Abstract
Objective: The purpose of this study was to detect and enumerate T cells secreting type 1 and 2 cytokines in the peripheral blood of patients with oral lichen planus (OLP) and in healthy controls.
Subjects and Methods: The study group consisted of 80 OLP patients and 80 healthy individuals. Cytokine secreting T cells were detected using ELISPOT assay.
Results: There was a statistically significant decrease (p<0.05) in the number of IFN– and IL–12 secreting cells in the peripheral blood of patients with OLP compared to the controls. No statistical difference was observed in the number of IL–2 and TNF–a secreting cells between OLP patients and controls (p>0.05). Also there was no significant difference in the numbers of IFN–γ, IL–12, IL–2 and TNF–a secreting cells between reticular and erosive forms of OLP (p>0.05).
As regards type 2 cytokines, the number of IL–5 and IL–10 secreting cells was significantly decreased in OLP patients compared to the healthy control group (p<0.05). No statistical difference was observed in the number of IL–6 secreting cells between OLP patients and control group (p>0.05). Similarly, no statistical difference was observed in the number of IL–4 secreting cells between OLP patients and controls (p>0.05). No significant difference was also found in the numbers of IL–4, IL–5, IL–10 and IL–6 secreting cells between reticular and erosive OLP group.
Conclusion: These data suggest decreased type 1 and type 2 cytokine production (except IL–4) in OLP patients.
Keywords: T helper cells, type 1 cytokine, type 2 cytokines, peripheral blood, oral lichen planus
Oral lichen planus (OLP) is a chronic inflammatory disease of unknown etiology1. It is one of the most common pathological conditions in patients referred to Oral Medicine clinics2–4. There is consensus that in OLP there is a chronic, cell–mediated, immune damage to basal keratinocytes in the oral mucosa, that are recognized as being antigenically foreign or altered, although the initial event in OLP lesion formation and the factors that determine OLP susceptibility are still unknown5–7. Studies on peripheral blood indicate an alteration in the immune response of OLP patients but do not identify abnormalities which may characterize the disease8–10. Cytokines play a critical role in determining the strength, the nature and the duration of immune responses and have been a major focus in the study of the pathogenesis of autoimmunity11– 13. In general, cytokines are distinguished in type 1 cytokines (i.e. IL–2, IL–12, IFN–γ, TNF–a) that are involved as pro–inflammatory cytokines in cell–mediated immunity and type 2 cytokines (i.e. IL–4, IL–5, IL–6, IL–10, IL–13) that promote humoral immunity14.
The purpose of the present investigation was to detect and enumerate type 1 and type 2 cytokine producing cells in the peripheral blood of patients with OLP, to compare them with those of healthy controls and to discuss the role that these cells may play in the pathogenesis of OLP.
Subjects and Methods
Patients and samples
The study group consisted of 80 OLP patients (49 men, 31 women, mean age 55.6 ± 13.3 years, range 18–76 years) who were referred to the Clinic of Oral Medicine and 80 healthy individuals (40 men, 40 women, mean age 50 ± 6.1 years, range 20–65 years). All OLP patients and controls were free from any systemic diseases and they did not take any medication during the previous three months. Patients with lichenoid reactions to drugs, amalgam fillings, or topical allergic reactions of unknown origin were excluded from this study. Clinical evaluation of OLP patients was based on the classification that was proposed by Andreasen (1963)15 who described six clinical forms of OLP (reticular, plaque, papular, atrophic, bullous and erosive form). Of 80 OLP patients, 40 manifested the reticular form (50%), 32 the erosive form (40%), 1 the atrophic form (1.2%) and 7 the plaque–like form of the disease (8.7%). The criteria used for histopathologic diagnosis were the presence of hyperkeratosis, degenerative alterations of the basal cells as well as inflammatory infiltrates in the lamina propria consisting mainly of lymphocytes and histiocytes. Peripheral blood samples were obtained from healthy volunteers and OLP patients by venepuncture in the active stage of the disease. The whole study was conducted according to Helsinki declaration.
