been introduced; only few of them have been validated, mostly in small studies and for specific disorders.11,12 Hence, the availability of an appropriate tool for assessment and quantification of fatigue, which can be used in different disorders, is important for clinical and research purposes.
A recent bibliographic study of fatigue measurement scales has shown that the FSS is the most commonly used fatigue specific questionnaire.13 The Fatigue Severity Scale (FSS) is a 9-item self-report questionnaire scale developed in 1989; it was applied in 25 MS patients, 29 patients with systemic lupus erythematosus, and in 20 healthy controls.11 The simple and timesaving application of the FSS is probably the main reason for its high general acceptance. However, its use has been mainly limited to MS patients; large studies including patients with other sleep-wake and neurological disorders are lacking. These limitations may be the reason that there is no clearly defined FSS cut-off to discriminate normal from pathological results.1
Our aim in this study was to validate the FSS for the first time in a large sample size, by applying it to healthy controls and selected disorders frequently associated with fatigue.
MATERIAL AND METHODS
The study was conducted at the Neurology and Pulmonary Departments of the University Hospital of Zurich, Switzerland, between December 2005 and April 2007. The study protocol was approved by the local ethics committee.
German Translation of the FSS
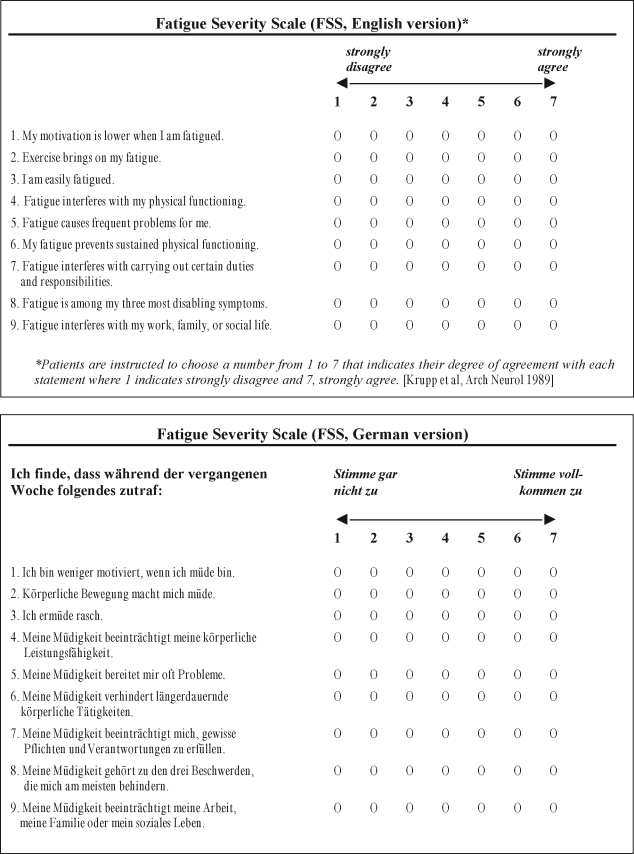
The FSS is a self-administered questionnaire with 9 items (questions) investigating the severity of fatigue in different situations during the past week. Grading of each item ranges from 1 to 7, where 1 indicates strong disagreement and 7 strong agreement, and the final score represents the mean value of the 9 items. We initially translated the 9 items of the FSS from English into German. Thereafter, a bilingual neurologist who was blinded with respect to the original version translated it backwards into English. Finally, the German version was adapted according to this procedure (Figure 1).
Figure 1.
The original English and the German version of the Fatigue Severity Scale (FSS).
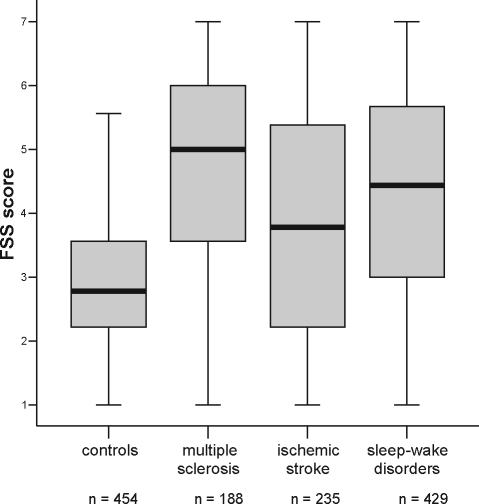
Figure 2.
