Abstract
It has been well established that bacterial superantigens lead to the induction and aggravation of chronic inflammatory skin diseases. We investigated the clinical significance of serum specific immunoglobulin E (lgE) to the staphylococcal superantigens staphylococcal enterotoxin A (SEA), staphylococcal enterotoxin B (SEB), and toxic shock syndrome toxin (TSST)-1 in patients with chronic urticaria (CU), focusing on the differences in these prevalences between aspirin-intolerant CU (AICU) and aspirin-tolerant CU (ATCU) patients. Aspirin sensitivity was confirmed by oral aspirin provocation test. There were 66 patients AICU and 117 patients ATCU in the study. Serum IgE antibodies specific for SEA, SEB, and TSST-1 were measured by the ImmunoCAP test and the patients were compared with 93 normal controls (NC). The prevalences of serum specific IgE to staphylococcal superantigens were significantly higher in CU than in NC patients (IgE to SEA, 13.7% vs. 5.4%; IgE to SEB, 12.0% vs. 4.3%; IgE to TSST-1, 18.0% vs. 6.5%; p< 0.05, respectively). The patients with specific IgE to SEA, SEB, and TSST-1 had higher serum total IgE levels and higher rates of atopy. Significant associations were noted between the prevalence of specific IgE to SEA and SEB and the HLA DQB1*0609 and DRB1*1302 alleles in the AICU group. We confirmed that a sub-population of patients with CU possesses serum IgE antibodies to SEA, SEB, and TSST-1. Particularly, the IgE immune response to TSST-1 is associated with aspirin sensitivity in CU patients.
Keywords: Aspirin Hypersensitivity, Specific IgE, Superantigens, Chronic Urticaria
INTRODUCTION
Chronic urticaria (CU) is a common skin disorder that is characterized by a 6-week or longer history of widespread, transient, itchy cutaneous swellings, with individual lesions lasting less than 24 hr (1). The pathogenic mechanisms of CU are still poorly understood. However, it is now widely accepted that CU is triggered by multiple factors, which include autoreactivity to the high affinity immunoglobulin E receptor (FcεR1α) or to immunoglobulin E (IgE) itself (1, 2), genetic predisposition, (3, 4) hypersensitivity to drugs or food additives (5, 6), infectious processes (7), malignancy, and endocrine diseases (8).
Aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) aggravate 20-30% of patients with CU (6). The pathogenesis of aspirin sensitivity probably involves selective inhibition of cyclooxygenase-1 and increased leukotriene synthesis. The prevalence of atopy and the levels of serum total IgE in NSAID-intolerant patients was found to be increased in previous challenge-proven studies (9).
Superantigens, predominantly those derived from Staphylococcus aureus, can activate T cells, induce IgE synthesis by B cells, and directly affect the activities of inflammatory leukocytes, such as eosinophils. Specific IgE to staphylococcal superantigens has recently been associated with the pathogenesis of various allergic conditions, e.g., atopic dermatitis (AD), nasal polyposis, and allergic rhinitis (10, 11). In atopic dermatitis, >50% of the patients are colonized on the skin by superantigen-secreting S. aureus strains. Furthermore, patients with AD often mount a systemic IgE immune response against these exotoxins that correlates with disease severity (12, 13). Antibodies to staphylococcal antigens have been found in chronic urticaria (14). Potaczek et al. (15) have reported that 11 out of 34 allergic patients, including those with urticaria and asthma, had serum IgE antibodies against the staphylococcal enterotoxins staphylococcal enterotoxin A (SEA), staphylococcal enterotoxin B (SEB), and toxic shock syndrome toxin (TSST)-1. However, there are no published data on the prevalence of specific IgE antibodies against staphylococcal superantigens in CU patients or on the association between the presence of such antibodies and the clinical phenotype of CU.
