Abstract
Study Objectives:
Frequently disrupted and restricted sleep is a common problem for many people in our Western society. In the long run, insufficient sleep may have repercussions for health and may sensitize individuals to psychiatric diseases. In this context, we applied an animal model of chronic sleep restriction to study effects of sleep loss on neurobiological and neuroendocrine systems that have been implied in the pathophysiology of depression, particularly the serotonergic system and the hypothalamic-pituitary-adrenal (HPA) axis.
Design:
Adult rats were exposed to a schedule of chronic partial sleep deprivation allowing them only 4 h of sleep per day. Sleep restriction was achieved by placing the animals in slowly rotating drums. To examine the regulation and reactivity of the HPA axis, blood samples were collected to measure adrenocorticotropin (ACTH) and corticosterone (CORT) responses.
Measurements and Results:
While one day of restricted sleep had no significant effect on HPA axis stress reactivity, sleep restriction for a week caused a blunted pituitary ACTH response in a conditioned fear paradigm. Despite this lower ACTH response, adrenal CORT release was normal. The blunted pituitary response may be related to reduced sensitivity of serotonin-1A receptors and/or receptors for corticotropin-releasing hormone (CRH), since sleep restricted rats showed similar reductions in ACTH release to direct pharmacological stimulation with a serotonin-1A agonist or CRH.
Conclusions:
Chronic sleep restriction may lead to changes in neurotransmitter receptor systems and neuroendocrine reactivity in a manner similar to that seen in depression. This experimental study thus supports the hypothesis that disrupted and restricted sleep may contribute to the symptomatology of psychiatric disorders.
Citation:
Novati A; Roman V; Cetin T; Hagewoud R; den Boer JA; Luiten PGM; Meerlo P. Chronically restricted sleep leads to depression-like changes in neurotransmitter receptor sensitivity and neuroendocrine stress reactivity in rats. SLEEP 2008;31(11):1579–1585.
Keywords: Sleep deprivation, sleep disturbance, insomnia, stress reactivity, serotonin, HPA axis, mood disorders, depression
RESTRICTED OR DISRUPTED SLEEP IS A WIDESPREAD AND SERIOUS PROBLEM IN OUR WESTERN SOCIETY.1,2 MANY PEOPLE EXPERIENCE REGULAR SLEEP LOSS due to our modern around-the-clock lifestyle, increased work pressure, and psychosocial stress. In the long run, insufficient sleep may have many yet unknown repercussions for health and well being. Controlled studies have shown that acute sleep deprivation affects cognitive performance and emotionality.3 Recent experimental studies in healthy subjects show that successive nights of restricted sleep result in a gradually accumulating decline in cognitive function.4,5 Whereas subjects may initially recover from these effects after subsequent sleep, frequent or chronic sleep loss may induce neurobiological changes that are not immediately evident but accumulate over time, ultimately with serious health consequences. Indeed, sleep complaints and restricted sleep have been identified as risk factors for various diseases including psychiatric disorders.6–11
Although sleep disturbances associated with psychiatric disorders are traditionally considered as a symptom of the disease, several studies suggest that the relationship between sleep changes and mood disorders is more complex and may work in the other direction as well. Instead of being a symptom, disrupted and restricted sleep may also be a causal factor that sensitizes individuals and contributes to the development of mood disorders.12,13 Consistent with this, primary insomnia often precedes and predicts the onset of a new depressive episode.6–8,10,11 However, in a clinical setting, cause and consequence are often difficult to separate, and the mechanisms by which disrupted sleep might contribute to the development of mood disorders are unknown.
In this context, we applied an animal model to establish the consequences of chronically disrupted and restricted sleep. We focused our attention on neurobiological and neuroendocrine systems that have been implicated in the pathophysiology of depression, particularly the serotonergic system and the hypothalamic-pituitary-adrenal (HPA) axis.14–16
The HPA axis is an important neuroendocrine stress system, and depression is often described as a condition with HPA axis overactivity on the basis of elevated CRH and cortisol levels.17,18 On the other hand, depressed patients often display a blunted pituitary ACTH response.19,20 Perhaps the chronically elevated CRH levels gradually desensitize the CRH receptors, which in turn may be responsible for the attenuated pituitary responsiveness. Alternatively, the attenuated pituitary ACTH response may also be a result of reduced sensitivity of serotonin receptors and reduced serotonergic neurotransmission. The serotonin-1A receptors in particular are involved in regulating ACTH release, not only directly at the level of the pituitary, but also at the level of the paraventricular nucleus of the hypothalamus.21,22 Several lines of evidence indicate that serotonergic neurotransmission is impaired in depression.23–25 A decrease in serotonin-1A receptor-mediated signalling in depressed patients has been shown by pharmacological challenges26–28 and positron emission tomography (PET) studies.29–32 Although postmortem studies have yielded various results, some of them are consistent with a decrease in serotonin-1A receptor function in depression.25
The altered HPA axis regulation in depressed patients is often taken as an indication that depression is a disorder of stress. However, the question of whether changes in regulation and responsivity of the HPA axis are related to disrupted sleep has received little attention.33
The first aim of this study was to establish the effects of restricted sleep on neuroendocrine stress reactivity, particularly the reactivity of the HPA axis. Rats were subjected to chronic partial sleep deprivation for 7 d, after which they were exposed to a stressor to measure pituitary ACTH and adrenal CORT responses. To examine effects of sleep restriction on the HPA axis response to different kinds of stressors, the animals were subjected to a fear conditioning protocol, which consists of a stressor with a clear physical component (footshocks) and a more emotional stressor (reexposure to the shock box, which is associated with a conditioned fear response).
