Abstract
Introduction:
Although wrist actigraphy-derived sleep indices correlate with adverse health outcomes, it is unclear whether these indices identify specific sleep disorders.
Methods:
Overnight polysomnography and ≥ three 24-h periods of wrist actigraphy were performed in the Study of Osteoporotic Fractures (SOF) (n = 455, age: 73–96 y). Actigraphy identified those with reduced sleep efficiency (SE, < 70%) and decreased sleep duration (≤ 5 h). Sleep disorders considered were: (1) sleep-disordered breathing (SDB): respiratory disturbance index ≥ 15 and (2) periodic limb movement disorder (PLMD): periodic limb movement-arousal index ≥ 5. Multivariable logistic regression analyses modeled each sleep disorder as the dependent variable with wrist actigraphy measures, age, race, medication use, depression, body mass index, activity, mental status, and comorbidity as independent variables.
Results:
In multivariable models, poor SE derived from wrist actigraphy was associated with 2.4-fold higher odds of SDB (OR = 2.43, 95% CI: 1.43–4.14) and PLMD (OR = 2.36, 95% CI: 1.34–4.15). Reduced sleep duration was associated with 3.2-fold higher odds of SDB (OR = 3.18, 95% CI: 1.51–6.68), and a 3.8-fold higher odds of PLMD (OR = 3.77, 95% CI: 1.78–17.95).
Conclusions:
In elderly women, wrist actigraphy-ascertained reduced SE and sleep duration are associated with objective measures of SDB and PLMD. Thus, although not able to discriminate between the different sleep disorders, variations in wrist actigraphy measures collected in epidemiologic studies may identify individuals at higher risk of SDB or PLMD.
Citation:
Mehra R; Stone KL; Ancoli-Israel S; Litwack-Harrison S; Ensrud KE; Redline S. Interpreting wrist actigraphic indices of sleep in epidemiologic studies of the elderly: the study of osteoporotic fractures. SLEEP 2008;31(11):1569–1576.
Keywords: Actigraphy, sleep-disordered breathing, periodic limb movement disorder
SLEEP DISRUPTION IS AN EXTREMELY COMMON PROBLEM IN THE ELDERLY POPULATION ASSOCIATED WITH DECREMENTS IN QUALITY OF LIFE AND medical health status.1–8 Epidemiologic data indicate that 12% to 54% of older individuals report symptoms of sleep difficulties on a consistent basis, with a higher percentage of self-reported sleep disruption in women compared to their male counterparts.6,7,9 For example, in a study of nearly 2400 older individuals, the prevalence of insomnia was 54% in women and 36% in men.7 Subjective symptoms of sleep problems have been associated with a wide variety of adverse health outcomes. In the Study of Osteoporotic Fractures (SOF), women with self-reported increased napping and longer sleep had a greater risk of falls and fractures.10 These findings are further corroborated by results from a separate study demonstrating an association between disrupted sleep and falls reporting an overall higher risk of falls in women compared to men with sleep difficulties even after consideration of confounding factors.11 Also, results from the National Sleep Foundation 2003 survey highlight increased napping in the elderly which is associated with the diagnosis of depression.12 Prospective, population-based data also support a relationship between daytime naps in the elderly with increased mortality.13 Self reported sleep problems may represent manifestations of underlying sleep disorders such as sleep-disordered breathing (SDB) and/or periodic limb movement disorder (PLMD). These data underscore the importance of understanding the basis for poor sleep in older individuals, in particular elderly women, as they are at increased risk of adverse outcomes including increased mortality.5,7,8,10–22
In epidemiological studies, objective measures of poor sleep have been quantified largely by use of actigraphy, which employs a watch-like device typically worn on the wrist used to record a digitally integrated measurement of gross motor activity as indicators of sleep wake parameters. Compared to standard single-night polysomnography, actigraphy has the attributes of decreased obtrusiveness and minimal individual burden as well as the ability to record continuously over several weeks or longer providing data over the course of multiple sleep periods. Actigraphy thereby takes into consideration night-to-night variability of collected sleep measures. Impaired sleep determined by wrist actigraphy has been reported to be associated with falls, fractures, impaired cognition, and increased mortality.10,15,23,24 In the SOF cohort, women with short sleep (total sleep time [TST] ≤5 h and those with reduced sleep efficiency (SE, defined as the amount of sleep given the amount of time in bed) as defined by wrist actigraphy, experienced an increased mortality risk compared to those with longer sleep or higher SE.24 Actigraphically defined reduced SE, prolonged sleep latency, increased wakefulness after sleep onset and increase in naps have been shown to be associated with impaired cognition in a cohort of elderly females.15
Despite the growing use of actigraphy in epidemiologic studies for characterizing and quantifying sleep problems, the bases for actigraphically ascertained poor sleep are uncertain; and may reflect primary sleep disorders such as SDB and/or PLMD. It is also unclear whether individuals with actigraphically determined sleep disruption have underlying primary sleep disorders or other comorbid factors such as depression that predispose to or may account for reduced quality of sleep. Poor sleep determined by wrist actigraphy defined as reduced SE or reduced TST may in essence be a non-specific “marker” for other comorbidity and not represent specific sleep disorders. We chose to capitalize on the assembly of both standardized polysomnography and wrist actigraphy data from the SOF study, a longitudinal cohort of elderly women, to better understand whether actigraphic measures of reduced SE and decreased TST are associated with polysomnography-determined SDB and/or PLMD symptoms in older women.
