Abstract
It has been speculated that human leukocyte antigen (HLA) alleles are associated with the outcome of hepatitis B virus (HBV) infection although the data obtained from various populations have shown some inconsistencies. A total of 464 HBV-infected Korean individuals (80 spontaneously recovered [SR] and 384 chronically infected [CI]) were selected to investigate the association of HLA class II alleles with the viral clearance and persistence. Our results showed that: 1) multiple HLA class II alleles and haplotypes were associated with viral clearance (DRB1*1302, DRB1*1502, DQB1*0302, DQB1*0609, and related-haplotypes) and persistence (DRB1*0701, DQB1*0301, and related-haplotypes); 2) DRB1*1302 and DQB1* 0609 were more strongly associated with viral clearance. And the association of DQB1*0609 (pc=0.0084; OR, 7.24) with vial clearance was much stronger than previously recognized, DRB1*1302 (pc=0.0038; OR, 4.34); and 3) linkage to a specific DPB1 allele in a haplotype strengthened the association with viral clearance, although DPB1 itself was not associated with the outcome. These results indicate the existence of multiple factors controlling viral clearance in the HLA class II gene region. Further extended investigation on the genetic factors related to the outcome of HBV infection will provide valuable insights into the understanding of the mechanisms involved.
Keywords: Hepatitis B Virus, Clearance, Persistence, DQB1*0609, DRB1*1302
INTRODUCTION
Infection with human hepatitis B virus (HBV) can lead to high morbidity and mortality due to the development of end-stage liver diseases. It is estimated that more than 350 million people worldwide are infected with HBV (1). Among infected individuals, about 5-10% of adults and more than 90% of children become persistently infected by the virus and develop liver diseases. The clinical outcome of the infection is variable and may result in spontaneous recovery, inactive HBV surface antigen (HBsAg) carrier state, chronic hepatitis, liver cirrhosis, or hepatocellular carcinoma (2, 3). The precise mechanisms leading to these various outcomes are not clearly defined yet but it has been perceived that host immune responsiveness is the critical factor.
It has been demonstrated that vigorous CD4+ and CD8+ T lymphocyte responses to various HBV antigens were associated with HBV self-elimination, while insufficient CD4+ T cell help and defective CD8+ T cell repertoire at the early stages of infection were connected to viral persistence (4, 5). Due to the specific antigen presenting function of human leukocyte antigens (HLA) in immune responsiveness, the contribution of HLA to the outcome of HBV infection has been studied extensively in different populations. However, data have shown some inconsistencies with regard to HLA effects on HBV clearance or persistence in different ethnic and racial groups. Extensive HLA polymorphism contributes to the selection of antigenic peptides for presentation to T lymphocytes resulting in different immune responses to infection amongst individuals. Furthermore, HLA molecules differing only at 1-2 amino acids showed different peptide binding preference (6). This implies that HLA analysis for disease association studies requires high resolution typing to better understand the mechanisms involved.
Korea is an endemic area for HBV infection and about 5% of the population has been shown to be chronically infected (7). Two studies regarding HLA association in Koreans have been reported and consistently demonstrated that DR13 was associated with self-elimination of HBV (8, 9). However, these studies to clarify the HLA association in the population were limited in several respects. They examined only HLA-A, -B, and -DR at low resolution and used patients with organ transplants as their study subjects. Therefore, the purpose of this study was to further investigate the HLA association with the outcome of HBV infection using high resolution typing of HLA class II loci (HLA-DRB1, -DQB1, and -DPB1). The impact of HLA diversity was evaluated on viral clearance and persistence.