Histologic analysis of the lesions of CU patients reveals perivascular infiltrates that contain mononuclear cells, especially CD4+ T cells, as well as variable numbers of neutrophils and eosinophils (16, 17). These findings are very similar to those observed in the allergen-induced skin late-phase reaction, with the exception of increased IFN-γ expression, irrespective of the presence of anti-FcεR1α or anti-IgE autoantibodies (17, 18). Notably, recent studies have indicated that even the uninvolved skin of CU subjects presents an apparently latent inflammatory state that is characterized by lymphocytic and granulocytic infiltrates (19). Sperantigens function by simultaneously interacting with class II major histocompatibility complex (MHC) and T cell receptor (TCR) molecules on antigen presenting cells and T lymphocytes, respectively. Considering engagement of TCR and MHC molecules by superantigens is quite different from the group of which superantigens belong to. This study was aimed also to find any associations between specific IgE to superantigens and HLA markers of aspirin senstitivity in patients with CU (20).
Therefore, we evaluated the clinical significance of serum specific IgE to the staphylococcal superantigens SEA, SEB, and TSST-1 in patients with CU, focusing on the differences in these prevalences between aspirin-intolerant CU (AICU) and aspirin-tolerant CU (ATCU) patients.
MATERIALS AND METHODS
Subjects
This study included 183 CU patients (89 males and 94 females, age range 15-74 yr mean age 39 yr) who were followed in the outpatient Allergy & Rheumatology Clinics of Ajou University Medical Center. The AICU were defined as patients who had a definite history of urticaria/angioedema development after the ingestion of more than two types of NSAID and positive responders in the oral aspirin challenge test, classified as the cross-reacting group by Sanchez-Borges et al. (9) In addition, NSAID sensitivity could be confirmed, as the patients presented at our Allergy Clinics or emergency rooms with current urticaria/angioedema after taking NSAIDs. Patients who manifested both aspirin-intolerant asthma (AIA) and aspirin intolerant urticaria (AIU) were excluded from this study. In all, 93 normal control (NC) subjects (39 males and 54 females, age range 18-67 yr, mean age 35 yr) who had no personal or family history of allergic diseases, and no past history of aspirin or other drug hypersensitivity, were recruited from the general population. Informed consent was obtained from each patient and the study protocol was approved by the ethics committee of Ajou University Medical Center, Suwon, Korea.
Allergy skin prick test
Skin prick tests were performed with 50 common aeroallergens (Bencard, Brentford, U.K.), which included Dermatophagoides pteronyssinus, D. farine, cat, dog, cockroach, tree pollen mixture, grass pollen mixture, mugwort, ragweed, Hummulus japonicus, Aspergillus, Alternaria, histamine, and a saline control. The reactions were read after 15 min, and the wheal was measured in two directions. A positive reaction was defined as a mean wheal diameter of ≥3 mm. Atopy was defined as a positive skin test response to at least one common inhalant allergen.
Oral provocation test with aspirin
All medications, including anti-histamines, steroids, and leukotriene receptor antagonists, were stopped 72 hr before the procedure. After the placebo challenge, 500 mg of aspirin (Rhonal®; KunWha Pharmaceutical Co., Seoul, Korea) in tablet form was administered orally, and the patients were observed for 4 hr, with monitoring of urticaria, angioedema, and changes in lung function every 30 min. The appearance of urticaria within 4 hr without any change in FEV1 was considered a positive result.
Measurements of total and specific IgE
The levels of total IgE and specific IgE directed against SEA, SEB, and TSST-1 were measured by the ImmunoCAP system (Pharmacia Diagnostics, Uppsala, Sweden) according to the manufacturer's instructions.
Genomic DNA preparation and high-resolution human leukocyte antigen (HLA) genotyping
HLA DRB1 and DQB1 typing was carried out by direct DNA sequencing only in AICU group. The procedures and primers for PCR amplification and sequencing of the HLA-DRB1 and DQB1 alleles have been described previously (21).
Statistical analysis
Intergroup comparisons of the prevalences of specific IgE antibodies to SEA, SEB, and TSST-1, and for categorical clinical parameters, were performed using the chi-square test or Fisher's exact tests, with adjustments for multiple comparisons. Analysis of variance (ANOVA) followed by post-hoc Dunnett's T3 was applied to compare the demographic data and mean levels of total IgE among the three study groups. Continuous variables that did not have a normal distribution, such as the levels of serum total IgE, were log-transformed. For comparisons of clinical parameters and HLA markers according to the results of specific IgE against superantigens in patients with AICU, the chi-square test or Fisher's exact test was used. A p value ≤0.05 was regarded as being statistically significant. The statistical analyses were performed using the SPSS 12.0 for Windows software (SPSS Inc., Chicago, IL, U.S.A.).
