Abstract
FLT3 mutations are common genetic changes, and are reported to have prognostic significance in acute myeloid leukemia (AML). The FLT3 internal tandem duplication (ITD) and the D835 activating mutation in the tyrosine kinase domain (TKD) were analyzed by polymerase chain reaction (PCR) in the genomic DNA of Korean patients with AML at diagnosis and during follow-up. There were 226 patients with AML enrolled between March 1996 and August 2005. The incidence of ITD and TKD at diagnosis was 13% (29/226) and 3% (6/226). When compared to Western and other Asian patients with AML, Korean patients had a lower frequency by about two-thirds of ITD and TKD. Among the non-M3 cases (N=203), the patients with an ITD had a significantly shorter event-free survival when compared with those without an ITD (p=0.0079). Among 54 relapsed patients, 9 patients had the FLT3 ITD at diagnosis. Six patients demonstrated a reappearance of the ITD and 3 patients remained negative at relapse. One patient, among 45 patients who relapsed, had a negative baseline ITD but acquired a de novo ITD at relapse. There were 101 samples from 93 patients in remission; they were all negative for an ITD. Among 34 patients who failed to achieve a remission, five patients had a persistent ITD and one patient had a de novo ITD. These results support the concept of resistance of FLT3 ITD leukemic clones to chemotherapy. Therefore, effective therapy with FLT3 targeting agents may improve the prognosis of non-M3 AML patients with the FLT3 mutation.
Keywords: FLT3 Mutations; Internal Tandem Duplication; Tyrosine Kinase Domain Mutation; Leukemia, Myeloid, Acute
INTRODUCTION
FLT3 mutations are common cytogenetic changes in acute myeloid leukemia (AML). Internal tandem duplication (ITD) of exon 14 or 15 is detected in 20-30% of patients (1-3) and a mutation of D835 in exon 20 in 7% of patients (4-7). Many clinical studies have shown that patients with an ITD at diagnosis have frequent disease relapse and a short duration of survival when compared to patients without an ITD (8-17). Two prior Korean trials included 165 adult (14) and 61 pediatric (16) patients with AML, and showed similar results; patients with an ITD at diagnosis had reduced event-free survival and similar overall survival when compared to patients without an ITD. The incidence of ITD in the 2 Korean studies was 35.2% and 6.6%, respectively (14, 16). A second mutation of FLT3 involves the tyrosine kinase domain (TKD) at D835. Its incidence is relatively low and its prognostic value has not been determined (8, 14, 18). However, one of several studies on TKD showed that the survival of patients with TKD at diagnosis was longer than those without TKD (18).
Several studies have investigated the variability of the FLT3 mutation in patients with AML during treatment (13, 17, 19-22). When compared to diagnosis, leukemic relapse occurred with persistent, newly developed or disappeared ITD. Because of the small number of patients in prior studies, the understanding of the FLT3 changes is incomplete. In addition, the role of the FLT3 status in disease remission or persistence during therapy has not been evaluated. Therefore, we studied FLT3 mutations (ITD and TKD) at diagnosis and evaluated their variability during disease remission relapse or persistence with therapy in Korean patients with AML.
MATERIALS AND METHODS
Eligibility
From March 1996 to August 2005, 226 patients from three centers were diagnosed with AML and underwent induction chemotherapy according to the center's protocol usually including anthracycline and cytarabine. Among them, 33 patients did not receive chemotherapy. Twenty-four patients did not receive chemotherapy due to co-morbid conditions or advanced age, and pretreatment mortality occurred in nine patients. All patients' bone marrow (BM) samples at diagnosis were available, but samples after induction were available for 111 patients. BM samples were classified into four groups: Group A samples at diagnosis, Group B first relapse or more, Group C first remission or more, and Group D first induction failure or more. The number of patient samples available at diagnosis and relapse was 54 for A/B, 91 for A/C and 28 for A/D. All patients provided informed consent and the individual institutional review boards at each center approved the study protocol.
