Abstract
The members of the S100 protein family are multifunctional proteins with a regulatory role in a variety of cellular processes. They exert their actions usually through calcium binding, although Zn2+ and Cu2+ have also been shown to regulate their biological activity.
The most studied member, protein S100B, exhibits neurotrophic (at physiologic concentration) or neurotoxic (at higher concentration) activity and its immunohistochemical expression or serum levels have been determined in various clinical disorders. S100B has been well documented as a marker of astrocytic activation mediating its effects via interaction with receptor for advanced glycation end products (RAGE).
We herein provide a wide range of information concerning their clinical application in traumatic brain injuries, Alzheimer disease, subarachnoid haemorrhage and other neurologic disorders, malignant melanoma and several other neoplasms (as S100B has been shown to down-regulate p53), as well as inflammatory diseases. Also its use on predicting neurologic outcome after resuscitation for cardiac arrest or in intrauterine growth retardation newborns is discussed.
Keywords: S100 protein family, protein S100B, traumatic brain injury, neurodegenerative disorders, malignant melanoma
S100 protein structure and functions
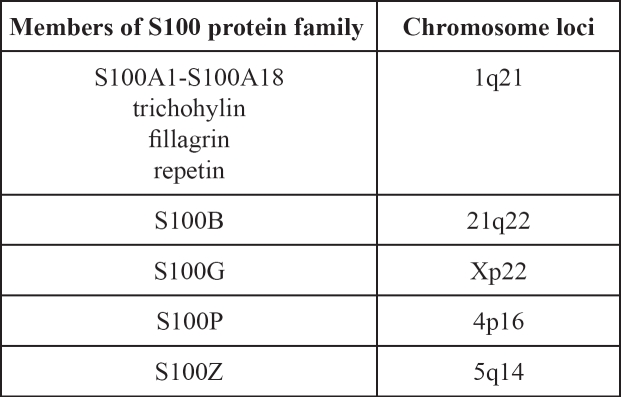
S100 protein family consists the largest subgroup of the Ca2+-binding EF-hand (helix E-loop-helix F) protein group. These proteins are called S100 because of their solubility in a 100%-saturated solution with ammonium sulphate at neutral pH. They were first identified by B.W. Moore in 19651. S100 proteins are small, acidic proteins of 10-12kDa and contain two distinct EF-hands, 4 α-helical segments, a central hinge region of variable length and the N- and C- terminal variable domains. In contrast to the abundance of S100 genes in vertebrates, they are completely absent in invertebrates. At present, at least 25 proteins have been identified as belonging to the S100 protein family, 21 of them having genes clustered at chromosome locus 1q21, known as the epidermal differentiation complex (Table 1) 2–4.
Table 1: The members of S100 protein family and the location of their specific genes.
The members of the S100 protein family are multifunctional proteins expressed in a diverse spectrum of tissues. Through their interaction with several effector proteins within cells they are involved in the regulation of a variety of cellular processes such as contraction, motility, cell growth and differentiation, cell cycle progression, transcription, structural organization of membranes, dynamics of cytoskeleton constituents, protection from oxidative cell damage, protein phosphorylation and secretion5. This variety of functions appears due to:
broad diversification of the different members (25 members in human)
different metal ion-binding properties of the individual S100 proteins
spatial distribution in specific intracellular compartments or extracellular space, and
their ability to form non-covalent homo- and hetero- dimers, allowing for dynamic exchange of the S100 subunits.
S100 proteins do not have intrinsic catalytic activity. They are generally thought to be – that – in a manner similar to calmodulin and troponin C – undergo conformational changes and modulate biological activity via calcium binding6. Upon calcium binding, the helices of S100 proteins rearrange, revealing a cleft, which forms the target protein binding site7. In addition, some S100 members have been shown to bind Zn2+ and Cu2+, suggesting the possibility that their biological activity in some cases might be regulated by Zn2+ and/or Cu2+, rather than by Ca2+ 8–10. n example of a Ca2+-independent but Zn2+-dependent target protein recognition is the interaction of S100B with tau protein (inhibition of tau protein phosphorylation by protein kinase II). Also Cu2+ binding to S100B might have a neuroprotective function11,12. Calcium and zinc binding proteins S100A9 and S100A8, abundant in myeloid cells, are considered to play important roles in both calcium signaling and zinc homeostasis, but they do neither serve as calcium nor as zinc buffering proteins in granulocytes13.
Several S100 proteins, such as S100B, SA4, S100A8, S100A9, S100A12, and S100A13, are secreted and act in a cytokine-like manner. For example, the S100A8/ A9 heterodimer acts as a chemotactic molecule in inflammation14, S100B exhibits neurotrophic (at physiologic concentration: nanomolar levels) or neurotoxic (at higher concentration: micromolar levels) activity15,16 and S100A4 has angiogenic effects17.
Methods of measurement
Analytical methods such as immunoradiometric assay (IRMA)18, mass spectroscopy, western blot, ELISA (enzyme linked immunosorbent assay), electrochemiluminence and quantitative PCR, can detect S100 changes in immunohistochemical expression or in serum concentration with high sensitivity, providing an important tool in clinical diagnosis19.
Protein S100B (homodimer of the β subunit) has a molecular weight of 21kD and is coded on the long arm of chromosome 21 (21q22.3). The biological half-life of S100B approximates 30 minutes. This implies that any persistent elevation of its serum levels reflects continuous release from affected tissues. S100B is mainly eliminated by the kidneys.