MATERIALS AND METHODS
Study subjects
A total of 464 Koreans who had been exposed to HBV as detected by the presence of antibody to HBV core antigen (anti-HBc) IgG, were enrolled from the outpatient clinics of the Gastroenterology Department of Ajou University Hospital (Suwon, Korea) between March and December 2002. Study subjects were divided into two groups based on the status of HBV serologic markers exhibited for more than 6 months. The spontaneously recovered (SR) group included 80 individuals (60 males and 20 females, aged 19-70 yr, mean±SD, 47.9±10.3) who were HBsAg negative, anti-HBc positive and antibody to HBV surface antigen (anti-HBs) positive, and had no evidence of liver function abnormalities and of previous chronic liver disease based on their medical history. The chronically infected (CI) group included 384 individuals (283 males and 101 females, aged 16-77 yr, mean±SD, 41.0±10.3) who were HBsAg and anti-HBc positive, and anti-HBs negative. They were regularly followed with blood tests for serum alanine aminotransferase (ALT)/aspartate aminotransferase (AST), hepatitis B e antigen (HBeAg)/antibody to hepatitis B e antigen (anti-HBe) and alpha fetoprotein (AFP), and with radiographic tests of the liver (computed tomography or ultrasonography) at an interval of every 6 months for more than a year. HBV-DNA was detected by Digene Hybrid Capture II Assay (Digene Diagnostics, Beltsville, MD, U.S.A.); the detection limit of the test was 0.5 pg/mL. None of the patients had hepatocellular carcinoma. Patients who were positive for anti-HBs and negative for anti-HBc IgG, and patients with other types of chronic liver disease such as alcoholic liver disease, chronic hepatitis C, steatohepatitis, and Wilson's disease, were excluded from this study. Informed consents were obtained from each subject, and the Institutional Review Board of Human Research of Ajou University Hospital approved the study protocol.
HLA allelic typing
Genomic DNA was extracted from peripheral blood mononuclear cells of the study subjects using the method of Miller and colleagues (10). Allele-level genotypes of the HLA-DRB1, -DQB1, and -DPB1 genes from each study sample were obtained by direct DNA sequence analysis based on the procedures described previously (11-13). Briefly, the polymorphic exon 2 of the gene was amplified using group-specific (HLA-DRB1) or locus-specific (HLA-DQB1, and -DPB1) primer sets. Automated cycle sequencing using dye terminator chemistry (v3.1, Applied Biosystems, Foster City, CA, U.S.A.) was performed on the amplified gene product and the amplicon was analyzed on an ABI3100 Genetic Analyzer (Applied Biosystems). Data analysis was performed with Sequence Navigator and MatchTool software (PE-Applied Biosystems Inc., U.S.A.). Homozygosity was assumed when no other HLA-DRB1, -DQB1, and -DPB1 allele was detected upon direct DNA sequence analysis.
Statistical analysis
Statistical analyses were done using SAS (version 8.01, SAS institute, Cary, NC). Haplotype reconstruction was implemented by PHASE (14). Hardy-Weinberg equilibrium for all loci and linkage disequilibrium for all pairs of loci were tested using Arlequin (version 2.0, Genetics and Biometry Laboratory, University of Geneva, Switzerland). Phenotype frequencies of HLA-DRB1, -DQB1, and -DPB1 alleles as well as haplotype frequencies of DRB1-DQB1, DQB1-DPB1, and DRB1-DQB1-DPB1 in two study groups were compared. Alleles (<3%) and haplotypes (<2%) with low frequencies were not analyzed due to inadequate statistical power. Some general characteristics were compared between the two study groups using t-test and chi-square test. Odd ratios (ORs) with 95% confidence intervals (95% CI) and p values for alleles and haplotypes associated with the outcome of HBV infection were calculated by logistic regression analysis adjusting for age and sex. When one element in the 2×2 table was zero, the p value was calculated by a chi-square test in haplotype analysis. Statistical significance was assumed at p<0.05. When the p value was less than 0.05, a Bonferroni correction was applied by multiplying the p value by the number of comparisons made.
RESULTS
Characteristics of study groups
Among HBV-exposed subjects including SR group (n=80) and CI group (n=384), the SR group (47.9±10.3 yr old) was somewhat older than the CI group (41.0±10.3 yr old) (p<0.0001). However, regarding gender, there was no significant difference between the SR group (% male=75.0) and the CI group (% male=73.7) (p>0.1).
Alleles associated with HBV clearance or persistence
Using direct DNA sequencing analysis, class II allele genotypes at the four-digit level were obtained from the study subjects. A total of 31 HLA-DRB1, 16 HLA-DQB1, and 23 HLA-DPB1 alleles were identified. Distribution of the alleles for all loci were consistent with Hardy-Weinberg equilibrium in the SR and CI groups, respectively (p>0.05).