Boxplots showing the median and interquartile range of FSS scores in healthy controls and patients with MS, previous ischemic stroke, and sleep-wake disorders.
Subjects
We included 3 different patient groups (MS, ischemic stroke, and sleep-wake disorders), in which the prevalence of fatigue is known to be high. We sent the FSS by postal service to 314 patients with clinically definite MS and to 490 patients with previous ischemic stroke; these consecutive patients were examined in our neurological clinic between January 2005 and January 2007. A total of 188 patients (60%) with MS and 235 patients (48%) with ischemic stroke filled in and returned the questionnaire. The intervals between disease onset and the completion of this study were 11.1 ± 9.8 (mean ± SD, range 0.5–58) years for MS patients and 1.2 ± 0.6 (range 0.33–2) years for stroke patients. In addition, we prospectively included 429 consecutive sleep-wake disordered patients referred to our neurological (n = 345) and pulmonary (n = 84) sleep clinics since December 2005. These patients were diagnosed with narcolepsy with cataplexy (n = 22), RLS (n = 79), sleep apnea (n = 108), insomnia (n = 62), parasomnia (n = 25), EDS/hypersomnia of other origin (n = 84), and other sleep-wake disorders (n = 49).
The FSS was also administered to 454 healthy control subjects recruited by the authors among relatives and friends. We attempted to obtain a control group that was representative of the general population with an equal distribution of different age categories and educational status (defined as highest educational degree attained). Subjects with a diagnosed sleep-wake disorder, previous sleep studies, or other diseases known to cause fatigue (e.g., advanced cancer, HIV infection, heart failure, rheumatic disorders and depression) were excluded.
Questionnaire
Apart from the FSS, all subjects had to quantify their fatigue on a Visual Analog Scale (VAS), where 0 indicates “very alert” and 10 “extremely fatigued.” Furthermore, the German version of the Epworth Sleepiness Scale (ESS) and questions regarding bedtime were simultaneously administered.14
Statistical Methods
We used SPSS (version 12.0), STATA (version 9), and R (version 2.4.1) for statistical analysis. Group data are described by means and standard deviations (SD). We calculated comparisons between patient groups by means of a linear regression model, including thorough residual analysis. We performed correlation analyses between FSS and continuous demographic variables with the Pearson correlation coefficient; for binary comparisons we used the t-test. We considered statistical test results to be significant at a level of P < 0.05. Reliability was estimated through stability (test-retest) and internal consistency assessment. The test-retest variability of the scale was evaluated by Lin's correlation coefficient of concordance,15 which takes departures from the 45° angle bisector into account, and which is typically lower than the corresponding correlation coefficient of Pearson. Internal consistency of the FSS was assessed by calculating the item to total correlation and the Cronbach α statistics.16 Cronbach α measures how well a set of items (or variables) measures a single unidimensional latent construct. In our case, α reflected how the 9 items of the FSS scale measured overall fatigue in patients or healthy subjects.
RESULTS
Subjects
The demographic and clinical characteristics of the patient groups and the control group are listed in Table 1.
Table 1.
