Abstract
Study Objectives:
Both basic and clinical data suggest a potential significant role for GABA in the etiology and maintenance of primary insomnia (PI). Proton magnetic resonance spectroscopy (1H-MRS) can non-invasively determine GABA levels in human brain. Our objective was to assess GABA levels in unmedicated individuals with PI, using 1H-MRS.
Design and Setting:
Matched-groups, cross-sectional study conducted at two university-based hospitals.
Participants:
Sixteen non-medicated individuals (8 women) with PI (mean age = 37.3 +/− 8.1) and 16 (7 women) well-screened normal sleepers (mean age = 37.6 +/− 4.5).
Methods and Measurements:
PI was established with an unstructured clinical interview, a Structured Clinical Interview for DSM-IV (SCID), sleep diary, actigraphy and polysomnography (PSG). 1H-MRS data were collected on a Varian 4 Tesla magnetic resonance imaging/spectroscopy scanner. Global brain GABA levels were averaged from samples in the basal ganglia, thalamus, and temporal, parietal, and occipital white-matter and cortex.
Results:
Average brain GABA levels were nearly 30% lower in patients with PI (.18 +/− .06) compared to controls (.25 +/− .11). GABA levels were negatively correlated with wake after sleep onset (WASO) on two independent PSGs (r = −0.71, p = 0.0024 and −0.70, p = 0.0048).
Conclusions:
Our preliminary finding of a global reduction in GABA in non-medicated individuals with PI is the first demonstration of a neurochemical difference in the brains of those with PI compared to normal sleeping controls. 1H-MRS is a valuable tool to assess GABA in vivo, and may provide a means to shed further light on the neurobiology of insomnia.
Citation:
Winkelman JW; Buxton OM; Jensen JE; Benson KL; O'Connor SP; Wang W; Renshaw PF. Reduced brain GABA in primary insomnia: preliminary data from 4T proton magnetic resonance spectroscopy (1H-MRS). SLEEP 2008;31(11):1499–1506.
Keywords: primary insomnia, magnetic resonance spectroscopy, polysomnographic sleep measures
CHRONIC INSOMNIA AFFECTS ROUGHLY 10% OF ALL ADULTS IN INDUSTRIALIZED COUNTRIES AND IS THE MOST COMMON SLEEP DISORDER. IT IS ASSOCIATED with high comorbidity with a variety of medical disorders,1 impaired quality of life,2 and consistent evidence of an increased risk of incident mood disorders.3 In the absence of an adequate understanding of its pathophysiology, insomnia is divided into primary and comorbid forms, the latter diagnosed when coexisting medical, sleep or psychiatric disorders are present. Roughly 25% of those with insomnia are considered to have primary insomnia (PI).
Three distinct lines of evidence point to a potential significant role of GABA in the etiology and/or persistence of PI: 1) benzodiazepine receptor agonists (BzRAs), which are efficacious in the treatment of insomnia, increase activity at GABA neurons, the most prevalent inhibitory neurotransmitters in the CNS4; 2) physiological, neuroimaging, and cognitive methods demonstrate hyperarousal in PI, which may relate to an imbalance of excitatory and inhibitory CNS influences, in which GABA may potentially play a role5; and 3) neurons in the ventrolateral preoptic nucleus (VLPO), which contain GABA, promote sleep in lower animals through suppression of CNS arousal systems in the tuberomammillary nucleus (TMN) and the brainstem monoaminegic systems.6
Recent progress in the non-invasive evaluation of GABA levels in human brain has been achieved by means of proton magnetic resonance spectroscopy (1H-MRS).7–12 These methods estimate the relative concentrations of brain neurotransmitters and metabolites from their resonance spectra in stimulated brain areas. Due to the low concentration of these metabolites in brain tissue, 1H-MRS averages spectra from larger anatomical areas than MRI to increase the signal:noise ratio.
We now report the results of the first study of in vivo GABA levels in unmedicated individuals with PI, using 1H-MRS.
METHODS
Subjects
Young and middle-aged (25–55 years) subjects were recruited from advertisements for a study of glucose metabolism and neuroimaging in DSM-IV defined PI (307.42) at Brigham and Women's Hospital and McLean Hospital from January 2007 to May 2008. Insomnia subjects had to have had greater than 6 months of difficulty initiating or maintaining sleep with resulting daytime distress or dysfunction. Specifically, they had to report a total sleep time ≤ 6.5 hrs and a) sleep onset latency > 45 minutes or b) wake after sleep onset > 45 minutes or c) total wake time during the sleep period (sleep latency + wake after sleep onset) > 60 minutes. Controls without sleep complaints were also recruited by advertising.
Additional evaluations in all subjects included assessment with an unstructured clinical interview for history of medical and sleep disorders, and interview for lifetime history of psychiatric disorders with the Structured Clinical Interview for DSM-IV (SCID). The Pittsburgh Sleep Quality Inventory (PSQI) was self-administered by all subjects, and the Beck Depression Inventory (BDI), and Insomnia Severity Index (ISI) by all subjects with PI. Laboratory assessment included electrolytes, CBC, liver and thyroid functions, pregnancy testing, reproductive hormone testing and a toxicology screen for illicit substances.