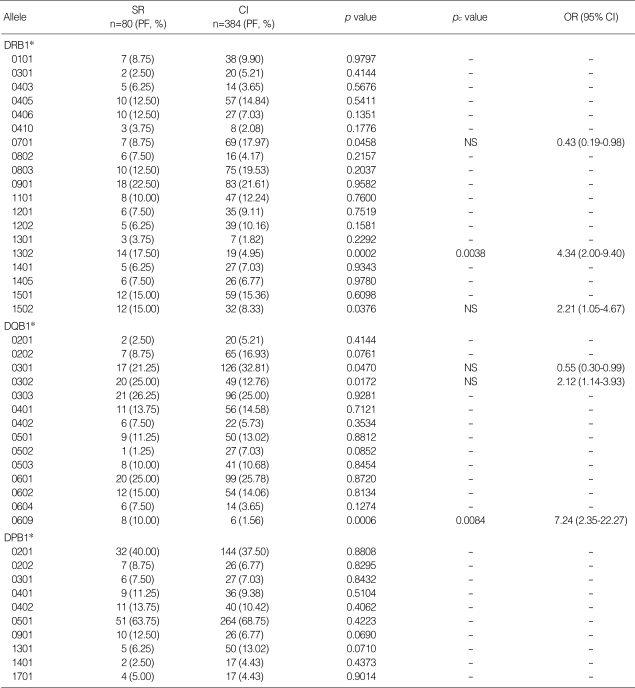
Phenotype frequencies of HLA class II alleles in the two study groups are listed in Table 1. The frequencies of DRB1* 1302 (17.50% vs. 4.95%, p=0.0002, OR, 4.34) and DQB1*0609 (10.00% vs. 1.56%, p=0.0006, OR, 7.24) were significantly higher in the SR group compared to the CI group. Their statistical significance was maintained after correction of the p values (pc=0.0038 and pc=0.0084, respectively). The frequencies of DRB1*1502 and DQB1*0302 were higher in the SR group, while the frequencies of DRB1*0701 and DQB1*0301 were higher in the CI group. However, p values were not statistically significant after the correction (Table 1).
Table 1.
Comparison of HLA class II phenotype frequencies between HBV spontaneously recovered (SR) and chronically infected (CI) groups
PF, phenotype frequency of HLA allele is presented in percentage; pc value, p value corrected by multiplying the number of alleles tested (19 for DRB1, 14 for DQB1); OR, odds ratio (SR group vs. CI group); CI, confidence interval; NS, statistically not significant after correction.
Alleles with a phenotype frequency >3% are listed.
Only significant pc values (<0.05) are presented.
Comparison of the impact of DRB1*1302 and DQB1*0609 on HBV clearance
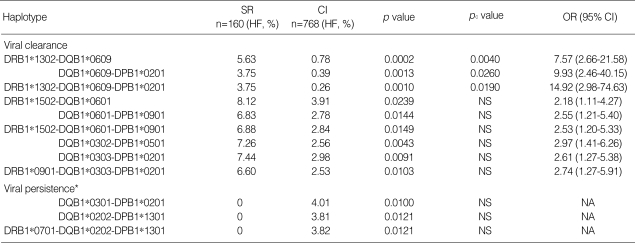
As described above, two alleles (DRB1*1302 and DQB1* 0609) were significantly associated with HBV clearance. Interestingly, all of the individuals carrying DQB1*0609 were DRB1*1302-positive in this study. This is due to the strong linkage disequilibrium between these two alleles in Koreans as previously described (15). Thus, the statistics for these two alleles might be distorted and an additional analysis was necessary to estimate the impact of the two alleles on HBV clearance. Fortunately, DRB1*1302 also showed strong linkage disequilibrium with DQB1*0604 in the study population (15). About 42% of DRB1*1302+ Korean individuals carry DQB1*0609 and the remainder (58%) carry DQB1* 0604. Thus, the significance of those two clearance alleles were indirectly evaluated by comparing haplotype frequencies of DRB1*1302-DQB1*0609 and DRB1*1302-DQB1* 0604 in the two study groups. As shown in Table 2, the frequency of DRB1*1302-DQB1*0609 was much higher in the SR group than in the CI group with a statistical significance (5.63% vs. 0.78%, p=0.0002, OR, 7.57), and its significance remained after correcting the p value (pc=0.0040). Although it was still elevated in the SR group (3.75% vs. 1.82%), the frequency of the second haplotype, DRB1*1302-DQB1*0604, was not statistically different between the two groups. In conclusion, DQB1*0609 was the primary allele associated with HBV clearance. The observed association of clearance with DRB1*1302 might be due to the DRB1 allele itself and/or to the linked DQB1*0609.