S100 expression in related diseases
Diseases associated with altered expression levels of S100 proteins can be classified into four categories:
1. Neurologic disorders
As protein S100B is primarily produced by astrocytes in CNS, its increased expression – as well as that of glial fibrillary acidic protein (GFAP) – represents a hallmark of astrocytic activation. It is less astrocyte-specific than GFAP, as it is localized in many neural cell types20. S100B protein's autocrine effects on astrocytes (upregulation of IL-6, TNF-alpha expression) are mediated through its interaction with RAGE (Receptor for Advanced Glycation End products)5,21. Secretion of S100B is an early process during the glial response to metabolic injury (oxygen, serum and glucose deprivation)22. The relationship between stress conditions (brain traumas, blood-brain barrier disruption, ischemia) and serum levels of S100B seems to be glucocorticoid independent23.
Traumatic brain injuries (TBI) result in an increase in S100B levels in blood and CSF. After mild traumatic brain injuries, increased concentrations of S100B and S100A1B occur in 31% and 48% of the patients, without significant association with symptoms or signs of cognitive impairment24. Furthermore, the serum levels of S100B and C-tau protein are not reliable predictors of the long outcome in such cases25, particularly in children with TBI26. However, with a cutoff limit of 0.1µg/l, the measurement of S100B has been shown to help to identify the subgroup of patients with trauma-relevant intracerebral lesions on CT scan with a sensitivity of 99% and a specificity of 30%. So, due to its excellent negative predictive value, the addition of S100B test to the clinical decision rules for the evaluation of patients with a mild head injury helps avoiding 30% of unnecessary CT scans27. Opposing results have also been presented, suggesting that S100B determinations can not replace the physical examination or CT scan (which has an indisputable role in assessing moderate or severe TBI) in the approach of patients with mild head injury28. The separate analysis of the two dimers S100A1B and S100BB (which account for the total measured concentration of S100B) does not offer any essential advantage29. As extracerebral sources of S100B, particularly adipocytes or chondrocytes, contribute to its serum levels, caution is needed when interpreting serum S100B increase as a clinical marker of brain damage30. It should also be noted that serum levels of S100B depend on the integrity of the blood-brain barrier. So, its early increase after TBI can be attributed either to mechanical discharge from a destroyed blood-brain barrier or to active expression by a brain involved in systemic inflammatory reaction. The role of S100 in TBI poses some challenging issues, as recent research provides growing evidence that S100B may decrease neuronal injury and/or contribute to repair following TBI, triggering wound repair events in the border of trauma and exerting paracrine trophic actions on the adjacent regions31,32.
In newborns with birth asphyxia, serum S100B levels provide a biochemical indication for the presence of hypoxic-ischemic encephalopathy33. Also, significantly higher S100B levels in cord artery have been noted in neonates with acidosis and pathological patterns (leading to appropriate interventions) in cardiotocography and fetal electrocardiography during labour34.
The increased levels of S100B and GFAP after spontaneous subarachnoid haemorrhage correlate well with the clinical and neuroimaging severity of the disease, contributing to the better initial assessment and follow–up35 and are good predictors of the outcome36. S100B serum levels above 0.3µg/l on admission of such patients are associated with unfavourable outcome.
Such elevated levels are also observed in patients suffering from chronic neurodegenerative disorders such as Alzheimer's disease37–39. Elevated levels of S100B originating from necrotic tissues might enhance or even amplify neurodegeneration by S100B-induced apoptosis.
S100B serum levels can also provide independent information about the individual risk of a patient with acute stroke experiencing intracerebral haemorrhagic complications under thrombolytic therapy (intravenous recombinant tissue plasminogen activator). But its low diagnostic accuracy (parallel to that of metalloproteinase- 9 or fibronectin which have also been investigated in this context) is a limiting factor for it to function as a reliable biomarker in acute stroke management40.
Interestingly, in patients suffering from amyotrophic lateral sclerosis, the serum concentration of S100B is decreased41, whereas the expression of S100A6 is greatly increased42. No clinically meaningful fluctuations of S100B serum levels have been noted in multiple sclerosis (MS) patients43. In MS patients, S100B cerebrospinal fluid (CSF) level is increased and correlates with CSF ferritin44.
It has been reported that serum and CSF S100 level is raised in systemic lupus erythematosus with neuropsychiatric involvement (i.e. organic brain syndrome, seizures, cerebral vascular accident, psychosis) and in obstructive sleep apnea syndrome, reflecting the ongoing neurological damage45–47. Also, the elevated and persistent expression of S100B induced by hyperammonemia may contribute to the brain impairment observed in hepatic encephalopathy48. Finally, significant increase of serum S100B is observed during exacerbations of bipolar disorder (episodes of mania and depression), but not during the remission phases of the disease49.
The above described wide range of applications has led to the consideration of S100B measurement in neurologic disorders as analogous to that of CRP in systemic inflammation50.
2. Neoplastic disorders
Different forms of cancer exhibit dramatic changes in the expression of S100 proteins such as S100B, S100A2, S100A4, S100A6, and S100P51. The S100-RAGE signalling pathway plays an important role in linking inflammation and cancer and in tumour cell survival and malignant progression (RAGE-deficient tumours are characterized by accelerated apoptosis, reduced activation of NFκB and significantly impaired proliferation).