RESULTS
Clinical characteristics of the study subjects
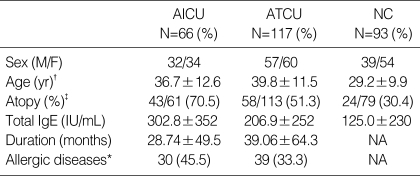
For the AICU patients, the atopy rate and the mean total serum IgE level were 70.5% and 302.8 IU/mL, respectively, which were significantly higher than the values for the ATCU patients (p=0.016 and p=0.043, respectively). However, there was no significant difference in the prevalence of allergic diseases and the duration of urticaria according to aspirin sensitivity. The characteristics of the subjects are shown in Table 1.
Table 1.
Demographics of the study groups
*Allergic diseases indicate that the subject had one or more of associated diseases including allergic asthma and allergic rhinitis; †, p<0.05 for NC vs. AICU or ATCU (ANOVA); ‡, p<0.016 for AICU vs. ATCU and p<0.01 for NC vs. AICU or ATCU (chi-square test).
AICU, aspirin-intolerant chronic urticaria; ATCU, aspirin-tolerant chronic urticaria; NC, normal control; IgE, immunoglobin E; NA, not assessable.
Prevalences of superantigen-specific IgE
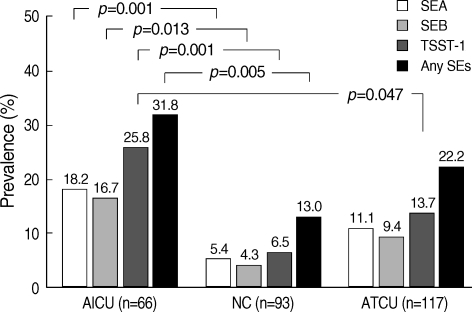
Specific IgE for SEA, SEB, and TSST-1 was detected in 14.6%, 13.1%, and 19.8% of the CU patients and in about 5% of the NC patients (p=0.041, p=0.049, and p=0.001, respectively). When the prevalences of specific IgE to the three staphylococcal superantigens were compared among the ATCU, AICU, and NC groups, the highest values were noted for the AICU patients (18.2% for SEA, 16.7% for SEB, 25.8% for TSST-1), followed by ATCU (11.1% for SEA, 9.4% for SEB, 13.7% for TSST-1), and NC (5.4% for SEA, 4.3% for SEB, 6.5% for TSST-1). Moreover significant differences were noted between the AICU and NC groups (p=0.001 for SEA and TSST-1, p=0.013 for SEB; Fig. 1). The specific IgE to TSST-1 was the most prevalent in AICU patients, and statistical significance was noted in comparison with ATCU patients (p=0.047). However, no significant difference in the prevalence of specific serum IgE to superantigens was noted in the comparison of the ATCU and NC groups.
Fig. 1.
Prevalences of serum specific IgE to staphylococcal superantigens When the prevalences of specific IgE to the three staphylococcal superantigens were compared among the AICU, ATCU and NC groups, the highest values were noted for the AICU patients (18.2% for SEA, 16.7% for SEB, 25.8% for TSST-1, 31.8% for any SEs). Moreover significant differences were noted between the AICU and NC groups (p=0.001 for SEA and TSST-1, p=0.013 for SEB). The specific IgE to TSST-1 was the most prevalent in AICU patients, and statistical significance was noted in comparison with ATCU patients (p=0.047).
AICU, aspirin-intolerant chronic urticaria; ATCU, aspirin-tolerant chronic urticaria; NC, normal control; SEA, staphylococcal enterotoxin A; SEB, staphylococcal enterotoxin B; TSST-1, toxic shock syndrome toxin-1; SEs, staphylococcal enterotoxins; NS, not significant.
