Abstract
Background: Abdominal compartment syndrome (ACS) has been recognized as an entity, affecting cardiovascular, pulmonary, and cerebral function, while it is often complicated with sepsis. Goal of the study was the evaluation of brain oxygenation during ACS alone and in combination with endotoxinemia.
Materials and Methods: Sixteen pigs, undergone intra–abdominal hypertension, were allocated to receive intravenous administration of either saline or endotoxin. Pigs were evaluated regarding brain tissue oxygenation (PbrO2), systemic oxygenation (PaO2) and regional cerebral blood flow (rCBF).
Results: Statistical analysis revealed significant reduction of PbrO2 over time for sepsis group, after endotoxin administration. Regarding differences between groups, sepsis group experienced lower PbrO2 values, compared to saline group, only after endotoxin administration.. Both groups experienced reduction in arterial oxygenation, with greater pertubations seen after sepsis induction. Regarding rCBF, septic pigs showed greater flow values, while ACS alone did not influence rCBF. ACS has no deleterious effects in cerebral oxygenation and flow, provided systemic oxygenation and CPP are maintained above normal value.
Conclusions: Combined sepsis–ACS lead to perturbations in cerebral oxygenation, in conjunction with greater rCBF values. The latter could be ascribed to abnormalities in oxygen extraction.
Keywords: abdominal compartment syndrome, sepsis, cerebral oxygenation, pigs
Intra–abdominal hypertension (IAH) has been recognized as a cause of organ dysfunction in critically ill patients, contributing to overall morbidity and mortality. Intra–abdominal hypertension is defined as the presence an intra–abdominal pressure (IAP) of 12 mmHg or greater or an abdominal perfusion pressure (APP: mean arterial pressure minus intra–abdominal pressure) of 60 mm Hg or less. Based on the severity of intra–abdominal hypertension, a grading system from grade I to grade IV is used, with grade I corresponding to 12–15 mmHg IAP, grade II corresponding to 16–20 mmHg IAP and grade III and IV corresponding to IAP of 21–25 and >25 mmHg respectively1. The term abdominal compartment syndrome (ACS), an entity first noted from Marey and Burt in 1863 and 1870 respectively, describes a situation characterized by the presence an IAP of 20 mmHg or greater with or without APP below 50 mmHg, in conjunction with single or multiple organ system failure, that was not previously present1. The syndrome is caused by abnormal increase of intra–abdominal pressure, as a consequence of abdominal trauma, aortic aneurysm rupture, retroperitoneal haemorrhage, ascites, pancreatitis or liver transplantation. Any pathologic condition that may lead to acute increase in the volume of abdominal contents, may contribute to the development of ACS2.
Previous studies have shown that intra–abdominal hypertension has a negative impact on organ function, especially renal, gastrointestinal, cardiovascular and pulmonary function3–6. On the other hand, trauma victims constitute one of the most common subsets of patients to experience intra–abdominal hypertension and the ACS1. Patients with multiple traumas often suffer from combined brain and abdominal injuries. Nevertheless, intra–abdominal hypertension has also been shown to increase intracranial pressure6. Furthermore, treatment of these patients could also be complicated with sepsis due to disruption of intestinal–blood barrier and subsequent bacterial translocation7. Sepsis has been documented to deteriorate cerebral oxygenation and lead to septic encephalopathy8.
In the present study, the impact of intra–abdominal hypertension on brain oxygenation in a porcine model was investigated. Furthermore, it was studied the potential synergistic effect of endotoxemia on brain oxygenation in pigs with intra–abdominal hypertension.
Materials and Methods
Attention was paid to minimizing pain and discomfort of the animals. Experiments were conducted in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC) and the "Principles of laboratory animal care" (NIH publication No. 85–23, revised 1985) and approved by the Animal Care and Use Committee of the National Veterinary Institute, and by the ethical committee of the School of Medicine of Aristotle University of Thessaloniki.
