THE CRITICAL TOPICS FORUM IN THIS ISSUE OF SLEEP FOCUSES ON THE NEUROCHEMICAL MECHANISMS CAUSING THE MOTOR ATONIA OF RAPID EYE movement (REM) sleep. I thank Editor-in-Chief David Dinges for the invitation to serve as editor for this Forum, and the authors for their didactic contributions. A recently published paper entitled: “Glycinergic and GABAA-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia”1 is the focus of this Forum. The mechanisms causing the atonia of REM sleep are complex, but the relevance of state-dependent motor control justifies the effort that will be required by readers to parse the commentaries and rebuttal. The following paragraphs provide a brief context by highlighting clinical, functional, and anatomical details pertinent to this Forum.
Motor control has special significance for every aspect of sleep research. Many sleep disorders include omission of appropriate movements, or commission of movements that are not appropriate to sleep. These movement disorders may be expressed in specific muscle groups that exhibit failure to suppress muscle activity during sleep (e.g., restless leg syndrome) or expressed as a sleep-dependent inability to maintain motor tone (e.g., upper airway collapse). Sleep related movement disorders may also involve the entire organism. A cardinal sign of narcolepsy, for example, includes the sudden loss of tone in postural (antigravity) muscles. Dreaming mentation is often organized around imagined movements, and the affective valence of dreams is inseparable from the motor acts of dreams.
Advances in sleep medicine have been characterized by a dynamic exchange between basic and clinical research. At its best, such an exchange reveals mechanisms that underlie a clinical disorder, and knowledge of the underling cause promotes rational development of therapies that enhance patient care. One of the best examples of a viable exchange between preclinical and clinical research is the discovery of REM sleep without atonia. Mark Mahowald and Carlos Schenk have described how the preclinical finding of REM sleep without atonia is relevant for understanding REM behavior disorder and other parasomnias.2,3 The Internet (http://www.sleeprunners.com) provides a compelling description of complex behaviors that can occur during sleep.
Muscle atonia occurs when motoneurons are not generating action potentials. During the tonic periods of REM sleep, motoneurons do not generate action potentials. Motoneuron action potentials arise at the initial segment of the cell's axon, close to its soma, and result from a summation of currents that are generated at synapses on the soma and dendrites. If the voltage produced by these currents is above a certain threshold, an action potential is triggered. Research by the authors contributing to this forum has focused on three processes as likely contributors to the decreased discharge of motoneurons during REM sleep: (1) postsynaptic inhibition, (2) disfacilitation (i.e., withdrawal of excitatory input), and (3) presynaptic inhibition of muscle afferents. Some combination of these three processes is also possible.
Previous studies obtained intracellular recordings from motoneurons during states of sleep and wakefulness in order to determine the basis for atonia during REM sleep. Direct measures of motoneuron excitability allow one to differentiate between postsynaptic inhibition and disfacilitation. In addition to recording motoneuron membrane potential, intracellular recording can measure input resistance, capacitance, and changes in induced excitatory postsynaptic potentials, antidromic spikes, and other membrane properties of a cell. These data have been used to identify mechanisms responsible for the suppression of motoneuron excitability during REM sleep.
As these commentaries review, it is also important to determine the neurotransmitters responsible for the inhibition of motoneurons during REM sleep. Previous studies have microiontophoretically applied strychnine (a glycine receptor antagonist), picrotoxin, and bicuculline (GABAA receptor antagonists) adjacent to the cell body of motoneurons while recording intracellularly during sleep and wakefulness. Microdialysis has revolutionized neurochemistry by making it possible to deliver drugs to specific brain regions while quantifying drug effects on endogenous neurotransmitters, neuronal firing rate, or, as described in this Forum, on muscle tone. All methods have strengths and limitations, and the Forum considers these methodological issues in detail.