Detection of FLT3 ITD and TKD
Mononuclear cells from the BM aspirate were isolated using Histopaque-1077 (Sigma, St. Louis, MO, U.S.A.) and then DNA was extracted using the QIAmp DNA Blood Mini Kit (Qiagen, Hilden, Germany). Polymerase chain reaction (PCR) for the ITD was carried out as described previously (1). We used a 100 ng sample of each patient's DNA, which was amplified in a 36-cycle PCR reaction at an annealing temperature of 56℃. We used 10 µM/L of two reverse primers (11R of 5'-ACTCTA AATTTTCTCT-3', and 12R of 5'-CTTTCAGCATTTTGACGGCAACC-3') for each reaction and 10 µM/ L of a common forward primer (3'-GCAATTTAGGTATGAAAGCCAGC-5'). The PCR for TKD was performed as described previously (3). We amplified exon 17 of the FLT3 gene by the genomic PCR method using the primers (17F of 5'-CCGCCAGGAACGTGCTTG-3', and 17R of 5'-GCAG-CCTCACATTGCCCC-3'). Amplified products were digested with Eco RV; then they were subjected to electrophoresis on an agarose gel.
Statistical analysis
The correlation of the clinical characteristics and the FLT3 ITD or TKD mutations at diagnosis was analyzed in 226 patients with AML, including or excluding those with M3 disease. Differences in the median variables of age, peripheral white blood cell (WBC) counts, platelet counts and the serum lactate dehydrogenase (LDH) concentration were analyzed by the Mann-Whitney U test. The analysis of data frequencies was performed using the Fisher's exact test for 2 × 2 tables or the chi-square test for larger tables. Survival probabilities were estimated by the Kaplan-Meyer method, and differences in the survival distributions were evaluated by the log-rank test. These statistical analyses were performed with SPSS software, version 13.0 (Chicago, IL, U.S.A.). For all analyses, the p values were 2-tailed, and a p value of less than 0.05 was considered statistically significant.
RESULTS
Clinical characteristics of AML patients with or without FLT3 mutations
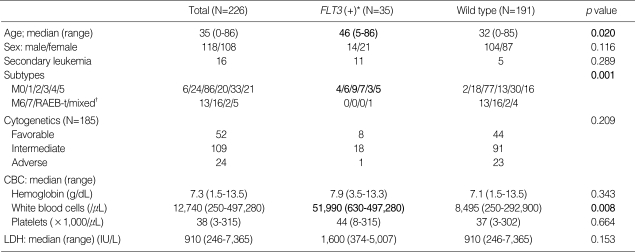
Among the 226 AML patients, 29 (12.8%) had an ITD, 6 (2.7%) had a TKD and 191 (84.5%) had neither (wild type). No patient had an ITD and TKD concomitantly. When compared to patients with the wild type, the patients with an ITD or a TKD were significantly older (p=0.020) and had higher WBC (p=0.008) counts. Their subtypes were most frequently from M0 to M5. The presence of FLT3 mutations was not related to gender, previous hematological disease, cytogenetic changes, or high serum LDH (Table 1).
Table 1.
Patient and disease characteristics according to FLT3 mutational status
*, FLT3 internal tandem duplication (ITD) or D835 point mutation of tyrosine kinase domain (TKD) was identified in this group; †, mixed means biphenotypic or bilineage leukemia.
RAEB-t, refractory anemia with excess blast in transformation; CBC, complete blood count; LDH, latacte dehydrogenase.
The effect of FLT3 mutations on remission and survival
Among the 193 patients receiving induction chemotherapy, 182 patients were available to evaluate for remission and 151 patients achieved complete remission; the remission rate (RR) was 78%. Nine patients died of therapy-related complications within 30 days. Among 22 patients with an ITD at diagnosis, 19 patients achieved remission. There was no significant difference in the RR between patients with an ITD and the wild type (p=0.651). Therefore, the presence of a FLT3 mutation did not influence the RR (p=0.824).
