Abstract
Although it is not rare to find sputum that is positive acid-fast bacilli (AFB) smear but subsequent culture fails to isolate mycobacteria in clinical practice, the incidence and clinical implication of those sputa from new patients has not been clearly elucidated. The aim of this study was to determine the incidence and clinical implication of sputum with positive AFB smear but negative in mycobacterial culture. All sputa that were positive AFB smear requested during diagnostic work up for new patients visiting Seoul National University Hospital from 1 January 2005 through 31 December 2006 were included. Sputa producing a positive AFB smear but negative mycobacterial culture were classified into one of four categories: laboratory failure to isolate mycobacteria, false positive AFB smear, pathogen may show a positive AFB smear other than mycobacteria, and indeterminate results. Out of 447 sputa with a positive AFB smear, 29 (6.5%) failed to culture any organism. Among these 29 sputa, 18 were caused by laboratory failure to isolate mycobacteria, six were false positive smears, and five indeterminate. Although most sputum with a positive AFB smear but negative culture could be classified as a laboratory failure, clinicians should consider the possibility of false positive AFB smear.
Keywords: Tuberculosis; Diagnosis; Hospital Laboratories, Hospital
INTRODUCTION
Despite longstanding intense efforts to conquer pulmonary tuberculosis (TB), this disease remains an expanding global health crisis. According to the World Health Organization (WHO), about 8.8 million individuals develop active TB disease and 1.6 million die from TB every year (1).
To confirm the diagnosis of pulmonary TB, we should culture Mycobacterium tuberculosis from the sputum. However, because mycobacterial culture requires expensive equipment and the long turn-around time (2), the simpler and easier acid-fast bacilli (AFB) smear is used widely in the diagnosis of TB and evaluation of treatment responses.
In clinical practice, it is not rare to find sputum that is positive AFB smear but subsequent culture fails to isolate mycobacteria (3-7). Some sputum can be positive in the AFB smear but negative in mycobacterial culture because of nonviable bacilli during successful anti-TB medication, and the possibility of a false-positive AFB smear or alternative diagnosis other than mycobacterial diseases cannot be excluded (4, 6-8). However, the incidence and clinical meaning of sputum that is positive AFB smear but negative in mycobacterial culture from new patients has not been clearly elucidated. The aim of this study was to determine the incidence and clinical implication of sputum with positive AFB smear but negative in mycobacterial culture from new patients in a tertiary referral hospital in South Korea.
MATERIALS AND METHODS
Study populations
All sputa that were positive AFB smear requested during diagnostic work up for new patients visiting Seoul National University Hospital, a university-affiliated tertiary referral hospital, from 1 January 2005 through 31 December 2006 were included in this retrospective analysis. We checked the results of the mycobacterial culture of each sputum sample that was positive in the AFB smear. We also reviewed the demographic characteristics, clinical features, and radiographic findings of patients with a positive AFB smear but negative mycobacterial cultures. The protocol of this study was approved by the institutional review board of Seoul National University Hospital.
AFB smear and mycobacterial culture
Sputa were decontaminated with 4% NaOH, homonized, and concentrated with centrifugation (3,000 g, 20 min). The processed sediment was stained using the Ziehl-Neelsen method (9). The results of AFB smear was graded according to the American Thoracic Society/Center for Disease Control and Prevention (ATS/CDC) as follows (10): grade 0, no bacilli in 300 fields; trace, 1-2 bacilli in 300 fields; grade 1, 1-9 bacilli in 100 fields; grade 2, 1-9 bacilli in 10 fields; grade 3, 1-9 bacilli in 1 field; and grade 4, >9 bacilli in 1 field. Sputa with trace to grade 4 positive for AFB smear were included for the analysis. Cultures were performed using 3% Ogawa media with concentrated specimen and observed every weeks till 9 weeks after inoculation. Once cultured, differentiation between M. tuberculosis and nontuberculous mycobacteria was performed using Gen-probe method (Gen-Probe®, San Diego, CA, U.S.A.) (11). Sputum was considered contaminated if other bacteria without characteristics of mycobacteria were cultured overwhelmingly.
