Abstract
Relational memory theory holds that the hippocampus supports, and amnesia following hippocampal damage impairs, memory for all manner of relations. Unfortunately, many studies of hippocampal-dependent memory have either examined only a single type of relational memory or conflated multiple kinds of relations. The experiments reported here employed a procedure in which each of several kinds of relational memory (spatial, associative, and sequential) could be tested separately using the same materials. In Experiment 1, performance of amnesic patients with medial temporal lobe (MTL) damage was assessed on memory for the three types of relations as well as for items. Compared to the performance of matched comparison participants, amnesic patients were impaired on all three relational tasks. But for those patients whose MTL damage was limited to the hippocampus, performance was relatively preserved on item memory as compared to relational memory, although still lower than that of comparison participants. In Experiment 2, study exposure was reduced for comparison participants, matching their item memory to the amnesic patients in Experiment 1. Relational memory performance of comparison subjects was well above amnesic patient levels, showing the disproportionate dependence of all three relational memory performances on the integrity of the hippocampus. Correlational analyses of the various task performances of comparison participants and of college-age participants showed that our measures of item memory were not influenced significantly by memory for associations among the items.
Keywords: relational memory, hippocampus, amnesia
Introduction
The critical participation of the medial temporal lobe (MTL), and especially the hippocampus, in memory has been well established since the earliest reports of profound amnesia following MTL resection in the patient H.M. (Scoville and Milner, 1957). Since that seminal report, attempts to characterize its role with more precision have been undertaken by many researchers, and a variety of different accounts have emerged. One account has emphasized spatial memory as the chief function of the MTL, starting with O'Keefe and Nadel (1978), arguing that the central role of this system is in the formation and use of rich, flexible cognitive maps of the environment. Although initially inspired by lesion and electrophysiological studies of rodents, this view has a number of adherents who cite recent neuroimaging and neuropsychological studies in humans for support (see Bird and Burgess, 2008 for a recent review). Neuroimaging data collected during performance of spatial tasks almost universally reveal MTL activation, frequently in hippocampus and parahippocampal cortex (e.g., Hartley et al., 2003; Pine et al., 2002; Spiers and Maguire, 2006). Work with neurological patients with MTL damage has documented many examples of spatial memory impairments, including deficits in memory for arbitrary object locations (Crane and Milner, 2005; Holdstock et al., 2002, 2005), spatial arrangements among items in scenes (Hannula et al., 2006, submitted; Ryan et al., 2000), spatial lay-outs of residences occupied for years following the onset of amnesia (Bayley and Squire, 2005), and spatial navigation of complex large-scale places (Maguire et al., 2006).
Other theorists have identified the MTL with declarative memory, critical for supporting the acquisition and retention of information about facts and events (e.g., Cohen, 1984; Cohen and Eichenbaum, 1993; Eichenbaum and Cohen, 2001; Squire et al., 2004). Cohen, Eichenbaum, and their colleagues have stressed the fundamentally relational nature of declarative memory, arguing that this system is principally involved in relational memory, i.e., in representing the arbitrary or accidental relations among the constituent elements of events or scenes (Cohen and Eichenbaum, 1993; Eichenbaum and Cohen, 2001). These arbitrary links could be, for example, spatial in nature, such as between a building and its location in a city or relative to a second building; associative, such as a name and a face or the simple coinciding of two stimuli on a computer screen; or temporal, such as meeting one person and then later another. Associations are typically formed by the co-occurrence of two items, although this is not always the case; they could be related across a span, such as seeing a face and later learning the name that goes with it. Memory for relations can be distinguished from memory for the items themselves that are bound together, such as the buildings, city coordinates/locations, names, faces, or stimuli. This view is broader than the spatial memory or cognitive mapping account in emphasizing that the MTL is critical in memory for all manner of relations, and not just spatial relations. Substantial empirical support comes from multiple converging lines of work, including animal models of amnesia, human neurological patients, and functional neuroimaging.
For example, work with rats shows that hippocampal system lesions disrupt transitive inference and transverse patterning performance, requiring hierarchical or other (non-spatial) relational representations among pairs of odors (Dusek and Eichenbaum, 1997, 1998), and memory for sequences of odors (Fortin et al., 2002); while hippocampal neuronal firing has been shown to encode not only place information, but also other relational information within a given place (Wood et al., 2000) and, more generally, various relations among items (for reviews see Eichenbaum and Cohen, 1988; Eichenbaum et al., 1999), including sequential or temporal context relations (Agster et al., 2002; Manns et al., 2007). Human amnesic patients with hippocampal lesions have exhibited selective deficits in various tests of non-spatial relational memory, including for pairs of words (Giovanello et al., 2003), two-syllable words (Kroll et al., 1996), and face–scene (Hannula et al., 2006, 2007), face–word (Turriziani et al., 2004), and face–face (Kroll et al., 1996; Turriziani et al., 2004) pairings, as well as item–context relations (for review see Ryan and Cohen, 2003). Finally, neuroimaging investigations have contributed findings of selective hippocampal activation in relational conditions using various stimuli (for reviews see Cohen et al., 1999; Davachi, 2006), including: word triplets (Davachi and Wagner, 2002); cue-driven mental imagery (Davachi et al., 2003); word pairs (Giovanello et al., 2004), face–scene (Henke et al., 1997) or face–occupation pairings (Degonda et al., 2005; Henke et al., 2003); word–font pairings (Prince et al., 2005); word–color mental imagery (Staresina and Davachi, 2006); as well as memory for items in relation to particular learning experiences (e.g., Davachi et al., 2003; Ranganath et al., 2004), and sequential relations among items (Kumaran and Maguire, 2006). One important issue that should be noted, and will be further addressed later, is that the majority of these studies examine a single type of relational memory (e.g., associative) or compare at most two kinds of memory.
One additional tenet of the relational memory theory is that the MTL is functionally heterogeneous, with different memory functions associated with different structures. It is proposed that the formation of new relational memories is dependent on the hippocampus specifically, whereas memory for single objects or items is proposed to be dependent on MTL cortical regions, such as perirhinal cortex (e.g., Eichenbaum and Cohen, 2001; Eichenbaum et al., 1994). This claim has also received substantial support from multiple converging lines of work. Many studies of human neurological patients with MTL damage largely limited to hippocampus have shown deficits in relational memory, for both spatial and non-spatial relations, in the absence of or disproportionate to deficits in memory for items (e.g., Giovanello et al., 2003; Hannula et al., 2006, submitted; Holdstock et al., 2002, 2005; Kan et al., 2007; Mayes et al., 2004; Ryan et al., 2000; Turriziani et al., 2004). Likewise, a growing number of neuroimaging findings point to increased hippocampal activation when evaluating or forming representations of relationships among items, or of items in relation to a particular learning experience, rather than encoding of items individually (e.g., Davachi and Wagner, 2002; Davachi et al., 2003; Henke et al., 1997; Ranganath et al., 2004), whereas activity in the MTL cortical regions, especially perirhinal cortex, has been associated with processing and (familiarity-based) memory for items individually (e.g. Davachi and Wagner, 2002; Davachi et al., 2003; Kohler et al., 2005; Ranganath et al., 2004).
Not all the extant findings support the proposal that distinct memory processes (e.g., relational and item memory) are uniquely associated with particular MTL structures (e.g., hippocampus and MTL cortical regions, respectively), however. Several studies by Squire and colleagues are in conflict with some of the reported dissociation findings. Stark and Squire (2003) tested amnesic patients and normal comparison participants with two-syllable words, object–object pairs, and face–house pairs, and reported that none of the amnesic performances constituted specific associative memory impairment. Rather, amnesic performance was argued to qualitatively resemble the uniformly decremented performance of neurologically normal comparison participants rather than the selective decrementing of associative memory. Gold et al. (2006) reported the successful matching of amnesic performance with that of comparison participants in an impoverished study condition, using stimuli consisting of words and associated self-generated source information, for both item and source (relational) memory. The same publication also reported functional imaging findings in which no differences in hippocampal activation for items recalled with or without accompanying source information was observed, contrary to the findings of Davachi et al. (2003). On the basis of these and related findings in their laboratory, these investigators argue for distributed MTL memory function, in which putative functional dissociations arise more due to differences in the information available to particular structures than from tight yoking of function to structure (see Squire et al., 2004 for a review).