The second aim was to establish potential neurobiological mechanisms underlying sleep restriction-induced changes in HPA axis stress reactivity. In a previous study we showed that restricted sleep causes a gradual desensitization of the serotonin-1A receptor system.15 Similar to depressed patients, chronically sleep restricted rats had a blunted temperature response to direct stimulation of serotonin-1A receptors with a 1A agonist.15,16 In the present experiment we studied whether changes in HPA axis reactivity might be related to this serotonin-1A receptor desensitization. In addition, we examined whether changes in HPA axis reactivity might be directly related to altered CRH sensitivity. We therefore injected sleep restricted and control rats with a serotonin-1A agonist or CRH and measured their ACTH and CORT responses.
METHODS
Animals and Housing
We used adult male Wistar rats (Harlan, Horst, The Netherlands) weighing approximately 350 g at the start of the experiments. Animals were housed under a 12 h light/12 h dark cycle, with lights on from 09:00 h to 21:00 h. Temperature in the room was maintained at 21±1°C. Rats were provided with food and water ad libitum in all experiments. Experiments were approved by the Ethical Committee of Animal Experiments of the University of Groningen.
Experimental Design
Two experiments were performed with different groups of animals. In both experiments, animals were subjected to a protocol of sleep restriction, allowing them 4 h of sleep each day. Since rats normally sleep about 10 to 12 h each day, 4 h of sleep may not be sufficient to fully recover from 20 h of wakefulness. In previous studies we have shown that rats survive well on this protocol but it does result in gradual neuroendocrine and neurobiological changes.14,15 For example, although one day of sleep restriction had no significant effects on the systems that were studied, 8 d of restricted sleep caused a significant reduction in serotonin-1A receptor sensitivity. In the first experiment of the present study, we examined the effect of sleep restriction on HPA axis stress responsivity. Rats were subjected to a fear conditioning protocol after 1 or after 7 days of sleep restriction. In the second experiment, we examined the effects of sleep restriction on serotonin-1A and CRH receptor sensitivity, particularly in relation to HPA axis responsivity. The HPA axis response to direct stimulation of serotonin-1A receptors and CRH receptors was measured after 7 and 8 d of restricted sleep, respectively.
Sleep Restriction and Forced Activity
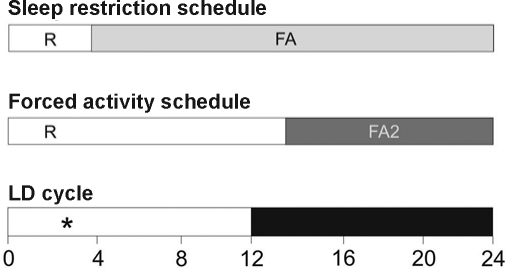
Rats were subjected to a protocol of repeated partial sleep deprivation for 8 d, allowing them to sleep 4 h per day at the beginning of the light phase (09:00–13:00) in their home cage.14–16 The remainder of the time, animals were kept awake by placing them in slowly rotating wheels (40 cm in diameter) driven by an engine at constant speed (0.4 m/min). Since the sleep deprivation procedure includes mild forced locomotion, we used forced activity control rats to test whether effects of sleep restriction might be caused by forced activity rather then sleep loss per se. Animals of the forced activity group were placed in the same plastic drums as the ones that were used for sleep restriction. However, these wheels rotated at double speed (0.8 m/min) for half the time (10 h). Therefore, the rats of the forced activity control group walked the same distance as sleep restricted ones, but had sufficient time for sleep (14 h). Animals were subjected to forced activity during the last 10 h of the dark phase, i.e., their circadian activity phase (Figure 1). Before starting the experiments, all rats were habituated to the experimental apparatus by placing them in the wheels for 1–2 h on 3 successive days.
Figure 1.
Experimental set-up of sleep restriction protocol and forced activity control. Top bar: rats were sleep restricted by forced locomotion (FA) for 20 h each day (grey section of the bar) and were allowed 4 h of rest (R) in their home cage (first 4 h of the light phase). Middle bar: rats were subjected to a protocol of forced activity at double speed (FA2) for half the time. Animals were subjected to the 10 h of forced activity in one block (dark grey section of the bar) which coincided with the last 10 h of the dark phase of the light-dark cycle. Lower bar depicts the 24-h light-dark cycle. As indicated by (*), the stress exposure in the first experiment (fear conditioning on day 1 / 2 or day 7 / 8) and the pharmacological challenges in the second experiment (8-OH-DPAT and CRH on day 7 and 8, respectively) took place between the third and fourth hour of the light phase.