Sixteen domestic female pigs, weighted 20–25 kg were used. All experimental animals used were individually caged, had free access to water but were food deprived for 12 hours before the commencement of the procedure.
All surgical procedures were conducted under general anaesthesia conditions. Pigs were initially sedated with droperidol 0.25 mg/kg, fentanyl 5 µg/kg and midazolam 0.5 mg/kg, given intramuscularly, 25 minutes prior to induction of general anaesthesia. Afterwards, an intravenous catheter (20 G, 2 in) was placed in the left earflap vein. The animal was placed on the surgical table and general anaesthesia was induced after thiopental (10 mg/kg, iv) and fentanyl (5 µg/kg, iv) administration. Muscle paralysis was achieved after vecuronium bromide (0.3 mg/kg, iv) administration. The animal was ventilated with a Mapleson C breathing circuit via facemask No 2, administering 100% oxygen. During facemask manual ventilation, a tracheostomy was performed and a cuffed tracheostomy tube (I.D: 6mm) was inserted. Controlled mechanical ventilation (Cesar ventilator, Taema, France) was applied with a tidal volume of 10–15 ml/kg and respiratory rate of 10–14 /min in order a PaCO2: 35–40mmHg to be maintained. General anaesthesia was maintained with a continuous intravenous infusion of midazolam (1–2 mg/kg/h), fentanyl (5 µg/kg/h) and vecuronium (2 mg/kg/h) for muscle relaxation.
Systolic, mean (mean arterial pressure was an automated reading from Datex Cardiocap monitor, extracted from systolic and diastolic pressure measurements) and diastolic arterial pressures were continuously measured via a 20 G catheter, placed in the right common femoral artery and connected to pressure transducer (Transpac III, Abbott Laboratories Ltd, North Chicago, IL, USA). This catheter was also used for intermittent blood sampling.
Cerebral microcirculation estimation was achieved via laser–flowmetry. For that purpose, a laser–Doppler microprobe (PF319, single optical fibre, diameter: 0.5mm, length: 300 mm) was inserted in the right frontal cortex, after mini craniotomy. The laser–Doppler microprobe was then connected to a flowmeter (Periflux PF2B, Perimed, Sweden) via a master–probe (PF318).
The partial pressure of O2 in the cerebral tissue (PbrO2) was measured by a Clark type polarographic microcatheter (Tissutrack, Pfizer Biomedical sensors, Ltd, England) with an outer diameter of 0.55 mm, inserted into the right frontal lobe of the brain. Catheter placement was guided by an introducer and then fixed tightly in a scull screw. The catheter was connected with the Tissutrack device that measures the partial pressure of tissue oxygen (Tissutrack, Diametrics Medical, Ltd, England). The insertion depth of the probe (measured from the bone surface to the catheter tip) was about 50 mm into the frontal lobe cortex.
After systemic blood pressure, regional cerebral flow and cerebral tissue oxygen monitors were connected to the animal, stabilized and subsequent recordings were obtained, a Veres laparoscopic needle was introduced under direct vision into the peritoneal cavity, while the abdominal cavity was closed air tight in two layers. Pneumoperitoneum was then induced using helium administration via a laparoscopic surgical device (Wisap, Germany) and intra–abdominal pressure was maintained at 25 mmHg until the end of the experiment. Inta–abdominal pressure was automatically measured, via laparoscopic surgical device.
The animals were randomly assigned into two groups (n: 8 animals per group), using a computer generated random numbers table. Group A pigs (n: 8) received 250 ml of saline iv, one hour after pneumoperitoneum induction. Group B pigs (n=8) received 100 µg/kg of endotoxin (lipopolysacharide, LPS E. Coli, 111:B4, Difco, Detroit, MI, USA), diluted in 250 ml of saline as a slow intravenous infusion. The research fellow participated in the infusions wasn't aware about the kind of infusate.