METHODS
Study Population
The sample was derived from the SOF study, an ongoing cohort study examining risk factors for osteoporotic fractures in women. Women who were community-dwellers, aged ≥65 years, able to walk unassisted and had no previous bilateral hip replacement were recruited from population-based listings in 4 US areas: Baltimore County, Maryland; Minneapolis, Minnesota; Portland, Oregon; and the Monongahela Valley, near Pittsburgh, Pennsylvania. A total of 9,704 Caucasian women were enrolled between September 1986 and October 1988,25 and 662 African American women were enrolled between February 1997 and February 1998.26
Objective and subjective measures of sleep were added to the protocol during the most recent biannual visit (the 8th examination) which took place from January 2002 to April 2004. A total of 4727 women (age: 84.1 ± 4.1 [mean ± SD], 70–100 (range)) participated in this visit, of whom 3137 visited a clinic for wrist actigraphy, performance measures, anthropometry, and a clinic interview. Of these participants, 1051 had self-administered questionnaire data only, and 539 consented to a limited exam done in the home. This home visit exam was performed by clinic staff and included height, weight, pulse, questionnaire data, wrist actigraphy, medication information, cognitive tests, functional status, grip strength, chair stands and walking speed if possible. Wrist actigraphy data were collected on all participants who completed a clinic or home visit. Questionnaire information regarding sleep habits was asked of all women. Unattended overnight 12-channel in-home polysomnography was completed in a subset of 455 women recruited from 2 clinical centers (Minnesota and Pittsburgh). The current study is based upon data from these 455 participants. The institutional review boards at each site approved the study, and informed consent was obtained from the participants.
Wrist Actigraphy and Polysomnography Data Collection
Wrist actigraphy was performed using the Sleepwatch-O (Ambulatory Monitoring, Inc., Ardsley, NY) worn on the wrist of the nondominant hand over a minimum of three 24-h periods during which sleep diaries were self-completed. The actigraph measures movement using a piezoelectric biomorph-ceramic cantilevered beam which generates a voltage when the actigraph is moved, and the voltages are collected continuously and stored in 1-min epochs. The Action W-2 software (Ambulatory Monitoring, Inc.)27 was used to analyze the raw data, and the University of California San Diego scoring algorithm28 was used for data collected. Data were collected simultaneously in 3 modes as follows: proportional integration mode (PIM), which calculates the areas under the curve for each epoch; time above threshold (TAT), which counts the amount of time per epoch that the signal is above a set threshold; and zero crossing mode (ZCM), which counts the number of times per epoch that the signal crosses a threshold (set very close to zero). In recently published data from the SOF study involving subset of 68 women with concurrently performed wrist actigraphy and polysomnography, the ICC between wrist actigraphic data derived from PIM analysis and polysomnography was high: 0.76 (95% CI 0.64 to 0.84) for TST and 0.61(95% CI 0.44 to 0.74) for SE.29 Given this high correlation, the analyses of the current study are focused upon PIM actigraphy. However, exploratory analyses also examined actigraphy data analyzed using TAT and ZCM. Collection of wrist actigraphy was usually initiated the day of the clinic exam and continued for a minimum of ≥ three 24-h periods. Primary actigraphy data were edited to indicate in-bed intervals. For this, the time in and out of bed were identified from self-report from the sleep diaries. A “quality” variable was also developed which is a grading of how closely the self-report interval point (in and out of bed) corresponded to the interval points scored in the file. SE determined by wrist actigraphy was defined as the percentage of time scored as sleep during the in-bed interval. The points for these intervals are set based on self-report information from the sleep diaries. TST determined by wrist actigraphy was defined as the total number of minutes scored as sleep in the in-bed interval. Interscorer reliability has been reported as excellent in the same actigraphy scoring group (intraclass coefficient [ICC] of 0.84 and 0.95 respectively for SE and TST).30
At the time of the clinic examination, a home visit, for the purpose of performing single-night polysomnography, was also scheduled. Generally, polysomnography and actigraphy occurred within 3 months of one another. The majority (63%) of these sleep studies were performed within one month of each other, and 14.9% of the participants had their polysomnograms concurrent with actigraphy collection. In-home polysomnography (Compumedics Siesta Unit, Abbotsville, Australia) was performed by trained and certified research staff as described before were performed.31 During an evening home visit, sensors were attached to provide the following recording montage: 2 central electroencephalograms (C3/A2, C4/A1), bilateral electrooculogram, chin electromyogram, thoracic and abdominal respiratory effort, airflow (nasal-oral thermocouple and nasal pressure), finger pulse oximetry, electrocardiogram, body position, and bilateral leg movements (piezoelectric sensors). The sleep studies were transferred to the Case Reading Center, Cleveland, Ohio where they were scored by certified scorers.32 Apneas were identified as a complete or almost complete cessation of airflow (by thermocouple) >10 sec associated with ≥3% oxygen desaturation. Obstructive apneas were scored if effort was observed on either abdominal or thoracic effort channels, and central apneas were scored if there was no effort on either of these channels. Hypopneas were noted as a clearly discernible, at least 30%, reduction in respiratory channels > 10 sec associated with a ≥ 3% oxygen desaturation. Inter- and intra-scorer reliability for scoring of respiratory events by the same scoring group is excellent (ICC range of 0.76–0.99).33 The respiratory disturbance index (RDI) was defined as the number of apneas and hypopneas per hour of sleep. Sleep stages and arousals were scored by certified scorers using standard criteria. Leg movements were scored according to American Academy of Sleep Medicine criteria (> 4 consecutive 0.5- to 5-sec movements, each separated by 5–90 sec).34 Leg movements that occurred at the termination of respiratory events were not considered in the calculation of periodic limb movements in sleep unless they were part of a cluster of ≥ 4 leg movements in which ≥ 2 leg movements occurred independently of respiratory event termination.