Classification of a positive AFB smear but with negative mycobacterial culture
Sputum producing a positive AFB smear but negative mycobacterial culture was classified into one of four categories based on clinical features and radiographic findings.
Laboratory failure to isolate mycobacteria
Sputum temporally between others with positive culture results in the same patient, or sputum from a patient in whom mycobacteria were cultured in samples obtained from other organs simultaneously.
False positive AFB smear
Sputum from a patient without any clinical evidence of active TB or nontuberculous mycobacterial (NTM) diseases and with a definite alternative diagnosis such as bacterial pneumonia. Patients included in this category were followed up at least 3 months.
Pathogen may show a positive AFB smear other than mycobacteria
A positive AFB smear resulting from other bacteria that may have a positive AFB smear, such as Nocardia, Actinomyces, Rhodococcus, Legionella micdadei or cysts of Cryptosporidium species (12, 13).
Indeterminate
Sputum from a patient with respiratory symptoms and radiographic lesions but for whom the diagnosis of mycobacterial diseases or other alternative diagnoses was not definite based on clinical features and treatment responses.
RESULTS
Incidence of sputum that is positive AFB smear but negative mycobacterial culture
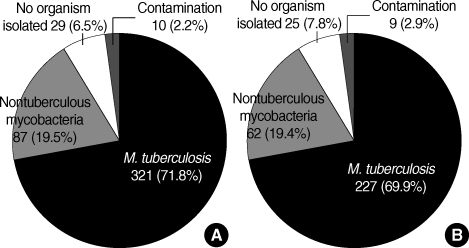
A total of 447 sputa from 319 patients were analyzed. Of these 447 sputa with a positive AFB smear, 321 (71.8%) yielded M. tuberculosis, 87 (19.5%) NTM, and 10 (2.2%) were contaminated during culture process. The other 29 (6.5%) failed to culture any organism (Fig. 1); these 29 sputa without mycobacterial culture were produced from 25 patients. In addition, 1 to 3 times of AFB smear with their sputa (median 2 times) were requested in these 25 patients.
Fig. 1.
Results of mycobacterial culture from 447 specimen with positive AFB smear in terms of (A) numbers of specimen and (B) numbers of patients.
Classification of positive AFB smear but negative mycobacterial culture
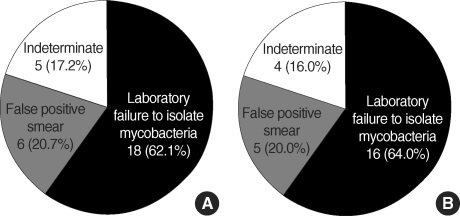
Of the 29 sputa that were positive in the AFB smears but negative in culture, 18 (62.1%) were caused by laboratory failure to isolate mycobacteria, six (20.7%) were false positive smears, and five (17.2%) were indeterminate. No disease was caused by a pathogen that showed a positive AFB smear other than pulmonary TB or NTM (Fig. 2). Among these 29 sputa, 11 were collected within one week of the initiation of anti-TB treatment. Nine out of them were classified as laboratory failures.
Fig. 2.
Classification of AFB smear among 29 sputa with negative mycobacterial culture in terms of (A) numbers of specimen and (B) numbers of patients.
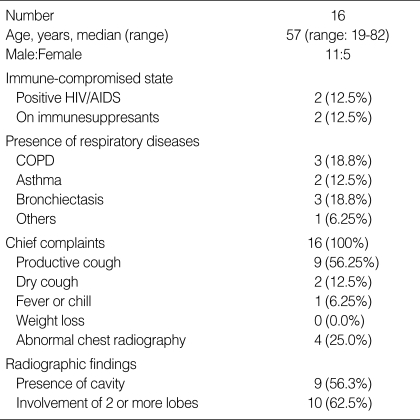
Ten of 16 patients with a laboratory failure to isolate mycobacteria had active pulmonary TB, and the other six patients had NTM lung diseases or colonization. Nine of these 16 patients had underlying lung diseases such as chronic obstructive pulmonary disease, asthma, and bronchiectasis (Table 1).
Table 1.
Demographic and clinical characteristics of 16 patients with laboratory failure to isolate mycobacteria
HIV, human immunodeficiency virus; AIDS, acquired immune deficiency syndrome; COPD, chronic obstructive pulmonary disease.