For example, elevated levels of S100A4 (metastasin) are associated with poor survival rates in breast cancer patients and have been found to induce metastasis in mouse models. Increased serum concentration of S100A4 is also found in esophageal squamous and colon carcinoma, invasive pancreatic carcinoma, non small cell lung cancer, bladder carcinoma and correlates with a worse outcome and more aggressive disease. S100A4 interacts with annexin II, an endothelial plasminogen co-receptor, and accelerates tPA-mediated plasminogen activation. The resulting local plasmin formation could contribute to tumor-induced angiogenesis and metastasis52. Also, S100A4 at nanomolar levels stimulates matrix metalloproteinase 13 release from chondrocytes in a RAGE-mediated manner53.
There is a high (stage-dependent) secretion of S100B in malignant melanoma, reflecting tumour load, stage and prognosis54. S100B serum levels are widely used for the early detection of recurrence or metastases in combination with MIA (Melanoma-Inhibiting Activity) protein and TA90-IC (TA90-Immune Complex)55. A cutoff of 0.12µg/l has been suggested and is associated with a sensitivity and specificity of 0.29 and 0.93, respectively. Due to the possibility of false positive S-100B tumour marker determinations, repetition of its measurement is recommended if elevated S-100B levels occur during the follow up of high risk melanoma patients56. In such cases, FDG-PET/CT can be used for the accurate identification of lymph node or distant metastases and/or the reliable exclusion of metastases, reaching an accuracy of 98%. Also, S100B remains the most sensitive immunohistochemical marker of melanoma, despite the emergence of newer markers (HMB-45, MART-1/Melan-A, tyrosinase, MITF, etc) reflecting differentiation, proliferation, immunomodulation and other relevant processes57.
Concerning the detection of brain metastases, serum concentration of S100B has a good negative predictive value comparable to radiologic investigations. As its levels may also reflect the existence of cerebrovascular ischemic changes without infiltrating tumour, it may be used in conjunction with proApolipoprotein A1 for a sufficiently specific serum-based diagnosis of the presence of metastatic brain tumours58.
Recent studies have shown that S100A2 is highly expressed in tumours such as:
non-small lung cancer (in stage I NSCLC overexpression of S100A2 is associated with a significantly lower overall survival and disease-specific survival rate)59–62
gastric / oesophageal squamous cancer63
lymphoma
granular cell tumours of the gastrointestinal tract (in correlation with nestin, a cytoskeletal filament protein expressed in neuroectodermal stem cells and skeletal muscle progenitor cells)64
renal tumours (specially in papillary renal cell carcinomas and oncocytomas)65
papillary and anaplastic thyroid carcinomas (while it is not expressed in follicular carcinomas)
On the contrary, in early-stage oral squamous cell carcinoma patients at high risk of recurrence, down regulation of S100A2 is significantly associated with shorter disease free survival66.
There is accumulating evidence that BRCA1 negative breast carcinomas exhibit increased expression of S100A767.
S100A8 and S100A9 form a heterodimer complex implicated in regulating cell proliferation and in the metastatic process (increasing the motility of cancer cells and facilitating the homing of migrating cells to pre-metastatic "niches" within the target tissues)68.
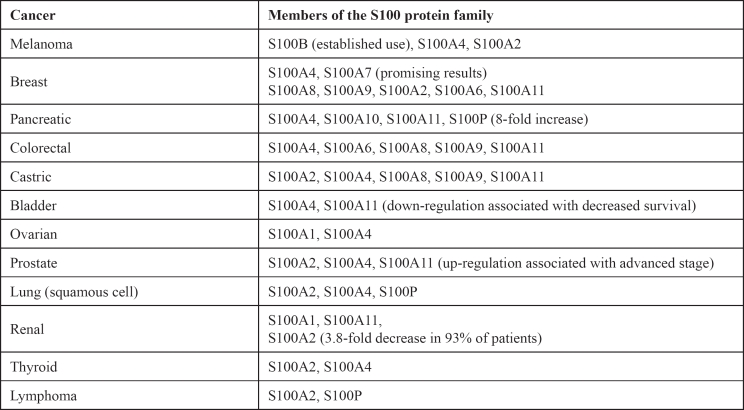
The associations between members of the S100 protein family and several types of cancer are summarised in Table 2.
Table 2: Summary of the links that have been noted between various types of cancer and members of the S100 protein family.
Although in most cases the function of S100 proteins in cancer cells is still unknown, the specific expression patterns of these proteins can be used as a valuable prognostic tool. S100A4 and S100B proteins bind to p53 tumour suppressor gene and inhibit its phosphorylation69, leading to p53 down-regulation in a calcium dependent manner70. Efforts are made to restore functional wildtype p53 in neoplasms with elevated S100B through the inhibition of the S100B-p53 interaction71. Other S100 proteins exert different effects on p53 activity (S100A2 promotes p53 transcriptional activity, etc).
3. Cardiac diseases
S100A1 is specifically and highly expressed in the mammalian myocardium, where it modulates contractile performance of the heart via interaction with contractile filaments and with proteins of the sarcoplasmic reticulum (SR). It also increases the release of calcium from the sarcoplasmic reticulum by interacting with the ryanodine receptor. S100A1 is up-regulated in right ventricular hypertrophy72 and down-regulated in end-stage heart failure73, indicating a correlation between S100A1 expression and contractile performance. In patients with acute myocardial ischemia, a rise in the plasma concentration of S100A1 was observed, which might be associated with a role of S1001 as a cardioprotective factor with antiapoptotic function74,75.
The combination of Glasgow Coma Scale (< 6 points) with elevated serum concentrations of NSE (> 65ng/ml) and S100 (> 1.5µg/l) 48-72h after cardiopulmonary resuscitation for cardiac arrest, lead to prediction of a poor neurological outcome and cognitive impairment with a specificity of 100% (sensitivity around 42%)76,77. Elevation of S100 alone increases the risk of death and persistent vegetative state 12.6- fold78.