Blood Sampling and Hormone Assays
In the first experiment on HPA axis reactivity in a fear conditioning paradigm, blood samples were collected by making a small incision at the end of the tail.14 Although brief handling was required for this blood sampling procedure, it likely did not interfere with neuroendocrine responses, since it coincided with the beginning and end of the stress sessions. Any effect of the mild handling stress would be obscured by the more severe stress of the footshock conditioning protocol. In the second experiment, we aimed to measure the HPA axis response after direct stimulation of CRH and serotonin-1A receptors without stress exposure. In this case, we made use of permanent heart catheters that allowed stress-free and frequent blood sampling in unrestrained and freely moving animals.34 Rats were provided with a polyethylene catheter in the right atrium of the heart under isoflurane/N2O/O2 inhalation anaesthesia. The catheter was inserted through the right jugular vein and externalized on top of the head according to techniques described earlier.34 After surgery, rats were allowed ≥10 d of recovery before the start of experiments. During this period, animals were habituated to handling and blood sampling procedures. In all experiments, blood was collected in chilled centrifuge tubes (0°C) containing EDTA. Blood samples were centrifuged at 4°C for 15 min at 2600 g, and the supernatant was stored at −80°C for later analysis. ACTH and CORT concentrations were determined by radioimmunoassay (ICN Biomedicals, Costa Mesa, CA).
Conditioned Fear Challenge
To establish the effect of restricted sleep on HPA axis stress reactivity, rats were subjected to a fear conditioning paradigm after 1 or after 7 d of sleep restriction. The experiments after 1 and 7 days were done in separate groups of rats (in each experiment: 8 sleep restriction, 8 forced activity control, 8 home cage control). On these days, rats returned to their home cage after the daily sleep deprivation session and were exposed to a fearful environment approximately 3 h later. Rats were placed in a shock box (25 × 25 × 15 cm) for a half-hour, during which they received 3 shocks at t = 5, 15, and 25 min (2 sec, 0.8 mA each). After the shock session, rats were returned to their home cage. The next day, approximately 24 h later, the rats were reexposed to the shock box for another 30-min period, this time without shocks, to establish HPA axis response to an emotional stressor. Sleep deprivation was not continued between the initial shock session and the shock box reexposure. Importantly, we have previously shown that some effects of chronic sleep restriction persist for many days, even with unrestricted recovery sleep.15 Therefore, we anticipated that changes in HPA axis reactivity would persist as well and not normalize with a single day of recovery sleep. Blood samples were collected to measure plasma levels of ACTH and CORT at the beginning of the stress session, the end of the stress session, and after 45 min of recovery in the home cage (t = 0, 30, and 75 min). On both days, the shock box was thoroughly cleaned and dried between the tests of successive animals.
Serotonergic Challenge
In order to examine the effect of sleep restriction on serotonin-1A receptor sensitivity and serotonergic modulation of HPA axis function, the rats were pharmacologically challenged with the serotonergic 1A receptor agonist (±)-8-hydroxy-2-(di-n-propyl-amino) tetralin hydrobromide (8-OH-DPAT; Sigma, St. Louis, MO). The experiment was done with a total of 30 rats (10 sleep restriction, 10 forced activity control, 10 home cage control). The challenge test took place after 7 d of sleep restriction, during the 4-h rest period in the home cage, between the third and fourth hour of the light phase. Each rat received a tube for blood sampling and infusion of the agonist, which was connected to the permanent heart catheter that externalized on top of the head. After ≥1.5 h, when any handling effect would have disappeared, rats received an intravenous injection of 8-OH-DPAT through the catheter (0.1 mg/kg body weight, dissolved in saline). The concentration of 8-OH-DPAT was based on earlier studies and was chosen to cause intermediate hormone responses.35 To measure plasma levels of ACTH and CORT in response to serotonin-1A receptor activation, blood samples were taken shortly before as well as 5, 15, and 60 min after the 8-OH-DPAT injection. After the last blood sample, the rats were placed back in the rotating wheels to continue the sleep restriction regime.
CRH Challenge
In order to investigate whether sleep restriction alters CRH control of the HPA-axis, rats received an injection of ovine CRH (oCRH; American Peptide Company, Sunnyvale, CA). After 8 d of sleep restriction, i.e., one day after the 8-OH-DPAT challenge, the same rats were again connected to the sampling tubes while in their home cage during the daily 4-h resting phase. Between the third and fourth hour of the light phase, the rats received an intravenous injection of CRH through the jugular vein catheter (0.5 μg/kg body weight, dissolved in saline). The concentration of CRH was based on earlier studies and was known to induce intermediate ACTH and CORT responses.36 Blood samples were taken to assess the sensitivity of the pituitary gland to CRH. Blood sampling and hormone measurements for ACTH and CORT were carried out as described for the serotonin-1A challenge.
Data Analysis and Statistics
To test for effects of sleep restriction on the HPA axis responses to fear conditioning and to injections of 8-OH-DPAT and CRH, hormone data were subjected to analysis of variance (ANOVA) with repeated measures. When appropriate, post hoc Tukey test was applied to establish at which time points after stress exposure or pharmacological challenge the ACTH or CORT levels differed between experimental and control groups.