Other Measures
All participants completed questionnaire data, which included questions about self-reported health and medical history. Participants were asked to bring in all current medications used within the last 2 weeks, and a computerized medication coding dictionary was used to categorize the medications.35 During the home or clinic visits, body weight was measured with a standard balance beam scale, height with a wall-mounted Harpenden stadiometer (Holtain, England), and these measurements were used to calculate the body mass index (BMI, kg/m2). Self-reported information about daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS).36 The standard cutpoint of ESS > 10 was used to define excessive daytime sleepiness.37 The Geriatric Depression Scale was used to assess depressive symptoms, and the standard cutoff of ≥ 6 symptoms was used to define depression.38 During the home or clinic visit the Mini-Mental State Examination (MMSE) was administered to assess cognitive function (range 0 to 30), with higher scores representing better cognitive functioning.39 To assess function, women were asked whether they had difficulty performing any of 6 independent activities of daily living, which included walking 2–3 blocks, climbing or walking down 10 steps, preparing meals, performing heavy housework, and shopping.40,41
Statistical Analyses
Exploratory bivariate analyses, were initially performed assessing the relationships between SE categories (≥ 90, > 85–90, > 80–85, ≤ 75) and polysomnogram indices including RDI, percentage sleep time below 90% oxygen saturation, periodic limb movement index (PLM-I) and periodic limb movement arousal index (PLM-AI), and Pearson or Spearman correlations were performed on these variables considered as continuous measures. Clinically significant cutoffs for SE, TST, SDB, and PLMD were used in final models. The exposures of sleep quality considered were wrist actigraphy-based SE dichotomized at 70% and TST dichotomized at 5 h with decided cutoffs based on previously published data.10,15 The primary outcomes (polysomnogram-based) were SDB defined as RDI defined as RDI ≥ 15; PLMD defined as PLM-AI ≥ 5. These cutoffs were determined by clinical significance.31
Unadjusted logistic regression models are presented in addition to models adjusted for those variables that are potential confounders which could be associated with poor sleep determined by wrist actigraphy and polysomnography including subject characteristics, depression, activity level, medication use, and medical conditions. Logistic models were used when considering SE as the relevant exposure (dichotomized at 70%) and alternatively considering TST as the exposure (dichotomized at 5 h) with unadjusted and adjusted models presented as follows: Model 1 adjusted for age, race, antidepressant use, depression, BMI, activity level, > 1 instrumental activities of daily living impairments, long and slow acting benzodiazepine use, MMSE score, and > 1 of the following medical conditions (cancer, chronic obstructive pulmonary disease, diabetes mellitus, osteoarthritis, Parkinson disease, stroke) and Model 2 adjusted for the covariates noted in Model 1 in addition to PLMD (when SDB was considered as the relevant outcome) or for SDB (when PLMD was considered as the relevant outcome). We further assessed models of actigraphic exposures of poor sleep with the outcomes of either or both sleep disorders (SDB and PLMD). The odds ratios and 95% confidence intervals are presented.
Concordance statistics (C-statistics) were calculated to assess the degree to which wrist actigraphy measures (TST, SE) could discriminate between those with and without SDB and PLMD (unadjusted and adjusted models presented).42,43
Analyses were performed using SAS statistical software (version 9.1, SAS Institute, Inc., Cary, NC).
RESULTS
Sample Characteristics
As reported by Claman and colleagues, the subset considered for analysis in the current study was similar to the overall cohort except for slightly younger age (82.9 ± 3.5 versus 84.2 ± 4.1 y), higher BMI (27.9 ± 5.1 versus 26.8 ± 5.0 kg/m2), less antidepressant use (11% versus 15%), and higher cognitive functioning (28.3% ± 1.6% versus 27.6% ± 2.2%).31 The analytic sample consisted of a subset of elderly women participating in the SOF study who had both a minimum of three 24-h periods of wrist actigraphy and single night polysomnography performed within an average of 3 months of actigraphy (n = 455). This subset of women were older (82.9 ± 3.5 y), overweight (BMI: 27.9 ± 5.1 kg/m2), and predominantly Caucasian (91.8%) (Table 1).
Table 1.
Characteristics of SOF Participants According Sleep Measures Determined by Actigraphy
| All participants (n = 455) | Sleep Efficiency <70% (n = 84) | Sleep Efficiency ≥70% (n = 371) | Total Sleep Time ≤5 hours (n = 36) | Total Sleep Time >5 hours (n = 419) | |
|---|---|---|---|---|---|
| Age † | 82.9 ± 3.5 | 83.2 ± 4.0 | 82.8 ± 3.3 | 83.3 ± 3.9 | 82.9 ± 3.4 |
| Caucasian ‡ | 91.8 | 79.8* | 94.3 | 86.1 | 92.2 |
| Body mass index (kg/m2) † | 27.9 ± 5.1 | 29.0 ± 5.4* | 27.6 ± 5.0 | 30.2 ± 5.9* | 27.7 ± 4.9 |
| Walk for exercise ‡ | 34.4 | 27.4 | 35.7 | 16.7* | 35.7 |
| Depression ‡ | 12.8 | 20.2* | 11.1 | 19.4 | 12.2 |
| Antidepressant use ‡ | 11.1 | 19.1* | 9.4 | 16.7 | 10.7 |
| Benzodiazepine use ‡ | 8.0 | 9.5 | 7.8 | 5.6 | 8.4 |
| Functional Status > 1 (%) | 29.7 | 40.5* | 27.0 | 36.1 | 28.9 |
| Mini-Mental Status Examination † | 28.3 ± 1.6 | 27.8 ± 1.6* | 28.4 ± 1.6 | 27.6 ± 1.8* | 28.4 ± 1.6 |
| Medical condition 1+ ‡ | 70.3 | 79.8* | 67.9 | 69.4 | 70.2 |
| Cardiovascular event ‡ | 34.3 | 35.7 | 34.0 | 27.8 | 34.8 |
| Epworth Sleepiness Scale ≥ 10 ‡ | 16.1 | 19.1 | 15.4 | 36.1* | 14.3 |
| Respiratory disturbance index † | 15.7 ± 15.1 | 22.7 ± 19.9* | 14.2 ± 13.3 | 24.0 ± 18.4* | 15.1 ± 14.6 |
| Sleep disordered breathing (RDI ≥ 15) ‡ | 38.0 | 54.8* | 34.5 | 63.9* | 36.0 |
| Sleep disordered breathing (RDI ≥ 30) ‡ | 13.5 | 25.0* | 10.8 | 22.2 | 12.7 |
| Periodic limb movement index † | 28.5 ± 31.9 | 36.8 ± 37.3 | 26.8 ± 30.3 | 43.6 ± 46.7 | 27.4 ± 30.0 |
| Periodic limb movement disorder (PLM-AI ≥ 5) ‡ | 27.3 | 39.3* | 24.7 | 50.0* | 25.4 |
P-value < 0.05;
Expressed in Mean ± Standard Deviation;
Expressed in Percentage
The distribution of sleep variables, quantified by both polysomnography and wrist actigraphy are also presented (Table 1). In the overall sample, the median RDI (11.4) and mean PLM-I (28.5 ± 31.9) were both mild to moderately elevated and sleep stages showed a modest elevation in the arousal index (20.8 ± 11.9), although had percentages of SWS (20.8 ± 12.5) and REM sleep (18.5 ± 7.2) similar to what has been reported in other female populations.44 Other polysomnography-ascertained sleep characteristics were as follows: SE (%): 74.3 ± 13.2, TST (h): 5.8 ± 1.3, RDI: 15.8 ± 15.1, PLM-AI: 4.1 ± 6.1. Wrist actigraphy-based sleep characteristics were: SE (%): 78.2 ± 10.9 and TST: 6.7 ± 1.2.