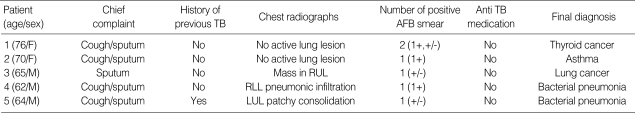
During the study period, five patients yielded 6 sputa with a false positive AFB smear. All of these patients complained of cough or sputum, although the cause of their symptoms was not pulmonary TB or NTM lung diseases. Asthma, lung cancer, pneumonia, or thyroid cancer infiltrating trachea could explain their symptoms (Table 2).
Table 2.
Clinical characteristics of 5 patients with sputa of false positive AFB smear
AFB, acid-fast bacilli; TB, tuberculosis; RUL, right upper lobe; LUL, left upper lobe.
Grade of the AFB smear in sputa with positive AFB sputa but negative mycobacterial culture
Of the 29 AFB smear-positive sputa that were negative in mycobacterial culture, the most common result was 1+ results in the AFB smear (nine sputa, 31.0%), followed by 2+ (seven sputa, 24.1%) and 3+ (seven sputa, 24.1%). Five (17.2%) sputa showed trace results, and one (3.6%) sputum revealed a 4+ positive result. The laboratory failure to isolated mycobacteria ranged from trace to 4+ positive results. However, false positive AFB smears were confined to below 2+ positive results.
DISCUSSION
The AFB smear is a rapid, economical, and practicable test for bacteriological diagnosis of TB. Because of the expensive equipment and long turn-around time for mycobacterial culture (2), we often rely on this procedure for the diagnosis and the evaluation of treatment responses. Although a concern has been raised about the low sensitivity of the AFB smear (14-16), less attention is given to the fact that a smear may appear positive without actually having mycobacteria (4, 17).
In our study, 29 (6.5%) of 447 sputa with a positive AFB smear during 2 yr failed to grow any organism in culture. This rate was in the range reported in previous studies (0.5-25.6%) (3, 4, 6, 8, 18, 19). Most sputa with a positive AFB smear but negative culture could be classified as a laboratory failure to isolated mycobacteria (18 sputa or 62.1%). However, false positive AFB smear were also observed in 6 sputa (20.7%) from 5 patients.
Eighteen sputa (62.1%) of the 29 positive AFB smears with negative culture specimens were classified as laboratory failure to isolated mycobacteria. Although the majority of these results were caused by technical failure during the mycobacterial culture, the possibility of non-culturable M. tuberculosis should be considered. Some bacteria may be viable but impossible to culture (20). This may also be applicable to M. tuberculosis (21). Tuberculous bacilli has low viability and may appear positive in a smear test because of the acid fast cell wall, but these organisms are still impossible to culture (22). In this context, some portion of the laboratory failure to isolated mycobacteria might be attributable to these kinds of Mycobacteria.
We also note the false positive AFB smear results observed in five patients without any evidence of pulmonary TB. Considering that all six sputa with false positive AFB smears from five patients were confined to 2+ positive results or less, the clinician should carefully interpret a weakly positive AFB smear in a patient with low probability of pulmonary TB. In fact, WHO recommends more stringent criteria for smear positivity than ATS/CDC does (9). The WHO classification 'scanty' matches ATS/CDC grade 1, and WHO '2+/3+' matches ATS/CDC grades 2, 3, and 4. Applying the WHO criteria instead of the ATS/CDC criteria for smear positivity reduces the numbers of false positive AFB smears; however, in that case, one must accept the lower sensitivity in the diagnosis of pulmonary TB based on a positive AFB smear.
Although other respiratory pathogens, such as Nocardia, Actinomyces, Rhodococcus, Legionella micdadei, and cyst of Cryptosporidium can cause positive AFB staining (12, 13, 23, 24), and one should be aware of the potential for misdiagnosis because of these organisms (25, 26), we found no false positive AFB smears caused by these organisms of the 447 sputa with positive AFB smears screened over two years. In this context, we believe that a false positive AFB smear is rarely caused by a micro-organism other than mycobacteria.