It is known that processing of pericardial shed blood with a cell-saving device helps to prevent lipid microembolization and to protect from neurocognitive dysfunction after cardiopulmonary bypass and this may be associated with the reduction of S100B release observed when this method is performed79.
4. Inflammatory diseases
S100A8, S100A9, and S100Al2, are predominant1y expressed in phagocytes and are strongly associated with pro-inflammatory functions. They are secreted especially at sites of inflammation. The serum concentrations of these S100 proteins correlate with inflammatory disease activity; high levels were identified in several inflammatory disorders such as rheumatoid arthritis, chronic bronchitis, and cystic fibrosis80,81. S100A7, S100A8, S100A9, and S100A12 are up-regulated in active psoriatic lesions82,83. Overexpression of S100A7 (acting as a keratinocyte- derived chemotactic agent for immune cells) is also seen in many epidermal inflammatory diseases, like atopic dermatitis, mycosis fungoides and Darier's disease7. Antiallergic drugs which bind to S100A12 might b1ock the S100 protein-RAGE interaction84, implying a promising approach to anti-inflammatory therapy85. Enteric glial-derived S100B (associated with the onset of inflammation) is increased in the duodenum of patients with celiac disease and contributes to nitric oxide production86.
Apart from the above mentioned clinical applications, S100B serum measurement may provide helpful information on the neuromuscular system activation induced by physical training, as S100B is released from the involved muscles and nerves87 and on the reliable monitoring of alcohol detoxification treatment88. Finally, increased urine S100B protein levels are found in intrauterine growth retardation newborns in the first week after birth suggesting the presence of brain damage. S100B protein measurements soon after birth contribute to the stratification of these patients concerning the risk of possible neurologic sequelae89.
Conclusion
The members of the S100 protein family through their interaction with several effector proteins are involved in the regulation of a diverse spectrum of cellular processes. Although their pathophysiologic implications still require further clarification, some of these proteins have already been successfully investigated in clinical context.
S100B protein has gained a role as a complementary specific index of early diagnosis and prognosis judgement after TBI. Unraveling the mechanism of S100B neurotoxicity and assessment of the therapeutic effect of S100B protein are promising research directions for accomplishing the optimal clinical treatment of TBI.
S100B is already widely used in malignant melanoma patients with a particular help in staging, in assessing the therapeutic outcome and in the early detection of recurrence.
As S100A4 has molecular interactions involved in tumourigenesis and metastasis, it would seem feasible that it may be used as a complementary staging and prognostic tool in patients with breast, colorectal and gastric carcinomas.
Finally, other members of the S100 protein family may prove to be useful biomarkers in future applications and may S100 protein-targeted therapies emerge as useful opportunities in specific clinical settings90.
References
- 1.Moore BW. A soluble protein characteristic of the nervous system. Biochem Biophys Res Commun. 1965;19:739–744. doi: 10.1016/0006-291x(65)90320-7. [DOI] [PubMed] [Google Scholar]
- 2.Marenholz I, Heinzman CW, Fritz G. S100 protein in mouse and man: from evolution to function and pathology. Biochem Biophys Res Commun. 2004;322:1111–1122. doi: 10.1016/j.bbrc.2004.07.096. [DOI] [PubMed] [Google Scholar]
- 3.Michetti F, Gazzolo D. S100B protein in biological fluids: a tool for perinatal medicine. Clin Chem. 2002;48:2097–2104. [PubMed] [Google Scholar]
- 4.Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60:540–551. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- 5.Santamaria–Kisiel L, Rintala–Dempsey AC, Shaw GS. Calcium dependent and independent interactions of the S100 protein family. Biochem J. 2006;396:201–214. doi: 10.1042/BJ20060195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikura M. Calcium binding and conformational response in EFhand proteins. Trends Biochem Sci. 1996;21:14–17. [PubMed] [Google Scholar]
- 7.Eckert RL, Broome A-M, Ruse M, Robinson N, Ryan D, Lee K. S100 proteins in the epidermis. J Invest Dermatol. 2004;123:23–33. doi: 10.1111/j.0022-202X.2004.22719.x. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa T, Lee ISM, Shiraishi T, Ishikawa T, Ohta Y, Nishikimi M. Identification of S100b protein as copper-binding protein and its suppression of copper-induced cell damage. J Biol Chem. 1997;272:23037–23041. doi: 10.1074/jbc.272.37.23037. [DOI] [PubMed] [Google Scholar]