Stratification for sensitization to staphylococcal enterotoxins
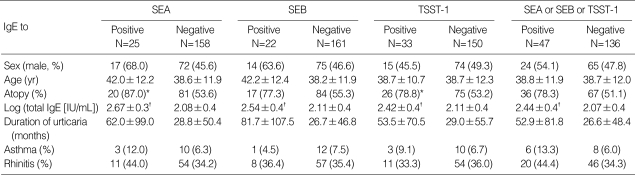
Irrespective of their AICU and ATCU statuses, all 183 subjects were stratified for the presence or absence of serum IgE specific for SEA, SEB, and TSST-1 (Table 2). Subjects who were positive for IgE specific for three superantigens did not differ from those who were negative for each superantigen-specific IgE in terms of gender ratio, age, and the duration of urticaria. However, the subjects with IgE to superantigens had remarkably higher levels of total IgE (p<0.001, for all three superantigens). The subjects who had specific IgE to SEA or TSST-1 had significantly higher atopy rates compared to those subjects who were negative for specific IgE to SEA or TSST-1 (p=0.003 for SEA and p=0.010 for TSST-1).
Table 2.
Comparison of clinical parameters according to specific IgE to Staphylococcal superantigens in 183 chronic urticaria patients
*p<0.05 for positive IgE to SEA and TSST-1 compared with the respective negative counterpart (chi-square test); †p<0.001 for positive IgE to SEA, SEB, and TSST-1 compared with the respective negative counterpart (chi-square test).
SEA, staphylococcal enterotoxin A; SEB, staphylococcal enterotoxin B; TSST-1, toxic shock syndrome toxin-1.
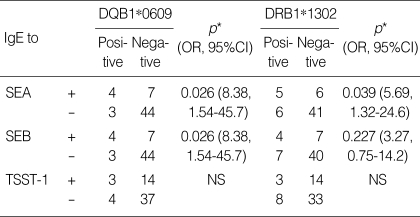
Association between IgE to staphylococcal enterotoxins and the presence of HLA DQB1*0609 and DRB1*1302 alleles in patients with AICU
The presence of specific IgE to SEA was significantly associated with the presence of HLA DQB1*0609 (p=0.026, odds ratio=8.38) and HLA DRB1*1302 (p=0.039, odds ratio [OR], 5.69) in AICU patients (Table 3). A significant association was also noted for specific IgE to SEB and HLA DQB1*0609 (p=0.026, OR, 8.38). However, no significant associations were found between the presence of IgE to TSST-1 and HLA DRB1*1302 or DQB1*0609 (p>0.05 in each case).
Table 3.
Association between HLA alleles and specific IgE to staphylococcal superantigens in 58 aspirin-intolerant chronic urticaria (AICU) patients
*p values were determined by the chi-square test with continuity correction or Fisher's exact test.
SEA, staphylococcal enterotoxin A; SEB, staphylococcal enterotoxin B; TSST-1, toxic shock syndrome toxin-1; HLA, human leukocyte antigen; IgE, immunoglobulin E; OR, odds ratio; CI, confidence interval; NS, not significant.
DISCUSSION
This study is the first to demonstrate that the sera from 25.7% of CU patients contain specific IgE for one or more of the staphylococcal superantigens SEA, SEB, and TSST-1. In particular, compared with healthy controls, a significantly greater proportion of the AICU patients had serum IgE to either enterotoxins or TSST-1. However, some of the ATCU patients had specific IgE to superantigens, the prevalences of which did not differ from those of normal controls. With respect to IgE to individual staphylococcal toxins, IgE to TSST-1 was most prevalent in all three groups. A significant difference was noted for the prevalence of IgE to TSST-1 in AICU patients, compared with NC or ATCU patients. The evidence of IgE antibodies to these staphylococcal superantigens in the sera of CU patients, and the notable prevalence of these antibodies in the AICU group, suggest a link between allergy and infection in this disease. Since HLAs play a major role in regulating immune responses, a significant association between IgE to SEA or SEB and AICU-related HLA markers, which include HLA DQB1*0609 and DRB1*1302, strengthen this hypothesis. However, IgE to TSST-1, which was the most prevalent form in AICU patients, was not associated with these two HLA alleles. Given that we selectively targeted these two HLA alleles based on a previous study on the association of HLA alleles with aspirin-intolerant urticaria in a Korean population (21), it is possible that the IgE immune response to TSST-1 is influenced by other HLA molecules. As proven that more than 30 distinct superantigens are identified. Although they share a conserved tertiary structure, there exist key differences in how the characterized representatives for each superantigen engage their host receptors. Among superantigens, TSST-1 is the unique in that it binds MHC through an N-terminal, low-affinity binding domain that is peptide-dependent. However, this study has some limitations to identify the association of specific IgE to staphylococcal superantigens and their recognition by immune system. The first our analysis was performed about two HLA markers, HLA DRB1*1302 and HLA DQB1*0609. The second, only the subjects with AICU group were involved in genotyping of HLA markers. Therefore, further study involving other HLA markers particularly associated with TSST-1 and expanded patients population to ATCU would be necessary to clarify the association between HLA markers and IgE immune response to staphylococcal superantigens.