Isolation of T cells
T cells were purified by density centrifugation (2000 rpm for 30 min, at 5°C) on Ficoll, were isolated and centrifuged three more times at 2500 rpm, for 10 min, at 5°C with RPMI–1640 and were supplemented with 10% heat inactivated Fetal Bovine Serum, 1% Hepes Buffer 10Mm, 1% penicillin 1U/ml, 1% streptomycin 1µg/ml and 1% L–glutamine 2Mm. All reagents were purchased by Biochrom KG, Berlin, Germany. T cells were finally resuspended in RPMI–1640 medium at a concentration of 1 x 105 cells/ml for IL–2 assay, 1x 105 cells/ml for IL–12 assay, 2 x 104 cells/ml for TNF–a assay and 5 x 104 cells/ ml for IFN–γ assay.
Cytokine ELISPOT (Elisa spots) assay.
Commercially available ELISPOT kits were used to measure IL–2, IL–12, TNF–a, IFN–γ, IL–4, IL–5, IL–6, and IL–10 producing cells (Diaclone Research, Besançon, France). Each kit contained concentrated solutions of capture and detection antibodies, concentrated solution of streptavidin conjugated alkaline–phosphatase and BCIP/ NBT substrate. Assays were performed according to the protocol that was included in each ELISPOT kit. The 96– well PVDF–bottomed–well plates (Millipore Multiscreen, France) were coated with100 µl/well of monoclonal anticytokine detection antibody into phosphate buffer saline (PBS) (Sigma, St Louis, USA). For each plate 100 µl of capture antibody into 10 ml of PBS was used. The antibody was kept into the plates overnight at 4°C.
Plates were then washed with PBS and consequent dilutions of cells suspension, starting with 5–10 x 104 cells/ ml (for IL–2 assay), 1–10 x 105 cells/ml (for IL–12 assay), 2–4 x104 cells/ml (for TNFµa assay) and 2µ5 x 104 cells/ ml (for IFNµγ assay) in RPMIµ1640 medium and were incubated on cytokineµcoated plates at 37°C for 15µ20 hours in a humidified environment with 5% C02. Plates were washed three times with PBSµ 0.1%, Tween 20 and were overlaid with 100 µl/well of biotinylated detection anticytokine antibody into PBS, containing 1% BSA (for each plate we used 100 µl of reconstituted detection antibody into 10 mL of PBS containing 1% BSA) and were incubated for 1 hour 30 min at 37°C. Plates were then washed again three times with PBSµ0.1%, Tween 20 and treated with 1:1000 dilution of streptavidinµconjugated alkaline phosphatase into PBSµ1% BSA for 1 hour at 37°C and were washed three times with PBSµ0.1% Tween 20. The cytokine secreted by single cells was visualized by the addition of BCIP/NBT in wells. The colorimetric reaction was halted after 20 minutes by washing with distilled water and the number of spots in the wells, each representing one cytokineµproducing cell, was evaluated using a light microscope. The numbers of cytokine producing cells were counted under a stereo microscope and were expressed as cells/well.
Statistical analysis
Statistical differences between the numbers of cytokine producing cells of OLP patients and control group were determined by two tailed t–test. Statistical differences between the numbers of cytokine producing cells of reticular type of OLP patients group and erosive type of OLP patients group were determined by two tailed t–test. Results were expressed as mean ± S.E.M for each group.
Results
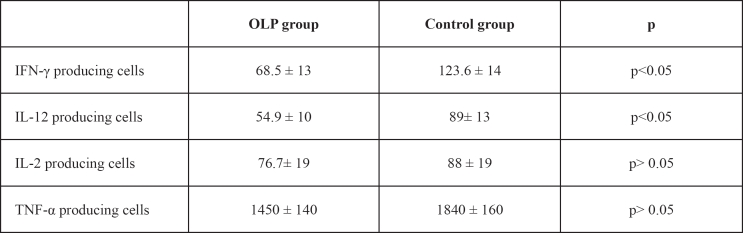
The numbers of IFN–γ and IL–12 producing cells were significantly lower (p<0.05) in OLP patients (68.5 ± 13 IFN-γ producing cells/well and 54.9 ± 10 IL-12 producing cells/well) than in control group (123.6 ± 14 IFN–γ producing cells/ml and 89 – 13 IL–12 producing cells/ well, respectively). No statistical difference (p>0.05) was observed in the numbers of IL–2 and TNF–a producing cells between OLP patients (76.7 ± 19 IL–2 producing cells/well and 1450 ± 140 TNF–a producing cells/well) and controls (88 ± 19 IL–2 producing cells/well and 1840 ± 160 TNF–a producing cells/ well) (Table 1).