- 9.Schafer BW, Fritschy JM, Murmann P, et al. Brain S100A5 is a novel calcium-zinc-, and copper ion-binding protein of the EFhand superfamily. J Biol Chem. 2000;275:30623–30630. doi: 10.1074/jbc.M002260200. [DOI] [PubMed] [Google Scholar]
- 10.Heizmann CW, Cox JA. New perspectives on S100 proteins: a multifunctional Ca(2+)-, Zn(2+)-, and Cu(2+)- -binding protein family. Biometals. 1998;11:383–397. doi: 10.1023/a:1009212521172. [DOI] [PubMed] [Google Scholar]
- 11.Yu WH, Fraser PE. S100b interaction with tau is promoted by zinc and inhibited by hyperphosphorylation in Alzheimer's disease. J Neurosci. 2001;21:2240–2246. doi: 10.1523/JNEUROSCI.21-07-02240.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiraishi N, Nishikimi M. Suppression of copper-induced cellular damage by copper sequestration with S100b protein. Arch Biochem Biophys. 1998;357:225–230. doi: 10.1006/abbi.1998.0802. [DOI] [PubMed] [Google Scholar]
- 13.Nacken W, Mooren FC, Manitz MP, Bond G, Sorg C, Kerkhoff C. S100A9 deficiency alters adenosine-5'-triphosphate induced calcium signalling but does not generally interfere with calcium and zinc homeostasis in murine neutrophils. Int J Biochem Cell Biol. 2005;37:1241–1253. doi: 10.1016/j.biocel.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Newton RA, Hogg N. The human S100 protein MRP-14 is a novel activator of the b2 integrin Mac-1 on neutrophils. J Immunol. 1998;160:1427–1435. [PubMed] [Google Scholar]
- 15.Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem. 2000;275:40096–40105. doi: 10.1074/jbc.M006993200. [DOI] [PubMed] [Google Scholar]
- 16.Korfias S, Stranjalis G, Papadimitriou A, et al. Serum S-100B protein as a biochemical marker of brain injury: a review of current concepts. Curr Med Chem. 2006;13:3719–3731. doi: 10.2174/092986706779026129. [DOI] [PubMed] [Google Scholar]
- 17.Ambartsumian N, Klingelhofer J, Grigorian M, et al. The metastasis associated Mts1 (S100A4) protein could act as an angiogenic factor. Oncogene. 2001;20:4685–4695. doi: 10.1038/sj.onc.1204636. [DOI] [PubMed] [Google Scholar]
- 18.AB Sangtec Medical; Sangtec 100 IRMA. Immunoradiometric assay for the quantification of protein S100B. Instruction for use. 2000.
- 19.Wild D. The immunoassay handbook. 2nd ed. London: Nature Publishing Group; 2001. 660 pp. [Google Scholar]
- 20.Steiner J, Bernstein HG, Bielau H, et al. Evidence for a wide extra-astrocytic distribution of S100B in human brain. BMC Neurosci. 2007;8:2. doi: 10.1186/1471-2202-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponath G, Schettler C, Kaestner F, et al. Autocrine S100B effects on astrocytes are mediated via RAGE. J Neuroimmunol. 2007;184:214–222. doi: 10.1016/j.jneuroim.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Gerlach R, Demel G, Konig HG, et al. Active secretion of S100B from astrocytes during metabolic stress. Neuroscience. 2006;141:1697–1701. doi: 10.1016/j.neuroscience.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Scaccianoce S, Del Bianco P, Pannitteri G, Passarelli F. Relationship between stress and circulating levels of S100B protein. Brain Res. 2004;1004:208–211. doi: 10.1016/j.brainres.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 24.de Boussard CN, Lundin A, Karlstedt D, Edman G, Bartfai A, Borg J. S100 and cognitive impairment after mild traumatic brain injury. J Rehabil Med. 2005;37:53–57. doi: 10.1080/16501970410015587. [DOI] [PubMed] [Google Scholar]
- 25.Bazarian JJ, Zemlan FP, Mookerjee S, Stigbrand T. Serum S-100B and cleaved-tau are poor predictors of long-term outcome after mild traumatic brain injury. Brain Inj. 2006;20:759–765. doi: 10.1080/02699050500488207. [DOI] [PubMed] [Google Scholar]
- 26.Piazza O, Storti MP, Cotena S, et al. S100B is not a reliable prognostic index in paediatric TBI. Pediatr Neurosurg. 2007;43:258–264. doi: 10.1159/000103304. [DOI] [PubMed] [Google Scholar]
- 27.Biberthaler P, Linsenmeier U, Pfeifer KJ, et al. Serum S-100B concentration provides additional information for the indication of computed tomography in patients after minor head injury: a prospective multicenter study. Shock. 2006;25:446–453. doi: 10.1097/01.shk.0000209534.61058.35. [DOI] [PubMed] [Google Scholar]
- 28.Muller K, Townend W, Biasca N, et al. S100B serum level predicts computed tomography findings after minor head injury. J Trauma. 2007;62:1452–1456. doi: 10.1097/TA.0b013e318047bfaa. [DOI] [PubMed] [Google Scholar]
- 29.Nylen K, Ost M, Csajbok LZ, et al. Serum levels of S100B, S100A1B and S100BB are all related to outcome after severe traumatic brain injury. Acta Neurochir (Wien) 2008;150:221–227. doi: 10.1007/s00701-007-1489-2. [DOI] [PubMed] [Google Scholar]
- 30.Netto CB, Conte S, Leite MC, et al. Serum S100B protein is increased in fasting rats. Arch Med Res. 2006;37:683–686. doi: 10.1016/j.arcmed.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Kleindienst A, Ross Bullock M. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J Neurotrauma. 2006;23:1185–1200. doi: 10.1089/neu.2006.23.1185. [DOI] [PubMed] [Google Scholar]