To appreciate these observation and analysis properly, several weak points of this study should be addressed. First of all, this study was performed retrospectively. Evaluation of patients prospectively with a same protocol could provide more reliable data. Second, complementary tests for AFB smear such as nucleic acid amplification of mycobacteria had not always used for sputa included in this study. If the results of complementary tests were available, the analysis of the data might be more accurate.
In conclusion, although most sputum with a positive AFB smear but negative culture could be classified as a laboratory failure to isolated mycobacteria, false positive AFB smear were also observed in patients without any clinical evidence of active pulmonary TB or NTM diseases. Clinicians should interpret these results cautiously in the context of the patient's clinical characteristics, radiographic findings, and grade of smear positivity.
References
- 1.WHO. WHO Report 2007. 2007. Global tuberculosis control. Surveillance, planning, financing. [Google Scholar]
- 2.Joh JS, Lee CH, Lee JE, Park YK, Bai GH, Kim EC, Han SK, Shim YS, Yim JJ. The interval between initiation of anti-tuberculosis treatment in patients with culture-positive pulmonary tuberculosis and receipt of drug-susceptibility test results. J Korean Med Sci. 2007;22:26–29. doi: 10.3346/jkms.2007.22.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warring FC, Jr, Sutramongkole U. Nonculturable acid-fast forms in the sputum of patients with tuberculosis and chronic pulmonary disease. Am Rev Respir Dis. 1970;102:714–724. doi: 10.1164/arrd.1970.102.5.714. [DOI] [PubMed] [Google Scholar]
- 4.Kothadia SN, Deshmukh S, Saoji AM. Evaluation of direct microscopy as a screening test in the diagnosis of pulmonary tuberculosis. Indian J Pathol Microbiol. 1990;33:68–73. [PubMed] [Google Scholar]
- 5.Rieder HL. Sputum smear conversion during directly observed treatment for tuberculosis. Tuber Lung Dis. 1996;77:124–129. doi: 10.1016/s0962-8479(96)90026-x. [DOI] [PubMed] [Google Scholar]
- 6.Vidal R, Martin-Casabona N, Juan A, Falgueras T, Miravitlles M. Incidence and significance of acid-fast bacilli in sputum smears at the end of antituberculous treatment. Chest. 1996;109:1562–1565. doi: 10.1378/chest.109.6.1562. [DOI] [PubMed] [Google Scholar]
- 7.Al-Moamary MS, Black W, Bessuille E, Elwood RK, Vedal S. The significance of the persistent presence of acid-fast bacilli in sputum smears in pulmonary tuberculosis. Chest. 1999;116:726–731. doi: 10.1378/chest.116.3.726. [DOI] [PubMed] [Google Scholar]
- 8.Kim TC, Blackman RS, Heatwole KM, Kim T, Rochester DF. Acid-fast bacilli in sputum smears of patients with pulmonary tuberculosis. Prevalence and significance of negative smears pretreatment and positive smears post-treatment. Am Rev Respir Dis. 1984;129:264–268. [PubMed] [Google Scholar]
- 9.Laboratory services in tuberculosis control, Part II: Microscopy. Geneva, Switzerland: 1998 World Health Organization; [Google Scholar]
- 10.American Thoracic Society. Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 11.Bergmann JS, Yuoh G, Fish G, Woods GL. Clinical evaluation of the enhanced Gen-Probe Amplified Mycobacterium Tuberculosis Direct Test for rapid diagnosis of tuberculosis in prison inmates. J Clin Microbiol. 1999;37:1419–1425. doi: 10.1128/jcm.37.5.1419-1425.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaude GS, Hemashettar BM, Bagga AS, Chatterji R. Clinical profile of pulmonary nocardiosis. Indian J Chest Dis Allied Sci. 1999;41:153–157. [PubMed] [Google Scholar]