The reasons for this discrepancy are not entirely clear, but may involve specific methodological and paradigmatic choices that differ across investigators working with different populations. A comprehensive reading of this rapidly growing literature makes evident that current theorizing about the precise role in memory of the hippocampus and other MTL regions depends upon synthesizing results from across many different methods, paradigms, stimulus materials, and species or research populations, creating significant challenges. Concerns about the heavy reliance of the field on evidence of dissociations in memory drawn from contrasts across tasks and stimulus materials have been noted elsewhere (see Ryan and Cohen, 2003). While a number of studies (e.g., Giovanello et al., 2003) have compared relational to item memory, only a few recent studies have investigated multiple forms of relational memory. Kohler et al. (2005), for example, tested memory for both spatial and co-occurrence (or associative) relations in an fMRI paradigm and found significant hippocampal involvement; however, an item memory test in the same article was carried out with a different paradigm in a different group of participants. Pihlajamaki et al. (2004) tested memory for novel spatial arrangements and novel objects, but confounded novel object memory with new item–location and item–item co-occurrence memory. Kumaran and Maguire (2005) directly compared spatial relational memory to “social” relational memory; contrasting navigation through a spatial layout with navigation through a social network; however, there was no item memory test and the relations involved friends of the participants and thus are hard to compare to other studies that use more experimentally-novel materials. In general, these experiments reflect a recent welcome trend of testing multiple kinds of relations (spatial and non-spatial) among items. Most such studies have found similar hippocampal dependence or hippocampal involvement for both spatial and non-spatial relational memory (Hannula et al., 2006; Holdstock et al., 2002, 2005; Kohler et al., 2005; Pihlajamaki et al., 2004), whereas Kumaran and Maguire (2005) found hippocampal activity only for spatial relationships and not for social relationships. But, regardless of the outcomes, it should be noted that the comparisons among conditions nearly universally involved different paradigms or participant groups which, as noted above, poses significant challenges.
A better approach would be one in which each of several kinds of relational memory could be tested separately using the same materials in the same experiment. This is the approach taken in the work to be reported here. By using the same stimuli to test multiple forms of relational memory as well as item memory within the same participant, and notably by attempting to unconfound the different relations as much as possible, we hoped to clarify the pattern of results and expand on previous work comparing only two tasks. Furthermore, neurological patients with damage to MTL structures and neurologically normal participants were tested in order to permit conclusions to be drawn about regional specificity in the MTL of relational memory of different kinds and of item memory.
Experiment 1
Materials and methods
Participants
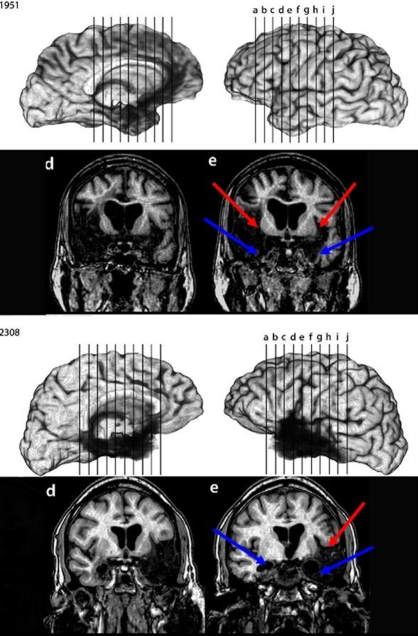
Participants were seven amnesic patients (two women; five men) drawn from the Patient Registry of the Division of Cognitive Neuroscience at the University of Iowa and 10 neurologically intact comparison participants. In five of the patients, amnesia occurred due to an anoxic/hypoxic episode due to cardiac arrest. In the other two, amnesia resulted from herpes simplex encephalitis. All patients completed neuropsychological testing to confirm memory impairment disproportionate to deficits in general cognitive or intellectual functioning. Each patient showed severe memory impairment on standardized tests of memory: Their performance on the Wechsler Memory Scale-III was at least 25 points lower than their performance on the Wechsler Adult Intelligence Scale-III, and the average delay score on the memory scale was more than two standard deviations below population means. Patient 2563 was ineligible for MRI examination because of a pacemaker, but hippocampal damage was confirmed with a CT scan (see Hannula et al., 2006, for details). Structural MRI findings from patients 1606, 1846, 2144, and 2363 are detailed in Allen et al. (2006), documenting reduced hippocampal volumes in each case, at least two standard deviations smaller than normal controls. Patient 1606 is an exception to the other anoxic cases in that perirhinal cortex damage could not be ruled out. The MTL damage in the two post-encephalitic patients was found to extend beyond the hippocampus to include other MTL structures; scans of these patients are shown in Figure 1 (from Duff, Hengst, Cohen, and Tranel, personal communication). Patient 1951 has damage outside of the hippocampus in bilateral entorhinal cortex, amygdala, and temporal pole. The lesion is more extensive in the right temporal lobe, with left perirhinal cortex being mostly spared. Patient 2308, on the other hand, has more extensive left MTL damage. The right hemisphere lesion is more anterior, including the hippocampus and amygdala, while the left hemisphere is more widespread and includes the entorhinal and perirhinal cortices. Table 1 contains information on each of the patients; those with damage beyond the hippocampus are labeled “MTL”, although it should be noted that damage extends beyond the temporal lobe in these two patients (as can be seen in Figure 1). The patients were also evaluated on several tests of executive function; these results are presented in Table 2. The frontal lobes have been implicated in tasks that require more attention or increased memory search (c.f. Fletcher and Henson, 2001). While a number of the patients display at least one impaired score across the 10 measures, there is no systematic pattern of impairment. Furthermore, the hippocampal and MTL patients do not differ in their scores; in fact, the two patients who did not show impairment on any tests of executive function included one from each patient group. The comparison group consisted of four women and six men matched to individual patients on gender, age, and education. All comparison participants were recruited from the Champaign-Urbana community. Comparison participants received $10 per hour for their participation while amnesic patients were compensated by the University of Iowa. Testing was carried out under the guidelines and with the approval of the Human Subjects Committee and the Institutional Review Boards of the University of Illinois Urbana-Champaign and of the University of Iowa.
Figure 1.
MRI scans of post-encephalitic patients 1951 and 2308. Arrows point to regions of extensive tissue loss in the temporal lobe.
Table 1.
Demographics and memory scores for the amnesic patients at the time of testing.
| Participant | Gender | Age | WAIS-III full-scale IQ score | WMS-III general memory index | Etiology/Damage |
|---|---|---|---|---|---|
| 1606 | Male | 58 | 91 | 66 | Anoxia/MTL |
| 1846 | Female | 44 | 84 | 57 | Anoxia/hippocampus |
| 1951 | Male | 53 | 121 | 75 | Encephalitis/MTL |
| 2144 | Female | 56 | 99 | 56 | Anoxia/hippocampus |
| 2308 | Male | 49 | 87 | 45 | Encephalitis/MTL |
| 2363 | Male | 49 | 98 | 73 | Anoxia/hippocampus |
| 2563 | Male | 48 | 102 | 75 | Anoxia/hippocampus |
Table 2.
Scores on various tests of frontal function for each patient.
| Patient | Trail making | WCS | COWA | Tower of London | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | Categories | Pers. errors | Move | Correct | Initiation | Execution | Problem solving | ||
| 1606 | 7 (32%) | 5 (9%) | 6 (>16%) | 26 (14%) | 43 (72%) | 100 (44–51%) | 88 (19%) | 86 (1–5%) | 102 (32–44%) | 100 (32–39%) |
| 1846 | 7 (7%) | 9 (18%) | 6 (>16%) | 6 (73%) | 24 (8 %) | 100 (44–51%) | 100 (46%) | 102 (71–73%) | <60 (1%) | 62 (3–4%) |
| 1951 | 10 (52%) | 11 (70%) | 6 (>16%) | 16 (5%) | 40 (63%) | 110 (68–69%) | 100 (46%) | 98 (59–62%) | 106 (55–68%) | 106 (35–55%) |
| 2144 | 10 (70%) | 10 (70%) | 6 (>16%) | 6 (79%) | 33 (34%) | 94 (34–37%) | 94 (31%) | 120 (88–90%) | 110 (75–79%) | 68 (4–8%) |
| 2308 | 5 (2%) | 5 (2%) | NA | NA | 16 (<1%) | 114 (79–82%) | 106 (62%) | 96 (55–58%) | 94 (25–26%) | 96 (27–29%) |
| 2363 | 7 (5%) | 5 (1%) | 6 (>16%) | 12 (23%) | 26 (12%) | 88 (21–24%) | 106 (62%) | 116 (84–86%) | 66 (4–5%) | 62 (3–4%) |
| 2563 | 5 (2%) | 7 (8%) | 5 (11–16%) | 32 (2%) | 21 (3%) | 114 (79–82%) | 118 (85%) | 140 (99%) | 100 (30–32%) | 88 (14–19%) |
Scores are presented as scaled score (percentile). Bolded scores are impaired, defined as 2 standard deviations from the mean on each test. WCS, Wisconsin card sorting test; Pers. errors, perseverative errors; COWA, Controlled Oral Word Association; COWA score is summed over F, A, and S.