Table 1: Elispot results. Numbers of T cells secreting type 1 cytokines in the peripheral blood of OLP patients and control group. Results are expressed as the number of cytokine producing cells/ well (mean±S.E.M.).
As for IFN–γ, IL–12, IL–2 and TNF–a producing cells in reticular and erosive OLP patients, no significant difference was observed between the two groups (p>0.05) (Table 2).
Table 2: ELISPOT results. Type 1 cytokine–producing cells from peripheral blood of patients with reticular and erosive OLP. Results are expressed as the number of cytokine producing cells/well (mean ± S.E.M.).
As for Type 2 cytokines, the numbers of IL–5 and IL– 10 producing cells were found to be significantly lower (p<0.05) in OLP patients (48.2 ± 4 IL–5 producing cells/ well and 730 ± 100 IL–10 producing cells/well) compared to control group (88.2 ± 9 IL–5 producing cells/well and 1930 ± 290 IL–10 producing cells/well, respativaly). No statistical difference (p>0.05) was observed in the number of IL–6 producing cells between OLP and control group (661 ± 95 vs. 804 ± 85 IL–6 producing cells/well, respectively). Similarly, no statistical difference (p>0.05) was observed in the number of IL–4 producing cells between OLP patients and controls (117 ± 22 vs. 73.1 ± 11 IL–4 producing cells/well, respectively) (Table 3).
Table 3: Elispot results. Numbers of T cells secreting type 2 cytokines in the peripheral blood of OLP patients and control group. Results are expressed as the number of cytokine producing cells/well (mean±S.E.M.).
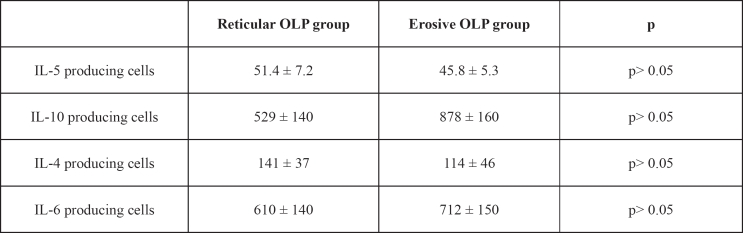
As for IL–4, IL–5, IL–10 and IL–6 producing cells in reticular and erosive OLP patients, no significant difference (p>0.05) was observed between the two groups (Table 4).
Table 4: ELISPOT results. Type 2 cytokine–producing cells from the peripheral blood of patients with reticular and erosive OLP. Results are expressed as the number of cytokine producing cells/ well (mean ± S.E.M.).
Discussion
In this study, we detected and compared the IL–2, IFN– γ, IL–12, TNF–a, IL–4, IL–5, IL–10 and IL–6 producing cells in the peripheral blood of OLP patients and healthy controls. ELISPOT assays were used to detect cytokine producing cells from peripheral blood in both groups. The results have shown a decrease of IFN–γ, IL–12, IL–5, IL– 10 and IL–6 producing cells in patients with OLP.
Cytokines play an important role in the immune system as they mediate and regulate immune and inflammatory reactions16. Type 1 cytokines primarily promote cell mediated immunity. In our study, the numbers of IFN–γ and IL–12 producing cells in peripheral blood were found to be significantly decreased in OLP group compared to healthy controls (p<0.05), indicating a suppressed cell mediated immune response in OLP patients (Th1 response). IFN–γ inhibits the differentiation and effector functions of type 2 cytokines secreting cells and can lead to a dominant Th1 response17. The antigen presenting cell (APC) –derived cytokine IL–12, strongly drives the differentiation of type 1 cytokine secreting cells, in vivo and in vitro, partly through its potent induction of IFN–γ production17–20.