The potential role of superantigens in immune-mediated inflammatory skin disease is an area of intense investigation. Superantigens potently and specifically activate a large proportion of T cells, resulting in the release of T-helper (Th) 2 cytokines as well as increased IgE synthesis and eosinophilia (22). Leung et al. (23) have demonstrated that 57% of AD patients have circulating IgE to staphylococcal superantigens and that their own basophils release histamine upon exposure to the relevant enterotoxins but not in response to enterotoxins, against which there is no IgE response. While colonization by S. aureus has been reported for 2-25% of normal skin sites (24) and patients with psoriasis experience frequent exacerbation due to staphylococcal infection, IgE specific for staphylococcal enterotoxins has not been detected in the sera of these patients (23). These findings support the notion that IgE antibodies to superantigens induce the degranulation of mast cells and basophils, with consequent release of mediators, cytokines, and chemokines, which results in sustained tissue inflammation in a subpopulation of CU patients, as seen for a subset of AD patients. However, our preliminary experiment which evaluated the degree of colonization with S. aureus from the skin of AICU patients (n=13) showed no significant difference from that of healthy controls (n=9) (p>0.05, Fisher's exact test). We performed bacterial swabs from the skin and the anterior nares of subjects. Three of the patients with AICU harbored S. aureus in their nares and none of them had cutaneous colonization of the strain. Among healthy controls, three harbored the strain in their nares and two had cutaneous colonization. But, there was no significant association between the degree of colonization and the presence of serum IgE specific for staphylococcal superantigens (data not shown), suggesting that skin colonization of S. aureus could not affect on IgE sensitization to staphylococcal superantigens in AICU patients.
In the present study, the patients with IgE specific for staphylococcal superantigens had higher total IgE levels and higher rates of atopy than patients without serum IgE specific for superantigens. These significant differences in total IgE levels and atopy rates were found in comparisons of AICU and ATCU patients. On that point, this study has some limitations that the atopic status of patients might affect on IgE sensitization to staphylococcal superantigens than the effect of aspirin sensitivity. However, after adjustment for the potential confounding effect of total serum IgE levels and atopic status, the prevalence of IgE specific for TSST-1 was still significantly associated with the AICU phenotype. Moreover, there were no significant differences in the prevalence of allergic diseases including allergic asthma, allergic rhinitis and atopic dermatitis between AICU and ATCU groups in this study. We could not find any significant difference in the prevalence of allergic diseases according to the presence or absence of specific IgE to superantigens. These results suggested that in CU subjects their accompanying allergic diseases did not affect on IgE immune responses to staphylococcal superantigens.
There is no evidence to suggest that aspirin sensitivity is directly related to an immune response to superantigens, previous studies on the pathogenesis of nasal polyposis in aspirin-sensitive subjects provide some clues (25, 26). We have previously demonstrated the presence of IgE specific for staphylococcal superantigens in nasal polyp tissues, and shown that the IgE levels correlate with eosinophil-related markers. Moreover, the levels of superantigen-specific IgE were markedly elevated in the nasal polyps of subjects with AIA, as compared to patients with aspirin-tolerant asthma (ATA) (26). Recently, it has been reported that cysteinyl leukotriene production is up-regulated in the polyp tissues of patients with IgE to superantigens, and that these levels correlate with the presence of an eosinophilic inflammation marker and specific IgE to staphylococcal enterotoxins (27). On the other hand, histologic examinations of CU patients have demonstrated perivascular infiltrations of mixed Th1/ Th2 cells, eosinophils, and increased IL-4, IL-5, and IFN-γ mRNA expression, similar to what has been observed in chronic AD skin (18). Furthermore, deposition of major basic protein (MBP) and eosinophil cationic protein (ECP) has been observed outside of eosinophils in skin specimens from patients with CU, as has also been seen for involved tissue specimens from patients with various allergic diseases, including asthma, AD, and nasal polyps. Though this study could not evaluate how the presence of specific IgE to staphylococcal superantigens modified the clinical course, such as the severity or exacerbation rate, of CU, because there has been no standard severity index for patients with CU. Taken together, our results suggest that IgE directed against staphylococcal superantigens trigger and perpetuate eosinophilic and T cell-mediated allergic inflammation in some CU patients, as has been observed previously for patients with AD, nasal polyps, and asthma.