Paradigm
The paradigm examined memory in successive study-then-test phases. Each study phase was the same regardless of subsequent memory test conditions. At study, each participant was presented with a series of trials, each trial consisting of a set of three stimuli presented sequentially. Each stimulus in a set was a novel visual object presented in one of three spatial locations (upper left, upper right, or lower center) and in one of three temporal slots (1st, 2nd, or 3rd). In each test phase, memory for the studied stimulus sets was probed in one of four recognition conditions, in separate blocks, testing memory for: individual stimulus items; spatial relations among stimuli within a set; temporal sequences or relations among stimuli within a set; and associative relations of stimuli within a set. Each of the test conditions was designed to probe one type of memory or form of representation unconfounded by the others.
Stimuli and design
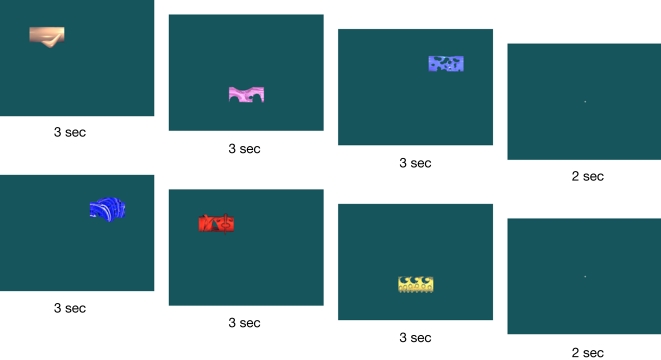
The stimulus materials were novel visual objects (Warren and Cohen, in preparation) created using Bryce software. Examples of stimulus sets are shown in Figure 2. The stimuli were created so as to ensure that participants had no prior exposure to, and thus no pre-existing representations of, them. This is critical when attempting to contrast item memory with relational memory, because testing memory for items such as words or pictures that have been viewed many times in a participant's history becomes confounded with relational memory, necessarily involving memory for item–context relations (i.e., of all the times you saw this item, did you see it on this particular test list?).
Figure 2.
Two sample study trials. Each individual stimulus was displayed for 3 s before being replaced by the next. Each set of three stimuli was separated by a 2-s fixation screen.
Trials were presented in blocks, each block including a study phase then a test phase. Each block contained 16 study trials, consisting of 8 unique stimulus sets presented twice, followed by 8 test trials. Study trials were separated by a fixation cross. Each test phase included four repeat and four manipulated trials.
For the item task, repeat trials consisted of three previously viewed stimuli, whereas manipulated trials consisted of one studied stimulus and two never-studied stimuli, all presented simultaneously rather than sequentially, in positions at the center of the screen not occupied at all during study, so as to minimize the possible contributions at test of memory for sequential relations or spatial relations among the studied stimuli.
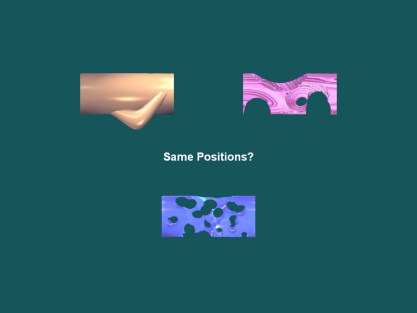
For the spatial relations task, test displays always consisted of three studied items from a given stimulus set; repeat trials presented the stimuli in their original locations, whereas manipulated trials swapped the locations of two stimuli. The stimuli were presented simultaneously rather than sequentially, so memory for the original sequential relations would not be helpful at test; and all test displays involved intact stimulus sets, so memory for associative relations would not be discriminating at test.
For the sequential relations task, test displays always consisted of three studied items from a given stimulus set; repeat trials presented the stimuli in their original order, whereas manipulated trials presented the stimuli in a different order. The stimuli were presented in a position at the center of the screen never occupied during study, so memory for the original spatial relations would not be helpful at test. Also, because test displays in this condition always involved intact stimulus sets, memory for associative relations would not be discriminating at test.
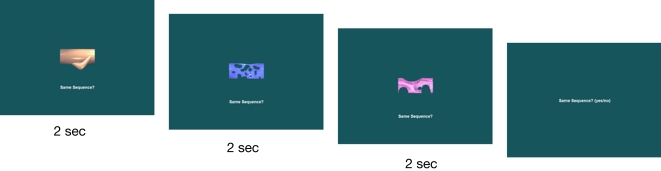
Finally, for the associative relations task, test displays always consisted of three previously viewed stimuli; but whereas repeat trials consisted of three stimuli that had co-occurred in a given set at study, manipulated trials consisted of stimuli taken from two different stimulus sets. By presenting the stimuli simultaneously rather than sequentially, in positions at the center of the screen not occupied at all during study, any contributions of memory for the temporal slot or spatial position in which a given item appeared would be minimized. Examples of each test type are shown in Figures 3–6.
Figure 3.
A sample item memory test trial using items from Figure 2. The first trial is a repeat trial, consisting of old stimuli from Figure 2. The second is a manipulated trial, consisting of one old stimulus from Figure 2 and two novel stimuli.
Figure 4.
A sample manipulated associative relations test trial based on Figure 2.
Figure 5.
A sample manipulated sequential relations test trial based on Figure 2. This is the only type of test trial in which the three stimuli were presented sequentially rather than simultaneously.
Figure 6.
A sample manipulated spatial relations test trial based on Figure 2.
For counterbalancing of stimuli, individual stimulus items were grouped into triplets, and thus always studied together or not studied and available as novel lures for manipulated item trials. Triplets were counterbalanced across participants such that each triplet appeared equally in each condition and block.
Procedure
Each participant was tested individually on a PC using Presentation software. After obtaining informed consent, a participant was given instructions about the different types of blocks. Prior to the item task, the participant was told to study stimuli as they appeared and to remember them as well as possible. There was no mention of locations or sets; participants were only told to pay attention to the items. For the various relational tasks, the participant was told to treat the three stimuli occurring between fixation screens as a group or set that went together, and that after studying a number of such stimulus sets they would receive a test of which stimuli were in the same set together, or of the places or locations they appeared on the screen, or of the order in which the stimuli within a set were presented. Participants were not told which relational test would follow a given study block; instead they were told that all three kinds of information should be remembered. The item task was given as a separate task, followed by the relational tasks. The order of the relational tasks was counterbalanced across participants.
After each study phase, a task-appropriate question was presented on the monitor and verbal instructions were given to remind the participant of the information to be tested. In the item task, participants were to respond “yes” if all three stimuli had been presented during the study block, and “no” if any of the stimuli were new. For the associative relations task, participants were to respond “yes” if all three stimuli had been studied together in the same set, or “no” if they were mixed together from different sets. For the spatial relations task, participants were to respond “yes” if each stimulus was presented in the same location in which it had been studied, or “no” if the stimuli were moved around. For each of these tasks, if the participant responded within 6 s, a fixation appeared for 2 s and the next trial was presented. If 6 s elapsed before a response was given, the items were replaced on the monitor by a reminder question and participants had as long as necessary to respond. Finally, during the sequential relations task, a single stimulus was presented in the center of the screen, followed by a second stimulus, then a third stimulus, each presented for 2 s. After the third item had been presented, a question screen appeared until a response was given. Participants were to wait until the question screen appeared, and then respond “yes” if the stimuli were presented in the same order as when they had been studied, or “no” if they were presented in a different order. Breaks were allowed between study-test blocks as necessary.
Each amnesic participant completed six blocks of each task, some across two sessions. Each comparison participant completed three blocks of each relational task and six blocks of the item task (except for two who did not complete the item task); two performed the task twice in separate sessions separated by several months.
Analysis
Responses were categorized as hits, misses, false alarms, or correct rejections, used to calculate a d′ (d-prime) value for each participant for each task. A correction of 0.5 was added to each bin to eliminate any cases of zero hits or false alarms (in order to avoid infinite d′ scores). Hit-minus-false-alarm scores were also calculated as a measure of adjusted accuracy; hit and false alarm rates are presented for completeness. All reported results were comparable for both measures. Planned comparisons of amnesic group versus comparison group performance were conducted for each task as well as comparisons of performances across tasks within each group. Further analyses involved dividing the amnesic group into those patients whose MTL damage was largely limited to the hippocampus (hereafter “hipp”) versus those with MTL damage extending beyond the hippocampus (hereafter “MTL”). Because of the small sample sizes for the amnesic subgroups, initial ANOVAs were run in a nonparametric fashion (similar to Berryhill et al., 2007; Olson et al., 2006). An initial F value was calculated using obtained scores on each task for each subject. Scores were then held constant while group (patient or comparison), damage (hipp or MTL), and task (item, associative, sequence, and spatial) were randomly rotated across the scores. A new ANOVA was run and the resulting F values recorded. This procedure was repeated 100 000 times to generate a nonparametric distribution of F values for main effects as well as interactions; an effect in the original data was considered significant if the obtained F value fell in the top 5% of the distribution for that effect. It should be noted that while the comparison group has no brain damage by which to be categorized into “hipp” or “MTL”, for the purposes of conducting the exploratory ANOVAs they are grouped according to the individual patient they are matched to. Trends are noted along with the reporting of significant results.