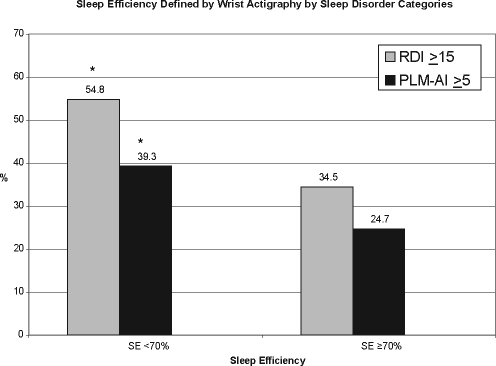
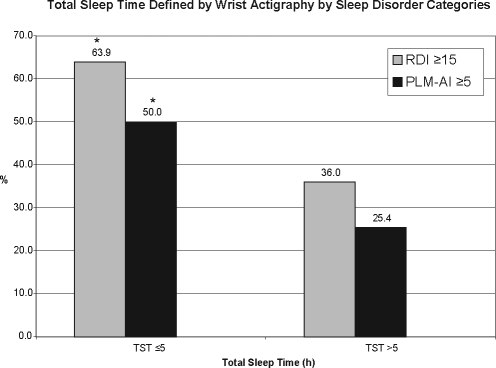
By wrist actigraphy, 18.5% of the sample had a low SE (< 70%) and 7.9% were short sleepers (TST averaged ≤ 5 h). Women with wrist actigraphy-based SE < 70% included a significantly higher proportion of individuals with SDB and with PLMD (P-values < 0.05) (Figure 1). Similarly, those with wrist actigraphy-based TST < 5 h included a higher percentage with SDB and PLMD (P-values < 0.05) (Figure 2)
Figure 1.
The bar graph depicts the percentage of those individuals with SDB and PLMD with sleep efficiency of <70% versus ≥70%. Stars denote categories of sleep disorders which are statistically different (P < 0.05).
Figure 2.
The bar graph depicts the percentage of those individuals with SDB and PLMD with total sleep time of ≤5 h versus >5 h. Stars denote categories of sleep disorders which are statistically different (P < 0.05).
Associations Between Wrist Actigraphy-Based Sleep Efficiency With Sleep Disorders Defined by Polysomnography
Logistic regression analyses were performed to quantify the strength of the association between wrist actigraphy-based SE (dichotomized at 70%) with each sleep disorder. When assessing wrist actigraphy analyzed using the PIM, statistical models showed that women with SE < 70% had a 2.3-fold higher unadjusted odds of SDB (defined as RDI ≥ 15; OR = 2.30, 95% CI: 1.42–3.71) compared to those with SE ≥ 70%, which remained significant in the multivariable Model 1 (OR = 2.43, 95% CI: 1.43–4.14) and in Model 2 after further adjustment for PLMD (OR = 2.47, 95% CI: 1.44–4.24). In unadjusted analyses, a significant association was noted between SE and PLMD (OR = 1.98, 95% CI: 1.20–3.25), which persisted after multivariable adjustment (Model 1) (OR = 2.36, 95% CI: 1.34–4.15) and also after further adjustment for SDB (OR = 2.35, 95%CI: 1.32–4.17) (Table 2). Reduced SE by wrist actigraphy analyzed by PIM was associated with 4-fold increased odds of either SDB or PLMD, and similarly 4-fold increased odds in the presence of both sleep disorders (Table 3).
Table 2.