Pneumoperitoneum was maintained at 25 mmHg intra– abdominal pressure for three hours. During that period, mean blood pressure (MAP) was recorded in both groups just prior to pneumoperitoneum induction (t: 0) and every 20 minutes thereafter until the end of the experiment. Cerebral regional blood flow (rCBF) to the right frontal cortex was also estimated at the same time intervals.
Cerebral tissue oxygen partial pressure (PbrO2) was measured just prior to pneumoperitoneum induction (t: 0) and every 20 minutes for a time period of 180 min.
Blood samples from arterial line were intermittently obtained and arterial oxygen partial pressure (PaO2) was measured (ABL 510; Radiometer, Copenhagen, Denmark) just prior to pneumoperitoneum induction (t: 0) and every 60 min thereafter until the end of the experiment.
At the end of each experiment animals were sacrificed under deep anaesthesia with thiopental, 500 mg iv and KCL, 20 ml of a 10% solution iv. The brain of each animal was dissected and the localization of brain catheters was confirmed.
Data were analyzed using statistical software, SPSS 13.0 (Chicago, IL, USA). One–way within subjects ANOVAs were conducted in order to evaluate within group differences across time regarding PbrO2, PaO2, and rCBF. Pairwise comparisons (Holms Bonferroni procedure, controlling for familywise error across the tests at the 0.05 level) were also conduced. Furthermore, any between groups differences for the parameters mentioned above, were evaluated using MANOVA. ANOVAs were then conducted as follow–up tests to the MANOVA. The Bonferroni method was also applied.
Differences between groups, regarding animal's age and weight were evaluated using t–test. Differences were considered to be statistically significant for p < 0.05.
Results
Groups were comparable according to age and animals' body weight.
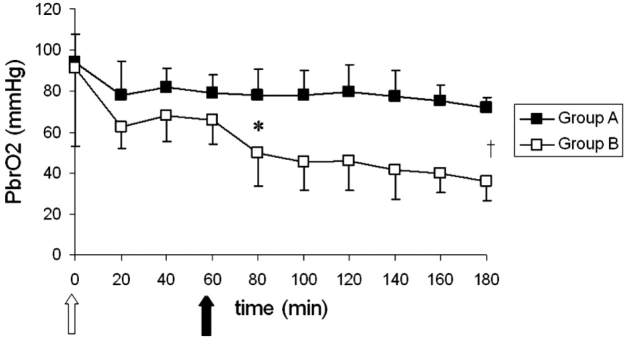
The means and standard deviations of PbrO2 for both groups over time are shown in Figure 2. Within groups' comparisons showed a significant time effect only for group B. Post hoc comparisons showed significant differences between 60 min and 180 min for group B. Between groups' comparisons showed significant differences. Follow up tests showed significant differences between groups at all time intervals studied after endotoxin administration.
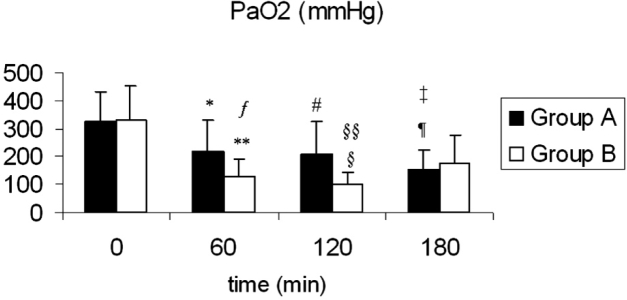
Figure 2: Means and standard deviations for PaO2 values over time for both groups. *: p =0.001 between t: 0 min and t: 60 min, <sup>#</sup> : p = 0.004 between t: 0 min and t: 120 min, ¶ : p =0.001 between t: 0 min vs. t: 180 min.: **p <0.001 between t: 0 min vs. t: 60 min. § : p <.001 between t: 0 min vs. t: 120 min. §§ : p<.001 between t: 60 min vs. t: 120 min. : p = 0.003 for group A vs. group B, ╪: P<0.01 for group A vs. group B.