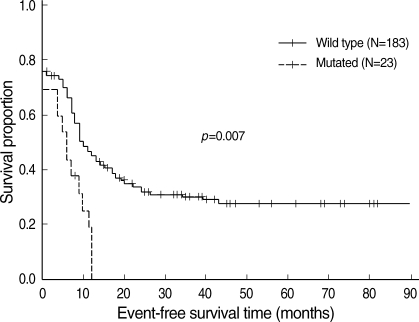
At a median follow-up of 61 (11-123) months, 90 of the 236 patients (39.8%) were still alive. The median overall survival (OS) was 14 (95% CI, 12-17), 14 (4-23) and 14 (10-18) months in all patients, those with an ITD and those with the wild type, respectively (p=0.530). The median event-free survival (EFS) was 10 (8-12), 7 (2-11) and 11 (7-14) months for all patients, those with an ITD and those with the wild type, respectively (p=0.094). When excluding the M3 types (N=20), comparison of the OS between the ITD and wild type patients showed no difference; however, the EFS with an ITD was significantly shortened (p=0.0079, Fig. 1). In the multivariate analysis in treated non-M3 patients (N=176), the significant factors influencing the OS and the EFS were cytogenetic risk group (23), the presence of a complete remission after the first induction and post-remission transplantation either autologous or allogeneic (Table 2). However, neither a FLT3 mutation nor an ITD affected survival in this analysis.
Fig. 1.
Event-free survival according to FLT3-ITD in 206 patients with non-M3 AML.
Table 2.
Multivariate analysis of the significant factors for event-free (EFS) and overall survival (OS) in treated non-M3 patients (N=176)
HR, hazard ratio; Cyto, cytogenetic 3 risk groups consisted of favorable, intermediate, and adverse risk groups; 1st CR, complete hematologic remission to the first induction treatment; SCT, stem cell transplantation.
Variability of the FLT3 mutation
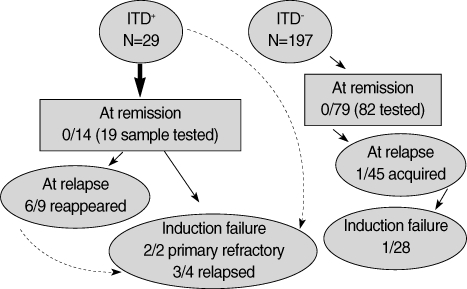
The changes of the FLT3 ITD are summarized in Fig. 2. At hematological remission, all samples were negative for an ITD even if there was an initially positive ITD (N=14). The ITD reappeared with relapse in 6 out of 9 patients, and was de novo in one of 45 patients. Induction failures with a persistent ITD were common. One patient had a lately occurred ITD at the second induction failure. The TKD changes were evaluated in patients from one center. Among 23 samples at relapse, two had a TKD. One patient had a TKD initially, and it reappeared on relapse. The other patient had an ITD only at diagnosis, and showed a newly appeared TKD at remission, which persisted at relapse and during the refractory period despite negative conversion of ITD.
Fig. 2.
Changes of FLT3-ITD during follow-up.
ITD+, FLT3-ITD positive, and ITD-, FLT3 wild type. A (numeral)/B (numeral) means a number of patients with FLT3-ITD/tested patients.
DISCUSSION
Our study showed a low frequency of FLT3 ITD and TKD, 12.8% and 2.7%, respectively, in Korean AML patients. This frequency was about two-thirds lower than reports from other Asian and Western patients. (1, 2, 4, 8-13, 19, 24). A recent Korean study on M3 disease showed a relatively low frequency of ITD and TKD, 12.0% and 9.3% (25). Another unpublished Korean report also showed a low frequency of the FLT3 mutation in AML patients, the ITD was 10.4% and the TKD was 9.1% (26). To determine the association with age, we separated the patients into 148 adults and 72 pediatric patients. The adult group had the FLT3 mutation in 16% of cases and the pediatric group in 6%. Previous reports showed the FLT3 mutation in 20-30% of adults and in 9-11.5% of pediatric aged patients (27, 28). Therefore, the information to date shows that the frequency of the FLT3 mutation, especially the ITD, is low in Korean AML patients in both the adult and pediatric age groups.