Adjusted and Unadjusted Odds Ratios Relating Actigraphy-Based Sleep Efficiency and Total Sleep Time with Sleep Disordered Breathing and Periodic Limb Movement Disorder
| Sleep Disordered Breathing (RDI ≥ 15) |
Periodic Limb Movement Disorder (PLM-AI ≥ 5) |
|||||
|---|---|---|---|---|---|---|
| Unadjusted | Model 1* | Model 2** | Unadjusted | Model 1* | Model 2† | |
| Proportional Integration Mode | ||||||
| Reduced sleep efficiency (<70%) | 2.30 (1.42, 3.71) | 2.43 (1.43, 4.14) | 2.47 (1.44, 4.24) | 1.98 (1.20, 3.25) | 2.36 (1.34, 4.15) | 2.35 (1.32, 4.17) |
| Reduced total sleep time (≤5 h) | 3.14 (1.55, 6.38) | 3.18 (1.51, 6.68) | 3.26 (1.54, 6.94) | 2.93 (1.47, 5.85) | 3.77 (1.78, 7.95) | 3.80 (1.78, 8.11) |
| Time Above Threshold Mode | ||||||
| Reduced sleep efficiency (<70%) | 1.79 (1.10, 2.91) | 1.96 (1.14, 3.36) | 1.97 (1.14, 3.39) | 1.55 (0.92, 2.59) | 1.82 (1.02, 3.25) | 1.82 (1.02, 3.25) |
| Reduced total sleep time (≤5 h) | 2.64 (1.36, 5.13) | 2.16 (1.07, 4.34) | 2.18 (1.08, 4.41) | 1.88 (0.96, 3.67) | 2.39 (1.15, 4.96) | 2.38 (1.14, 4.95) |
| Zero Crossing Mode | ||||||
| Reduced sleep efficiency (<70%) | 1.84 (1.26, 2.70) | 1.72 (1.14, 2.60) | 1.71 (1.12, 2.61) | 1.87 (1.23, 2.84) | 2.16 (1.36, 3.44) | 2.16 (1.36,3.44) |
| Reduced total sleep time (≤5 h) | 1.85 (1.21, 2.83) | 1.77 (1.13, 2.79) | 1.80 (1.13, 2.85) | 1.74 (1.11, 2.73) | 2.06 (1.26, 3.37) | 2.06 (1.25, 3.37) |
Adjusted for age, race, antidepressant use, depression, body mass index, walk for exercise, >1 instrumental activities of daily living impairments, long acting and slow acting benzodiazepine use, mini mental status examination score, and >1 selected medical conditions
Adjusted for Model 1 covariates and periodic limb movement disorder (PLM-AI ≥5)
Adjusted for Model 1 covariates and sleep disordered breathing (respiratory disturbance index ≥15)
Odds ratios and 95% confidence intervals presented
Table 3.
Odds Ratios Relating Actigraphy-Based Sleep Efficiency and Total Sleep Time with Sleep Disordered Breathing and Periodic Limb Movement Disorder Outcomes
| SDB or PLMD | SDB and PLMD | |
|---|---|---|
| Reduced sleep efficiency (<70%)* | 2.79 (1.65, 4.73) | 2.58 (1.36, 4.89) |
| Reduced total sleep time (≤5 h)* | 4.59 (1.87, 11.25) | 4.25 (1.95, 9.30) |
Determined by proportional integration mode wrist actigraphy
Similar, albeit somewhat weaker associations were observed when actigraphy was analyzed using either the TAT or ZCM modes of analysis. For example, the adjusted odds ratios for SDB were 1.96 (95% CI: 1.14–3.36) and 1.72 (95% CI: 1.14–2.60), for SE derived using the TAT and ZCM modes, respectively. For PLMD, the adjusted odds ratios for actigraphy defined low SE were 1.82 (95% CI: 1.02–3.252.16 (95% CI: 1.36–3.44), for TAT and ZCM modes, respectively (Table 2).
Wrist Actigraphy-Based Total Sleep Time with Sleep Disorders Defined by Polysomnography
Similar logistic regression models were constructed to understand the associations between wrist actigraphy TST and each PSG-based sleep disorder. Participants with TST ≤ 5 h had 3.1-fold higher unadjusted odds of SDB (OR = 3.14, 95% CI: 1.55–6.38) compared to those with TST > 5 h, which persisted after multivariable adjustment in Model 1 (OR = 3.18, 95% CI: 1.51–6.68) and further adjustment for PLMD in Model 2 (OR = 3.26, 95% CI: 1.54–6.94). In unadjusted analyses, a significant association was noted between reduced TST and PLMD (OR = 2.93, 95% CI: 1.47–5.85), and persisted after multivariable adjustment (Model 1) (OR = 3.77, 95% CI: 1.78–7.95) and adjustment for SDB (Model 2) (OR = 3.80, 95% CI: 1.78–8.11) (Table 2). Reduced TST by actigraphy was associated with 2-fold increased odds of either SDB or PLMD, and similarly 2-fold increased odds in presence of both sleep disorders (Table 3).
Similar although somewhat weaker associations were observed when short sleep duration was defined using the TAT or ZCM actigraphy analyses modes (e.g., adjusted odds rations ranging from 1.77 to 2.39 for the same associations as described above; Table 2).
Predictive Value of Wrist Actigraphy
Although significant associations were observed between wrist actigraphy determined TST or SE with SDB and PLMD, the C-statistics, describing the ability of SE to discriminate individuals with SDB and PLMD were low at 0.57 and 0.56 in unadjusted models and 0.64 and 0.65 in adjusted models, respectively. Similarly, the C-statistics for TST to predict SDB and PLMD were 0.54 and 0.55 in unadjusted models and 0.65 and 0.65 in adjusted models, respectively.
Correlation between Wrist Actigraphy-Based Sleep Efficiency and Total Sleep Time and Polysomnography-Based Indices
Finally, to further understand the association between continuous measures from wrist actigraphy (determined over multiple days) and single night polysomnography, we computed either Pearson or Spearman correlation coefficients depending on the normality of the variables. There were modest correlations between wrist actigraphy-based SE and polysomnography-based SE (r = 0.24) and TST (r = 0.21). Spearman correlations revealed modest inverse associations between wrist actigraphy-based SE and RDI (r = −0.21) as well as wrist actigraphy-based SE and percent sleep time with <90% oxygen saturation (r = −0.18). Weak inverse correlations were also noted between wrist actigraphy-based SE and PLM-AI (r = −0.18) as well as wrist actigraphy-based SE and PLM-I (r = −0.14) (Table 4).