Various studies have indicated that urticaria has a complex physiopathology that involves multiple inflammatory pathways. However, it is generally considered that the interaction between IgE-bound mast cells and allergens is unlikely to be the mechanism by which urticaria develops in most patients. Most studies on the pathogenesis of CU have focused on the autoimmune mechanisms and features, including the anti-FcεR1α, anti-IgE, and anti-thyroid autoantibodies. However, these autoimmune responses have been documented in only about 50% of patients with CU. Previous studies have indicated that the atopic condition may represent an important risk factor for the development or aggravation of urticaria in response to aspirin or NSAIDs. In particular, the results of our recent study on FcεR1α promoter polymorphisms suggest that prolonged exposure to IgE induces the persistent accumulation and activation of mast cells and basophils via increased surface expression of high-affinity IgE receptors in AICU patients (28). Furthermore, recent studies have demonstrated that TSST-1, which is a prototypic superantigen, has an adjuvant effect on IgE synthesis through several mechanisms, such as the up-regulation of the B7.2 molecule and CD40 ligand on B and T cells, respectively (29, 30). TSST-1 is unique among staphylococcal toxins in its ability to cross mucosal surfaces, due its high content of hydrophobic amino acids (31). This is consistent with the results of the present study in that IgE specific for TSST-1 was the most prevalent IgE type in the urticaria groups. In this regard, aspirin sensitivity may have synergistic effects on leukotrienes and eosinophil-mediated inflammation, as well as on mast cell activation in CU patients who carry serum IgE to TSST-1.
From the literature, it is clear that the prevalence of infections, whether bacterial, viral, parasitic or fungal, appears not different from the general population. However, there are several reports that demonstrate the benefit of eradicating the infectious process (7). As data on the beneficial effects for AICU patients of leukotriene receptor antagonist in combination with antihistamines is increasing, the use of antibiotics to decrease staphylococcal colonization, with concomitant reductions in the levels of staphylococcal toxins, may be useful in the treatment of CU patients with serum IgE to superantigens.
In conclusion, we have identified a subset of CU patients with serum IgE antibodies to staphylococcal superantigens. Although the statistical significance was not found, the prevalences of specific IgE to superantigens were higher in patients with AICU than in ATCU patients. We propose that the IgE immune response to TSST-1 is associated with aspirin sensitivity in CU patients. Further study is necessary to confirm how these IgE antibodies with specificity for staphylococcal superantigens are involved in the pathogenesis of CU.
Footnotes
This study was supported by grants from the Korea Health 21 R&D Project of the Ministry of Health & Welfare, Republic of Korea (03-PJ10-PG13-GD01-0002 and A050571).