Sleep diaries were completed by all subjects, supplemented by daily call-ins at bedtime and waketime for insomniacs, to assess the timing of sleep and wake onset, total sleep time and awakenings within the sleep episode in all subjects. For insomniacs, inclusion criteria described above for sleep onset latency, wake after sleep onset and total sleep time had to be met during the two-week screening period prior to the 1H-MRS scan.
Exclusion criteria for all subjects included current or recent (within the preceding year) diagnosis of a DSM-IV Axis I disorder (including alcohol or drug dependence/abuse) other than Primary Insomnia; symptoms, diagnosis, or history of any sleep disorder other than primary insomnia; history of significant head trauma or loss of consciousness > 30 minutes; BMI >32 or < 19.8; regular treatment (>1 time/wk) with CNS active agents within 3 months of the first visit; current smoking of more than 10 cigarettes per day, consumption of > 2 caffeinated beverages per day, or more than 2 standard alcoholic drinks per day for a period > 1 month within the preceding year; and work history of swing shift, night shift, or rotating shift within the preceding year.
Actigraphy
A wrist-worn actigraphy unit (Actiwatch AW-64, Minimitter Inc., Bend OR) was used to continuously monitor rest-activity patterns for at least two weeks prior to the 1H-MRS scan. Daily call-ins with bedtimes and waketimes were used to assist with estimation of actigraphically-derived wake and sleep times. These data were primarily used to verify sleep-wake diary information and not for independent assessment of inclusion and exclusion criteria. Actigraphy data was not available for 3 controls due to loss of information due to device and scheduling limitations.
Polysomnography
Insomnia subjects who met initial screening criteria for insomnia underwent one night of attended in-laboratory screening polysomnography (PSG) to rule out primary sleep disorders (Sleep Screen) and one additional night for assessment of sleep architecture (Inpatient PSG). These two PSGs were performed an average of 24.3 (range 1–49) days apart. All sleep studies were generally performed within two weeks of the 1H-MRS acquisition. One PI subject did not have either PSG study performed and one additional subject did not have the Inpatient PSG.
PSGs were conducted using either Vitaport 3 or Alice IV digital sleep recorders. Surface electrodes (Beckman Instrument Company, Schiller Park, IL) were applied for recordings of central (C3 and C4) and occipital (O1 and O2) electroencephalogram (EEG), electrooculogram (EOG: LOC and ROC), anterior tibialis and submentalis electromyogram (EMG), electrocardiogram (ECG). Respiratory measures were conducted via oximetry and respiratory effort (abdominal and thorax), flow and nasal pressure. Anterior tibialis and respiratory recordings were only performed on the sleep screen. All sleep recordings were visually scored in 30-second epochs according to the method of Rechtschaffen and Kales. Lights out occurred at the subjects' usual time and all subjects were studied for 8 hours. All records were scored by the same, experienced registered PSG technologist. A cutoff of 15 apnea + hypopneas or 20 periodic limb movements per hour of sleep led to exclusion from the study. Similarly, a sleep efficiency greater than 90% combined with a report of sleep similar to that at home was exclusionary, as potential evidence of paradoxical insomnia. Other than these exclusionary criteria, the results of PSG were not used to confirm a diagnosis of PI.
The study was approved by the Institutional Review Boards of Partners Healthcare, the parent organization of Brigham and Women's Hospital, and McLean Hospital. All subjects were compensated for their participation in this study.
Proton Magnetic Resonance Spectroscopy
Data acquisition
All MRI/MRS data were collected on a Varian UnityINOVA, 4 Tesla (T) whole body magnetic resonance imaging/spectroscopy scanner using a volumetric, TEM-design (transverse electromagnetic) radiofrequency (RF) coil operating at 170.3MHz for proton (1H). A set of T1-weighted anatomical images were acquired in sagittal to guide the position of the 3cm thick CSI slab (TE/TR = 6.2/11.4ms, field-of-view (FOV) = 24cm × 24cm, readout-duration = 4ms, receive bandwidth = ±32kHz, in-plane matrix size = 128×256, in-plane resolution = 0.94×0.94mm, readout points = 512, axial-plane matrix size = 16, axial-plane resolution = 2.5mm sagittal, scan time = 1 minute, 15 seconds). Once complete an axial set of 32 images using the same sequence acquired a set of T1-weighted axial images of very high resolution (TE/TR = 6.2/11.4ms, field-of-view (FOV) = 24cm × 24cm, readout-duration = 4ms, receive bandwidth = ±32kHz, in-plane matrix size = 256×256, in-plane resolution = 0.94×0.94mm, readout points = 512, axial-plane matrix size = 32, axial-plane resolution = 2.5mm sagittal, scan time = 2 minutes, 30 seconds).