The prognostic value of the FLT3 mutation in AML was evaluated in a group of patients with normal karyotypes excluding those with known prognostic cytogenetic changes. About half of the AML patients had a normal karyotype or an intermediate cytogenetic risk (24, 29). The FLT3 ITD at diagnosis in these patients was associated with a significantly shortened EFS and OS (8-17). Therefore, intensified consolidation including transplantation or clinical trials targeting the FLT3 mutation should be considered in patients with an ITD (30, 31). The variability of the FLT3 ITD, during treatment in this study, supports a pattern of reappearance of the ITD with disease relapse and persistent ITD in refractory disease, which suggests chemo-resistance of the ITD-positive leukemic clones.
Our study included only 20 patients with M3. Recently several studies reported that M3 patients with ITD had a poor survival (25, 32). Clinical trials with FLT3-targeting agents to ATRA and anthracycline-based treatment may provide more information on the role of FLT3 in M3.
Although a more sensitive method for detection of ITD, such as the genescan technique, (21) was not used in our trial, all samples at remission were negative for ITD. In a follow-up to our study (data not included) genescan detected ITD in three out of 101 samples at remission. Therefore, future studies should use the more sensitive methods now available instead of the PCR.
In summary, this study included the largest number of Korean patients with AML to date, and showed a relatively low frequency of FLT3 ITD consistent with prior reports. ITD in Korean non-M3 AML patients was associated with poor EFS. In addition, the variability of ITD, during treatment follow-up, suggested chemo-resistance of the ITD-positive leukemic clones. Therefore, aggressive therapeutic approaches should be considered in AML patients with ITD at diagnosis.
ACKNOWLEDGMENTS
We particularly thank Hee Geun Kang, R.N. and Jung In Jue, R.N. for assistance with the data management.
References
- 1.Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, Sonoda Y, Fujimoto T, Misawa S. Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia. 1996;10:1911–1918. [PubMed] [Google Scholar]
- 2.Yokota S, Kiyoi H, Nakao M, Iwai T, Misawa S, Okuda T, Sonoda Y, Abe T, Kahsima K, Matsuo Y, Naoe T. Internal tandem duplication of the FLT3 gene is preferentially seen in acute myeloid leukemia and myelodyplastic syndrome among various hematological malignancies. A study on a large series of patients and cell lines. Leukemia. 1997;11:1605–1609. doi: 10.1038/sj.leu.2400812. [DOI] [PubMed] [Google Scholar]
- 3.Kiyoi H, Naoe T. FLT3 in human hematologic malignancies. Leuk Lymphoma. 2002;43:1541–1547. doi: 10.1080/1042819021000002866. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, Asou N, Kuriyama K, Yagasaki F, Shimazaki C, Akiyama H, Saito K, Nishimura M, Motoji T, Shinagawa K, Takeshita A, Saito H, Ueda R, Ohno R, Naoe T. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood. 2001;97:2434–2439. doi: 10.1182/blood.v97.8.2434. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Duhier FM, Goodeve AC, Wilson GA, Care RS, Peake IR, Reilly JT. Identification of novel FLT-3 Asp835 mutations in adult acute myeloid leukaemia. Br J Haematol. 2001;113:983–988. doi: 10.1046/j.1365-2141.2001.02850.x. [DOI] [PubMed] [Google Scholar]
- 6.Spiekermann K, Bagrintseva K, Schoch C, Haferlach T, Hiddemann W, Schnittger S. A new recurrent activating length mutation in exon 20 of the FLT3 gene in acute myeloid leukemia. Blood. 2002;100:3423–3425. doi: 10.1182/blood-2002-03-0953. [DOI] [PubMed] [Google Scholar]
- 7.Stirewalt DL, Radich JP. The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer. 2003;3:650–665. doi: 10.1038/nrc1169. [DOI] [PubMed] [Google Scholar]