Results
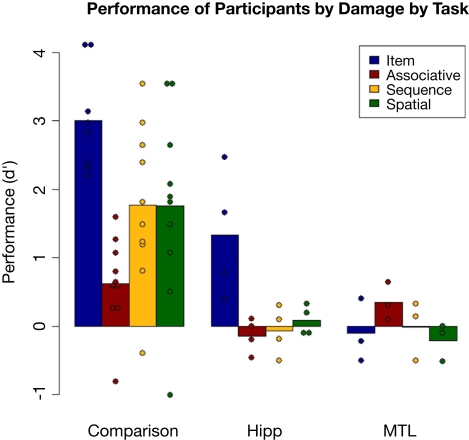
Results for each group on each task can be seen in Figure 7. The proportion of hits and false alarms is presented in Table 3. The nonparametric ANOVA procedure found main effects of group (p < 0.001) and task (p = 0.013), as well as a trend of a three-way interaction (p = 0.055).
Figure 7.
Performance on each task by group; dots represent individual participants' scores.
Table 3.
Hit and false alarm rates for controls and patients on each task in Experiment 1.
| Item | Cooc | Sequence | Spatial | |||||
|---|---|---|---|---|---|---|---|---|
| HR | FAR | HR | FAR | HR | FAR | HR | FAR | |
| Controls | 0.82 | 0.17 | 0.66 | 0.47 | 0.71 | 0.25 | 0.75 | 0.29 |
| Hipp patients | 0.73 | 0.43 | 0.62 | 0.62 | 0.51 | 0.53 | 0.56 | 0.53 |
| MTL patients | 0.61 | 0.63 | 0.67 | 0.59 | 0.53 | 0.52 | 0.48 | 0.54 |
Looking at more specific analyses, first at the comparison participants considered as group, a significant effect of task was found [F(3,41) = 7.796, p < 0.001]. Follow-up Tukey tests showed that performance on the item task was significantly higher than on each of the relational tasks (all p's < 0.05). None of the relational tasks were performed significantly differently from the others, although performance on the associative task was marginally poorer than other relational task performances.
Comparing performance of amnesic patients as a group (ignoring the hipp/MTL distinction) versus comparison participants, the comparison group performed significantly better than the patient group on every task (Bonferroni-adjusted t-tests; all p's < 0.05).
Performance by each group was tested against chance (d′ = 0) by Bonferroni-corrected t-tests. The comparison group performed above chance on every task (all p's < 0.05). By contrast, the only patient group score (marginally) above chance was the performance of the hipp group on the item task [t(3) = 2.87, p = 0.06]. Thus the hipp patients were at chance on all relational tasks, but not on the item task, while the MTL patients were at chance on all tasks. A direct comparison of the two patient subgroups found that the numerical difference in performance on the item task (hipp > MTL) mirrored the comparison to chance [t(5) = 2.42, p = 0.06], while a planned comparison of the two patient groups using the nonparametric process found a trend of a main effect of damage (p = 0.058) and a significant two-way interaction between damage and task (p = 0.02) reflecting the difference between the flat performance across tasks by the MTL group as opposed to the selectively better performance on the item task in the hipp group.
Summary
Amnesic patients were profoundly impaired, performing at chance levels, on all three relational memory tasks. This finding obtained even in those amnesic patients whose MTL damage was limited to the hippocampus. Thus, it does not require damage extending into MTL cortical regions in order to see deficits in relational memory, and the deficits extend to memory for all manner of relations tested here.
Amnesic patients were also impaired on the test of item memory. However, there were significant differences between amnesic subgroups on the item task that were not seen on the relational tasks and that have important implications for understanding the roles of different MTL regions in memory. Patients whose MTL damage was limited to the hippocampus performed above chance levels on the item memory task, and above their performance levels for any of the relational memory tasks, whereas those patients whose MTL damage extended beyond hippocampus to include MTL cortical regions were at chance for item memory as they were for relational memory. The findings here that item memory was relatively spared compared to relational memory after restricted hippocampal damage conforms with previous findings from our lab (Hannula et al., 2006; Ryan et al., 2000) and others (e.g., Giovanello et al., 2003; Kan et al., 2007; Turriziani et al., 2004), and supports our claim that the hippocampus is specialized for relational memory (Cohen and Eichenbaum, 1993; Eichenbaum and Cohen, 2001). Furthermore, the additional finding that when MTL damage extends beyond the hippocampus it is accompanied by additional deficits that extend to item memory supports theoretical claims that attribute relational and item memory to hippocampus and MTL cortical regions, respectively (Eichenbaum and Cohen, 2001; Eichenbaum et al., 1994; also see Brown and Aggleton, 2001; Eichenbaum et al., 2007).
Yet, it should be noted that performance on the test of item memory was not entirely normal in the hippocampal patients. In an attempt to examine the disparity in performance between the comparison group and hippocampal amnesics, we retested the comparison participants on a harder version of the task. In previous studies, researchers have attempted to equate performance on one task to look for a differential impairment across tasks between two groups. Stark et al. (2002), for example, tested a comparison group and an amnesic group on their ability to remember either face–house pairs or single items (a face or a house). They found an equal impairment in the amnesic group for both the item and paired condition. However, the patients were not significantly above chance on either task. They then gave the patient group the same task but with eight study repetitions to raise performance, and found that the patients performed as well as the comparison group on both tasks. However, it is possible that the amnesics' raised performance was due to some kind of fused representation of the face–house pairs after repeated viewings, perhaps via unitization, i.e., treating them as single compound items (Cohen et al., 1997; Eichenbaum et al., 1994; Giovanello et al., 2006; Quamme et al., 2007). So instead of attempting to raise amnesic performance, in the next experiment we attempted to lower comparison group performance (e.g., see Gold et al., 2006). Finding that the comparison group performed similarly to the hippocampus-only patient group on the item task but above chance on the relational tasks would provide stronger evidence that hippocampal damage differentially impairs relational memory.
Experiment 2
Materials and methods
Participants
The participants consisted of eight individuals matched to the amnesic patients in Experiment 1. Seven of the eight had also participated in Experiment 1, but at least 1 year passed between the two testing sessions. Each participant received $10 for completing the experiment.
Paradigm
The same basic paradigm was used as in Experiment 1.
Stimuli and design
The stimuli and design were the same as Experiment 1, except that there was only a single study exposure to each stimulus set (thus study blocks consisted of 8 trials instead of 16), and items were presented for 2 s rather than 3 s. In addition, item task blocks were intermixed with relational task blocks (each participant performed three blocks of each task).
Procedure
Each participant was tested individually, using the same procedure as in Experiment 1 (with the exception that confidence ratings were also collected on each test trial, although the ratings are not reported here). Participants were tested on a different counterbalancing of stimulus sets to conditions than in Experiment 1 to ensure that specific stimulus sets were not tied to any particular type of relations.
Analysis
Analyses were performed on the calculated d′ scores, testing for differences of each task performance from chance levels, differences between performances on the different tasks, and differences between performance of Experiment 2 comparison participant versus patient scores from Experiment 1. The nonparametric ANOVA procedure was again used for guiding later t-tests.
Results
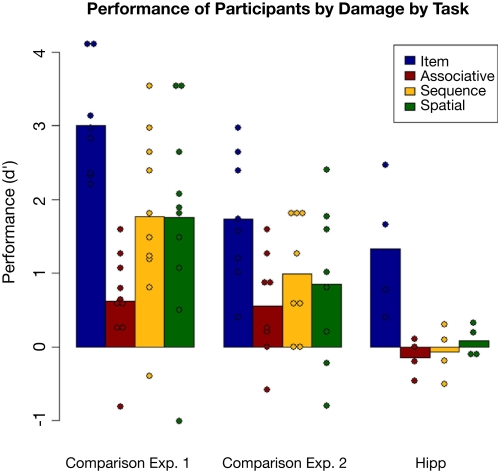
Performance of the comparison participants on each of the four tasks in Experiment 2 is shown in Figure 8, plotted along with performance by the comparison and patient groups from Experiment 1. Hit and false alarm rates for this experiment are in Table 4. It is important to ensure that performance was reduced but above chance, so first performance on each task in Experiment 2 was compared to chance (d′ = 0) by Bonferroni-corrected t-tests. Performance was significantly above chance on the item and sequential relations tasks [t(7) = 5.60 and 3.52, p = 0.001 and 0.01 respectively], and at trend levels for the associative and spatial relations tasks [t(7) = 2.2, p = 0.06 for each] likely due to the small number of subjects; numerically, performance was far from chance. Tukey pair-wise comparisons found that the only significant difference in performance among tasks was higher performance on the item task than on the associative relations task (p = 0.05). Performance of the comparison group in Experiment 2 versus Experiment 1 reveals that the more difficult version of the task reduced performance levels while still keeping them above chance.