The 1H-CSI acquisition utilized a rapid spectroscopic imaging sequence combined with J-resolved-MRS to yield a large amount of spectral information in a relatively short scan period (20 minutes). The 2D slab-selective, spin-echo sequence, referred to as J-resolved Echo-Planar Spectroscopic Imaging (JEPSI) sampled 24 TE-steps per phase-encode step, where the k-space sampling scheme was a standard 1616 grid (256 k-space points in total). The 2D, 1H-CSI JEPSI slab was positioned exactly coplanar with the original T1-weighted mpFLASH3D images as described above. A manual slab-shim was performed over the JEPSI slab in the absence of readout gradients and water suppression to achieve a typical unsuppressed slab water linewidth of 8–12 Hz. Water-suppression employed a WET water-suppression scheme.13 The final parameters for this JEPSI sequence were TR = 1.5s; tip-angle = 90/180 degrees (spin-echo); spectral-bandwidth = ±1kHz; complex-points = 2048; readout duration = 512ms; pre-pulses = 4; field of view (FOV) = 24×24cm; nominal volume = 6.75cc; sampled matrix = 16×16 and scan duration was 19 minutes.
Data Processing / Analysis
1H-MRSI grids were aligned such that all of the relevant neural anatomy was covered and was consistent between subjects. A sub-matrix of 8×8 1H voxels was chosen from the central region of the brain, encompassing the basal ganglia, thalamus, frontal, temporal, parietal, and occipital white-matter and cortex. Only voxels within these sub-matrices were analyzed.
The 1H-CSI JEPSI data were first re-ordered and re-combined into conventional Cartesian k-space format, then read into a zero-padded 16×16 matrix for each sample echo-time (24 in total). Each re-ordered k-space JEPSI dataset (24 in total) were spatially-filtered using a Hanning window to reduce cross-voxel bleedthrough in the final CSI-image. The time-series of k-space images (24 in total) were zero-padded out to 64 k-space images and then Fourier Transformed for each time-point along the TE-dimension to produce 64, J-resolved K-space JEPSI datasets. Finally, each J-resolved JEPSI k-space dataset was Fourier Transformed to yield J and spatially-resolved spectral time-FIDS which were stored as binary data.
Spectral Fitting
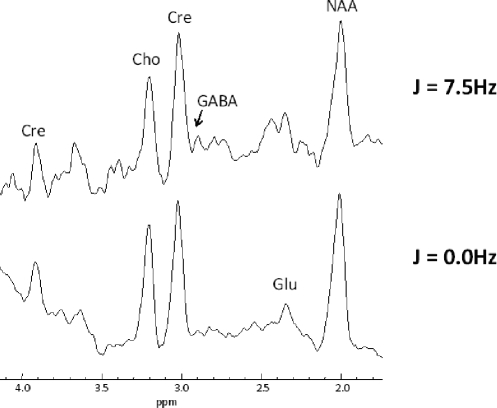
All 1H-CSI spectra were fitted with the commercial, proton-dedicated fully automated fitting software LCModel. The metabolite basis sets for 4T in vivo proton spectral quantitation were simulated using software developed on site. The metabolites included in our basis set were: Choline (total), Creatine (total), N-acetyl-asparte (NAA), N-acetyl-aspartyl-glutamate (NAAG), Glutamate, Glutamine, Glycine, GABA, Myo-inositol and Taurine. For our 2D-JEPSI spectra, we fitted the J = 7.5HZ extraction for GABA and the J = 0.0Hz extraction for creatine to use as an internal reference, in keeping with our prior publications.11,12
Data Quality Control
For the proton data, there were several levels of voxel selection. Firstly, the automated program which extracts and fits the proton spectra with LCModel filters out all spectra deemed of insufficient quality by the following criteria: excessive contamination by sub-cutaneous lipid signal-bleed, excessive residual water linewidth and residual water shift of > 20Hz off-resonance. Prior testing of this algorithm with the current cutoff parameters ensures that voxels immediately adjacent to the skull are omitted due to excessive lipid signal. Voxels from the frontal regions, anterior to the caudate, were removed due to susceptibility from the underlying sinus cavity. Thus, only proton voxels in the basal ganglia, thalamus and parietal, occipital, and temporal white matter and cortical regions were used and fitted (see Figure 1). The second stage of proton voxel filtering involved visual inspection of each LCModel-fitted spectrum by an experienced MRS-expert (JEJ). Proton spectra in which the following resonances were clearly fitted: Cr (3.9ppm, 3.0ppm), Cho (3.2ppm), NAA (2.0ppm), Glu (2.35ppm) and Myo (3.6ppm) were included and all other spectra in which the LCModel fit was deemed of insufficient quality were discarded (see Figure 2). The final stage of filtering involved a statistical filter applied after all measured proton metabolites were normalized to total creatine: any metabolite-to-creatine value that fell outside of 3 standard deviations of the mean for each group was omitted. After this 3-stage filtering process, the average number of proton voxels utilized per subject was 18.3 ± 6.8 and 14.1 ± 6.6 for the PI and control groups, respectively. A “global” average over the 8×8 sub-matrix was then computed for each subject.