- 8.Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, Wermke M, Bornhauser M, Ritter M, Neubauer A, Ehninger G, Illmer T. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood. 2002;99:4326–4335. doi: 10.1182/blood.v99.12.4326. [DOI] [PubMed] [Google Scholar]
- 9.Kiyoi H, Naoe T, Nakano Y, Yokota S, Minami S, Miyawaki S, Asou N, Kuriyama K, Jinnai I, Shimazaki C, Akiyama H, Saito K, Oh H, Motoji T, Omoto E, Saito H, Ohno R, Ueda R. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–3080. [PubMed] [Google Scholar]
- 10.Boissel N, Cayuela JM, Preudhomme C, Thomas X, Grardel N, Fund X, Tigaud I, Raffoux E, Rousselot P, Sigaux F, Degos L, Castaigne S, Fenaux P, Dombret H. Prognostic significance of FLT3 internal tandem repeat in patients with de novo acute myeloid leukemia treated with reinforced courses of chemotherapy. Leukemia. 2002;16:1699–1704. doi: 10.1038/sj.leu.2402622. [DOI] [PubMed] [Google Scholar]
- 11.Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA, Walker H, Wheatley K, Bowen DT, Burnett AK, Goldstone AH, Linch DC. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood. 2001;98:1752–1759. doi: 10.1182/blood.v98.6.1752. [DOI] [PubMed] [Google Scholar]
- 12.Kainz B, Heintel D, Marculescu R, Schwarzinger I, Sperr W, Le T, Weltermann A, Fonatsch C, Haas OA, Mannhalter C, Lechner K, Jaeger U. Variable prognostic value of FLT3 internal tandem duplications in patients with de novo AML and a normal karyotype, t(15;17), t(8;21) or inv(16) Hematol J. 2002;3:283–289. doi: 10.1038/sj.thj.6200196. [DOI] [PubMed] [Google Scholar]
- 13.Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C, Loffler H, Sauerland CM, Serve H, Buchner T, Haferlach T, Hiddemann W. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood. 2002;100:59–66. doi: 10.1182/blood.v100.1.59. [DOI] [PubMed] [Google Scholar]
- 14.Kim YK, Lee JJ, Lee YR, Lee IK, Lee HN, Kim NY, Park KS, Yang DH, Park MR, Kim HJ. The presence of FLT3/ITD mutations is an independent prognostic factor in acute myeloid leukemia patients with normal karyotype. Blood. 2004;104:3008a. [Google Scholar]
- 15.Moreno I, Martin G, Bolufer P, Barragan E, Rueda E, Roman J, Fernandez P, Leon P, Mena A, Cervera J, Torres A, Sanz MA. Incidence and prognostic value of FLT3 internal tandem duplication and D835 mutations in acute myeloid leukemia. Haematologica. 2003;88:19–24. [PubMed] [Google Scholar]
- 16.Kang HJ, Hong SH, Kim IH, Park BK, Han KS, Cho HI, Shin HY, Ahn HS. Prognostic significance of FLT3 mutations in pediatric non-promyelocytic acute myeloid leukemia. Leuk Res. 2005;29:617–623. doi: 10.1016/j.leukres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Nakano Y, Kiyoi H, Miyawaki S, Asou N, Ohno R, Saito H, Naoe T. Molecular evolution of acute myeloid leukaemia in relapse: unstable N-ras and FLT3 genes compared with p53 gene. Br J Haematol. 1999;104:659–664. doi: 10.1046/j.1365-2141.1999.01256.x. [DOI] [PubMed] [Google Scholar]
- 18.Mead A, Linch D, Hills R, Wheatley K, Burnett A, Gale R. Favourable prognosis associated with FLT3 tyrosine kinase domain mutations in AML in contrast to the adverse outcome associated with internal tandem duplications. Blood. 2005;106:334a. doi: 10.1182/blood-2006-04-015826. [DOI] [PubMed] [Google Scholar]
- 19.Shih LY, Huang CF, Wu JH, Lin TL, Dunn P, Wang PN, Kuo MC, Lai CL, Hsu HC. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: a comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood. 2002;100:2387–2392. doi: 10.1182/blood-2002-01-0195. [DOI] [PubMed] [Google Scholar]