References
- 1.Sabroe RA, Fiebiger E, Francis DM, Maurer D, Seed PT, Grattan CE, Black AK, Stingl G, Greaves MW, Barr RM. Classification of anti-FcεRI and anti-IgE autoantibodies in chronic idiopathic urticaria and correlation with disease severity. J Allergy Clin Immunol. 2002;110:492–499. doi: 10.1067/mai.2002.126782. [DOI] [PubMed] [Google Scholar]
- 2.Tong LJ, Balakrishnan G, Kochan JP, Kinet JP, Kaplan AP. Assessment of autoimmunity in patients with chronic urticaria. J Allergy Clin Immunol. 1997;99:461–465. doi: 10.1016/s0091-6749(97)70071-x. [DOI] [PubMed] [Google Scholar]
- 3.Oztas P, Onder M, Gonen S, Oztas MO, Soylemezoglu O. Is there any relationship between human leucocyte antigen class II and chronic urticaria? (chronic urticaria and HLA class II) Yonsei Med J. 2004;45:392–395. doi: 10.3349/ymj.2004.45.3.392. [DOI] [PubMed] [Google Scholar]
- 4.Kim SH, Ye YM, Lee SK, Park HS. Genetic mechanism of aspirin-induced urticaria/angioedema. Curr Opin Allergy Clin Immunol. 2006;6:266–270. doi: 10.1097/01.all.0000235899.57182.d4. [DOI] [PubMed] [Google Scholar]
- 5.Zembowicz A, Mastalerz L, Setkowicz M, Radziszewski W, Szczeklik A. Safety of cyclooxygenase 2 inhibitors and increased leukotriene synthesis in chronic idiopathic urticaria with sensitivity to nonsteroidal anti-inflammatory drugs. Arch Dermatol. 2003;139:1577–1582. doi: 10.1001/archderm.139.12.1577. [DOI] [PubMed] [Google Scholar]
- 6.Grattan CE. Aspirin sensitivity and urticaria. Clin Exp Dermatol. 2003;28:123–127. doi: 10.1046/j.1365-2230.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 7.Wedi B, Raap U, Kapp A. Chronic urticaria and infections. Curr Opin Allergy Clin Immunol. 2004;4:387–396. doi: 10.1097/00130832-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Rumbyrt JS, Schocket AL. Chronic urticaria and thyroid disease. Immunol Allergy Clin North Am. 2004;24:215–223. doi: 10.1016/j.iac.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Borges M, Capriles-Hulett A. Atopy is a risk factor for non-steroidal anti-inflammatory drug sensitivity. Ann Allergy Asthma Immunol. 2000;84:101–106. doi: 10.1016/S1081-1206(10)62748-2. [DOI] [PubMed] [Google Scholar]
- 10.Bachert C, Gevaert P, van Cauwenberge P. Staphylococcus aureus superantigens and airway disease. Curr Allergy Asthma Rep. 2002;2:252–258. doi: 10.1007/s11882-002-0027-9. [DOI] [PubMed] [Google Scholar]
- 11.Zollner TM, Wichelhaus TA, Hartung A, Von Mallinckrodt C, Wagner TO, Brade V, Kaufmann R. Colonization with superantigen-producing staphylococcus aureus is associated with increased severity of atopic dermatitis. Clin Exp Allergy. 2000;30:994–1000. doi: 10.1046/j.1365-2222.2000.00848.x. [DOI] [PubMed] [Google Scholar]
- 12.Skov L, Baadsgaard O. Bacterial superantigens and inflammatory skin diseases. Clin Exp Dermatol. 2000;25:57–61. doi: 10.1046/j.1365-2230.2000.00575.x. [DOI] [PubMed] [Google Scholar]
- 13.Leung DY. Atopic dermatitis and the immune system: the role of superantigens and bacteria. J Am Acad Dermatol. 2001;45:S13–S16. doi: 10.1067/mjd.2001.117024. [DOI] [PubMed] [Google Scholar]
- 14.Kuokkanen K, Sonck CE. Antistreptolysin and antistaphylolysin titres in allergic skin conditions. Observations on 6104 patients suffering from various eczemas and urticaria. Acta Allergol. 1973;28:260–282. doi: 10.1111/j.1398-9995.1973.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 15.Potaczek DP, Sanak M, Mastalerz L, Setkowicz M, Kaczor M, Nizankowska E, Szczeklik A. The alpha-chain of high-affinity receptor for IgE (FcεRIα) gene polymorphisms and serum IgE levels. Allergy. 2006;61:1230–1233. doi: 10.1111/j.1398-9995.2006.01195.x. [DOI] [PubMed] [Google Scholar]
- 16.Mekori YA, Giorno RC, Anderson P, Kohler PF. Lymphocyte subpopulations in the skin of patients with chronic urticaria. J Allergy Clin Immunol. 1983;72:681–684. doi: 10.1016/0091-6749(83)90629-2. [DOI] [PubMed] [Google Scholar]