Demographic and Clinical Characteristics of Healthy Subjects and Patients in this Study
| Healthy subjects (n = 454) | Multiple sclerosis (n = 188) | Previous ischemic stroke (n = 235) | Sleep-wake disorders (n = 429) | |
|---|---|---|---|---|
| Age (years, mean ± SD, range) | 47 ± 18 (13–94) | 45 ± 13 (20–79) | 63 ± 14 (21–87) | 52 ± 15 (16–86) |
| Gender: female (%) | 60 | 67 | 31 | 35 |
| Education status, n (%) | ||||
| Primary school degree | 43 (10) | 63 (36) | 59 (28) | 80 (28) |
| Second school degree | 128 (29) | 57 (32) | 105 (49) | 113 (40) |
| College degree | 93 (21) | 26 (15) | 21 (10) | 34 (12) |
| University degree | 183 (41) | 30 (17) | 29 (14) | 59 (21) |
| Duration since disease onset (years, mean ± SD, range) | 11.07 ± 9.79 (0.5–58) | 1.21 ± 0.62 (0.33–2) | ||
| EDSS (mean ± SD) | 3.61 ± 2.26 | |||
| VAS fatigue (mean ± SD) | 3.47 ± 2.24 | 4.83 ± 2.49 | 4.65 ± 2.55 | 5.12 ± 2.45 |
| FSS score, mean ± SD | 3.00 ± 1.08 | 4.66 ± 1.64 | 3.90 ± 1.85 | 4.34 ± 1.64 |
| 95% CI | 2.90–3.10 | 4.42–4.89 | 3.66–4.14 | 4.19–4.50 |
| FSS score ≥4.0 | 18% | 69% | 49% | 62% |
SD: Standard Deviation, EDSS: Expanded Disability Status Scale, VAS: Visual Analog Scale, FSS: Fatigue Severity Scale, CI: Confidence Interval
FSS: Comparison between Groups
Mean FSS scores were 4.66 ± 1.64 in MS patients (≥ 4.0 in 69%), 3.90 ± 1.85 in patients with previous ischemic stroke (≥ 4.0 in 49%), 4.34 ± 1.64 in patients with sleep-wake disorders (≥ 4.0 in 62%), and 3.00 ± 1.08 in healthy controls (≥ 4.0 in 18%). The values for 95% confidence intervals (CI) are shown in Table 1. The results of the linear regression analysis showed a significantly higher FSS score for each of the 3 patient groups than healthy controls; the effects of each of the 4 groups on mean FSS can be found in Table 2. The residual analysis revealed symmetrically distributed residuals around zero, and only a minor departure from the model assumptions, as the distribution of the residuals showed a small ceiling and bottom effect. This means that slightly more observations lie in the far left and far right side of the histogram of the residuals than one would expect under a strict Gaussian distribution.
Table 2.
Results of the Linear Regression Model: Estimated Effects and Standard Errors of the 4 groups (Healthy Subjects, Patients with Sleep-Wake Disorder, MS, and Previous Ischemic Stroke) on the Mean FSS Score
| Coefficient | Estimate | Standard Error | P-value |
|---|---|---|---|
| Healthy subjects | 3.00 | 0.07 | < 0.01 |
| Sleep-wake disorder | 4.34 | 0.07 | < 0.01 |
| Multiple sclerosis | 4.66 | 0.11 | < 0.01 |
| Ischemic stroke | 3.90 | 0.10 | < 0.01 |
Normal Range and Cutoff of the FSS
Mean ± 2 SD of FSS scores in healthy controls ranged from 0.8–5.2. When using 5.2 as the cutoff for the presence of fatigue, we found the following frequencies of fatigue: 3.5% in healthy controls, 45% in MS, 31% in patients with previous ischemic stroke, and 36% in patients with sleep-wake disorders. When using a cutoff of 4 (as suggested by some authors17,21), we found fatigue in 18% of healthy controls, 69% of MS patients, 49% of stroke patients, and 62% of patients with sleep-wake disorders.
MS Patients (n = 188)
We observed a tendency towards higher FSS scores with increasing age (Pearson r = 0.18, P = 0.01). FSS scores were significantly correlated with physical disability (expanded disability status scale, EDSS) (r = 0.34, P < 0.01), but not with disease duration (r = 0.11, P = 0.13), gender (P = 0.86), or educational status (r = −0.05, P = 0.50).
Patients with Previous Ischemic Stroke (n = 235)
No correlation was found between FSS scores and age (P = 0.95), duration from disease onset (P = 0.91), gender (P = 0.82), or educational status (P = 0.96).
Patients with Sleep-Wake Disorders (n = 429)
FSS and ESS values for specific sleep-wake disorders are shown in Table 3. There are significant differences of mean FSS scores between sleep diagnoses (P = 0.04); patients with insomnia and narcolepsy had the highest scores (4.78 ± 1.51 and 4.75 ± 1.47, respectively), and patients with restless legs syndrome had the lowest FSS scores (4.02 ± 1.75). Overall, we observed a modest yet significant correlation between FSS and ESS (r = 0.34, P < 0.01). In the subgroup of patients with narcolepsy with cataplexy and sleep apnea we found the highest correlation (r = 0.71 and 0.48, respectively, P < 0.01), whereas the correlation was lowest for patients with insomnia (r = 0.21, P = 0.11).