RESULTS
Stress and Conditioned Fear Response
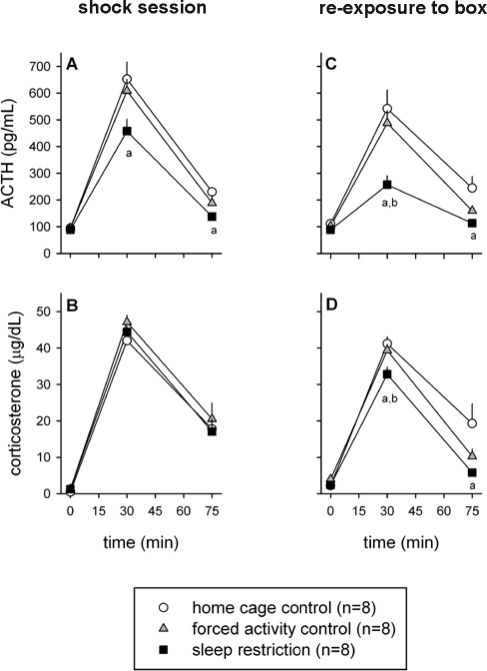
Both the 30-min footshock session and the reexposure to the shock box next day induced a pronounced HPA axis response. This response was not significantly altered after one day of sleep restriction (data not shown). However, 7 d of sleep restriction caused significant alterations in the HPA axis response (Figure 2). For the ACTH response to footshock, ANOVA revealed a significant treatment effect (F2,21 = 5.42, P = 0.013) and a significant treatment × time interaction (F4,42 = 2.99, P = 0.029). On average, the ACTH response was lower in sleep-restricted animals than it was in forced activity controls and home cage controls (Figure 2A). This difference only reached statistical significance for the comparison with home cage controls (post hoc Tukey test: P < 0.05 for t = 30 min and t = 75 min). The plasma levels of CORT after the footshock session were not different between the groups (Figure 2B).
Figure 2.
Effect of sleep restriction on the HPA axis response to stress. Sleep restricted and control rats were exposed to a 30-min session of footshocks after 7 d (left panels, A and B) and a 30-min reexposure to the shock box on day 8 (right panels, C and D). Significant differences in ACTH and CORT responses between sleep restricted and control animals: a = P < 0.05 compared to home cage control, b = P < 0.05 compared to forced activity control. All data are expressed as averages ± SEM.
Upon reexposure to the shock box next day, chronically sleep restricted animals had a significantly blunted ACTH response compared to both control groups (Figure 2C; ANOVA: treatment effect F2,21 = 10.73, P < 0.001 and treatment × time interaction F4,42 = 5.77, P < 0.001; post hoc Tukey test: P < 0.01 versus home cage controls at t = 30 min and t = 75 min, and P < 0.05 versus forced activity controls at t = 30 min). Also the CORT response upon reexposure to the shock box was slightly but significantly lower in sleep restricted animals (Figure 2D; ANOVA: treatment effect F2,21 = 5.54, P = 0.012 and treatment × time interaction F4,42 = 3.43, P = 0.016; post hoc Tukey test: P < 0.05 versus home cage controls at t = 30 min and t = 75 min, and P < 0.05 versus forced activity controls at t = 30 min).
Serotonergic Challenge
Due to partially blocked catheters, blood samples could not be drawn from 2 of 30 animals (both were sleep restricted rats). An IV injection of the serotonin-1A agonist 8-OH-DPAT induced a clear HPA axis response in all animals. On average, the ACTH response of the sleep restricted rats was lower than that of the home cage control animals, which in turn was lower than that of the forced activity control rats (Figure 3A). Repeated measures ANOVA revealed a significant treatment effect (F2,25 = 6.16, P = 0.007) and a significant treatment × time interaction (F6,75 = 4.32, P = 0.001). Post hoc Tukey test indicated that the response of sleep restricted animals was significantly lower than that of forced activity control animals (P < 0.01 for t = 15 min and P < 0.05 for t = 60 min). In contrast, the CORT response to 8-OH-DPAT was not significantly different between sleep restricted and control rats (Figure 3B).
Figure 3.
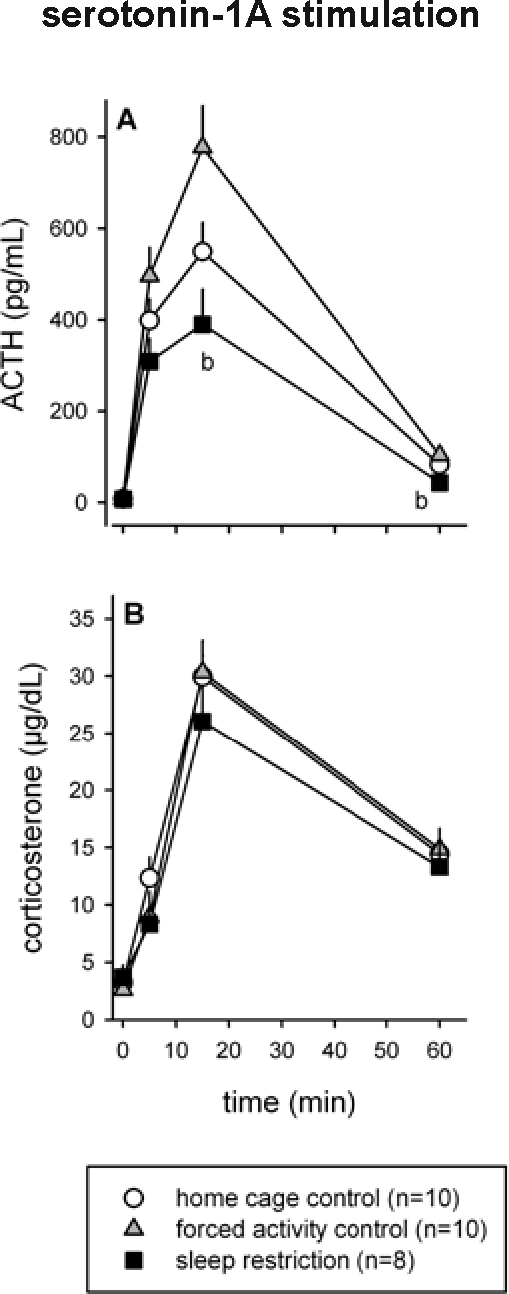
Effect of sleep restriction on serotonin-1A mediated HPA axis responsivity. After 7 d of sleep restriction, rats received an IV injection of the serotonin-1A receptor agonist 8-OH-DPAT and blood samples were taken to establish ACTH and CORT responses (A and B, respectively). Significant differences between sleep restricted and control animals: b = P < 0.05 compared to forced activity control. All data are expressed as averages ± SEM.