The means and standard deviations of PaO2 for both groups over time are shown in Figure 2. Within groups comparisons indicated a significant time effect for both groups. Furthermore, statistical analysis using MANOVA revealed significant differences between groups. ANOVAs conducted as follow up tests, revealed significant differences between groups on the PaO2 levels on t: 60 min and on t: 120 min. Furthermore, hypoxemic conditions were recorded in just one case of septic pig (PaO2: 66 mmHg, at t: 120 min).
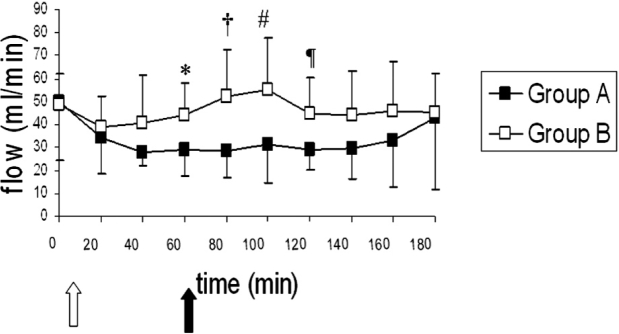
The means and standard deviations of rCBF for both groups are shown in Figure 3. Comparisons between baseline values (t:0) and subsequent time intervals' values didn't show significant different values for either group A or group B over time. Between groups comparisons showed significant lower rCBF values for group A, for the time intervals from t: 60 till t: 120.
Figure 3: Means and standard deviations for rCBF values over time for both groups. The white arrow shows the induction of pneumoperitoneum, while the black arrow shows the induction of sepsis in group B. *: p=0.038 for group A vs. group B, †: p=0.007 for group A vs. group B, #: p=0.028 for group A vs. group B, ¶: p=0.021 for group A vs. group B.
Discussion
The present work focused on the investigation of brain oxygenation alterations during two different situations, seen in clinical practice, i.e. abdominal compartment syndrome alone and in combination with sepsis. In order to create a model of ACS, we used a helium–induced pneumoperitoneum with an intra–abdominal pressure of as high as 25 mmHg. The selection of the specific level of intra–abdominal pressure was based on the known negative impact of 25 mmHg IAP on cardiac output and cardiac function1.
Cerebral oxygen monitoring was achieved via brain tissue oxygen tension measurement (PbrO2). Brain tissue oxygen tension measurement is considered to be the most accurate technique for monitoring cerebral oxygenation in clinical setting. PbrO2 is an expression of "oxygen pool" in brain tissue and is generally reflective of cerebral blood flow (CBF) although it may reflect oxygen extraction as well9–11.
Previous studies have shown the deleterious effects of both ACS and sepsis on pulmonary function and oxygenation8. Our study also revealed significant aggravation on systemic oxygenation, expressed as PaO2 values, from both ACS and combined ACS–sepsis. Nevertheless, it is well known that systemic hypoxemia reduces cerebral vascular resistance leading to alterations in CBF12. Consequently, during animals' mechanical ventilation, an inspiratory oxygen fraction (FiO2) equal to 0.5 was chosen on our study design, in order hypoxemic conditions and consequent CBF alterations to be avoided. Under these experimental conditions, systemic oxygenation was maintained above 100 mmHg in both groups for all the time intervals studied, except for one septic pig at t: 120 (Figure 2). Provided that cerebral oxygen tension is strongly depended on CBF, potential alterations in cerebral oxygenation would not be ascribed to hypoxemia induced CBF alterations. Consequently, high–inspired oxygen ventilation leaded to PbrO2 and SjvO2 values above normal limits.