- 32.Ellis EF, Willoughby KA, Sparks SA, Chen T. S100B protein is released from rat neonatal neurons, astrocytes, and microglia by in vitro trauma and anti-S100 increases trauma-induced delayed neuronal injury and negates the protective effect of exogenous S100B on neurons. J Neurochem. 2007;101:1463–1470. doi: 10.1111/j.1471-4159.2007.04515.x. [DOI] [PubMed] [Google Scholar]
- 33.Murabayashi M, Minato M, Okuhata Y, et al. Kinetics of serum S100B in newborns with intracranial lesions. Pediatr Int. 2008;50:17–22. doi: 10.1111/j.1442-200X.2007.02506.x. [DOI] [PubMed] [Google Scholar]
- 34.Stuart A, Edvinsson L, Kallen K, Olofsson P, Hellsten C, Amer-Wahlin I. Fetal electrocardiographic monitoring during labor in relation to cord blood levels of the brain-injury marker protein S-100. J Perinat Med. 2008;36:136–141. doi: 10.1515/JPM.2008.019. [DOI] [PubMed] [Google Scholar]
- 35.Vos PE, van Gils M, Beems T, Zimmerman C, Verbeek MM. Increased GFAP and S100beta but not NSE serum levels after subarachnoid haemorrhage are associated with clinical severity. Eur J Neurol. 2006;13:632–638. doi: 10.1111/j.1468-1331.2006.01332.x. [DOI] [PubMed] [Google Scholar]
- 36.Oertel M, Schumacher U, McArthur DL, Kastner S, Boker DK. S-100B and NSE: markers of initial impact of subarachnoid haemorrhage and their relation to vasospasm and outcome. J Clin Neurosci. 2006;13:834–840. doi: 10.1016/j.jocn.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Wang J, Sheng JG, et al. S100 b increases levels of b-amyloid precursor protein and its encoding mRNA in rat neuronal cultures. J Neurochem. 1998;71:1421–1428. doi: 10.1046/j.1471-4159.1998.71041421.x. [DOI] [PubMed] [Google Scholar]
- 38.Sheng JG, Mrak RE, Griffin WS. S100 b protein expression in Alzheimer disease: potential role in the pathogenesis of neuritic plaques. J Neurosci Res. 1994;39:398–404. doi: 10.1002/jnr.490390406. [DOI] [PubMed] [Google Scholar]
- 39.Van Eldik LJ, Griffin WS. S100 b expression in Alzheimer's disease: relation to neuropathology in brain regions. Biochim Biophys Acta. 1994;1223:398–403. doi: 10.1016/0167-4889(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 40.Foerch C, Wunderlich MT, Dvorak F, et al. Elevated serum S100B levels indicate a higher risk of hemorrhagic transformation after thrombolytic therapy in acute stroke. Stroke. 2007;38:2491–2495. doi: 10.1161/STROKEAHA.106.480111. [DOI] [PubMed] [Google Scholar]
- 41.Sussmuth SD, Tumani H, Ecker D, Ludolph AC. Amyotrophic lateral sclerosis: disease stage related changes of tau protein and S100 b in cerebrospinal fluid and creatine kinase in serum. Neurosci Lett. 2003;353:57–60. doi: 10.1016/j.neulet.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 42.Hoyaux D, Decaestecker C, Heizmann CW, et al. S100 proteins in corpora amylacea from normal human brain. Brain Res. 2000;867:280–288. doi: 10.1016/s0006-8993(00)02393-3. [DOI] [PubMed] [Google Scholar]
- 43.Koch M, Mostert J, Heersema D, Teelken A, De Keyser J. Plasma S100beta and NSE levels and progression in multiple sclerosis. J Neurol Sci. 2007;252:154–158. doi: 10.1016/j.jns.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 44.Rejdak K, Petzold A, Stelmasiak Z, Giovannoni G. Cerebrospinal fluid brain specific proteins in relation to nitric oxide metabolites during relapse of multiple sclerosis. Mult Scler. 2008;14:59–66. doi: 10.1177/1352458507082061. [DOI] [PubMed] [Google Scholar]
- 45.Schenatto CB, Xavier RM, Bredemeier M, et al. Raised serum S100B protein levels in neuropsychiatric lupus. Ann Rheum Dis. 2006;65:829–831. doi: 10.1136/ard.2005.048330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braga CW, Martinez D, Wofchuk S, Portela LV, Souza DO. S100B and NSE serum levels in obstructive sleep apnea syndrome. Sleep Med. 2006;7:431–435. doi: 10.1016/j.sleep.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 47.Yang XY, Lin J, Lu XY, Zhao XY. Expression of S100B protein levels in serum and cerebrospinal fluid with different forms of neuropsychiatric systemic lupus erythematosus. Clin Rheumatol. 2008;27:353–357. doi: 10.1007/s10067-007-0722-y. [DOI] [PubMed] [Google Scholar]
- 48.Leite MC, Brolese G, de Almeida LM, Pinero CC, Gottfried C, Goncalves CA. Ammonia-induced alteration in S100B secretion in astrocytes is not reverted by creatine addition. Brain Res Bull. 2006;70:179–185. doi: 10.1016/j.brainresbull.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Andreazza AC, Cassini C, Rosa AR, et al. Serum S100B and antioxidant enzymes in bipolar patients. J Psychiatr Res. 2007;41:523–529. doi: 10.1016/j.jpsychires.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 50.Sen J, Belli A. S100B in neuropathologic states: the CRP of the brain? J Neurosci. 2007;85:1373–1380. doi: 10.1002/jnr.21211. [DOI] [PubMed] [Google Scholar]