Table 2.
Comparison of DRB1*1302-related haplotype frequencies between the SR and CI groups
SR, spontaneously recovered; CI, chronically infected; HF, haplotype frequency is presented in percentage; pc value, p value corrected by multiplying the number of haplotypes tested (20 for DRB1-DQB1); OR, odds ratio (SR group vs. CI group); CI, confidence interval.
Only significant pc values (<0.05) are presented.
Haplotypes associated with HBV clearance or persistence
Two- and three-locus HLA class II haplotypes associated with viral clearance and persistence are listed in Table 3. All of the haplotypes, except two (DQB1*0303-DPB1*0201, DRB1*0901-DQB1*0303-DPB1*0201), carried at least one of the outcome-related alleles (Table 1). For example, three DRB1*1302- and DQB1*0609-related haplotypes (DRB1*1302-DQB1*0609 (pc=0.0040), DQB1*0609-DPB1*0201 (pc=0.0260), DRB1*1302-DQB1*0609-DPB1*0201 (pc=0.0190)) were strongly associated with viral clearance as DRB1*1302 and DQB1*0609 themselves were. The haplotypes maintained statistical significances after correction of the p values. Other haplotypes associated with viral clearance were DRB1*1502- and DQB1*0302-related haplotypes. Haplotypes associated with viral persistence were DQB1*0301- and DRB1*0701-related haplotypes. However, p values for those haplotypes were not statistically significant after the correction (Table 3).
Table 3.
Two- and three-locus haplotypes associated with viral clearance and persistence
SR, spontaneously recovered; CI, chronically infected; HF, frequency of allele or haplotype is presented in percentage; pc value, p value corrected by multiplying the number of haplotypes tested (20 for DRB1-DQB1; 20 for DQB1-DPB1; 19 for DRB1-DQB1-DPB1); OR, odds ratio (SR group vs. CI group); CI, confidence interval; NS, statistically not significant after correction (pc->0.05); NA, not applicable.
*, p value was calculated by chi-square test.
Although none of the DPB1 allele appeared to be associated with the outcome of HBV infection (Table 1), there was an indirect association of DPB1 with viral clearance observed at the haplotype level (Table 3). On a haplotype (DQB1* 0303-DPB1*0201, p=0.0091, OR, 2.61) associated with viral clearance with a statistical significance, neither DQB1* 0303 (26.25% vs. 25.00%) nor DPB1*0201 (40.00% vs. 37.50%) alone was associated with viral clearance as shown in Table 1. However, a haplotype consisting of these two alleles appeared to be associated with viral clearance. Furthermore, the OR value of its 3-locus haplotype, DRB1*0901-DQB1* 0303-DPB1*0201 (OR, 2.74), was similar to that of DQB1* 0303-DPB1*0201 (OR, 2.61). We could observe a similar phenomenon in another example. DQB1*0609 (OR, 7.24) was strongly associated with viral clearance as described above. However, linking this allele to statistically insignificant DPB1*0201 again in a haplotype DQB1*0609-DPB1*0201 increased the OR value (OR, 9.93). In a three-locus haplotype, DRB1*1302-DQB1*0609-DPB1*0201, the OR value was even higher (OR, 14.92).
DISCUSSION
From the study of the HLA association with the outcome of HBV infection, we found that two HLA class II alleles are strongly associated with viral clearance. The first allele was DRB1*1302 and this result was concordant with two previous Korean studies that demonstrated that DR13 was associated with self-elimination of HBV (8, 9). In addition, DRB1*1302 has been consistently reported as a factor associated with protection against persistent HBV infection in two other racial groups, Africans (16) and Caucasians (17, 18). Identification of the same HLA allele and/or serologic type from various populations strongly suggests that there is at least one common element restricting HBV in humans. A beneficial effect of DR13 on HBV infection has also been confirmed by functional assay. Diepolder et al. demonstrated that DR13-positive individuals showed a more vigorous CD4+ T cell response to HBV core antigen (HBcAg) than DR13-negative patients (19). DR13-restricted CD4+ T cell epitopes of HBcAg were later discovered (20).