CRH Challenge
Because of blocked catheters, blood samples could not be taken from 2 animals (1 home cage control and 1 sleep restricted rat). The IV injection of CRH resulted in a clear ACTH and CORT response. However, the magnitude of the ACTH response differed between the groups (Figure 4A). Repeated measures ANOVA indicated an overall treatment effect (F2,25 = 3.54, P = 0.044) and a treatment × time interaction (F6,75 = 4.03, P = 0.001). Post hoc Tukey test showed that ACTH release in sleep restricted animals was significantly lower than that in forced activity controls (P < 0.05 for t = 5 min) and lower than that in the home cage controls (P < 0.05 for t = 15 min). Yet, despite a lower ACTH response, the adrenal CORT response was not significantly different between the treatment groups (Figure 4B).
Figure 4.
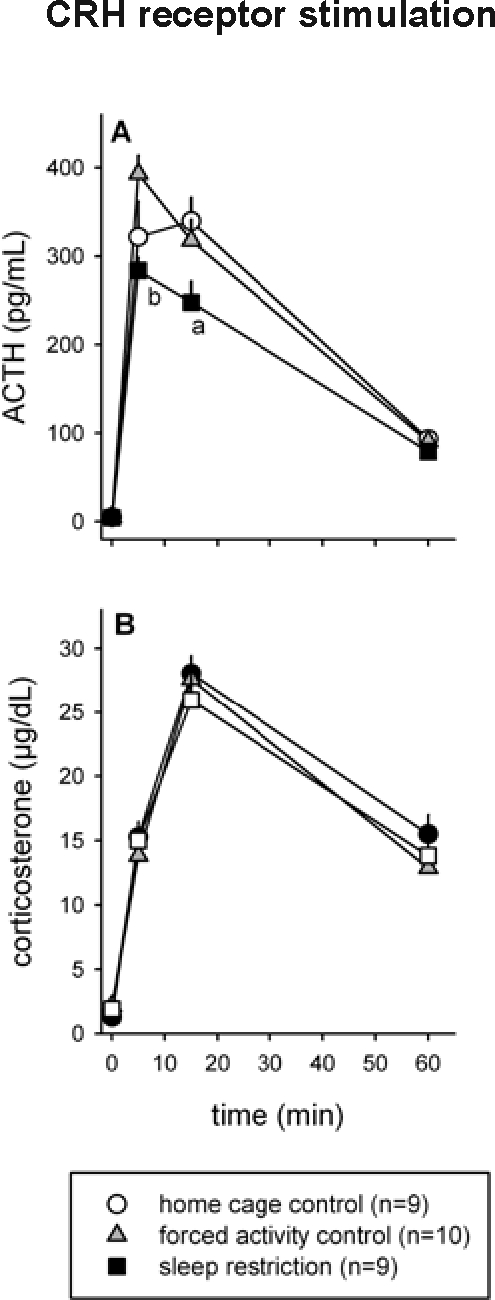
Effect of sleep restriction on CRH-induced HPA axis responses. After 8 d of sleep restriction, rats received an IV injection of CRH, and blood samples were taken to establish ACTH and CORT responses (A and B, respectively). Significant differences between sleep restricted and control animals: a = P < 0.05 compared to home cage control, b = P < 0.05 compared to forced activity control. All data are expressed as averages ± SEM.
DISCUSSION
This experimental study in rats shows that chronic sleep restriction may lead to alterations in neurotransmitter receptor systems (the serotonin-1A receptor and CRH receptor system) and neuroendocrine stress systems (the HPA axis), which are quite similar to changes that have been reported for major depression. While one day of restricted sleep had no significant effect on the HPA axis response to stress, sleep restriction for a week caused a blunted pituitary ACTH response in a conditioned fear paradigm. This blunted pituitary response may, in part, be related to a reduced sensitivity of the CRH and/or serotonin-1A receptors since sleep restricted rats showed a similar reduction in ACTH release to direct pharmacological stimulation with CRH and the serotonin-1A agonist 8-OH-DPAT.