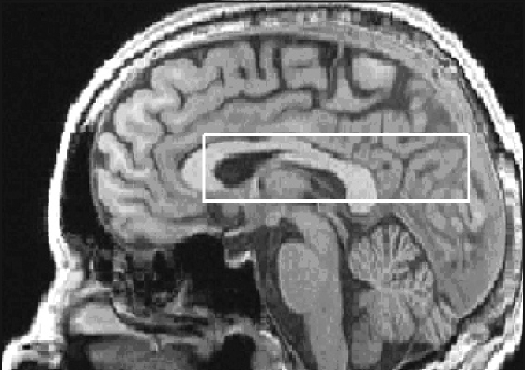
Figure 1.
Sagittal image at 4 T depicting the placement of the 30mm thick MRSI slab.
Figure 2.
J-resolved spectra from the parieto-occipital region of a healthy control subject. Spectra are exponentially-filtered with 4Hz filtering and the J = 7.5Hz spectrum is exaggerated in vertical scale for comparison to the J = 0.0Hz.
To assess whether any systematic differences between groups in acquisition variables may have influenced our results, creatine and field homogeneity measures were computed. Creatine values for the two groups were nearly identical (control = 0.966 +/− 0.312, insomnia = 0.921 +/− 0.304, P = NS). Unsuppressed global and slab water linewidth were tested to assess the degree of field homogeneity, and were also indistinguishable between groups (controls: global shim = 26.4 +/− 5.7, slab shim = 16.6 +/− 1.8; insomnia: global shim = 27.6 +/− 6.6, slab shim = 17.6 +/− 3.3, all P = NS).
JEPSI Test-retest Reliability of Measurement
Three healthy male subjects underwent repeated scans 1 week apart using the same protocol, analysis, and system described above. The coefficient of variance for repeated global-slab measures for GABA was 13%.
Statistics
The a priori primary outcome variable was the difference in GABA/creatine ratio between the PI and control group, tested with a two-group t test. Regression analysis was used to examine whether age, gender and BMI had an effect on GABA levels. Secondary analyses used the Spearmann correlation coefficient to assess the relationship of GABA levels with key PSQI, ISI and PSG variables. Exploratory analyses assessed levels of other neurotransmitters and metabolites [Choline, Creatine, N-acetyl-asparte (NAA), N-acetyl-aspartyl-glutamate (NAAG), Glutamate, Glutamine, Glycine, Myo-inositol and Taurine] in PI and controls.
RESULTS
A total of 2470 subjects with an insomnia complaint were initially screened by telephone; 84 were potentially eligible and were invited for additional screening at Brigham and Women's Hospital. Of these, 37 were excluded as they did not meet the study's inclusion and exclusion criteria after further screening (e.g., from sleep diary and actigraphy, laboratory or PSG abnormalities, or diagnosis of comorbid insomnia), and 28 withdrew consent. For the normal sleeping controls, 25 subjects were initially evaluated; 3 were excluded for having other primary sleep disorders, 4 for current or past psychiatric disorders, and 1 due to metal implants. One insomnia subject was not scanned due to scheduling conflicts, and one was not scanned due to machine malfunction. One additional subject with insomnia and one control subject were scanned but were excluded from analysis due to inadequate acquisition of sufficient GABA data. The average clock times of MRS scans for the insomnia group was 15:34 +/− 3hr 13 min and for the control group was 15:28 +/− 3hr 15 min.
The insomnia group (n = 16) was comprised of 8 females, with a mean age of 37.3 +/− 8.1 years (range 25–55). Of this group, 94% were Caucasian, and 6% were Hispanic. All PI subjects had a continuous history of insomnia for at least 6 months, all but one for greater than one year, and 10/16 for at least 5 years. Their PSQI and ISI scores as well as the results of their two overnight sleep studies confirmed their insomnia (see Tables 1 and 2). None had a history of a mood or anxiety disorder; one had a distant history of probable alcohol abuse. Six of sixteen subjects with PI had taken at least one dose of a benzodiazepine receptor agonist in their lifetime, though none had used any of these medications continuously for longer than one month at any time. One subject had used lorazepam every ten days until one month prior to their 1H-MRS scan; otherwise, all others had had no use for at least four months prior to scanning. The control group (n = 16) consisted of 7 females, with a mean age of 37.6 +/− 4.5 years and age range 30–48. Of these, 88% were Caucasian, 6% were Asian and 6% Hispanic.
Table 1.
Demographic and Questionnaire Data in Primary Insomnia and Good Sleeper Control Groups
| PI N=16 mean (sd) |
Control N=16 mean (sd) |
|
|---|---|---|
| Gender, % female | 50 | 43.8 |
| Age, y | 37.3 (8.1) | 37.6 (4.5) |
| BMI, kg/m2 | 24.2 (2.9) | 25.5 (4.2) |
| Non-hispanic white, % | 93.8 | 87.5 |
| PSQI Global | 12.0 (2.6) | 2 (1.2) |
| PSQI sleep latency, min | 57.8 (29.4) | 12.4 (8.3) |
| PSQI total sleep time, hr | 4.5 (1.1) | 7.3 (0.7) |
| Beck Depression Inventory | 5.7 (4.8) | — |
| ISI | 17.4 (3.5) | — |
PI = primary insomnia, BMI = body mass index, PSQI = Pittsburgh Sleep Quality Inventory, ISI = Insomnia Severity Index. In the PI group, 15 subjects had evaluable data for the PSQI Global and PSQI sleep latency items. In the Control group, the Beck Depression Inventory and ISI were not conducted, 14 subjects had evaluable data for the PSQI items, and BMI data were only collected from 13 subjects.