Table 4.
Correlation Coefficients Between Sleep Measures Determined by Wrist Actigraphy and Polysomnography
| n = 455 | Sleep Efficiency‡ | Total Sleep Time‡ | Respiratory Disturbance Index‡ | % Sleep Time Sp02 <90% | Periodic Limb Movement Arousal Index‡ | Periodic Limb Movement Index‡ | Arousal Index‡ | Slow Wave Sleep‡ | REM Sleep‡ |
|---|---|---|---|---|---|---|---|---|---|
| Actigraphy- sleep efficiency† | 0.24 | 0.21 | −0.21 | −0.18 | −0.18 | −0.14 | −0.24 | −0.02 | 0.17 |
| (<0.001) | (<0.001) | (<0.001) | (<0.001) | (<0.001) | (0.004) | (<0.001) | (0.61) | (<0.001) | |
| Actigraphy-total sleep time† | 0.03 | 0.20 | −0.15 | −0.11 | −0.13 | −0.07 | −0.10 | −0.04 | 0.06 |
| (0.52) | (<0.001) | (<0.001) | (0.02) | (0.007) | (0.13) | (0.03) | (0.40) | (0.17) |
Sleep measures determined by proportional integration mode (PIM) wrist actigraphy
Sleep measures determined by polysomnography
Spearman-RDI, % slp time <90%, PLMIA, PLMI (the others use Pearson because the variables are not skewed)
There were modest correlations between wrist actigraphy-based TST and polysomnography-based TST (r = 0.20), but not with polysomnography-derived SE. Significant inverse correlations were also noted between wrist actigraphy-based TST and polysomnography-based RDI (r = −0.15) and PLM-AI (r = −0.13); however, associations were not as strong as those noted with wrist actigraphy-based SE (Table 4). Of note, in a subset of 68 women with concurrently performed actigraphy and polysomnography, the ICC between actigraphic data and polysomnography was 0.76 (95% CI 0.64 to 0.84) for TST and 0.61 (95% CI 0.44 to 0.74) for SE.29
DISCUSSION
Although wrist actigraphy has been widely used in epidemiologic studies, it has been unclear whether short sleep and low sleep efficiency measured by wrist actigraphy operate as nonspecific markers of poor sleep quality or represent markers of specific sleep disorders such as SDB or PLMD. The present analyses demonstrate that low SE or decreased TST as estimated by wrist actigraphy identify elderly women with an approximately 2.5- to 4-fold increased likelihood of SDB or PLMD. In addition, the strength of these associations does not substantively vary with adjustment for an extensive array of covariates postulated to be potential confounders, including measures of depression and activity. On the other hand, assessment of predictive ability showed only modest values for the C-statistic, a measure that weighs sensitivity and specificity to quantify diagnostic utility. In aggregate, these findings indicate that actigraphic indices of poor sleep in elderly women provide unique information and identify women at increased likelihood of having SDB or PLMD. The modest C-statistics; however, suggest that wrist actigraphy cannot reliably be used for specific diagnostic purposes, and that there are likely potential additional etiologies of poor sleep ascertained by actigraphy beyond SDB and/or PLMD.
Our data are consistent with prior research showing the limited predictive ability of actigraphic assessments to reliably identify patients with SDB,45 and we extend this observation to an older general population sample. Results from a recent study in children as well as another study performed in adults also demonstrate poorer reliability of wrist actigraphy-based sleep-wake inference among individuals with SDB.46,47 Similarly, other data report insufficient actigraphic reliability for detection of periodic limb movements which is consistent with our findings of lack of diagnostic value of wrist actigraphy in the setting of PLMD.48 The high prevalence of SDB (38.0%) and PLMD (27.3%) in this older cohort is consistent with findings in the existing literature1,2 and highlights the generalizability of these findings to geriatric populations.
Somewhat stronger relationships were observed for short TST with SDB or PLMD as compared to low SE. Whether this is due to lesser measurement error for TST compared to SE measured using several nights of actigraphy data, or if shorter sleep is a more specific marker for SDB or PLMD than is SE, is unclear. Somewhat stronger associations were observed for either TST or SE when the outcome included either or both SDB and PLMD, compared to one or the other outcome. The latter is consistent with the notion that each sleep disorder—SDB and PLMD—is associated with increased time awake during the sleep period.
Our findings also demonstrate that SDB and PLMD were associated with wrist actigraphically determined poor sleep regardless of the analysis mode considered (i.e., PIM, TAT, or ZCM). However, similar to the overall associations between PSG-determined TST and actigraphy determined TST,29 associations with SDB and PLMD in older women were stronger when the PIM mode was used. A potential explanation for these results may be implicit in the way these actigraphic modes analyze data; i.e., the PIM mode, but not the TAT or ZCM modes, is sensitive to the amplitude of movement which may be important in distinguishing between sleep and wake times in elderly individuals.
A strength of the current study includes the use of standardized, concurrent collection of wrist actigraphy in a variety of modes and polysomnography data. The study's internal validity is substantiated by the excellent reliability of wrist actigraphy and polysomnography scoring.30,33 This study is likely generalizable to the older female population which represents a group of individuals who have a high prevalence of sleep disorders and symptoms with related and possibly resultant increased morbidity and mortality. The current analysis, however, was not designed and cannot address whether SDB or PLMD cause poor SE and short TST or the converse. Also, as an epidemiological study, it was not designed to compare either wrist actigraphy or PSG to a gold standard clinical assessment.