In the present study brain tissue oxygenation, as measured by Clark electrode, worsened over time for both groups (Figure 1). Nevertheless, within–groups comparisons over time showed significant differences only for group B and only for the time interval t=60, which corresponds to just prior endotoxin injection, vs. t: 180, two hours after sepsis induction. Consequently, alterations in PbrO2 could not be ascribed to intra–abdominal hypertension alone since either group A PbrO2 comparisons over time or group B comparisons prior to endotoxin infusion did not show any difference. On the other hand, statistical analysis showed that sepsis induction in group B caused significant reduction in PbrO2 values, but that was a late phenomenon. PbrO2 reduction over time, seen in group B animals, may be the result of reduced rCBF or disturbed oxygen extraction. Since rCBF did not significantly change over time in group B animals, it is possible that PbrO2 reduction is mainly the result of oxygen extraction deficit.
Figure 1: Means and standard deviations for PbrO2 values, over time for both groups. The white arrow shows the induction of pneumoperitoneum, while the black arrow shows the induction of sepsis in group B animals. †: p= 0.02 between t: 60 and t: 180 for group B. *: p<0.01 between groups for all the time intervals from t: 80 to t: 180.
Considering between groups PbrO2 comparisons, statistical analysis revealed lower PbrO2 values for group B animals, after endotoxin administration and subsequent induction of sepsis, compared to group A animals. Nevertheless, regional blood flow measurements, obtained in the same brain region through laser Doppler flowmetry, showed greater values for flow in group B from t: 60 to t: 120, compared to group A pigs. Consequently, the possible fall of oxygen content, seen after sepsis induction, compared to control animals, may be due to disturbance of oxygen extraction, rather than cerebral microcirculation abnormalities. The present observations are in accordance with previous studies, which have shown alterations in oxygen extraction during septic conditions13.
Pneumoperitoneum induction, as an independent factor, did not seem to induce perturbations in cerebral oxygen content. According to authors' knowledge, there were not previous works in the literature, investigating the impact of intra–abdominal hypertension on brain tissue oxygenation. Despite this fact, many studies have shown the negative effect of intra–abdominal hypertension on cardiac output, systemic blood pressure, systemic oxygenation and intra–cranial pressure1,2,4,14. In the present study, systemic oxygenation also worsened over time for both groups (Figure 2). Nevertheless, systemic oxygenation aggravation did not cause significant perturbations in cerebral oxygen delivery, since high inspiratory oxygen fraction concentration leaded to full haemoglobin oxygen saturation and differences in dissolved oxygen (as expressed by PaO2) have minimal contribution to overall oxygen content. Furthermore, regarding group A animals, the absence of any statistically significant difference in rCBF over time, leads to the conclusion that abdominal compartment syndrome alone seemed to have no deleterious effects on cerebral oxygenation, as long as systemic oxygenation and rCBF are maintained within normal values. However, it is not clear from the present study whether the deleterious effects on brain oxygenation, recorded after endotoxin administration are due to sepsis alone or due to sepsis–abdominal compartment syndrome combination. However, the evaluation of impact of sepsis alone on brain oxygenation was beyond the scope of the study, as previous studies have proven the negative influence of sepsis on brain oxygenation13, whereas the significance of combined sepsis–ACS syndrome had never been evaluated.
Apart from PbrO2 alterations over time, the present study showed greater rCBF flows in septic pigs, after sepsis induction, compared to control animals. Provided that previous studies have documented the deleterious effects of sepsis on cerebral perfusion pressure (CPP: mean arterial pressure minus intra–cranial pressure)14, one can assume that either cerebral autoregulation is preserved under septic conditions, either sepsis induction is followed by extensive vasodilation. Despite higher rCBF, sepsis induction produces reduction in cerebral oxygen content.
The presented experimental study did not prove any negative effect of intra–abdominal hypertension and abdominal compartment syndrome on brain tissue oxygenation, provided that systemic oxygenation and cerebral perfusion pressure are maintained above lower normal values. Despite this fact, combined ACS–sepsis caused deteriorations on systemic oxygenation. On the other hand, combined ACS–sepsis induction lead to significant lower brain tissue oxygenation values. Furthermore, it is not documented whether this reduction was caused by sepsis alone or it is the complex effect of the pathophysiologic alterations emerging from ACS–sepsis syndrome.