- 20.Hovland R, Gjertsen BT, Bruserud O. Acute myelogenous leukemia with internal tandem duplication of the Flt3 gene appearing or altering at the time of relapse: a report of two cases. Leuk Lymphoma. 2002;43:2027–2029. doi: 10.1080/1042819021000015989. [DOI] [PubMed] [Google Scholar]
- 21.Tiesmeier J, Muller-Tidow C, Westermann A, Czwalinna A, Hoffmann M, Krauter J, Heil G, Ganser A, Serve H, Verbeek W. Evolution of FLT3-ITD and D835 activating point mutations in relapsing acute myeloid leukemia and response to salvage therapy. Leuk Res. 2004;28:1069–1074. doi: 10.1016/j.leukres.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Cloos J, Zwaan CM, Corthals SL, Goemans BF, Waisfisz Q, Creutzig U, Reinhardt D, Hahlen K, Kaspers GJL. FLT3/internal tandem duplication in paired presentation and relapse pediatric AML. Blood. 2004;102:334a. [Google Scholar]
- 23.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, Rees J, Hann I, Stevens R, Burnett A, Goldstone A. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The medical research council adult and children's leukaemia working parties. Blood. 1998;92:2322–2333. [PubMed] [Google Scholar]
- 24.Wang L, Lin D, Zhang X, Chen S, Wang M, Wang J. Analysis of FLT3 internal tandem duplication and D835 mutations in Chinese acute leukemia patients. Leuk Res. 2005;29:1393–1398. doi: 10.1016/j.leukres.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Yoo SJ, Park CJ, Jang S, Seo EJ, Lee KH, Chi HS. Inferior prognostic outcome in acute promyelocytic leukemia with alteration of FLT3 gene. Leuk Lymphoma. 2006;47:1788–1793. doi: 10.1080/10428190600687927. [DOI] [PubMed] [Google Scholar]
- 26.Kong SY, Ki CS, Lee SG, Hong SA, Kim JW, Kim SH. Analysis of FLT3-activiating mutations in 77 patients with acute myelogenous leukemia; association with WHO classification. Korean J Hematol. 2003;38:44a. [Google Scholar]
- 27.Zwaan CM, Meshinchi S, Radich JP, Veerman AJ, Huismans DR, Munske L, Podleschny M, Hahlen K, Pieters R, Zimmermann M, Reinhardt D, Harbott J, Creutzig U, Kaspers GJ, Griesinger F. FLT3 internal tandem duplication in 234 children with acute myeloid leukemia: prognostic significance and relation to cellular drug resistance. Blood. 2003;102:2387–2394. doi: 10.1182/blood-2002-12-3627. [DOI] [PubMed] [Google Scholar]
- 28.Lacayo NJ, Meshinchi S, Raimondi SC, Kuo DJ, Yu R, Chang MN, Willman CL, Tibshirani R, Ravindranath Y, Sikic BI, Weinstein, Dahl GV. FLT3 mutations determine the clinical outcome in children with de novo acute myelogenous leukemia (AML) and normal karyotype: Pediatric Oncology Group (POG) Study # 9421. Blood. 2004;104:570a. [Google Scholar]
- 29.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, Paietta E, Willman CL, Head DR, Rowe JM, Forman SJ, Appelbaum FR. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–4083. [PubMed] [Google Scholar]
- 30.Gale RE, Hills R, Pizzey AR, Kottaridis PD, Swirsky D, Gilkes AF, Nugent E, Mills KI, Wheatley K, Solomon E, Burnett AK, Linch DC, Grimwade D. NCRI Adult Leukaemia Working Party. Relationship between FLT3 mutation status, biologic characteristics, and response to targeted therapy in acute promyelocytic leukemia. Blood. 2005;106:3768–3776. doi: 10.1182/blood-2005-04-1746. [DOI] [PubMed] [Google Scholar]
- 31.Sternberg DW, Licht JD. Therapeutic intervention in leukemias that express the activated fms-like tyrosine kinase 3 (FLT3): opportunities and challenges. Curr Opin Hematol. 2005;12:7–13. doi: 10.1097/01.moh.0000147891.06584.d7. [DOI] [PubMed] [Google Scholar]
- 32.Kuchenbauer F, Schoch C, Kern W, Hiddemann W, Haferlach T, Schnittger S. Impact of FLT3 mutations and promyelocytic leukaemiabreakpoint on clinical characteristics and prognosis in acute promyelocytic leukaemia. Br J Haematol. 2005;130:196–202. doi: 10.1111/j.1365-2141.2005.05595.x. [DOI] [PubMed] [Google Scholar]