Indices of poor sleep by wrist actigraphy are associated with increased risk of falls, fractures and mortality.10,24 The findings from the current study indicate an association between wrist actigraphy-based reduced TST and SE with polysomnography-determined SDB and PLMD. These data support the role of wrist actigraphy in identifying elderly women at increased risk for sleep pathologies. Thus, inclusion of wrist actigraphy in epidemiologic studies provides additional information potentially not captured by symptoms alone and identifies individuals at increased risk for specific sleep disorders. The high prevalence of SDB and PLMD in women with short TST or low SE suggests the need to determine the extent to which adverse health outcomes associated with actigraphic indices of TST or SE reflect the influence of SDB or PLMD on health and functioning, or rather reflect the poor health of women with disturbed sleep due to unmeasured health or psychological conditions or to nonspecific etiologies. Since the clinical utility of wrist actigraphy as a diagnostic tool for SDB and PLMD is low, these results suggest that wrist actigraphy may identify individuals at high risk for sleep disorders, but will not serve as a valid method to diagnose specific sleep disorders in older women.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Stone hasp participated in a speaking engagement for sponsored by Sanofi-Aventis. Dr. Ancoli-Israel has received research support from Sepracor, Takeda and Litebook; is on the advisory board of Arena, Cephalon, Neurocrine Biosciences, Sanofi-Aventis, Sepracor, and Takeda; and has received the use of equipment from Litebook at a discount. Dr. Ensrud has received research support from BioNovo, Inc. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Investigators in the Study of Osteoporotic Fractures Research Group: San Francisco Coordinating Center (California Pacific Medical Center Research Institute and University of California San Francisco): S.R. Cummings (principal investigator), M.C. Nevitt (co-investigator), D.C. Bauer (co-investigator), D.M. Black (co-investigator), K.L. Stone (co-investigator), W. Browner (co-investigator), R. Benard, T. Blackwell, P.M. Cawthon, L. Concepcion, M. Dockrell, S. Ewing, C. Fox, R. Fullman, S.L. Harrison, M. Jaime-Chavez, L. Lui, L. Palermo, M. Rahorst, D. Robertson, C. Schambach, R. Scott, C. Yeung, J. Ziarno.
University of Maryland: M.C. Hochberg (principal investigator), L. Makell (clinic coordinator), M.A. Walsh, B. Whitkop.
University of Minnesota: K.E. Ensrud (principal investigator), S. Diem (co-investigator), M. Homan (co-investigator), D. King (Program Coordinator), N. Michels (Clinic Director), S. Fillhouer (Clinic Coordinator), C. Bird, D. Blanks, C. Burckhardt, F. Imker-Witte, K. Jacobson, D. King, K. Knauth, N. Nelson, M. Slindee.
University of Pittsburgh: J.A. Cauley (principal investigator), L.H. Kuller (co-principal investigator), J.M. Zmuda (co-investigator), L. Harper (project director), L. Buck (clinic coordinator), C. Bashada, W. Bush, D. Cusick, A. Flaugh, A. Githens, M. Gorecki, D. Moore, M. Nasim, C. Newman, N. Watson.
The Kaiser Permanente Center for Health Research, Portland, Oregon: T. Hillier (principal investigator), E. Harris (co-investigator), E. Orwoll (co-investigator), K. Vesco (co-investigator), J. Van Marter (project director), M. Rix (clinic coordinator), A. MacFarlane, K. Pedula, J. Rizzo, K. Snider, T. Suvalcu-Constantin, J. Wallace.
Case Western Reserve University Reading Center staff: Susan Surovec, Nancy Scott, Rawan Salem, Jean Arnold, Sinziana Seacian, Joanna Romaniuk
Grant support: Supported by Public Health Service Grants AG05407, AR35582, AG05394, AR35584, AR35583, AG08415, Association of Subspecialty Professors, CHEST Foundation of the American College of Chest Physicians T. Franklin Williams Geriatric Development Research Award, and NHLBI K23 HL079114-01A2, American Heart Association National Scientist Development Award (0530188N)
Subject Codes: 112. Sleep Disordered Breathing Pathophysiology
REFERENCES
- 1.Ancoli-Israel S. Sleep-disordered breathing in community-dwelling elderly. Sleep. 1991;14:486–95. doi: 10.1093/sleep/14.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S. Periodic limb movements in sleep in community-dwelling elderly. Sleep. 1991;14:496–500. doi: 10.1093/sleep/14.6.496. [DOI] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S. Sleep fragmentation in patients from a nursing home. J Gerontol. 1989;44:M18–21. doi: 10.1093/geronj/44.1.m18. [DOI] [PubMed] [Google Scholar]
- 4.Fetveit A. Sleep disturbances among nursing home residents. Int J Geriatr Psychiatry. 2002;17:604–9. doi: 10.1002/gps.639. [DOI] [PubMed] [Google Scholar]
- 5.Foley D. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Ford DE. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 7.Maggi S, et al. Sleep complaints in community-dwelling older persons: prevalence, associated factors, and reported causes. J Am Geriatr Soc. 1998;46:161–8. doi: 10.1111/j.1532-5415.1998.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB. Sleep disturbance, psychosocial correlates, and cardiovascular disease in 5201 older adults: the Cardiovascular Health Study. J Am Geriatr Soc. 1997;45:1–7. doi: 10.1111/j.1532-5415.1997.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 9.Mellinger GD. Insomnia and its treatment. Prevalence and correlates. Arch Gen Psychiatry. 1985;42:225–32. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- 10.Stone KL, et al. Self-reported sleep and nap habits and risk of falls and fractures in older women: the study of osteoporotic fractures. J Am Geriatr Soc. 2006;54:1177–83. doi: 10.1111/j.1532-5415.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 11.Brassington GS. Sleep problems as a risk factor for falls in a sample of community-dwelling adults aged 64–99 years. J Am Geriatr Soc. 2000;48:1234–40. doi: 10.1111/j.1532-5415.2000.tb02596.x. [DOI] [PubMed] [Google Scholar]