Table 2.
Polysomnography Data from Primary Insomnia Subjects on Sleep Screen and Inpatient PSGs
| Sleep Screen N=15 mean (sd) |
Inpatient N=14 mean (sd) |
|
|---|---|---|
| TST, min | 354.4 (58.7) | 384.3 (68.5) |
| Sleep efficiency, % | 74.7 (12.4) | 80.0 (14.1) |
| Sleep Latency, Stage 1 min | 32.2 (66.2) | 23.2 (33.3) |
| Sleep Latency, stage 2, min | 41.4 (70.1) | 26.5 (33.9) |
| WASO, min | 81.0 (44.0) | 64.3 (48.7) |
| RDI | 3.3 (4.0) | — |
| PLMSi | 2.6 (6.9) | — |
| Stage 1, % | 15.3 (7.8) | 6.6 (2.9) |
| Stage 2, % | 58.3 (10.4) | 51.9 (9.4) |
| Stage SW, % | 5.5 (6.3) | 17.8 (10.3) |
| Stage REM, % | 21.0 (4.0) | 23.7 (7.5) |
TST = total sleep time, WASO = wake time after sleep onset, RDI = respiratory disturbance index, PLMSi = periodic limb movement during sleep index, SW = slow wave, REM = rapid eye movement.
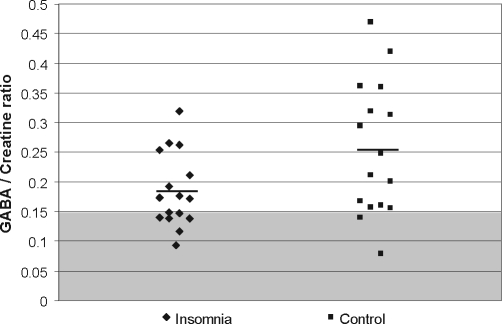
Average GABA levels from sampled brain regions were reduced by nearly 30% in patients with primary insomnia compared to controls (PI = 0.18 +/− 0.06; controls = 0.25 +/− 0.11, t = 2.16, P = 0.039) (see Figure 3). No significant effects of age (F1, 30 = 0.04, P = 0.846), gender (F1,30 = 0.74, P = 0.396), or BMI (F1,30 = 0.33, P = 0.570) on GABA levels were observed.
Figure 3.
GABA / Creatine ratios in Primary Insomnia and normal sleeping controls
Significant correlations between GABA levels and both subjective and objective sleep measures were observed. Across PI and control subjects, global sleep quality, as assessed by the PSQI question, “During the past month, how would you rate your sleep quality overall?” (0 = very good, 3 = very bad), was associated with GABA levels (r = −0.36, P = 0.052). Similarly, self-reported difficulty with sleep maintenance was correlated with GABA levels (r = −0.36, P = 0.05). In the PI group assessed alone, neither the PSQI or ISI global scores or global sleep quality correlated with GABA levels, though self-reported sleep duration from the PSQI was correlated with GABA levels (r = −0.59, P = 0.0158).
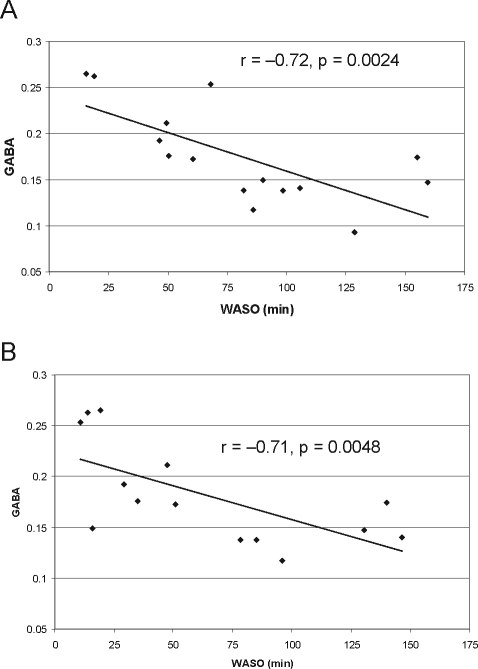
In the PI subjects who had PSGs (n = 15), wake after sleep onset (WASO) was correlated with GABA levels for each of the sleep recordings (sleep screen: r = −0.72, P = 0.0024; inpatient recording: r = 0.71, P = 0.0048) (Figure 4). Stage 1 percentage was correlated with GABA levels for the sleep screen (r = −0.61, P = 0.015) but not the inpatient recording. No significant correlations between GABA levels and sleep latency, sleep efficiency, or total sleep time at either sleep study were observed.
Figure 4.