The second allele associated with viral clearance was DQB1* 0609, which is in strong linkage disequilibrium with DRB1* 1302 in the study population. The impact of the DQB1* 0609 allele on HBV clearance was indirectly analyzed by comparing frequencies of the two DRB1*1302-positive haplotypes, DRB1*1302-DQB1*0609 and DRB1*1302-DQB1* 0604, between the two study groups. The result indicates that DQB1*0609 has a much stronger association with HBV clearance than DRB1*1302 and we believe that DQB1* 0609 is the primary class II allele associated with viral clearance. This hypothesis is supported by several previous studies. First, there has been a report that the phenotype frequency of DQw1 was significantly lower in Dutch patients with chronic active HBV infection (21) (DQB1*0609 is one of the alleles encoding the DQw1 molecule). In addition, a study in Gambians indicated that the frequency of DQB1* 06 linked to DRB1*1302 was significantly higher in children (11.9% vs. 6.5%, p=0.06) and adults (9.2% vs. 0%, p=0.05) who cleared the virus than in individuals who carried the virus persistently (16). Later, DQB1*0609 was found to be relatively frequent in Gambians (DQB1*0609=4.4%; DQB1*0604=0.7%) (22). Lastly, it has been demonstrated that DR13-positive individuals exhibited a more vigorous CD4+ T cell response to HBcAg than DR13-negative individuals during acute HBV infection (19). However, less than 10% of the HBc-specific CD4+ T cell clones derived from DR13-positive patients were DR13-restricted. This result left open the possibility that the majority of the CD4+ T cell clones (>90%) might be restricted to other HLA class II molecules including DQw1 encoded by a DQB1*06 allele in DR13-positive individuals.
Therefore, for the first time, we have shown that DQB1* 0609 is the primary allele associated with HBV clearance and its association is much stronger than previously found for DRB1*1302. In most of the previous association studies on HBV clearance, DQB1 high resolution typing was not performed. Only three studies so far analyzed DQB1 at allele resolution; however, the frequencies of DQB1*0609 might have been too low in the study populations to catch its statistical significance (18, 23, 24). That could be the reason why DQB1*0609 has not been identified thus far with HBV clearance. Our hypothesis needs to be confirmed by further studies in populations expressing a high frequency of this allele.
Several additional alleles were found to be associated with the outcome of HBV infection in this study, although the result did not reach statistical significance. The increased frequency of DRB1*1502 in the SR group was consistent with previous studies in Qartar (25) and China (26). Likewise, the higher frequencies of DRB1*0701 (9, 25) and DQB1*0301 (23, 24) in the CI group were consistent with previous reports. One of the previous studies analyzed a Korean population and found significant association of DR7 with HBV persistence (pc<0.001) (9). Another study in the same population found a high frequency of DR9 (p<0.001), instead of DR7, in the CI group (8). These discrepancies might stem from differences in the study subjects. In our study, the study samples were recruited from outpatient clinics based on the HBV serologic markers. Previous studies analyzed the compiled HLA typing data of individuals undergoing organ transplantation and their donors where HLA matching between patient and donor is beneficial. Thus, the HLA frequencies in those studies could have been biased. DQB1*0302, found in this study associated with HBV clearance, has not been identified previously.
During the analysis of HLA class II multi-locus haplotypes, we found several related haplotypes carrying outcome-related alleles. Interestingly, association with viral clearance is significantly intensified on DQB1-DPB1 haplotypes consisting of a DQB1 allele (either a statistically insignificant allele [e.g., DQB1*0303] or a clearance-associated allele [e.g., DQB1*0609]) and the statistically insignificant DPB1*0201, compared to OR values of the DQB1 allele alone. These results indicate that a DPB1 allele in combination with a DQB1 allele has either a cooperative (or synergistic) protective effect since DPB1*0201 (40.0% vs. 37.5%) alone has no effect. Alternatively, there might be another gene influencing the outcome of HBV infection located near the DPB1 gene. Several polymorphic genes, such as LMP, DM, and DO, whose gene products assist in antigen binding to the HLA molecules in a cell, are known to be located between DQB1 and DPB1 genes (27). Further extended investigation of these polymorphic genes might elucidate the cooperative (or synergistic) modification of immune responsiveness to HBV infection. Whether it is a cooperative (or synergistic) interaction or a closely linked gene, factor other than DR and DQ alleles might be involved in viral clearance.