Table 3.
Correlation Between Epworth Sleepiness Scale (ESS) and Fatigue Severity Scale (FSS) Values in Different Sleep-Wake Disorders (Mean ± SD)
| Sleep-wake disorder | n | ESS mean ± SD | FSS mean ± SD | r | Pearson** P-value |
|---|---|---|---|---|---|
| Narcolepsy with cataplexy | 22 | 15.00 ± 5.23 | 4.75 ± 1.47 | 0.71 | <0.01 |
| RLS | 79 | 8.59 ± 5.54 | 4.02 ± 1.75 | 0.28 | 0.01 |
| Sleep apnea | 108 | 10.76 ± 5.81 | 4.11 ± 1.64 | 0.48 | <0.01 |
| Insomnia | 62 | 7.39 ± 5.18 | 4.78 ± 1.51 | 0.21 | 0.11 |
| Parasomnia | 25 | 7.32 ± 3.96 | 4.64 ± 1.45 | 0.31 | 0.14 |
| EDS/hypersomnia of other origin | 84 | 10.47 ± 4.95 | 4.55 ± 1.70 | 0.33 | <0.01 |
| Other sleep-wake disorders* | 49 | 8.85 ± 4.79 | 4.18 ± 1.56 | 0.45 | <0.01 |
RLS: restless legs syndrome, EDS: excessive daytime sleepiness.
sleep related headache, nocturnal epilepsy, circadian rhythm disorders.
Correlation between ESS and FSS.
Healthy Subjects (n = 454)
We found no significant associations of FSS scores with age or education. There was a weak association between FSS score and gender: the mean difference of FSS in females compared to males was 0.21 (P = 0.04). Differences in bedtime of ≥ 2 hours between weekday and weekend—suggestive of behaviorally induced insufficient sleep syndrome (BIISS)—had no influence on the FSS score (BIISS: n = 97, FSS 3.07 ± 0.97; non-BIISS: n = 357, FSS 2.98 ± 1.11; P = 0.3). ESS scores, however, differed between the 2 groups (BIISS: 6.79 ± 3.53, non-BIISS: 5.93 ± 3.58, P = 0.04). In healthy subjects, there was a modest but significant correlation between FSS and ESS (r = 0.28, P < 0.01).
Correlation Between FSS and VAS
We observed a highly significant correlation (r = 0.69, P < 0.01) between FSS scores and fatigue as indicated on the VAS. This correlation was higher in patients (MS: r = 0.79, ischemic stroke: r = 0.70, sleep-wake disorders: r = 0.71) than in healthy subjects (r = 0.52).
Item Analysis, Internal Consistency, and Test-Retest Variability of the FSS
Cronbach coefficient α for the entire sample was 0.93, showing a high degree of internal consistency of the FSS (Table 4). Specific item analysis showed the lowest internal consistencies for items 1 and 2. A total of 104 healthy subjects filled in the FSS a second time after 21 days. Initial values (2.94 ± 0.90) did not differ from subsequent testing (2.90 ± 0.74) (P = 0.27). Lin's concordance measure rho was 0.88 (95% CI: 0.84 to 0.92). In 87 normal controls (84%) the difference of the total score was ≤0.5. The mean difference between test and retest was 0.04 ± 0.41.
Table 4.