GABA vs. WASO scatterplots in Primary Insomnia on Sleep Screen (A) and Inpatient (B) PSGs.
WASO = wake time after sleep onset.
Exploratory analyses revealed no differences in levels of other metabolites (aspartate, choline/phosphocholine, glutamine, glutamate, glycine, myo-inositol, N-acetyl-aspartate, and taurine) between PI and controls.
DISCUSSION
Our finding of reduced global brain GABA is the first demonstration of a specific neurochemical abnormality in patients with PI. The high inverse correlations between GABA levels and PSG-derived WASO in PI provide further confidence in the value of GABA measures. Our data is preliminary, in that it is from a small cohort of insomniacs, but is consistent with our limited understanding of possible mechanisms of insomnia pathophysiology. In addition, important confounders such as age and gender were controlled between groups, and all insomniacs were either benzodiazepine-naïve or currently untreated, and were carefully screened to be free of psychiatric and medical illnesses. Our data also emphasizes the value of 1H-MRS as a non-invasive instrument to measure in-vivo concentrations of GABA in patients with insomnia.
Barbiturates and benzodiazepines have been the most commonly used and effective agents for the treatment of insomnia for over 50 years. Their primary physiological function, to modulate GABA-A receptors, led to a longstanding interest in the role of GABA in the regulation of sleep and in the etiology of insomnia. The recent use of selective GABA-A animal knockouts has made clear the essential role of GABA-A receptors in the hypnotic function of BzRAs.14 Similarly, animal and cellular research has made substantial gains in elucidating the role of GABA in the regulation of sleep at the subcortical level. Activation of GABAergic cells in the basal forebrain and preoptic area appear to coordinate sleep onset through direct effects and/or through inhibition of cells promoting cortical activation.6 In fact, lesions of the ventrolateral preoptic area produce dramatic and longstanding reductions in sleep in rats.15 However, the direct relevance of these insights, derived from the molecular basis of hypnotic action and from brainstem control of sleep, to clinical insomnia, which has manifestations beyond sleep in both the physiological and cognitive domains, has not yet been established.
Advances in understanding the biology of clinical insomnia have also been made in the last twenty years. A number of findings have led to the concept that primary insomnia is a manifestation of a state of hyperarousal, which is present during both waking and sleep and at physiological and cognitive levels.16 Evidence for hyperarousal in insomnia comes from studies demonstrating that, when compared to non-insomnia controls, those with PI have elevated evening levels of cortisol,17 persistent fast EEG activity during early Non REM sleep,18 elevated whole body metabolic activity,19 increased inflammatory markers,20 and increased waking whole brain glucose metabolism.5 Although the underlying physiology of hyperarousal is unknown, at a general level it can be considered as an imbalance of excitatory and inhibitory influences in areas subserving alertness, metabolism, and emotional reactivity. As GABA is the most common inhibitory transmitter in the CNS and is involved in all of these processes, it would not be surprising if a reduction in GABA were implicated in such an imbalance. Our finding of a daytime reduction in brain GABA levels is thus consistent with the hyperarousal model of PI.
Reductions in brain GABA levels have also been observed with 1H-MRS in major depressive disorder (MDD) and anxiety disorders. In MDD, reduced GABA levels have been observed in both occipital and prefrontal areas.9,21 However, correlations between depression severity and GABA levels have generally not been observed in these studies.9,21,22 Reduced GABA levels have also been seen with 1H-MRS in panic disorder23 and social anxiety disorder.24 Similarly, PET has demonstrated globally reduced brain GABA-A binding in panic disorder.25 PI shares many features with anxiety and depressive disorders, including sleep disturbance, elevations in anxiety, impairments in concentration and energy, and hyperactivity of the HPA axis. In addition, PI is an important risk factor for incident mood and anxiety disorders.
There are a number of possible interpretations of the similar 1H-MRS-identified reductions in GABA in MDD and anxiety disorders, and PI. It is possible that these disorders share a similar underlying physiology. In fact, there is a strong association of insomnia and MDD both cross-sectionally as well as longitudinally.26–28 To further examine this association, future studies should assess the regional distribution of GABA in patients with PI compared to those with MDD. An alternate hypothesis for the reduction in GABA seen in our patients with PI is that they had either developing or subclinical depressive disorder. However, all our subjects were carefully screened to be free of anxiety and mood disorders. Similarly, the average duration of insomnia symptoms in our patients was over 10 years, and it is unlikely that these psychiatric disorders would not have developed during that time. As most patients with depressive and anxiety disorders exhibit substantial difficulty with sleep, another possibility is that the reductions in GABA seen in these patient groups are in fact based on disturbances in sleep rather than from the underlying psychiatric disorder. The finding that atypical depression (which is characterized by hypersomnia rather than insomnia) is not associated with decreased occipital GABA21 is consistent with this hypothesis. Similarly, the persistence of lowered GABA in one 1H-MRS study of subjects in full remission from depression29 may also be consistent with this hypothesis, as a substantial percentage of those with remitted depression continue to have disturbed sleep.30
The strong association between PSG measures of sleep continuity and GABA levels demonstrated in our PI group is noteworthy. It is also interesting that this association is stronger than that observed for sleep latency, sleep efficiency, and total sleep time. Is it possible that WASO is a more reliable manifestation of hyperarousal than other PSG-derived sleep variables? A previous study examining PSG-derived sleep measures did suggest that WASO was the most stable of PSG-derived measures in a 2-week study.31 Similarly, another, more recent, study demonstrated that of all the PSG-derived measures, WASO best predicted daytime performance deficits, suggesting that this measure may correlate most closely with daytime features of PI.32
The primary advantage of the MRSI-based technique that we employed in this study is the ability to collect information from multiple voxels in the brain in a single scan session, giving us the ability to average the weak GABA signal over all viable voxels and boost the signal-to-noise ratio. However, there are two main disadvantages of MRSI-based methods (especially when using rapid-imaging techniques): loss of spatial resolution and of spectral quality per voxel due to the necessity of having to optimize sequence parameters over the entire MRSI slab.