- 12.Foley DJ. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: findings from the National Sleep Foundation ,2003 Sleep in America` Poll. Am J Geriatr Psychiatry. 2007;15:344–50. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 13.Bursztyn M. The siesta in the elderly: risk factor for mortality? Arch Intern Med. 1999;159:1582–6. doi: 10.1001/archinte.159.14.1582. [DOI] [PubMed] [Google Scholar]
- 14.Bastien CH. Cognitive performance and sleep quality in the elderly suffering from chronic insomnia. Relationship between objective and subjective measures. J Psychosom Res. 2003;54:39–49. doi: 10.1016/s0022-3999(02)00544-5. [DOI] [PubMed] [Google Scholar]
- 15.Blackwell T, et al. Poor sleep is associated with impaired cognitive function in older women: the study of osteoporotic fractures. J Gerontol A Biol Sci Med Sci. 2006;61:405–10. doi: 10.1093/gerona/61.4.405. [DOI] [PubMed] [Google Scholar]
- 16.Ceolim MF. Sleep/wake cycle and physical activity in healthy elderly people. Sleep Res Online. 2000;3:87–95. [PubMed] [Google Scholar]
- 17.Cricco M. The impact of insomnia on cognitive functioning in older adults. J Am Geriatr Soc. 2001;49:1185–9. doi: 10.1046/j.1532-5415.2001.49235.x. [DOI] [PubMed] [Google Scholar]
- 18.Cummings SR, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–73. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 19.Foley DJ. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 20.Foley DJ. Associations of symptoms of sleep apnea with cardiovascular disease, cognitive impairment, and mortality among older Japanese-American men. J Am Geriatr Soc. 1999;47:524–8. doi: 10.1111/j.1532-5415.1999.tb02564.x. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan GA. Mortality among the elderly in the Alameda County Study: behavioral and demographic risk factors. Am J Public Health. 1987;77:307–12. doi: 10.2105/ajph.77.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Loughlin JL. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol. 1993;137:342–54. doi: 10.1093/oxfordjournals.aje.a116681. [DOI] [PubMed] [Google Scholar]
- 23.Schneider JL YK. Predicting onset of depression with self-reported sleep habits in older women. SLEEP abstracts. 2004;27:A124. [Google Scholar]
- 24.Stone KL BT, et al. Impaired sleep increases the short-term risk of mortality: preliminary results from a prospective actigraphy study. SLEEP abstracts. 2004;27:A123. [Google Scholar]
- 25.Cummings SR, et al. Appendicular bone density and age predict hip fracture in women. The Study of Osteoporotic Fractures Research Group. JAMA. 1990;263:665–8. [PubMed] [Google Scholar]
- 26.Vogt MT, et al. Lumbar spine listhesis in older African American women. Spine J. 2003;3:255–61. doi: 10.1016/s1529-9430(03)00024-x. [DOI] [PubMed] [Google Scholar]
- 27.Ardsley, NY: VAM, Inc.; Action-W User's Guide. [Google Scholar]
- 28.Jean-Louis G. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods. 2001;105:185–91. doi: 10.1016/s0165-0270(00)00364-2. [DOI] [PubMed] [Google Scholar]
- 29.Blackwell T, et al. Comparison of sleep parameters from actigraphy and polysomnography in older women: the SOF study. Sleep. 2008;31:283–91. doi: 10.1093/sleep/31.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackwell T. Actigraphy scoring reliability in the study of osteoporotic fractures. Sleep. 2005;28:1599–605. doi: 10.1093/sleep/28.12.1599. [DOI] [PubMed] [Google Scholar]
- 31.Claman DM, et al. Prevalence and correlates of periodic limb movements in older women. J Clin Sleep Med. 2006;2:438–45. [PubMed] [Google Scholar]
- 32.Rechtschaffen A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 33.Whitney CW, et al. Reliability of scoring respiratory disturbance indices and sleep staging. Sleep. 1998;21:749–57. doi: 10.1093/sleep/21.7.749. [DOI] [PubMed] [Google Scholar]
- 34.ASDA Atlas Task Force. Recording and scoring leg movements. The Atlas Task Force. Sleep. 1993;16:748–59. [PubMed] [Google Scholar]
- 35.Pahor M. Drug data coding and analysis in epidemiologic studies. Eur J Epidemiol. 1994;10:405–11. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- 36.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 37.Johns MW. Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the epworth sleepiness scale: failure of the MSLT as a gold standard. J Sleep Res. 2000;9:5–11. doi: 10.1046/j.1365-2869.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 38.Sheikh JI YJ. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontol. 1986;5:165–173. [Google Scholar]
- 39.Folstein MF. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 40.Fitti JE. The Supplement on Aging to the 1984 National Health Interview Survey. Vital Health Stat. 1987;1:1–115. [PubMed] [Google Scholar]
- 41.Pincus T. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–53. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 42.Bamber The area above the ordinal dominance graph and the area below the receiver operating characteristic graph. Journal of Mathematical Psychology. 1975;12:387–415. [Google Scholar]
- 43.Hanley JA. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 44.Redline S. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164:406–18. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- 45.Middelkoop HA. Wrist actigraphic assessment of sleep in 116 community based subjects suspected of obstructive sleep apnoea syndrome. Thorax. 1995;50:284–9. doi: 10.1136/thx.50.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyde M, et al. Validation of actigraphy for determining sleep and wake in children with sleep disordered breathing. J Sleep Res. 2007;16:213–6. doi: 10.1111/j.1365-2869.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 47.Hedner J. A novel adaptive wrist actigraphy algorithm for sleep-wake assessment in sleep apnea patients. Sleep. 2004;27:1560–6. doi: 10.1093/sleep/27.8.1560. [DOI] [PubMed] [Google Scholar]
- 48.Montgomery-Downs HE. Actigraphic recordings in quantification of periodic leg movements during sleep in children. Sleep Med. 2005;6:325–32. doi: 10.1016/j.sleep.2005.02.002. [DOI] [PubMed] [Google Scholar]