- 17.Sabroe RA, Poon E, Orchard GE, Lane D, Francis DM, Barr RM, Black MM, Black AK, Greaves MW. Cutaneous inflammatory cell infiltrate in chronic idiopathic urticaria: comparison of patients with and without anti-FcepsilonRI or anti-IgE autoantibodies. J Allergy Clin Immunol. 1999;103:484–493. doi: 10.1016/s0091-6749(99)70475-6. [DOI] [PubMed] [Google Scholar]
- 18.Ying S, Kikuchi Y, Meng Q, Kay AB, Kaplan AP. Th1/Th2 cytokines and inflammatory cells in skin biopsy specimens from patients with chronic idiopathic urticaria: comparison with the allergen-induced late-phase cutaneous reaction. J Allergy Clin Immunol. 2002;109:694–700. doi: 10.1067/mai.2002.123236. [DOI] [PubMed] [Google Scholar]
- 19.Caproni M, Giomi B, Volpi W, Melani L, Schincaglia E, Macchia D, Manfredi M, D'Agata A, Fabbri P. Chronic idiopathic urticaria: infiltrating cells and related cytokines in autologous serum-induced wheals. Clin Immunol. 2005;114:284–292. doi: 10.1016/j.clim.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Sundberg EJ, Deng L, Mariuzza RA. TCR recognition of peptide/MHC class II complexes and superantigens. Semin Immunol. 2007;19:262–271. doi: 10.1016/j.smim.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SH, Choi JH, Lee KW, Kim SH, Shin ES, Oh HB, Suh CH, Nahm DH, Park HS. The human leucocyte antigen-DRB1*1302-DQB1*0609-DPB1*0201 haplotype may be a strong genetic marker for aspirin-induced urticaria. Clin Exp Allergy. 2005;35:339–344. doi: 10.1111/j.1365-2222.2004.02197.x. [DOI] [PubMed] [Google Scholar]
- 22.Wehner J, Neuber K. Staphylococcus aureus enterotoxins induce histamine and leukotriene release in patients with atopic eczema. Br J Dermatol. 2001;145:302–305. doi: 10.1046/j.1365-2133.2001.04352.x. [DOI] [PubMed] [Google Scholar]
- 23.Leung DY, Harbeck R, Bina P, Reiser RF, Yang E, Norris DA, Hanifin JM, Sampson HA. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J Clin Invest. 1993;92:1374–1380. doi: 10.1172/JCI116711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahl MV. Staphylococcus aureus and atopic dermatitis. Arch Dermatol. 1983;119:840–846. [PubMed] [Google Scholar]
- 25.Perez-Novo CA, Kowalski ML, Kuna P, Ptasinska A, Holtappels G, van Cauwenberge P, Gevaert P, Johannson S, Bachert C. Aspirin sensitivity and IgE antibodies to staphylococcus aureus enterotoxins in nasal polyposis: studies on the relationship. Int Arch Allergy Immunol. 2004;133:255–260. doi: 10.1159/000076832. [DOI] [PubMed] [Google Scholar]
- 26.Suh YJ, Yoon SH, Sampson AP, Kim HJ, Kim SH, Nahm DH, Suh CH, Park HS. Specific immunoglobulin E for staphylococcal enterotoxins in nasal polyps from patients with aspirin-intolerant asthma. Clin Exp Allergy. 2004;34:1270–1275. doi: 10.1111/j.1365-2222.2004.02051.x. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Novo CA, Claeys C, Van Zele T, Holtapples G, Van Cauwenberge P, Bachert C. Eicosanoid metabolism and eosinophilic inflammation in nasal polyp patients with immune response to staphylococcus aureus enterotoxins. Am J Rhinol. 2006;20:456–460. doi: 10.2500/ajr.2006.20.2873. [DOI] [PubMed] [Google Scholar]
- 28.Bae JS, Kim SH, Ye YM, Yoon HJ, Suh CH, Nahm DH, Park HS. Significant association of FcεRα promoter polymorphisms with aspirin-intolerant chronic urticaria. J Allergy Clin Immunol. 2007;119:449–456. doi: 10.1016/j.jaci.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Jabara HH, Geha RS. The superantigen toxic shock syndrome toxin-1 induces CD40 ligand expression and modulates IgE isotype switching. Int Immunol. 1996;8:1503–1510. doi: 10.1093/intimm/8.10.1503. [DOI] [PubMed] [Google Scholar]
- 30.Hofer MF, Harbeck RJ, Schlievert PM, Leung DY. Staphylococcal toxins augment specific IgE responses by atopic patients exposed to allergen. J Invest Dermatol. 1999;112:171–176. doi: 10.1046/j.1523-1747.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 31.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]