Thus, an important limitation of this study, the lack of anatomical specificity of our findings of reduced GABA in PI, is a result of our 1H-MRSI technique. Voxels from the basal ganglia, thalamus, and temporal, parietal, and occipital white matter and cortex were averaged to create an overall average brain GABA level for each subject. As GABA is widely distributed in the brain, each of these regions certainly contributed to each subject's data. Similarly, we did not perform tissue segmentation into white and grey matter. Therefore, it is possible that differences in the anatomical distribution of sampled voxels in the PI and normal sleeping control groups may have contributed to the observed differences in GABA. Finally, although it is clear that GABA is predominantly found intracelluarly in the CNS, it is unclear whether the reductions in GABA we observed in PI represent reduced GABA in cells or a reduced number of GABA-containing cells. Although there is a recent finding of reduced hippocampal volumes in PI,33 the hippocampus was the only area in which brain volume reduction was observed in that study, and our 1H-MRS study of PI did not sample from the hippocampus. Future studies of GABA levels in PI can employ single voxel GABA-editing methods, which specify regions of interest, and offer the advantage of increased spectral and anatomical resolution, however at the loss of brain coverage.
Another important limitation of our data is that the behavioral state of the subjects at the time of GABA spectra acquisition with 1H-MRS is unknown. GABA data acquisition began roughly 20 minutes after entry into the magnet and occurred over a period of roughly 20 minutes. Without behavioral or EEG monitoring of subjects, it is unclear whether they were asleep or awake during GABA data collection. Differences between groups in brain GABA levels may thus potentially be accounted for by a systematic difference in sleep-wake state during 1H-MRS scans between groups. However, no differences were found on analysis of multiple secondary metabolites of interest (glutamate, phosphorus, aspartate, choline, glutamine, glycine, myo-inositol, NAA), suggesting that a gross difference in behavioral state was not present.
Due to the inclusion criteria of the study, our results are only generalizable to those with primary insomnia and to young and middle-aged adults. Evaluation of a wider age range and those with comorbid insomnia, particularly those with insomnia associated with anxiety and mood disorders, would be instructive. Similarly, our study used research volunteers rather than patients from sleep clinics, as a means to minimize medical and psychiatric comorbidity as well as the influence of pharmacotherapy. The diagnostic information regarding our good sleeper controls was more limited than what we obtained from the insomniacs, and it is possible that some of these subjects had primary sleep disorders that were not evaluable by history and sleep diaries but would have been identified by PSG.
In summary, our study provides the first evidence of a neurochemical difference in the brains of those with PI compared to normal sleeping controls. Our finding of a global reduction in daytime GABA levels in non-medicated individuals with PI is preliminary, and must be replicated. However, 1H-MRS is a valuable tool to assess GABA in vivo, and with use of alternate acquisition protocols that allow better anatomical resolution, may shed further light on the neurobiology of insomnia.
DISCLOSURE STATEMENT
This study was supported in part by Sepracor. Dr. Winkelman has received research support from Boehringer-Ingelheim, GlaxoSmithKline, UCB Pharma, and Sepracor; has been on the advisory board and/or consulted for Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, UCB Pharma, and Takeda; and has participated in speaking engagements for Boehringer Ingelheim, GlaxoSmithKline, Sanofi-Aventis, Sepracor, and Takeda. Dr. Buxton has received research support from Sepracor and Cephalon; is on the speaker's bureau for Takeda; and has consulted for Dinsmore, LLC. Dr. Renshaw has reseived research support from GlaxoSmithKline and Eli Lilly; has consulted for Novartis and Kyowa Hakko; and is founder and equity holder in PHB Labs. The other authors have indicated no other financial conflicts of interest.
ACKNOWLEDGMENTS
Supported by The Frank Gillis Fund, the Florence Petrlik Charitable Foundation, a research grant from Sepracor, GCRC grant M01-RR02635, and NIH grant MH58681.