References
- 1.Sugrue M. Abdominal compartment syndrome. Curr Opin Crit Care. 2005;11:333–338. doi: 10.1097/01.ccx.0000170505.53657.48. [DOI] [PubMed] [Google Scholar]
- 2.Eddy V, Nunn C, Morris JA., Jr Abdominal compartment syndrome. The Nashville experience. Surg Clin North Am. 1997;77:801–812. doi: 10.1016/s0039-6109(05)70585-5. [DOI] [PubMed] [Google Scholar]
- 3.Harman PK, Kron IL, McLachlan HD, Freedlender AE, Nolan SP. Elevated intra–abdominal pressure and renal function. Ann Surg. 1982;196:594–597. doi: 10.1097/00000658-198211000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes GE, Laine GA, Giam PY, Smith EE, Granger HJ. Cardiovascular responses to elevation of intra–abdominal hydrostatic pressures. Am J Physiol. 1985;248:R208–R213. doi: 10.1152/ajpregu.1985.248.2.R208. [DOI] [PubMed] [Google Scholar]
- 5.Bloomfield GL, Blocher CR, Fakhry IF, Sica DA, Sugerman HJ. Elevated intra–abdominal pressure increases plasma renin activity and aldosterone levels. J Trauma. 1997;42:997–1004. doi: 10.1097/00005373-199706000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Bloomfield GL, Ridings PC, Blocher CR, Marmarou A, Sugerman HJ. A proposed relationship between increased intra–abdominal, intrathoracic, and intracranial pressure. Crit Care Med. 1997;25:496–503. doi: 10.1097/00003246-199703000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg. 1999;178:449–453. doi: 10.1016/s0002-9610(99)00231-7. [DOI] [PubMed] [Google Scholar]
- 8.Baxter F. Septic shock. Can J Anaesth. 1997;44:59–72. doi: 10.1007/BF03014326. [DOI] [PubMed] [Google Scholar]
- 9.De Georgia MA, Deogaonkar A. Multimodal monitoring in the neurological intensive care unit. Neurologist. 2005;11:45–54. doi: 10.1097/01.nrl.0000149993.99956.09. [DOI] [PubMed] [Google Scholar]
- 10.Busija DW, Heistad DD. Factors involved in the physiological regulation of the cerebral circulation. Rev Physiol Biochem Pharmacol. 1984;101:161–211. doi: 10.1007/BFb0027696. [DOI] [PubMed] [Google Scholar]
- 11.Ogura H, Cioffi WG, Offner PJ, Jordan BS, Johnson AA, Pruitt BA., Jr Effect of inhaled nitric oxide on pulmonary function after sepsis in a swine model. Surgery. 1994;116:313–321. [PubMed] [Google Scholar]
- 12.Todd MM, Farrell S, Wu B. Cerebral blood flow during hypoxemia and hemodilution in rabbits. Different roles for nitric oxide? J Cereb Blood Flow Metab. 1997;17:1319–1325. doi: 10.1097/00004647-199712000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Porta F, Takala J, Kolarova A, et al. Oxygen extraction in pigs subjected to low–dose infusion of endotoxin after major abdominal surgery. Acta Anaesthesiol Scand. 2005;49:627–634. doi: 10.1111/j.1399-6576.2005.00683.x. [DOI] [PubMed] [Google Scholar]
- 14.Karakoulas KA, Vasilakos D, Grosomanidis V, Thomareis O, Goudas LC, Giala MM. Effects of pneumoperitoneum and LPS–induced endotoxemia on cerebral perfusion pressure in pigs. J Neurosurg Anesthesiol. 2006;18:194–199. doi: 10.1097/01.ana.0000211001.56151.96. [DOI] [PubMed] [Google Scholar]