Item Analysis of the FSS in Patients with MS, Ischemic Stroke, and Sleep-Wake Disorders, and in Healthy Controls
| Item number | mean±SD | corrected item to total correlation | Cronbach α if item deleted |
|---|---|---|---|
| a) multiple sclerosis (n = 188) | |||
| 1 | 5.28 ± 1.58 | 0.48 | 0.95 |
| 2 | 4.93 ± 1.91 | 0.72 | 0.93 |
| 3 | 4.69 ± 2.02 | 0.82 | 0.93 |
| 4 | 5.04 ± 1.91 | 0.82 | 0.93 |
| 5 | 4.45 ± 1.98 | 0.85 | 0.93 |
| 6 | 4.85 ± 2.06 | 0.85 | 0.93 |
| 7 | 3.99 ± 2.05 | 0.77 | 0.93 |
| 8 | 4.52 ± 2.20 | 0.79 | 0.93 |
| 9 | 4.19 ± 2.21 | 0.78 | 0.93 |
| Total | 4.66 ± 1.64 | 0.94 | |
| b) ischemic stroke (n = 235) | |||
| 1 | 4.65 ± 1.98 | 0.70 | 0.96 |
| 2 | 4.11 ± 2.00 | 0.77 | 0.96 |
| 3 | 4.03 ± 2.12 | 0.85 | 0.95 |
| 4 | 4.28 ± 2.13 | 0.86 | 0.95 |
| 5 | 3.69 ± 2.21 | 0.86 | 0.95 |
| 6 | 3.98 ± 2.26 | 0.85 | 0.95 |
| 7 | 3.53 ± 2.19 | 0.84 | 0.95 |
| 8 | 3.68 ± 2.34 | 0.85 | 0.95 |
| 9 | 3.51 ± 2.23 | 0.86 | 0.95 |
| Total | 3.90 ± 1.85 | 0.96 | |
| c) sleep-wake disorders (n = 429) | |||
| 1 | 5.07 ± 1.80 | 0.60 | 0.94 |
| 2 | 4.23 ± 1.90 | 0.60 | 0.94 |
| 3 | 4.28 ± 1.99 | 0.79 | 0.93 |
| 4 | 4.61 ± 1.92 | 0.83 | 0.92 |
| 5 | 4.41 ± 2.10 | 0.83 | 0.92 |
| 6 | 4.06 ± 2.05 | 0.77 | 0.93 |
| 7 | 3.90 ± 2.06 | 0.80 | 0.93 |
| 8 | 4.44 ± 2.21 | 0.79 | 0.93 |
| 9 | 4.16 ± 2.22 | 0.81 | 0.93 |
| Total | 4.34 ± 1.64 | 0.94 | |
| healthy controls (n = 454) | |||
| 1 | 5.09 ± 1.60 | 0.32 | 0.86 |
| 2 | 3.11 ± 1.66 | 0.41 | 0.85 |
| 3 | 2.75 ± 1.44 | 0.63 | 0.83 |
| 4 | 3.94 ± 1.71 | 0.50 | 0.84 |
| 5 | 2.66 ± 1.58 | 0.67 | 0.82 |
| 6 | 2.50 ± 1.57 | 0.66 | 0.83 |
| 7 | 2.41 ± 1.55 | 0.65 | 0.83 |
| 8 | 2.30 ± 1.73 | 0.65 | 0.83 |
| 9 | 2.16 ± 1.55 | 0.68 | 0.82 |
| Total | 3.00 ± 1.08 | 0.85 | |
| entire sample (n = 1306) | |||
| Total | 0.93 | ||
DISCUSSION
We show for the first time in a large sample size study that the FSS is a valuable tool to assess and quantify fatigue, as it differentiates between patients with various diseases and healthy subjects. Our data obtained in healthy subjects provide normal reference values with an upper limit of the normal range of 3.10. The FSS shows an excellent internal consistency, demonstrated by a Cronbach α coefficient of 0.93, which is higher than in the earlier small sample size studies in MS patients.11,13,17 The reliability of the FSS is further reflected by the high test-retest reliability. Based on our data, the minimal difference in FSS scores that can be reliably detected is 0.15. In addition, a high degree of correlation of FSS scores and VAS results could be demonstrated.
We found that the FSS is useful to distinguish frequency and severity of fatigue between healthy subjects and patients with MS, different sleep-wake disorders, and ischemic stroke.