REFERENCES
- 1.Pearson NJ. Insomnia, trouble sleeping, and complementary and alternative medicine: Analysis of the 2002 national health interview survey data. Arch Intern Med. 2006;166:1775–1782. doi: 10.1001/archinte.166.16.1775. [DOI] [PubMed] [Google Scholar]
- 2.LeBlanc M. Psychological and health-related quality of life factors associated with insomnia in a population-based sample. J Psychosom Res. 2007;63:157–166. doi: 10.1016/j.jpsychores.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Riemann D. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76:255–259. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 4.Gottesman C. GABA mechanisms and sleep. Neuroscience. 2002;111:231–239. doi: 10.1016/s0306-4522(02)00034-9. [DOI] [PubMed] [Google Scholar]
- 5.Nofzinger EA. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–2128. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 6.Saper CB. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 7.Ke Y. Assessment of GABA concentration in human brain using two-dimensional proton magnetic resonance. Psychiatry Res. 2000;100:169–178. doi: 10.1016/s0925-4927(00)00075-5. [DOI] [PubMed] [Google Scholar]
- 8.Rothman DL. Localized 1H NMR measurements of gamma-aminobutyric acid in human brain in vivo. Proc Natl Acad Sci USA. 1993;90:5662–5666. doi: 10.1073/pnas.90.12.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasler G. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64:193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 10.Martin WR. MR spectroscopy in neurodegenerative disease. Mol Imaging Biol. 2007;9:196–203. doi: 10.1007/s11307-007-0087-2. [DOI] [PubMed] [Google Scholar]
- 11.Jensen JE. Grey and white matter GABA level differences in the human brain using two-dimensional, J-resolved spectroscopic imaging. NMR Biomed. 2005;18:570–576. doi: 10.1002/nbm.994. [DOI] [PubMed] [Google Scholar]
- 12.Jensen JE. Two-dimensional, J-resolved spectroscopic imaging of GABA at 4 Tesla in the human brain. Magn Reson Med. 2005;54:783–788. doi: 10.1002/mrm.20644. [DOI] [PubMed] [Google Scholar]
- 13.Ogg RJ. WET, a T1- and B1-insensitive water-suppression method for in vivo localized 1H NMR spectroscopy. J Magn Reson B. 1994;104(1):1–10. doi: 10.1006/jmrb.1994.1048. [DOI] [PubMed] [Google Scholar]
- 14.Rudolph U. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- 15.Lu J. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–3842. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonnet MH. Hyperarousal and insomnia. Sleep Med Rev. 1997;1:97–108. doi: 10.1016/s1087-0792(97)90012-5. [DOI] [PubMed] [Google Scholar]
- 17.Vgontzas AN. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86:3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 18.Perlis ML. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet MH. 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep. 1995;18:581–588. doi: 10.1093/sleep/18.7.581. [DOI] [PubMed] [Google Scholar]
- 20.Vgontzas AN. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31:15–16. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 21.Sanacora G. Subtype-specific alterations of gamma-aminobutyric acid and glutamate in patients with major depression. Arch Gen Psychiatry. 2004;61:705–713. doi: 10.1001/archpsyc.61.7.705. [DOI] [PubMed] [Google Scholar]
- 22.Sanacora G. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]
- 23.Goddard AW. Reductions in occipital cortex GABA levels in panic disorder detected with 1h-magnetic resonance spectroscopy. Arch Gen Psychiatry. 2001;58:556–561. doi: 10.1001/archpsyc.58.6.556. [DOI] [PubMed] [Google Scholar]
- 24.Pollack MH. High-field MRS study of GABA, glutamate and glutamine in social anxiety disorder: response to treatment with levetiracetam. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:739–743. doi: 10.1016/j.pnpbp.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Malizia AL. Decreased brain GABA(A)-benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Arch Gen Psychiatry. 1998;55:715–720. doi: 10.1001/archpsyc.55.8.715. [DOI] [PubMed] [Google Scholar]
- 26.Ford DE. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 27.Roberts RE. Sleep complaints and depression in an aging cohort: A prospective perspective. Am J Psychiatry. 2000;157:81–88. doi: 10.1176/ajp.157.1.81. [DOI] [PubMed] [Google Scholar]
- 28.Ohayon MM. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37:9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 29.Bhagwagar Z. Low GABA concentrations in occipital cortex and anterior cingulate cortex in medication-free, recovered depressed patients. Int J Neuropsychopharmacol. 2008;11:255–260. doi: 10.1017/S1461145707007924. [DOI] [PubMed] [Google Scholar]
- 30.Nierenberg AA. Evolution of remission as the new standard in the treatment of depression. J Clin Psychiatry. 1999;60(Suppl 22):7–11. [PubMed] [Google Scholar]
- 31.Wohlgemuth WK. How many nights are enough? The short-term stability of sleep parameters in elderly insomniacs and normal sleepers. Psychophysiology. 1999;36:233–244. [PubMed] [Google Scholar]
- 32.Edinger JD. Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep. 2008;31:599–607. doi: 10.1093/sleep/31.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riemann D. Chronic insomnia and MRI-measured hippocampal volumes: a pilot study. Sleep. 2007;30:955–958. doi: 10.1093/sleep/30.8.955. [DOI] [PMC free article] [PubMed] [Google Scholar]