The DRB1*1302-DQB1*0609-DPB1*0201 haplotype shows the strongest protective effect against HBV (pc=0.0190, OR, 14.92). Based on the results obtained from this study, it is most likely that the strong protective effect of the haplotype against HBV persistence might derive from three different factors: DRB1*1302, DQB1*0609, and another unknown factor linked to DPB1 as just described. Further investigations should be made to identify this.
In summary, multiple HLA class II alleles and related haplotypes are associated with viral clearance and persistence in Koreans. In specific, DRB1*1302 and DQB1*0609 are strongly associated with viral clearance. However, unexpectedly, the association of DQB1*0609 is much stronger than that of DRB1*1302. In addition, the association with a statistically insignificant DPB1 allele in a haplotype strengthens the association with viral clearance, hinting that another unknown factor might be involved in viral clearance. These results may reflect the existence of multiple factors controlling viral clearance encoded in the HLA class II gene region. Further extended investigation on the genetic factors related to the outcome of HBV infection will provide valuable insights into the understanding of the mechanisms involved.
Footnotes
This research was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (A010383).
References
- 1.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–1745. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 2.Iino S. Natural history of hepatitis B and C virus infections. Oncology. 2002;62(Suppl 1):18–23. doi: 10.1159/000048271. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS, McMahon BJ, Practice Guidelines Committee, American Association for the Study of Liver Diseases Chronic hepatitis B. Hepatology. 2001;34:1225–1241. doi: 10.1053/jhep.2001.29401. [DOI] [PubMed] [Google Scholar]
- 4.Jung MC, Pape GR. Immunology of hepatitis B infection. Lancet Infect Dis. 2002;2:43–50. doi: 10.1016/s1473-3099(01)00172-4. [DOI] [PubMed] [Google Scholar]
- 5.Urbani S, Boni C, Amadei B, Fisicaro P, Cerioni S, Valli MA, Missale G, Ferrari C. Acute phase HBV-specific T cell responses associated with HBV persistence after HBV/HCV coinfection. Hepatology. 2005;41:826–831. doi: 10.1002/hep.20614. [DOI] [PubMed] [Google Scholar]
- 6.Davenport MP, Quinn CL, Chicz RM, Green BN, Willis AC, Lane WS, Bell JI, Hill AV. Naturally processed peptides from two disease-resistance-associated HLA-DR13 alleles show related sequence motifs and the effects of the dimorphism at position 86 of the HLA-DR beta chain. Proc Natl Acad Sci USA. 1995;92:6567–6571. doi: 10.1073/pnas.92.14.6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DH, Kim JH, Nam JJ, Kim HR, Shin HR. Epidemiological findings of hepatitis B infection based on 1998 National Health and Nutrition Survey in Korea. J Korean Med Sci. 2002;17:457–462. doi: 10.3346/jkms.2002.17.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahn SH, Han KH, Park JY, Lee CK, Kang SW, Chon CY, Kim YS, Park K, Kim DK, Moon YM. Association between hepatitis B virus infection and HLA-DR type in Korea. Hepatology. 2000;31:1371–1373. doi: 10.1053/jhep.2000.7988. [DOI] [PubMed] [Google Scholar]
- 9.Hwang SH, Sohn YH, Oh HB, Hwang CY, Lee SH, Shin ES, Lee KJ. Human leukocyte antigen alleles and haplotypes associated with chronicity of hepatitis B virus infection in Koreans. Arch Pathol Lab Med. 2007;131:117–121. doi: 10.5858/2007-131-117-HLAAAH. [DOI] [PubMed] [Google Scholar]
- 10.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotsch K, Wehling J, Blasczyk R. Sequencing of HLA class II genes based on the conserved diversity of the non-coding regions: Sequencing based typing of HLA-DRB genes. Tissue Antigens. 1999;53:486–497. doi: 10.1034/j.1399-0039.1999.530505.x. [DOI] [PubMed] [Google Scholar]