Figure 8.
Performance by the comparison group on each task in Experiment 2, plotted along with performances by the comparison and hippocampal groups from Experiment 1. Individual participant scores are again plotted over the means.
Table 4.
Hit and false alarm rates for control participants on each task in Experiments 1 and 2 as well as the hippocampal patients from Experiment 1.
| Task | Item | Cooc | Sequence | Spatial | ||||
|---|---|---|---|---|---|---|---|---|
| HR | FAR | HR | FAR | HR | FAR | HR | FAR | |
| Experiment 1 controls | 0.82 | 0.17 | 0.66 | 0.47 | 0.71 | 0.25 | 0.75 | 0.29 |
| Experiment 1 Hipp patients | 0.73 | 0.43 | 0.62 | 0.62 | 0.51 | 0.53 | 0.56 | 0.53 |
| Experiement 2 controls | 0.76 | 0.19 | 0.68 | 0.49 | 0.65 | 0.27 | 0.72 | 0.42 |
Comparing the current results to the entire patient group from Experiment 1, the nonparametric procedure found significant effects of task (p = 0.019) and group (p < 0.001), but no interactions. The comparison group performed better than the amnesic group on all tasks. A second nonparametric comparison of the controls to only the hipp group (taking out the MTL group) also found main effects of group (p = 0.002) and task (p = 0.004) but no interaction (p > 0.5). Direct comparison of task performance between the comparison group and the hipp patient group found no significant difference in item [t(10) = 0.755, p > 0.4] or spatial [t(10) = 1.38, p > 0.15] performance, but did find a trend in associative [t(10) = 1.86, p = 0.09] and a significant difference in sequence [t(10) = 2.51, p = 0.03] performance. Thus the comparison group, at reduced levels, performed equivalently to the hippocampal patients on the item task but generally better on the relational tasks.
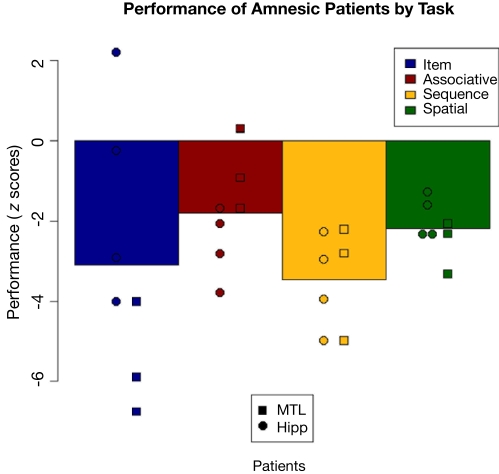
These effects are easier to understand if plotted as z-score differences from comparison performance for each patient, as shown in Figure 9. As a group, the amnesic patients were significantly impaired (corresponding to z-scores of greater than −1.96) on three of the four tasks, and were at trend levels on the associative task (−1.81) due largely to poor performance by the comparison group. Comparing the two amnesic groups, there was a numerical difference in associative task scores, but recall that performance did not differ significantly from chance in either group. The amnesic groups performed very similarly on the other relational tasks. The one test on which there was a difference between patient groups is on the item memory task. What had appeared to be an overall amnesic group deficit (z = −3.09) turned out to be driven by the MTL group. The hipp group was relatively unimpaired (z = −1.24), with one patient performing equivalent to the comparison group and another performing much better than average, whereas the MTL group was extremely impaired (z = −5.56) with all three patients having performed well below the impairment threshold.
Figure 9.
Performance decrements of the patients on each task, shown as deviation from comparison participants in z-scores. Shown are overall patient group means and individual patient scores with hippocampal patients as circles and MTL patients as squares.
Summary
The hippocampus-only group showed impairment on all tasks in Experiment 1. But, with performance so poor, and at chance levels across the relational tasks, it is difficult to confidently test the comparability of the impairment across tasks. Possible differences in performance might have been masked by a floor effect. In Experiment 2, the comparison group was retested on a more difficult version of the experiment that succeeded in bringing their item memory test scores down into the range of the amnesic patients, permitting fairer assessment of relative impairment on item versus relational memory tasks. Analyses of z-score differences of patient versus comparison performance showed that the hippocampus-only group was unimpaired on the item memory task while both groups of amnesic patients were significantly impaired on all three relational tasks. This pattern of results suggests a differential effect of hippocampal damage on relational as opposed to item memory, regardless of what kind of relations (spatial, sequential, or associative) were to be remembered.
One final issue to be considered concerns the finding from Experiment 1 that while the hippocampus-only patients performed above chance on the item task, they nonetheless performed more poorly than did the comparison group. One possible explanation for this may come from the way in which the item test trials were constructed. On repeat trials, all three items came from the same study set. This provides an opportunity for associative relational memory to contribute to performance if, at test time, associative information could be used to supplement whatever information was available about the items individually. For example, a single item could serve as the basis for associative pattern completion (e.g. Norman and O'Reilly, 2003), bringing forth the memory of another item in the set and aiding performance on the item task. Retrieving the spatial or temporal position of the item, on the other hand, should not influence the item memory decision. If indeed item memory performance was advantaged by the use of associative memory, it should be possible to see a correlation between item task performance and associative task performance. Accordingly, correlational analyses were conducted with the comparison participant performance from Experiment 1 and with the performance of a new set of control subjects, undergraduates at the University of Illinois Urbana-Champaign, in the next experiment.
Experiment 3
Materials and methods
Participants
The participants consisted of 22 undergraduate students at the University of Illinois Urbana-Champaign. Participants were paid $8 an hour for performing the experiment.
Paradigm
The same basic paradigm was used as in Experiments 1 and 2.
Stimuli and design
The stimuli and design were exactly the same as in Experiment 1.
Procedure
Each participant was tested individually, with the same procedure as in Experiment 2.
Analysis
Responses were again categorized as hits, misses, false alarms, and correct rejections, used to calculate d′ scores. The planned analysis for this experiment was to examine correlations between each of the tasks in this experiment and in the comparison group data from Experiment 1. Data from Experiment 2 were not examined because of the different presentation parameters used in that experiment and the lower performance levels that resulted.
Results
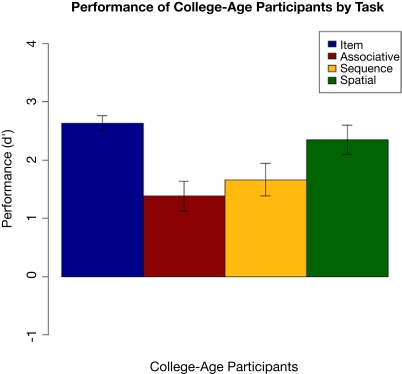
Performance by the college-age participants can be seen in Figure 10. Hit and false alarm rates are in Table 5. Performance on each task was compared using an ANOVA followed by Tukey pair-wise comparisons. There was a significant effect of task [F(3,84) = 6.310, p = 0.001]. Pair-wise comparisons showed that item performance was higher than associative and sequential relations task performance (both p < 0.025), and performance on the spatial task was better than on the associative relations task (p = 0.02). This pattern differs somewhat from that of the comparison participants in Experiment 1, where performance on the item memory task was superior to performance on any of the relational memory tasks, and performance on the associative relational task was even poorer than seen here.
Figure 10.
Performance of college-age participants on each task in Experiment 3, with standard error bars.
Table 5.