- 51.Hsieh HL, Schafer BW, Sasaki N, Heizmann CW. Expression analysis of S100 proteins and RAGE in human tumors using tissue microarrays. Biochem Biophys Res Commun. 2003;307:375–381. doi: 10.1016/s0006-291x(03)01190-2. [DOI] [PubMed] [Google Scholar]
- 52.Semov A, Moreno MJ, Onichtchenko A, et al. Metastasis-associated protein S100A4 induces angiogenesis through interaction with Annexin II and accelerated plasmin formation. J Biol Chem. 2005;280:20833–20841. doi: 10.1074/jbc.M412653200. [DOI] [PubMed] [Google Scholar]
- 53.Donato R. RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins. Current Mol Med. 2007;7:711–724. doi: 10.2174/156652407783220688. [DOI] [PubMed] [Google Scholar]
- 54.Von Schoultz E, Hansson LO, Djureen E, et al. Prognostic value of serum analyses of S100B protein in malignant melanoma. Melanoma Res. 1996;6:133–137. doi: 10.1097/00008390-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 55.Faries MB, Gupta RK, Ye X, et al. A Comparison of 3 tumor markers (MIA, TA90IC, S100B) in stage III melanoma patients. Cancer Invest. 2007;25:285–293. doi: 10.1080/07357900701208634. [DOI] [PubMed] [Google Scholar]
- 56.Strobel K, Skalsky J, Kalff V, et al. Tumour assessment in advanced melanoma: value of FDG-PET/CT in patients with elevated serum S-100B. Eur J Nucl Med Mol Imaging. 2007;34:1366–1375. doi: 10.1007/s00259-007-0403-8. [DOI] [PubMed] [Google Scholar]
- 57.Ohsie SJ, Sarantopoulos GP, Cochran AJ, Binder SW. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433–444. doi: 10.1111/j.1600-0560.2007.00891.x. [DOI] [PubMed] [Google Scholar]
- 58.Marchi N, Mazzone P, Fazio V, Mekhail T, Masaryk T, Janigro D. ProApolipoprotein A1: a serum marker of brain metastases in lung cancer patients. Cancer. 2008;112:1313–1324. doi: 10.1002/cncr.23314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nagy N, Hoyaux D, Gielen I, et al. The Ca2+-binding S100A2 protein is differentially expressed in epithelial tissue of glandular or squamous origin. Histol Histopathol. 2002;17:123–130. doi: 10.14670/HH-17.123. [DOI] [PubMed] [Google Scholar]
- 60.Heighway J, Knapp T, Boyce L, et al. Expression profiling of primary non-small cell lung cancer for target identification. Oncogene. 2002;21:7749–7763. doi: 10.1038/sj.onc.1205979. [DOI] [PubMed] [Google Scholar]
- 61.Wang H, Zhang Z, Li R, et al. Overexpression of S100A2 protein as a prognostic marker for patients with stage I non small cell lung cancer. Int J Cancer. 2005;116:285–290. doi: 10.1002/ijc.21035. [DOI] [PubMed] [Google Scholar]
- 62.Jassem E, Serkies K, Dziadziuszko R, et al. Prognostic value of S-100 immunostaining in tumour cells of non-small cell lung cancer. Biomarkers. 2006;11:262–269. doi: 10.1080/13547500600652277. [DOI] [PubMed] [Google Scholar]
- 63.El-Rifai W, Moskaluk CA, Abdrabbo MK, et al. Gastric cancers overexpress S100A calcium-binding proteins. Cancer Res. 2002;62:6823–6826. [PubMed] [Google Scholar]
- 64.Parfitt JR, McLean CA, Joseph MG, Streutker CJ, Al-Haddad S, Driman DK. Granular cell tumours of the gastrointestinal tract: expression of nestin and clinicopathological evaluation of 11 patients. Histopathology. 2006;48:424–430. doi: 10.1111/j.1365-2559.2006.02352.x. [DOI] [PubMed] [Google Scholar]
- 65.Li G, Gentil-Perret A, Lambert C, Genin C, Tostain J. S100A1 and KIT gene expressions in common subtypes of renal tumours. Eur J Surg Oncol. 2005;31:299–303. doi: 10.1016/j.ejso.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Tsai ST, Jin YT, Tsai WC, et al. S100A2, a potential marker for early recurrence in early-stage oral cancer. Oral Oncol. 2005;41:349–357. doi: 10.1016/j.oraloncology.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 67.Kennedy RD, Gorski JJ, Quinn JE, et al. BRCA1 and c-Myc associate to transcriptionally repress psoriasin, a DNA damageinducible gene. Cancer Res. 2005;65:10265–10272. doi: 10.1158/0008-5472.CAN-05-1841. [DOI] [PubMed] [Google Scholar]
- 68.Rafii S, Lyden D. S100 chemokines mediate bookmarking of premetastatic niches. Nat Cell Biol. 2006;8:1321–1323. doi: 10.1038/ncb1206-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fernandez-Fernandez MR, Veprintsev DB, Fersht AR. Proteins of the S100 family regulate the oligomerization of p53 tumor suppressor. Proc Natl Acad Sci USA. 2005;102:4735–4740. doi: 10.1073/pnas.0501459102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Markowitz J, Mackerell ADJr, Carrier F, Charpentier TH, Weber DJ. Design of Inhibitors for S100B. Curr Top Med Chem. 2005;5:1093–1108. doi: 10.2174/156802605774370865. [DOI] [PubMed] [Google Scholar]
- 71.Gieldon A, Mori M, Del Conte R. Theoretical study on binding of S100B protein. J Mol Model. 2007;13:1123–1131. doi: 10.1007/s00894-007-0231-6. [DOI] [PubMed] [Google Scholar]
- 72.Ehlermann P, Remppis A, Guddat O, et al. Right ventricular upregulation of the Ca(2+) binding protein S100A1 in chronic pulmonary hypertension. Biochim Biophys Acta. 2000;1500:249–255. doi: 10.1016/s0925-4439(99)00106-4. [DOI] [PubMed] [Google Scholar]