- 12.Van der Zwan A, Griffith B, Rozemuller E, Williams T, Tilanus MG. Sequence-based typing for HLA-DQB1 strategy for ABI sequencing equipment. In: Tilanus MGJ, Hansen JA, Hurley CK, editors. IHWG Technical Manual. Seattle: International Histocompatibility Working Group; 2000. pp. TM12A1–TM12A4. [Google Scholar]
- 13.Van der Zwan A, Rozemuller E, Tilanus MG. Sequence-based typing for HLA-DPB1 strategy for ABI equipment. In: Tilanus MGJ, Hansen JA, Hurley CK, editors. IHWG Technical Manual. Seattle: International Histocompatibility Working Group; 2000. pp. TM14A1–TM14A4. [Google Scholar]
- 14.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KW, Oh DH, Lee C, Yang SY. Allelic and haplotypic diversity of HLA-A, -B, -C, -DRB1, and -DQB1 genes in the Korean population. Tissue Antigens. 2005;65:437–447. doi: 10.1111/j.1399-0039.2005.00386.x. [DOI] [PubMed] [Google Scholar]
- 16.Thursz MR, Kwiatkowski D, Allsopp CE, Greenwood BM, Thomas HC, Hill AV. Association between an MHC class II allele and clearance of hepatitis B virus in the Gambia. N Engl J Med. 1995;332:1065–1069. doi: 10.1056/NEJM199504203321604. [DOI] [PubMed] [Google Scholar]
- 17.Höhler T, Gerken G, Notghi A, Lubjuhn R, Taheri H, Protzer U, Löhr HF, Schneider PM, Meyer zum Büschenfelde KH, Rittner C. HLA-DRB1*1301 and *1302 protect against chronic hepatitis B. J Hepatol. 1997;26:503–507. doi: 10.1016/s0168-8278(97)80414-x. [DOI] [PubMed] [Google Scholar]
- 18.Thio CL, Thomas DL, Karacki P, Gao X, Marti D, Kaslow RA, Goedert JJ, Hilgartner M, Strathdee SA, Duggal P, O'Brien SJ, Astemborski J, Carrington M. Comprehensive analysis of class I and class II HLA antigens and chronic hepatitis B virus infection. J Virol. 2003;77:12083–12087. doi: 10.1128/JVI.77.22.12083-12087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diepolder HM, Jung MC, Keller E, Schraut W, Gerlach JT, Grüner N, Zachoval R, Hoffmann RM, Schirren CA, Scholz S, Pape GR. A vigorous virus-specific CD4+ T cell response may contribute to the association of HLA-DR13 with viral clearance in hepatitis B. Clin Exp Immunol. 1998;113:244–251. doi: 10.1046/j.1365-2249.1998.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao T, Desombere I, Vanlandschoot P, Sallberg M, Leroux-Roels G. Characterization of HLA DR13-restricted CD4(+) T cell epitopes of hepatitis B core antigen associated with self-limited, acute hepatitis B. J Gen Virol. 2002;83:3023–3033. doi: 10.1099/0022-1317-83-12-3023. [DOI] [PubMed] [Google Scholar]
- 21.Van Hattum J, Schreuder GM, Schalm S. HLA antigens in patients with various courses after hepatitis B virus infection. Hepatology. 1987;7:11–14. doi: 10.1002/hep.1840070104. [DOI] [PubMed] [Google Scholar]
- 22.Begovich AB. HLA-DPA1, -DPB1, DQA1, and -DQB1 allele frequencies in a population from Gambia. Hum Immunol. 2004;65:944–945. [Google Scholar]
- 23.Thio CL, Carrington M, Marti D, O'Brien SJ, Vlahov D, Nelson KE, Astemborski J, Thomas DL. Class II HLA alleles and hepatitis B virus persistence in African Americans. J Infect Dis. 1999;179:1004–1006. doi: 10.1086/314684. [DOI] [PubMed] [Google Scholar]
- 24.Jiang YG, Wang YM, Liu TH, Liu J. Association between HLA class II gene and susceptibility or resistance to chronic hepatitis B. World J Gastroenterol. 2003;9:2221–2225. doi: 10.3748/wjg.v9.i10.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almarri A, Batchelor JR. HLA and hepatitis B infection. Lancet. 1994;344:1194–1195. doi: 10.1016/s0140-6736(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 26.Yang G, Liu J, Han S, Xie H, Du R, Yan Y, Xu D, Fan D. Association between hepatitis B virus infection and HLA-DRB1 genotyping in Shaanxi Han patients in northwestern China. Tissue Antigens. 2007;69:170–175. doi: 10.1111/j.1399-0039.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 27.Milner CM, Campbell D, Trowsdale J. Molecular genetics of the human major histocompatibility complex. In: Lechler R, Warrens A, editors. HLA in health and disease. London: Academic press; 2000. pp. 35–50. [Google Scholar]