The finding of a blunted pituitary ACTH response in a fear conditioning paradigm is consistent with an earlier study showing a chronic sleep restriction-induced attenuation of the pituitary response to restraint stress.14 In the present study, pituitary ACTH release was significantly reduced upon reexposure to the fearful environment in which the rats had previously received a series of shocks. On average, the ACTH response of sleep restricted rats to the initial footshock session itself was lower as well, but this reduction was smaller and statistically significant only in comparison with the home cage controls (but not the forced activity controls). It cannot be ruled out that the actual footshocks represented a stronger stressor, whereby a ceiling effect in the HPA axis response may have prevented larger differences between sleep-restricted animals and control groups. However, another explanation for the more pronounced effect of sleep restriction on the response to shock box reexposure versus the weaker effect on the response to the actual shock session may lie in the nature of the stressor. The footshocks are a direct and physical stimulus whereas reexposure to the shock box constitutes a more psychological stress. Perhaps effects of sleep loss on stress reactivity are more pronounced in case of psychological stressors.33 Although most stressors, both physical and emotional, are associated with the acute and typical increase in HPA axis activity, the magnitude of this response is regulated and modified by different brain circuits.37,38 Many brain regions are activated regardless of the nature of the stressor; for other regions there is some specificity in the activation depending on the stimulus. It may be that some of the brain regions and circuits that are specifically involved in the regulation and modulation of emotional stress responses are more sensitive to sleep loss than the circuits involved in physical stress.33
The second experiment of this study suggests that the blunted pituitary ACTH stress response of sleep restricted rats may at least partly be the result of a reduced sensitivity to serotonergic and CRH input. When serotonin-1A receptors or CRH receptors were stimulated directly, via an intravenous injection of an agonist, a similar attenuation of the ACTH response was found. However, for the serotonin-1A mediated ACTH response, this attenuation in sleep-restricted rats was statistically significant relative only to the forced activity controls but not the home cage controls. Interestingly, the ACTH response in sleep-restricted animals to both CRH and the serotonin-1A agonist 8-OH-DPAT was lower than that of the home cage control animals; whereas the response in the forced activity controls, if anything, was higher than that of home cage animals. In other words, restricted sleep and forced activity appeared to have opposite effects. The increase in ACTH responsivity in the forced activity control group may have been the result of mild stress experienced by these animals.15 Indeed, a similar increase in ACTH response to CRH was found in a model of social stress.36 Thus, mild forced activity involved in our sleep restriction procedure may have partly counteracted the effects of sleep loss per se. Sleep loss without forced activity might result in an even stronger attenuation of the pituitary ACTH response.
The attenuated ACTH release upon injection of CRH in sleep-restricted rats suggests a desensitization of CRH receptors in the pituitary gland. Such desensitization might be the result of prolonged activation of the CRH system itself. Although information is limited, a number of animal studies suggest that sleep deprivation is indeed associated with elevated expression and release of CRH.39–41 Especially in cases were disrupted and restricted sleep are a chronic condition, the corresponding overstimulation of CRH receptors by their own ligand might result in a downregulation of these receptors.
The attenuated ACTH response to serotonin-1A stimulation in sleep-restricted animals may be the result of changes at the level of pituitary or changes in other brain areas that provide input to the pituitary. Part of the attenuated serotonin-1A response may be an indirect effect related to the attenuated sensitivity to CRH discussed above. As injection of 8-OH-DPAT stimulates release of CRH from the paraventricular nucleus of the hypothalamus,22 the reduced ACTH response to 8-OH-DPAT may in part be a result of reduced sensitivity to CRH. It is also possible that the attenuated response to 8-OH-DPAT is a consequence of reduced sensitivity of the serotonin-1A receptors themselves at the level of the pituitary and the hypothalamus or even in other brain areas that innervate the HPA axis, such as the amygdala.22,42 In accordance with this, bilateral lesions of the central amygdaloid nucleus lead to a marked decrease in the ACTH release during stress.43,44 A reduction in serotonin-1A receptor sensitivity in sleep-restricted rats is supported by our earlier studies, showing that not only 1A receptor-induced ACTH release but also other1A-receptor mediated responses are decreased. In reaction to 8-OH-DPAT, chronically sleep-restricted rats displayed a significantly attenuated hypothermic response.15,16 The finding of a reduction in multiple physiological responses makes it likely that this attenuation is, at least partly, a consequence of a reduced sensitivity of the serotonin-1A receptor system itself. The mechanism underlying such a sleep-restriction induced serotonin-1A desensitization may be similar to the one proposed for a desensitization of the CRH receptors. Since the levels of serotonin are higher during wakefulness and sleep deprivation than they are during sleep,45 chronic sleep restriction and prolonged wakefulness may lead to overstimulation and ultimately downregulation of the serotonin receptors.15