- 73.Remppis A, Greten T, Schafer BW, et al. Altered expression of the Ca2+-binding protein S100A1 in human cardiomyopathy. Biochim Biophys Acta. 1996;1313:253–257. doi: 10.1016/0167-4889(96)00097-3. [DOI] [PubMed] [Google Scholar]
- 74.Kiewitz R, Acklin C, Minder E, Huber PR, Schafer BW, Heizmann CW. S100A1, a new marker for acute myocardial ischemia. Biochem Biophys Res Commun. 2000;274:865–871. doi: 10.1006/bbrc.2000.3229. [DOI] [PubMed] [Google Scholar]
- 75.Most P, Boerries M, Eicher C, et al. Extracellular S100A1 protein inhibits apoptosis in ventricular cardiomyocytes via activation of the extracellular signal-regulated protein kinase 1/2 (ERK1/2) J Biol Chem. 2003;278:48404–48412. doi: 10.1074/jbc.M308587200. [DOI] [PubMed] [Google Scholar]
- 76.Grubb NR, Simpson C, Sherwood RA, et al. Prediction of cognitive dysfunction after resuscitation from out-of-hospital cardiac arrest using serum neuron-specific enolase and protein S-100. Heart. 2007;93:1268–1273. doi: 10.1136/hrt.2006.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ekmektzoglou KA, Xanthos T, Papadimitriou L. Biochemical markers (NSE, S-100, IL-8) as predictors of neurological outcome in patients after cardiac arrest and return of spontaneous circulation. Resuscitation. 2007;75:219–228. doi: 10.1016/j.resuscitation.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 78.Pfeifer R, Borner A, Krack A, Sigusch HH, Surber R, Figulla HR. Outcome after cardiac arrest: predictive values and limitations of the neuroproteins neuron-specific enolase and protein S-100 and the Glasgow Coma Scale. Resuscitation. 2005;65:49–55. doi: 10.1016/j.resuscitation.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 79.Carrier M, Denault A, Lavoie J, Perrault LP. Randomized controlled trial of pericardial blood processing with a cell-saving device on neurologic markers in elderly patients undergoing coronary artery bypass graft surgery. Ann Thorac Surg. 2006;82:51–55. doi: 10.1016/j.athoracsur.2006.02.077. [DOI] [PubMed] [Google Scholar]
- 80.Frosch M, Strey A, Vogl T, et al. Myeloid-related proteins 8 and 14 are specifically secreted during interaction of phagocytes and activated endothelium and are useful markers for monitoring disease activity in pauciarticular-onset juvenile rheumatoid arthritis. Arthritis Rheum. 2000;43:628–637. doi: 10.1002/1529-0131(200003)43:3<628::AID-ANR20>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 81.Foell D, Wittkowski H, Hammerschmidt I, et al. Monitoring neutrophil activation in juvenile rheumatoid arthritis by S100A12 serum concentrations. Arthritis Rheum. 2004;50:1286–1295. doi: 10.1002/art.20125. [DOI] [PubMed] [Google Scholar]
- 82.Broome AM, Ryan D, Eckert RL. S100 protein subcellular localization during epidermal differentiation and psoriasis. J Histochem Cytochem. 2003;51:675–685. doi: 10.1177/002215540305100513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Semprini S, Capon F, Tacconelli A, et al. Evidence for differential S100 gene over-expression in psoriatic patients from genetically heterogeneous pedigrees. Hum Genet. 2002;111:310–313. doi: 10.1007/s00439-002-0812-5. [DOI] [PubMed] [Google Scholar]
- 84.Shishibori T, Oyama Y, Matsushita O, et al. Three distinct anti-allergic drugs, amlexanox, cromolyn and tranilast, bind to S100A12 and S100A13 of the S100 protein family. Biochem J. 1999;338:583–589. [PMC free article] [PubMed] [Google Scholar]
- 85.Morbini P, Villa C, Campo I, Zorzetto M, Inghilleri S, Luisetti M. The receptor for advanced glycation end products and its ligands: a new inflammatory pathway in lung disease? Mod Pathol. 2006;19:1437–1445. doi: 10.1038/modpathol.3800661. [DOI] [PubMed] [Google Scholar]
- 86.Esposito G, Cirillo C, Sarnelli G, et al. Enteric glial-derived S100B protein stimulates nitric oxide production in celiac disease. Gastroenterology. 2007;133:918–925. doi: 10.1053/j.gastro.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 87.Schulpis KH, Moukas M, Parthimos T, Tsakiris T, Parthimos N, Tsakiris S. The effect of alpha-Tocopherol supplementation on training-induced elevation of S100B protein in sera of basketball players. Clin Biochem. 2007;40:900–906. doi: 10.1016/j.clinbiochem.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 88.Liappas I, Tzavellas EO, Kariyannis C, et al. Effect of alcohol detoxification on serum S-100B levels of alcohol-dependent individuals. In Vivo. 2006;20:675–680. [PubMed] [Google Scholar]
- 89.Florio P, Marinoni E, Di Iorio R, et al. Urinary S100B protein concentrations are increased in intrauterine growth-retarded newborns. Pediatrics. 2006;118:e747–e754. doi: 10.1542/peds.2005-2875. [DOI] [PubMed] [Google Scholar]
- 90.Salama I, Malone PS, Mihaimeed F, Jones JL. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34:357–364. doi: 10.1016/j.ejso.2007.04.009. [DOI] [PubMed] [Google Scholar]