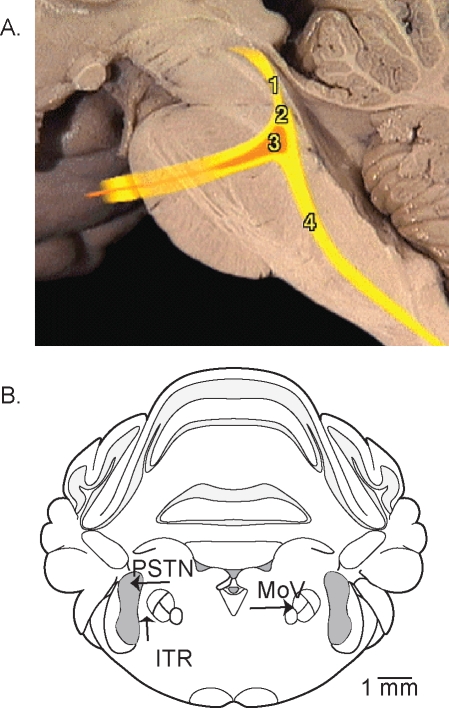
The human body contains approximately 700 muscles raising the question of which muscle groups are most appropriate for the study of REM sleep atonia. The focus article1 of this Critical Topics Forum describes studies of the masseter muscle, which is innervated by motoneurons in the trigeminal nucleus. Figure 1 will help readers visualize the trigeminal nuclear complex discussed in this Critical Topics Forum. The trigeminal nuclear complex extends from the midbrain to the medulla as illustrated by Figure 1A. The motor nucleus of the trigeminal nerve is located in the pontine brainstem, and the trigeminal motor nerve activates muscles of the mandible and 7 additional muscles. Lateral to the motor nucleus is the sensory trigeminal nucleus that receives proprioceptive, nociceptive, and tactile afferent input from the face and mouth. The space separating the sensory and motor trigeminal nuclei is referred to as the intertrigeminal region (Figure 1B). Adding to the complexity of the trigeminal nuclear complex are data showing that even the small intertrigeminal region contributes to the control of breathing.4,5
Figure 1.
Two views of the trigeminal nuclear complex. A. Sagittal view of the human brain stem with colored lines showing the location of the trigeminal nerve and trigeminal nuclear complex. Schematized components of the trigeminal nuclear complex include the mesencephalic (1), sensory (2), motor (3), and spinal (4) divisions. This image from “The Anatomy Project” (http://anatomy.med.umich.edu/atlas/n2a4p5.html) of the University of Michigan and used with permission of Dr. Thomas Gest. B. Coronal view of the rat brainstem marked to identify the trigeminal motor nucleus (MoV), the principal sensory trigeminal nucleus (PSTN), and the intertrigeminal region (ITR). Frame B was modified from the brain atlas of Paxinos and Watson.8
Finally, this Forum demonstrates the lineage between the topic of motor control, long considered canonical to neurophysiology, and the comparatively new and vibrant field of sleep research. Sherrington and Adrian shared the 1932 Nobel Prize in Physiology or Medicine for their work on the “functions of neurons.”6 Sherrington introduced his Nobel Lecture7 entitled “Inhibition as a Coordinating Factor” by noting “That a muscle on irritation of its nerve contracts had already long been familiar to physiology when the 19th century found a nerve which when irritated prevented its muscle from contracting. This observation seemed for a time too strange to be believed.” Yet stranger still is the evolutionary development of flexor and extensor muscle inhibition during sleep, a time when we are most vulnerable to predation.
DISCLOSURE STATEMENT
Dr. Lydic has indicated no financial conflicts of interest.
REFERENCES
- 1.Brooks PL. Glycinergic and GABAA-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia. J Neurosci. 2008;28:3535–45. doi: 10.1523/JNEUROSCI.5023-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahowald MW. Dissociated states of wakefulness and sleep. Neurology. 1992;42:44–52. [PubMed] [Google Scholar]
- 3.Mahowald MW. Evolving concepts of human state dissociation. Arch Ital Biol. 2001;139:269–300. [PubMed] [Google Scholar]
- 4.Chamberlin NL. A brainstem network mediating apneic reflexes in the rat. J Neurosci. 1998;18:6048–56. doi: 10.1523/JNEUROSCI.18-15-06048.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Radulovacki M. Effects of intertrigeminal region NMDA and non-NMDA receptors on respiratory responses in rats. Respir Physiol Neurobiol. 2007;156:40–6. doi: 10.1016/j.resp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Swazey JP. Reflexes and motor integration: Sherrington's concept of integrative action. Cambridge, MA: Harvard University Press; 1969. [Google Scholar]
- 7.Sherrington CS. Nobel Lectures, Physiology or Medicine 1922–1941. Amsterdam: Elsevier; 1965. [Google Scholar]
- 8.Paxinos G. The rat brain in stereotaxic coordinates. 4th ed. New York: Academic Press; 1998. [Google Scholar]