In both experiments on HPA axis reactivity, despite reduced pituitary ACTH release, the adrenal glucocorticoid response was not affected or only mildly affected. Upon reexposure to the shock box in the conditioned fear paradigm, the ACTH response of sleep-restricted animals was reduced by more than 60%, whereas there was only a minor, albeit significant, reduction of the CORT response. In reaction to pharmacological stimulation of the CRH or serotonin-1A receptors, the ACTH response of sleep restricted animals was attenuated, whereas the CORT response was not different from that of the control rats. An unchanged CORT response in the face of blunted ACTH levels can be explained by increased ACTH sensitivity in the adrenal cortex. This would imply that sleep restriction alters regulation of the HPA axis at multiple levels.14
It is noteworthy that the pharmacological CRH and serotonin-1A challenge tests that we applied in the present study have been used many times in clinical settings to investigate alterations in receptor sensitivity in psychiatric disorders such as depression. Very much like chronically sleep-restricted rats, depressed patients show blunted ACTH but normal cortisol responses to CRH injections.19,20 Also in response to serotonin-1A agonists, depressed subjects show blunted physiological responses.26–28 A reduction in serotonin-1A receptor binding capacity in depressed patients has been confirmed by several PET studies.29–32 In one of these imaging studies, decreased 1A receptor binding potential was associated with the occurrence of insomnia, underscoring the complex relationship between sleep disturbance, depression, and changes in serotonergic neurotransmission.32
In summary, chronic experimental reduction of sleep in laboratory rats causes gradual changes in neurotransmitter receptor systems and HPA axis regulation—changes similar to those seen in human depression. These data suggest that several important symptoms of depression, i.e., disturbed sleep, altered serotonergic neurotransmission, and changes in HPA axis regulation, may be interrelated. In fact, whereas changes in serotonin-1A receptor sensitivity may in part explain the alterations in HPA axis responsivity, sleep disturbance may be causal to both the neurobiological and neuroendocrine changes. On the basis of changes in the regulation and activity of neuroendocrine stress systems, depression is often considered a disorder of stress. However, our study suggests that some of the symptoms traditionally ascribed to stress may also be a result of insufficient sleep. This experimental study thus provides support for the hypothesis that sleep disturbance and insomnia may contribute to the symptomatology of psychiatric disorders. Chronically disrupted and restricted sleep may lead to alterations in neurobiological systems and altered regulation of stress systems, which may eventually sensitize individuals to stress-related disorders such as depression.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Jan Bruggink for his assistance with the surgeries, blood sampling and hormone analyses and Paulien Barf for her assistance with the experiments. This work was supported by the School of Behavioral and Cognitive Neurosciences and the Netherlands Organization for Scientific Research (VIDI grant 864.04.002 to P. Meerlo).
REFERENCES
- 1.Bonnet MH. We are chronically sleep deprived. Sleep. 1995;18:908–11. doi: 10.1093/sleep/18.10.908. [DOI] [PubMed] [Google Scholar]
- 2.Rajaratnam SMW. Health in a 24-h society. Lancet. 2001;358:999–1005. doi: 10.1016/S0140-6736(01)06108-6. [DOI] [PubMed] [Google Scholar]
- 3.Pilcher JJ. Effects of sleep deprivation on performance: a meta analysis. Sleep. 1996;19:318–26. doi: 10.1093/sleep/19.4.318. [DOI] [PubMed] [Google Scholar]
- 4.Dinges DF, et al. Cumulative sleepiness, mood disturbances and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20:267–77. [PubMed] [Google Scholar]
- 5.Van Dongen HP. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 6.Ford DE. Epidemiologic study of sleep disturbances and psychiatric disorders: An opportunity for prevention? JAMA. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 7.Breslau N. Sleep disturbance and psychiatric disorders: longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 8.Chang PP. Insomnia in young men and subsequent depression. The Johns Hopkins precursors study. Am J Epidemiol. 1997;146:105–14. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 9.Koren D. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159:855–7. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- 10.Neckelmann D. Chronic insomnia as a risk factor for developing anxiety and depression. Sleep. 2007;30:873–80. doi: 10.1093/sleep/30.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buysse DJ. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31:473–80. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riemann D. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76:255–9. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 13.Taylor DJ. Insomnia and depression. Sleep. 2008;31:447–8. doi: 10.1093/sleep/31.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meerlo P. Sleep restriction alters the hypothalamic-pituitary-adrenal response to stress. J Neuroendocrinol. 2002;14:1–11. doi: 10.1046/j.0007-1331.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- 15.Roman V. Too little sleep gradually desensitizes the serotonin 1A receptor system. Sleep. 2005;28:1505–10. [PubMed] [Google Scholar]
- 16.Roman V. Differential effects of chronic partial sleep deprivation and stress on serotonin-1A and muscarinic acetylcholine receptor sensitivity. J Sleep Res. 2006;15:386–94. doi: 10.1111/j.1365-2869.2006.00555.x. [DOI] [PubMed] [Google Scholar]