Based on our findings and on previous reports on the prevalence of fatigue, we suggest that a FSS score ≥ 4 be interpreted as indicative of fatigue.2–5,11,13,17 When using 5.2 (mean + 2 SD) as the cutoff for the presence of fatigue, we obtained fatigue prevalences that were clearly lower than previously published prevalences of fatigue. In MS for instance, the prevalence of fatigue is well documented and ranges from 76% to 92%,2–5 suggesting that a cutoff of 4 (obtained fatigue prevalence: 69%) would be more appropriate than 5.2 (obtained fatigue prevalence: 45%). Similarly, item 5 of the FSS (“Fatigue causes frequent problems for me”) indicates the presence of fatigue in tested subjects. The score of this item was ≥ 5 (affirmative answer) in 16% of healthy controls, 55% of MS patients, 36% of patients with previous ischemic stroke, and 51% of patients with sleep-wake disorders. This finding again is in favor of a cutoff of 4 for the upper limit of the normal range of the FSS.
The mean FSS scores for MS of this study were similar to those of the original validation study, a recent Turkish study, and other reports,11,13,17 indicating that the influences of language and cultural background are negligible in the FSS. Mean FSS scores for healthy controls (3.00 ± 1.08), however, were higher than in the study of Krupp et al. (2.3 ± 0.7). The main reason for this difference is probably our larger sample size of healthy subjects (454 vs. 20), which is probably more representative for the general population. Our results show that fatigue is frequent even in healthy controls, and that the prevalence of fatigue in the general population is largely independent of age, gender, and education. The reason for this finding remains unclear. Based on our questionnaires, chronic sleep deprivation (also referred to as behaviorally induced insufficiant sleep syndrome, BIISS, in the terminology of the American Academy of Sleep Medicine) did not account for fatigue in our large control group. In our study, however, BIISS was only assessed by questionnaires and not confirmed by actigraphy.
Fatigue was more pronounced in MS patients than patients with ischemic stroke and most patients with sleep-wake disorders. This underscores the high burden of fatigue in MS patients.18–20 In MS patients, severity of fatigue correlated with the degree of physical disability (EDSS score), but not with disease duration, age, or gender. Again, these results are in line with the findings of previous studies.18–20
Fatigue in patients with ischemic stroke was more pronounced than in healthy subjects, but less severe than in MS. Interestingly, we could not find a correlation between fatigue severity and the interval between stroke and study completion. This suggests that—in comparison to neurological symptoms, which may gradually improve after stroke—fatigue may persist as a stable complaint after stroke, at least during the first 2 years (observation time in this study).
Among patients with sleep-wake disorders, the highest FSS scores were found in patients with insomnia (4.78) and with narcolepsy (4.75), whereas lowest scores were found in RLS patients (4.02). Correlations between the ESS (assessment of subjective sleepiness) and the FSS were generally low and best in narcolepsy with cataplexy and sleep apnea. This confirms the concept that fatigue and excessive daytime sleepiness usually do not correspond but probably reflect different symptoms. This question, however, needs to be addressed with clinical sleep studies.
A limitation in our study was that the presence of depression was not assessed. In MS, several studies revealed a significant association between fatigue and depression,17,19,21 but in the validation study of Krupp et al., fatigue severity was largely independent of depressive symptoms.11 Similarly, a significant overlap between fatigue and depression exists in patients with recent ischemic stroke, but poststroke fatigue may also develop and persist in the absence of depression.8–10 The mutual interplay between fatigue, depression, and other disease-related factors remain unclear in most disorders and has to be elucidated in further studies.
In conclusion, the awareness and assessment of fatigue is essential for the management of affected patients. Currently, pharmacological therapy of fatigue is restricted to nonspecific wake-promoting agents (e.g., modafinil), amantadine, and various antidepressants. At the moment, however, fatigue remains an underrecognized problem, which may show negative interaction with performance levels and outcome measures (such as EDSS in MS patients, or Barthel Index in stroke patients for assessment of stroke-related disability), and therefore needs to be taken into consideration when caring for these patients. The scale allows detection and monitoring of disease-related fatigue and may indicate the need of appropriate interventions. In addition, reliable determination of fatigue severity and its variation during the time course of a disease are the prerequisite to develop future therapies to alleviate this troublesome symptom. The FSS constitutes a valid instrument to assess and quantify fatigue for such clinical and research purposes.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Blochhas received research support from Respironics, ResMed, and Weinmann AG and has had the free use of monitoring equipment from VivoMetrics.
ACKNOWLEDGMENTS
We are indebted to Dr. D. Weniger, Prof. Dr. F. Gutzwiller, Dr. T. Tchelidze, Dr. L. Bachmann, I. Leuthold-Wyss und M. Leuthold for their valuable collaboration.