Hit and false alarm rates for college-age participants on each task in Experiment 3.
| Task | Item | Cooc | Sequence | Spatial | ||||
|---|---|---|---|---|---|---|---|---|
| HR | FAR | HR | FAR | HR | FAR | HR | FAR | |
| Undergrads | 0.90 | 0.09 | 0.87 | 0.43 | 0.81 | 0.27 | 0.87 | 0.14 |
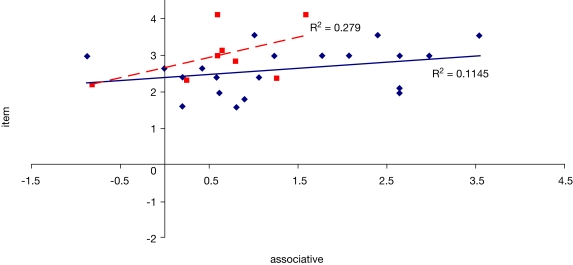
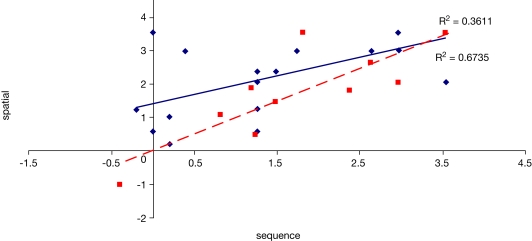
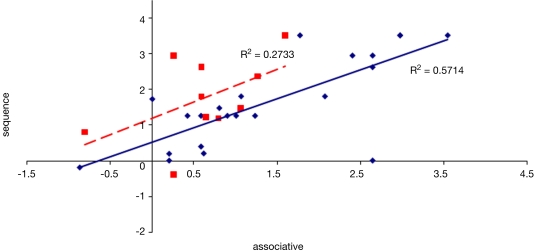
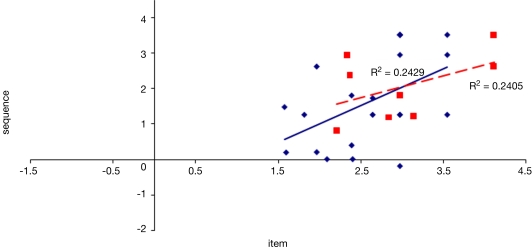
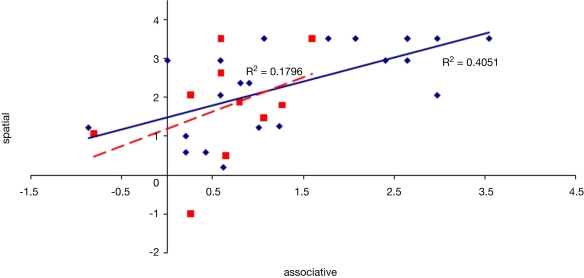
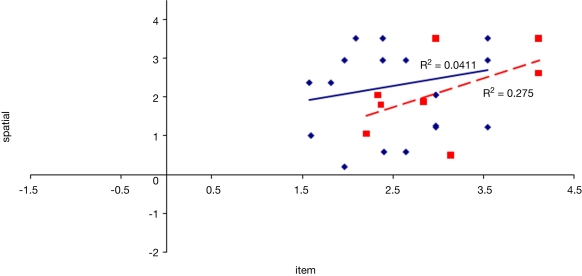
To examine the correlation between tasks, a regression analysis was carried out to find R-squared and significance values. The results are shown in scatter plot form in Figures 11–16, with significant (p < 0.05) correlations noted in the caption. In the college-age participants (Experiment 3), performance on each relational task correlated significantly with the others, while performance on the item task only correlated with the sequential relations task. In the comparison participants from Experiment 1, the only significant correlation was between spatial and sequence performance. The lack of significant correlations between other tasks in the control group is likely due to a lack of power. Notably, however, in neither dataset did item task performance correlate with associative relations task performance.
Figure 11.
Scatter plot of performance on the item and associative tasks with linear fit and R-squared values for control (Experiment 1, in red squares and dashed line) and college-age (Experiment 3, in blue circles and solid line) participants. Neither correlation was significant.
Figure 12.
Scatter plot of performance on the spatial and sequence tasks with linear fit and R-squared values for control (Experiment 1, in red squares and dashed line) and college-age (Experiment 3, in blue circles and solid line) participants. Both correlations are significant.
Figure 13.
Scatter plot of performance on the sequence and associative tasks with linear fit and R-squared values for control (Experiment 1, in red squares and dashed line) and college-age (Experiment 3, in blue circles and solid line) participants. The correlation is significant in college-age participants.
Figure 14.
Scatter plot of performance on the item and sequence tasks with linear fit and R-squared values for control (Experiment 1, in red squares and dashed line) and college-age (Experiment 3, in blue circles and solid line) participants. The correlation is significant in college-age participants.
Figure 15.
Scatter plot of performance on the spatial and associative tasks with linear fit and R-squared values for control (Experiment 1, in red squares and dashed line) and college-age (Experiment 3, in blue circles and solid line) participants. The correlation is significant in college-age participants.
Figure 16.
Scatter plot of performance on the item and spatial tasks with linear fit and R-squared values for control (Experiment 1, in red squares and dashed line) and college-age (Experiment 3, in blue circles and solid line) participants. Neither correlation is significant.
Summary
We considered whether the finding from Experiment 1 that item memory performance, while relatively preserved compared to relational memory in hippocampal amnesia but nonetheless inferior to that of the comparison participants, had been influenced in any way by memory for associative relations. Perhaps the comparison participants were able to make use of remembered information about associations, not available to the hippocampal amnesics, to aid their item memory task performance. However, we found no correlation between performance on the item task and the associative relations task in the comparison participants from Experiment 1. While the null finding may be due to a lack of power, no correlation was found in a larger group of college-aged participants either. Thus we found no evidence in support of a significant influence of associative relations information on item memory performance.
Discussion
The goals of the present work were (1) to develop a paradigm capable of testing memory for different types of relations independently, and also of testing item memory, all based on the same study trials with the same stimulus materials, and (2) using this paradigm to assess the status of these different aspects of relational and item memory in hippocampal amnesia. Using novel visual forms as stimuli, the paradigm involved a series of trials, each consisting of a set of three stimuli presented sequentially. By presenting the stimuli in sets, with each stimulus in one of three spatial locations and in one of three temporal slots, it was possible to subsequently test memory not only for which items had been studied (item memory), but also for which items had been presented together (memory for associative relations), the spatial locations of each set of items (memory for spatial relations), and the sequence of the items in each set (memory for sequential or temporal relations).
Previous work has tended to confound the different types of relations in testing relational memory, even while attempting to test only one. For example, in testing memory for relations in scenes or in grid-like displays, there are multiple types of information available, including about the spatial relations among items (item a is to the left and above item b), the item–location relations for the various items (item a is in coordinate position x, y or grid position n), and associative relations for which items occurred together regardless of their locations (item a with item b; item c with item d). In this paper, the term “associative” is reserved for information about mere co-occurrence or group membership, without regard to the more specific (temporal, spatial, or other) relations between them. Here, we have isolated different aspects or types of relational information, and have created recognition memory test trials that made it possible to assess each one unconfounded by the others. Using these separate probes, it was shown that all the different forms of relational memory were profoundly impaired, and indeed fell to chance levels of performance in hippocampal amnesia, disproportionate to any effect on item memory.
The present findings provide strong support for relational memory theory, confirming the prediction that memory for all manner of relations would be impaired in patients with hippocampal amnesia. That prediction came from considering many findings derived from different lines of work examining different aspects or types of relational memory separately, emphasizing either spatial memory or spatial navigation abilities, or explicit remembering and conscious recollection of previous episodes, or source memory, etc. Very few previous studies have examined multiple aspects of relational memory, as distinct from item memory, in the same work (but, see Kohler et al., 2005; Kumaran and Maguire, 2005). Most attempts to do so have used different paradigms with different materials and different displays, such as in our previous work testing memory for face–scene relations and for relations among items in scenes (Hannula et al., 2006). Here, by having a common set of study trials for all tests of relational memory, the present methods provide for more comparability across the various relational memory tests, and thereby permit a stronger test of the comparability of impairment in memory for all manner of relations.
The present findings also bear importantly on the debate about functional dissociations within the MTL. Although all patients performed at chance levels on the tests of relational memory, there were group differences in their item memory performance depending on extent of lesion. Patients whose MTL damage was limited to the hippocampus performed above chance on the item task while those with damage extending beyond the hippocampus fell to chance levels. While the damage in some MTL patients also extended beyond the temporal lobes, it is important to note that their scores on tests of executive function were similar to those in the hippocampal group, and those patients with the best frontal lobe/executive functioning did not perform any differently than the other amnesic patients on the memory tests reported here. Together with the additional finding that when a neurologically intact comparison group had their item memory performance matched to that of the patients with hippocampus-only damage the normal control group showed above-chance and better performance on the relational tasks, the findings support the idea that the hippocampus plays a special role in relational memory. With all task performance being at floor in the MTL group we cannot speak to further disproportionate deficits in that group; they could display further item deficits, relational deficits, or both. Similarly we are unable to comment on the possibility that some particular subset of relational memory is especially impaired in amnesia. For example, a weaker version of the spatial theory of hippocampal function could be that spatial processing is preferred or that other relations are derived from spatial information. This formulation is possible given our data, but importantly multiple kinds of relational memory were found to be impaired in patients with hippocampal damage, indicating that the relational memory theory is preferred to at least the strongest version of the spatial maps theory.