- 17.Nemeroff CB, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–4. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 18.Gillespie CF. Hypercortisolism and depression. Psychosom Med. 2005;67:S26–S28. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- 19.Holsboer F. Blunted corticotropin and normal cortisol response to human corticotropin-releasing factor in depression. N Engl J Med. 1984;311:1127. doi: 10.1056/NEJM198410253111718. [DOI] [PubMed] [Google Scholar]
- 20.Holsboer F. Blunted aldosteron and ACTH response after human CRH administration in depressed patients. Am J Psychiatry. 1987;144:229–31. doi: 10.1176/ajp.144.2.229. [DOI] [PubMed] [Google Scholar]
- 21.Fuller RW. The involvement of serotonin in regulation of pituitary-adrenocortical function. Front Neuroendocrinol. 1992;13:250–70. [PubMed] [Google Scholar]
- 22.Dinan TG. Serotonin and the regulation of hypothalamic-pituitary-adrenal axis function. Life Sci. 1996;58:1683–94. doi: 10.1016/0024-3205(96)00066-5. [DOI] [PubMed] [Google Scholar]
- 23.Cryan JF. 5-HT1A and beyond: the role of serotonin and its receptors in depression and the antidepressant response. Hum Psychopharmacol Clin Exp. 2000;15:113–35. doi: 10.1002/(SICI)1099-1077(200003)15:2<113::AID-HUP150>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 24.Sobczak S. Serotonergic dysregulation in bipolar disorders: a literature review of serotonergic challenge studies. Bipolar Disord. 2002;4:347–56. doi: 10.1034/j.1399-5618.2002.01217.x. [DOI] [PubMed] [Google Scholar]
- 25.Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. J Psychiatr Res. 2003;37:357–73. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 26.Lesch KP, et al. 5-HT1A receptor responsivity in unipolar depression: evaluation of ipsapirone-induced ACTH and cortisol secretion in patients and controls. Biol Psychiatry. 1990;28:620–8. doi: 10.1016/0006-3223(90)90400-v. [DOI] [PubMed] [Google Scholar]
- 27.Mann JJ. Blunted serotonergic responsivity in depressed inpatients. Neuropsychopharmacology. 1995;13:53–64. doi: 10.1016/0893-133X(95)00016-7. [DOI] [PubMed] [Google Scholar]
- 28.Shapira B. Blunted temperature and cortisol responses to ipsapirone in major depression: lack of enhancement by electroconvulsive therapy. Psychoneuroendocrinology. 2000;25:421–38. doi: 10.1016/s0306-4530(99)00067-0. [DOI] [PubMed] [Google Scholar]
- 29.Drevets WC, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–87. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 30.Sargent PA, et al. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635. Arch Gen Psychiatry. 2000;57:174–80. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 31.Drevets WC, et al. Serotonin- 1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34:865–77. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hirvonen J, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naïve patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-C]WAY-100635. Int J Neuropsychopharmacol. 2007;11:465–476. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- 33.Meerlo P. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12:197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Steffens AB. A method for frequent sampling of blood and continuous infusion of fluids in the rat without disturbing the animal. Physiol Behav. 1969;4:833–6. [Google Scholar]
- 35.Korte SM. Socially defeated male rats display a blunted adrenocortical response to a low dose of 8-OH-DPAT. Eur J Pharmacol. 1995;272:45–50. doi: 10.1016/0014-2999(94)00621-d. [DOI] [PubMed] [Google Scholar]
- 36.Buwalda B, et al. Long-lasting deficient dexamethasone suppression of hypothalamic-pituitary-adrenocortical activation following peripheral CRF challenge in socially defeated rats. J Neuroendocrinol. 1999;11:513–20. doi: 10.1046/j.1365-2826.1999.00350.x. [DOI] [PubMed] [Google Scholar]
- 37.Herman JP, et al. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Lopez JF. Neural circuits mediating stress. Biol Psychiatry. 1999;46:1461–71. doi: 10.1016/s0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- 39.Fadda P. Stress-induced sleep deprivation modifies corticotropin releasing factor (CRF) levels and CRF binding in rat brain and pituitary. Pharmacol Res. 1997;35:443–6. doi: 10.1006/phrs.1997.0155. [DOI] [PubMed] [Google Scholar]
- 40.Fujihara H. Short-term sleep disturbance enhances brain-derived neurotrophic factor gene expression in rat hippocampus by acting as internal stressor. J Mol Neurosci. 2003;21:223–32. doi: 10.1385/jmn:21:3:223. [DOI] [PubMed] [Google Scholar]
- 41.Koban M. Changes in hypothalamic corticotropin-releasing hormone, neuropeptide Y, and proopiomelanocortin gene expression during chronic rapid eye movement sleep deprivation of rats. Endocrinology. 2006;147:421–31. doi: 10.1210/en.2005-0695. [DOI] [PubMed] [Google Scholar]
- 42.Calogero AE. Mechanisms of serotonin receptor agonist-induced activation of the hypothalamic-pituitary-adrenal axis in the rat. Endocrinology. 1990;126:1888–94. doi: 10.1210/endo-126-4-1888. [DOI] [PubMed] [Google Scholar]
- 43.Beaulieu S. Control of ACTH secretion by the central nucleus of the amygdala: implication of the serotonergic system and its relevance to the glucocorticoid delayed negative feedback mechanism. Neuroendocrinology. 1986;44:247–54. doi: 10.1159/000124652. [DOI] [PubMed] [Google Scholar]
- 44.Herman JP. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuro-Psychopharm Biol Psychiatry. 2005;29:1201–13. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Portas CM. Serotonin and the sleep/wake cycle: special emphasis on microdialysis studies. Prog Neurobiol. 2000;60:13–35. doi: 10.1016/s0301-0082(98)00097-5. [DOI] [PubMed] [Google Scholar]