REFERENCES
- 1.Chaudhuri A. Fatigue in neurological disorders. Lancet. 2004;363:978–88. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- 2.Krupp LB, et al. Fatigue therapy in multiple sclerosis: results of a double-blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology. 1995;45:1956–61. doi: 10.1212/wnl.45.11.1956. [DOI] [PubMed] [Google Scholar]
- 3.Branas P, et al. Treatments for fatigue in multiple sclerosis: a rapid and systematic review. Health Technol Assess. 2000;4:1–61. [PubMed] [Google Scholar]
- 4.Zifko UA. Management of fatigue in patients with multiple sclerosis. Drugs. 2004;64:1295–1304. doi: 10.2165/00003495-200464120-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lichstein KL. Fatigue and sleep disorders. Behav Res Ther. 1997;35:733–40. doi: 10.1016/s0005-7967(97)00029-6. [DOI] [PubMed] [Google Scholar]
- 6.Hossain JL. Subjective fatigue and subjective sleepiness: two independent consequences of sleep disorders? J Sleep Res. 2005;14:245–53. doi: 10.1111/j.1365-2869.2005.00466.x. [DOI] [PubMed] [Google Scholar]
- 7.Ingles JL. Fatigue after stroke. Arch Phys Med Rehabil. 1999;80:173–8. doi: 10.1016/s0003-9993(99)90116-8. [DOI] [PubMed] [Google Scholar]
- 8.Choi-Kwon S, et al. Poststroke fatigue: characteristics and related factors. Cerebrovasc Dis. 2005;19:84–90. doi: 10.1159/000082784. [DOI] [PubMed] [Google Scholar]
- 9.Staub F. Fatigue after stroke: a major but neglected issue. Cerebrovasc Dis. 2001;12:75–81. doi: 10.1159/000047685. [DOI] [PubMed] [Google Scholar]
- 10.Bogousslavsky J. William Feinberg lecture 2002: emotions, mood, and behaviour after stroke. Stroke. 2003;34:1046–50. doi: 10.1161/01.STR.0000061887.33505.B9. [DOI] [PubMed] [Google Scholar]
- 11.Krupp LB. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–3. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 12.Mead G. Evaluation of fatigue scales in stroke patients. Stroke. 2007;38:2090–5. doi: 10.1161/STROKEAHA.106.478941. [DOI] [PubMed] [Google Scholar]
- 13.Hjollund NH. Assesssment of fatigue in chronic disease: a bibliographic study of fatigue measurement scales. Health Qual Life Outcomes. 2007;5:12. doi: 10.1186/1477-7525-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bloch KE. German version of the Epworth sleepiness scale. Respiration. 1999;66:440–7. doi: 10.1159/000029408. [DOI] [PubMed] [Google Scholar]
- 15.Lin L. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 45;1989:255–68. [PubMed] [Google Scholar]
- 16.Cronbach LJ. Coefficient alpha and the internal structure of test. Psychometrika. 1951;16:297–334. [Google Scholar]
- 17.Armutlu K, et al. The validity and reliability of the Fatigue Severity Scale in Turkish multiple sclerosis patients. Int J Rehabil Res. 2007;30:81–5. doi: 10.1097/MRR.0b013e3280146ec4. [DOI] [PubMed] [Google Scholar]
- 18.Flachenecker P, et al. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler. 2002;8:523–6. doi: 10.1191/1352458502ms839oa. [DOI] [PubMed] [Google Scholar]
- 19.Pittion-Vouyovitch S. Fatigue in multiple sclerosis is related to disability, depression and quality of life. J Neurol Sci. 2006;243:39–45. doi: 10.1016/j.jns.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 20.Bergamaschi R. Clinical aspects of fatigue in multiple sclerosis. Funct Neurol. 1997;12:247–51. [PubMed] [Google Scholar]
- 21.Kaynak H, et al. Fatigue and sleep disturbance in multiple sclerosis. Eur J Neurol. 2006;13:1333–9. doi: 10.1111/j.1468-1331.2006.01499.x. [DOI] [PubMed] [Google Scholar]