- 13.Talamo TS, Norbut AM, Kessler GF., Jr Opportunistic pneumonia caused by a new acid-fast bacterium. Am J Clin Pathol. 1980;74:842–845. doi: 10.1093/ajcp/74.6.842. [DOI] [PubMed] [Google Scholar]
- 14.Kanaujia GV, Lam PK, Perry S, Brusasca PN, Catanzaro A, Gennaro ML. Integration of microscopy and serodiagnostic tests to screen for active tuberculosis. Int J Tuberc Lung Dis. 2005;9:1120–1126. [PubMed] [Google Scholar]
- 15.Frimpong EH, Adukpo R, Owusu-Darko K. Evaluation of two novel Ziehl-Neelsen methods for tuberculosis diagnosis. West Afr J Med. 2005;24:316–320. doi: 10.4314/wajm.v24i4.28224. [DOI] [PubMed] [Google Scholar]
- 16.Laserson KF, Yen NT, Thornton CG, Mai VT, Jones W, An Do, Phuoc NH, Trinh NA, Nhung DT, Lien TX, Lan NT, Wells C, Binkin N, Cetron M, Maloney SA. Improved sensitivity of sputum smear microscopy after processing specimens with C18-carboxypropylbetaine to detect acid-fast bacilli: a study of United States-bound immigrants from Vietnam. J Clin Microbiol. 2005;43:3460–3462. doi: 10.1128/JCM.43.7.3460-3462.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipsky BA, Gates J, Tenover FC, Plorde JJ. Factors affecting the clinical value of microscopy for acid-fast bacilli. Rev Infect Dis. 1984;6:214–222. doi: 10.1093/clinids/6.2.214. [DOI] [PubMed] [Google Scholar]
- 18.Ito K. Pseudo-recurrence of lung tuberculosis based on the detection of smear AFB positive sputum due to excretion of necrotic material. Kekkaku. 2004;79:449–451. [PubMed] [Google Scholar]
- 19.Daddi G, Lucchesi M, Zubiani M. Some remarks on the recent bacteriological research on tuberculosis. Indian J Chest Dis Allied Sci. 1982;24:164–169. [PubMed] [Google Scholar]
- 20.Salina EG, Vostroknutova GN, Shleeva MO, Kaprel'iants AS. Cell-cell interactions during formation and reactivation of "nonculturable" mycobacteria. Mikrobiologiia. 2006;75:502–508. [PubMed] [Google Scholar]
- 21.Biketov S, Mukamolova GV, Potapov V, Gilenkov E, Vostroknutova G, Kell DB, Young M, Kaprelyants AS. Culturability of Mycobacterium tuberculosis cells isolated from murine macrophages: a bacterial growth factor promotes recovery. FEMS Immunol Med Microbiol. 2000;29:233–240. doi: 10.1111/j.1574-695X.2000.tb01528.x. [DOI] [PubMed] [Google Scholar]
- 22.Shleeva M, Mukamolova GV, Young M, Williams HD, Kaprelyants AS. Formation of 'non-culturable' cells of Mycobacterium smegmatis in stationary phase in response to growth under suboptimal conditions and their Rpf-mediated resuscitation. Microbiology. 2004;150:1687–1697. doi: 10.1099/mic.0.26893-0. [DOI] [PubMed] [Google Scholar]
- 23.Rogers BH, Donowitz GR, Walker GK, Harding SA, Sande MA. Opportunistic pneumonia: a clinicopathological study of five cases caused by an unidentified acid-fast bacterium. N Engl J Med. 1979;301:959–961. doi: 10.1056/NEJM197911013011802. [DOI] [PubMed] [Google Scholar]
- 24.Clavel A, Arnal AC, Sanchez EC, Cuesta J, Letona S, Amiguet JA, Castillo FJ, Varea M, Gomez-Lus R. Respiratory cryptosporidiosis: case series and review of the literature. Infection. 1996;24:341–346. doi: 10.1007/BF01716076. [DOI] [PubMed] [Google Scholar]
- 25.Hilton E, Freedman RA, Cintron F, Isenberg HD, Singer C. Acid-fast bacilli in sputum: a case of Legionella micdadei pneumonia. J Clin Microbiol. 1986;24:1102–1103. doi: 10.1128/jcm.24.6.1102-1103.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subhash HS, Christopher DJ, Roy A, Cherian AM. Pulmonary nocardiosis in human immunodeficiency virus infection: a tuberculosis mimic. J Postgrad Med. 2001;47:30–32. [PubMed] [Google Scholar]