The design here permitted comparing performances on each of the different relational memory tests to performance on a test of item memory, all based on the same study presentations. However, while item memory was tested separately from relational memory, the test trials on the item task involving intact (repeated) test trials consisted of three items from the same study set, opening the possibility that associative relational information could conceivably be used to contribute to performance on the item task. To address this potential concern, a second experiment was conducted and correlational analyses were undertaken on performance on the item and relational tasks for two independent experiments. If associative information were used to contribute to performance on the item task, then a correlation would be expected between item memory test performance and associative test performance: those participants who performed well on the associative task would be expected to also perform well on the item task. However, no significant correlation was found between item and associative performance in either the comparison group from Experiment 1 or the college-aged participants in Experiment 3, suggesting that there was little if any contribution of associative memory on performance in the item memory task.
Finally, there were also novel findings here in the data from the neurologically intact participants. Intriguingly, it was found that associative memory performance was the poorest of all relational memory task performances in both the college-aged and comparison groups. This could be a result of the spatial and sequential information being more salient or easier to encode in this particular paradigm, or it could reflect a more general property of memory systems such that associative memory is more poorly or more weakly represented in the brain. In either case, neither the general primacy nor the influence of a specific paradigm on the ease of creating different kinds of relational memories has been studied, to our knowledge. This area bears further research. Additionally, a comparison of performance by the control group in Experiment 1 and college-age students in Experiment 3 showed that the two groups performed very similarly; the only significant difference was on the associative task. The generally high performance of the older (comparison group) adults goes against typical research on relational memory and aging (see for example Naveh-Benjamin et al., 2004). This could be because our comparison group consists mostly of people in their 40s and 50s, matching our patient population, whereas most aging experiments look at a cohort in at least their 60s if not older. A different explanation is that most aging experiments use associative memory tasks, such as pairs of faces or face–name pairs, as opposed to other kinds of relations. Given that our younger and older groups performed most differently on the associative task, perhaps conclusions about aging would be very different if more spatial or sequence memory tasks were used. This area also bears much further research.
In summary, across three experiments designed to provide comparable tests of different aspects of relational memory as compared to item memory, we found support for the idea that the MTL is necessary for forming all manner of relational memories. In addition, the evidence supports more specific claims of relational memory theory that the hippocampus specifically is necessary for relational memory while other MTL structures, primarily the perirhinal cortex, add mediation of item memory.
Conflict of Interest Statement
The authors declare that the research was carried out in the absence of any financial relationships that could be construed as a conflict of interest.
Acknowledgments
We thank Lindsay Ellch and Kaitlin Morton for assistance in data collection and the rest of the Amnesia Research Laboratory and the Gonsalves Group for helpful discussions. This work was supported by NIMH grant MH062500 to NJC and Program Project Grant NINDS NS19632 to DT.
References
- Agster K. L., Fortin N. J., Eichenbaum H. (2002). The hippocampus and disambiguation of overlapping sequences. J. Neurosci. 22, 5760–5768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. S., Tranel D., Bruss J., Damasio H. (2006). Correlations between regional brain volumes and memory performance in anoxia. J. Clin. Exp. Neuropsychol. 28, 457–476 10.1080/13803390590949287 [DOI] [PubMed] [Google Scholar]
- Bayley P. J., Squire L. R. (2005). Failure to acquire new semantic knowledge in patients with large medial temporal lobe lesions. Hippocampus 15, 273–280 10.1002/hipo.20057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryhill M. E., Phuong L., Picasso L., Cabeza R., Olson I. R. (2007). Parietal lobe and episodic memory: bilateral damage causes impaired free recall of autobiographical memory. J. Neurosci. 27, 14415–14423 10.1523/JNEUROSCI.4163-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird C. M., Burgess N. (2008). The hippocampus and memory: insights from spatial processing. Nat. Rev. Neurosci. 9, 182–194 10.1038/nrn2335 [DOI] [PubMed] [Google Scholar]
- Brown M. W., Aggleton J. P. (2001). Recognition memory: what are the roles of the perirhinal cortex and the hippocampus? Nat. Rev. Neurosci. 2, 51–61 10.1038/35049064 [DOI] [PubMed] [Google Scholar]
- Cohen N. J. (1984). Preserved learning capacity in amnesia: evidence for multiple memory systems. In Neuropsychology of Memory, Squire L. R., Butters N., eds (New York, Guilford Press; ), pp. 83–103 [Google Scholar]
- Cohen N. J., Eichenbaum H. (1993). Memory, Amnesia, and the Hippocampal System. Cambridge, MIT Press [Google Scholar]
- Cohen N. J., Poldrack R. A., Eichenbaum H. (1997). Memory for items and memory for relations in the procedural/declarative memory framework. Memory 5, 131–178 10.1080/741941149 [DOI] [PubMed] [Google Scholar]
- Cohen N. J., Ryan J., Hunt C., Romine L., Wszalek T., Nash C. (1999). Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus 9, 83–98 [DOI] [PubMed] [Google Scholar]
- Crane J., Milner B. (2005). What went where? Impaired object–location learning in patients with right hippocampal lesions. Hippocampus 15, 216–231 10.1002/hipo.20043 [DOI] [PubMed] [Google Scholar]
- Davachi L. (2006). Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 16, 693–700 10.1016/j.conb.2006.10.012 [DOI] [PubMed] [Google Scholar]
- Davachi L., Mitchell J. P., Wagner A. D. (2003). Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc. Natl. Acad. Sci. U.S.A. 100, 2157–2162 10.1073/pnas.0337195100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L., Wagner A. D. (2002). Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J. Neurophysiol. 88, 982–990 [DOI] [PubMed] [Google Scholar]
- Degonda N., Mondadori C. R. A., Brossard S., Schmidt C. F., Besieger P., Nitsch R. M., Hock C., Henke K. (2005). Implicit associative learning engages the hippocampus and interacts with explicit associative learning. Neuron 46, 505–520 10.1016/j.neuron.2005.02.030 [DOI] [PubMed] [Google Scholar]
- Dusek J. A., Eichenbaum H. (1997). The hippocampus and memory for orderly stimulus relations. Proc. Natl. Acad. Sci. U.S.A. 13, 7109–7114 10.1073/pnas.94.13.7109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek J. A., Eichenbaum H. (1998). The hippocampus and transverse patterning guided by olfactory cues. Behav. Neurosci. 112, 762–771 10.1037/0735-7044.112.4.762 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H., Cohen N. J. (1988). Representation in the hippocampus – what do hippocampal neurons encode. Trends Neurosci. 11, 244–248 10.1016/0166-2236(88)90100-2 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H., Cohen N. J. (2001). From Conditioning to Conscious Recollection: Memory Systems of the Brain. New York, Oxford University Press [Google Scholar]
- Eichenbaum H., Dudchenko P., Wood E., Shapiro M., Tanila H. (1999). The hippocampus, memory, and place cells: is it spatial memory or a memory space? Neuron 23, 209–226 10.1016/S0896-6273(00)80773-4 [DOI] [PubMed] [Google Scholar]
- Eichenbaum H., Otto T., Cohen N. J. (1994). Two functional components of the hippocampal memory system. Behav. Brain Sci. 17, 449–517 [Google Scholar]
- Eichenbaum H., Yonelinas A. P., Ranganath C. (2007). The medial temporal lobe and recognition memory. Annu. Rev. Neurosci. 30, 123–152 10.1146/annurev.neuro.30.051606.094328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P. C., Henson R. N. A. (2001). Frontal lobes and human memory: insights from functional neuroimaging. Brain 124, 849–881 10.1093/brain/124.5.849 [DOI] [PubMed] [Google Scholar]
- Fortin N. J., Agster K. L., Eichenbaum H. (2002). Critical role of the hippocampus in memory for sequences of events. Nat. Neurosci. 5, 458–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello K. S., Keane M. M., Verfaellie M. (2006). The contribution of familiarity to associative memory in amnesia. Neuropsychologia 44, 1859–1865 10.1016/j.neuropsychologia.2006.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanello K. S., Schnyer D. M., Verfaellie M. (2004). A critical role for the anterior hippocampus in relational memory: evidence from an fMRI study comparing associative and item recognition. Hippocampus 14, 5–8 10.1002/hipo.10182 [DOI] [PubMed] [Google Scholar]
- Giovanello K. S., Verfaellie M., Keane M. M. (2003). Disproportionate deficit in associative recognition relative to item recognition in global amnesia. Cogn. Affect. Behav. Neurosci. 3, 186–194 [DOI] [PubMed] [Google Scholar]
- Gold J. J., Smith C. N., Bayley P. J., Shrager Y., Brewer J. B., Stark C. E. L., Hopkins R. O., Squire L. R. (2006). Item memory, source memory, and the medial temporal lobe: concordant findings from fMRI and memory-impaired patients. Proc. Natl. Acad. Sci. 103, 9351–9356 10.1073/pnas.0602716103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannula D. E., Ryan J. D., Tranel D., Cohen N. J. (2007). Rapid onset relational memory effects are evident in eye movement behavior, but not in hippocampal amnesia. J. Cogn. Neurosci. 19, 1690–1705 10.1162/jocn.2007.19.10.1690 [DOI] [PubMed] [Google Scholar]
- Hannula D. E., Tranel D., Cohen N. J. (2006). The long and the short of it: relational memory impairments in amnesia, even at short lags. J. Neurosci. 26, 8352–8359 10.1523/JNEUROSCI.5222-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T., Maguire E. A., Spiers H. J., Burgess N. (2003). The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron 37, 877–888 10.1016/S0896-6273(03)00095-3 [DOI] [PubMed] [Google Scholar]
- Henke K., Buck A., Weber B., Wieser H. G. (1997). Human hippocampus establishes associations in memory. Hippocampus 7, 249–256 [DOI] [PubMed] [Google Scholar]
- Henke K., Mondadori C. R. A., Treyer V., Nitsch R. M., Buck A., Hock C. (2003). Nonconscious formation and reactivation of semantic associations by way of the medial temporal lobe. Neuropsychologia 41, 863–876 10.1016/S0028-3932(03)00035-6 [DOI] [PubMed] [Google Scholar]
- Holdstock J. S., Mayes A. R., Gong Q. Y., Roberts N., Kapur N. (2005). Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus 15, 203–215 10.1002/hipo.20046 [DOI] [PubMed] [Google Scholar]
- Holdstock J. S., Mayes A. R., Roberts N., Cezayirli E., Isaac C. L., O'Reilly R. C., Norman K. A. (2002). Under what conditions is recognition spared relative to recall after selective hippocampal damage in humans? Hippocampus 12, 341–351 10.1002/hipo.10011 [DOI] [PubMed] [Google Scholar]
- Kan I. P., Giovanello K. S., Schnyer D. M., Makris N., Verfaellie M. (2007). Role of the medial temporal lobes in relational memory: neuropsychological evidence from a cued recognition paradigm. Neuropsychologia 45, 2589–2597 10.1016/j.neuropsychologia.2007.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S., Danckert S., Gati J. S., Menon R. S. (2005). Novelty responses to relational and non-relational information in the hippocampus and parahippocampal region: a comparison based on event-related fMRI. Hippocampus 15, 763–774 10.1002/hipo.20098 [DOI] [PubMed] [Google Scholar]
- Kroll N. E. A., Knight R. T., Metcalfe J., Wolf E. S., Tulving E. (1996). Cohesion failure as a source of memory illusions. J. Mem. Lang. 35, 176–196 10.1006/jmla.1996.0010 [DOI] [Google Scholar]
- Kumaran D., Maguire E. A. (2005). The human hippocampus: cognitive maps or relational memory? J. Neurosci. 25, 7254–7259 10.1523/JNEUROSCI.1103-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D., Maguire E. A. (2006). An unexpected sequence of events: mismatch detection in the human hippocampus. PLoS Biol. 4, 2372–2382 10.1371/journal.pbio.0040424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E. A., Nannery R., Spiers H. J. (2006). Navigation around London by a taxi driver with bilateral hippocampal lesions. Brain 129, 2894–2907 10.1093/brain/awl286 [DOI] [PubMed] [Google Scholar]
- Manns J. R., Howard M. W., Eichenbaum H. (2007). Gradual changes in hippocampal activity support remembering the order of events. Neuron 56, 530–540 10.1016/j.neuron.2007.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes A. R., Holdstock J. S., Isaac C. L., Montaldi D., Grigor J., Gummer A., Cariga P., Downes J. J., Tsivilis D., Gaffan D., Gong Q. Y., Norman K. A. (2004). Associative recognition in a patient with selective hippocampal lesions and relatively normal item recognition. Hippocampus 14, 763–784 10.1002/hipo.10211 [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M., Guez J., Kilb A., Reedy S. (2004). The associative memory deficit of older adults: further support using face–name associations. Psychol. Aging 19, 541–546 10.1037/0882-7974.19.3.541 [DOI] [PubMed] [Google Scholar]
- Norman K. A., O'Reilly R. C. (2003). Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol. Rev. 110, 611–646 10.1037/0033-295X.110.4.611 [DOI] [PubMed] [Google Scholar]
- O'Keefe J. A., Nadel L. (1978). The Hippocampus as a Cognitive Map. London, Oxford, Clarendon Press [Google Scholar]
- Olson I. R., Moore K. S., Stark M., Chatterjee A. (2006). Visual working memory is impaired when the medial temporal lobes are damaged. J. Cogn. Neurosci. 18, 1087–1097 10.1162/jocn.2006.18.7.1087 [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M., Tanila H., Kononen M., Hanninen T., Hamalainen A., Soininen H., Aronen H. J. (2004). Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. Eur. J. Neurosci. 19, 1939–1949 10.1111/j.1460-9568.2004.03282.x [DOI] [PubMed] [Google Scholar]
- Pine D. S., Grun J., Maguire E. A., Burgess N., Zarahn E., Koda V., Fyer A., Szeszko P. R., Bilder R. M. (2002). Neurodevelopmental aspects of spatial navigation: a virtual reality fMRI study. Neuroimage 15, 396–406 10.1006/nimg.2001.0988 [DOI] [PubMed] [Google Scholar]
- Prince S. E., Daselaar S. M., Cabeza R. (2005). Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. J. Neurosci. 25, 1203–1210 10.1523/JNEUROSCI.2540-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quamme J. R., Yonelinas A. P., Norman K. A. (2007). Effect of unitization on associative recognition in amnesia. Hippocampus 17, 192–200 10.1002/hipo.20257 [DOI] [PubMed] [Google Scholar]
- Ranganath C., Yonelinas A. P., Cohen M. X., Dy C. J., Tom S. M., D'Esposito M. (2004). Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia 42, 2–13 10.1016/j.neuropsychologia.2003.07.006 [DOI] [PubMed] [Google Scholar]
- Ryan J. D., Althoff R. R., Whitlow S., Cohen N. J. (2000). Amnesia is a deficit in relational memory. Psychol. Sci. 11, 454–461 10.1111/1467-9280.00288 [DOI] [PubMed] [Google Scholar]
- Ryan J. D., Cohen N. J. (2003). The contribution of long-term memory and the role of frontal-lobe systems in on-line processing. Behav. Brain Sci. 26, 756. 10.1017/S0140525X03510160 [DOI] [Google Scholar]
- Scoville W. B., Milner B. (1957). Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatr. 20, 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers H. J., Maguire E. A. (2006). Thoughts, behaviour, and brain dynamics during navigation in the real world. Neuroimage 31, 1826–1840 10.1016/j.neuroimage.2006.01.037 [DOI] [PubMed] [Google Scholar]
- Squire L. R., Stark C. E. L., Clark R. E. (2004). The medial temporal lobe. Annu. Rev. Neurosci. 27, 279–306 10.1146/annurev.neuro.27.070203.144130 [DOI] [PubMed] [Google Scholar]
- Staresina B. P., Davachi L. (2006). Differential encoding mechanisms for subsequent associative recognition and free recall. J. Neurosci. 26, 9162–9172 10.1523/JNEUROSCI.2877-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark C. E. L., Bayley P. J., Squire L. R. (2002). Recognition memory for single items and for associations is similarly impaired following damage to the hippocampal region. Learn. Mem. 9, 238–242 10.1101/lm.51802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark C. E. L., Squire L. R. (2003). Hippocampal damage equally impairs memory for single items and memory for conjunctions. Hippocampus 13, 281–292 10.1002/hipo.10085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turriziani P., Fadda L., Caltigirone C., Carlesimo G. A. (2004). Recognition memory for single items and associations in amnesic patients. Neuropsychologia 42, 426–434 10.1016/j.neuropsychologia.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Wood E. R., Dudchenko P. A., Robitsek R. J., Eichenbaum H. (2000). Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron 27, 623–633 10.1016/S0896-6273(00)00071-4 [DOI] [PubMed] [Google Scholar]