No statistical difference was observed in the numbers of IL–2 and TNF–a producing cells in the peripheral blood of patients with OLP compared with the control group. However a slight not statistically important decrease in the numbers of IL–2 and TNF–a producing cells was observed in the peripheral blood of patients with OLP. IL–2 is produced by T cells and acts as a growth factor for antigen–stimulated T lymphocytes. It is also responsible for T cell clonal expansion after antigen recognition21–23. IL–2 also regulates IFN–γ production by T cells24. Moreover, TNF–a is produced from macrophages, T cells and other types of cells when stimulated with IL–2, GM–CSF or TNF–a itself25. A possible explanation for the observed decreased number of type 1 cytokine producing cells in the peripheral blood of OLP group could be a deficiency at the level of co–operation between antigen–presenting cells (APCs) and T lymphocytes via MHC class II antigens and antigen/T cell receptor (TCR).
The decreased number of cytokine producing cells in peripheral blood in OLP group indicates an impairment of their effector functions. Although ineffective in peripheral blood lymphocytes of OLP patients have been described, the etiologic significance remains unclear26. Yamamoto et al11 have suggested that OLP patient's lymphocyte and neutrophil functions are impaired and that cellular immunosuppression is a pathologic characteristic of OLP. Previous studies demonstrated reduced spontaneous lymphocyte proliferation and lower IFN–γ secretion and MHC locus II antigen expression in OLP patients compared with controls8,9. Their results suggest a defect in OLP T cell activation localized between IL–2 receptor ligand binding and IFN–γ secretion.
Reduced mitogen–stimulated lymphocyte proliferation has also been reported but not in all OLP patients9,10,27. Furthermore, IL–2, IFN–γ and TNF–a production from PHA stimulated peripheral blood lymphocytes was found to be decreased in OLP patients compared to the control group27. Administration of phytohaemagglutinin (PHA) in low doses, restored OLP T cell proliferative response and cytokine production to normal levels, suggesting that the defect could not be attributed to numerical differences or deletion of T cell subsets but rather to T cell hyporesponsiveness8,28.
Measurements in the serum of OLP patients in some studies revealed that IL–2 levels were very low27, while TNF–a and IFN–γ levels were found slightly increased27.
In our study, type 1 cytokine expression was tested with Elispot assay exclusively in peripheral blood.
While OLP pathogenesis remains unclear, anti–keratinocytes auto–cytotoxic T cell clones in OLP lesions suggest a role for autoimmunity and cell–mediated cytotoxity in OLP29. Khan et al30 suggest that the development of a type 1 cytokine immune response in OLP lesions may promote CD8+ cytotoxic T cell activity in OLP. Thus, OLP may be considered as a hyper–reactive, immunostimulatory condition31.
Recent studies identified IFN–γ expression by T cells adjacent to basal keratinocytes in oral LP and IFN–γ production and secretion by oral LP lesional T cells in vitro32,33. More IL–2 was generated by tissue infiltrated mononuclear cells in OLP lesions (TIMC) compared to TIMC in the gingiva and peripheral blood34.
In contrary, the results in our study have shown decreased numbers of type 1 cytokine producing cells in peripheral blood of OLP patients. It could be hypothesized that tissue–infiltrated mononuclear cells are stimulated in situ and produce cytokines, that OLP is a localized autoimmune disease and that the inflammatory condition of OLP is determined by local cytokine network. The suppressed function of T helper cells in OLP may indicate an inability to respond to the immune reaction following antigen recognition in OLP lesion. Even though the proinflammatory cytokines – i.e. IFN–γ and TNF–a– play an important role in tissue destruction in autoimmune diseases35,36, several data suggest that they can also activate homeostatic mechanisms to suppress inflammation37,38. Previous studies have suggested that a defect in immunoregulation –specifically involving suppressor mechanisms– may facilitate cellular autoreactivity in OLP39,40.
Ragarding the role of type 2 cytokines, IL–10 is an inhibitory cytokine, produced by T cells, macrophages/ monocytes, B cells, eosinophils, mast cells and keratinocytes41. The major physiologic roles of IL–10 include limiting inflammation, preventing an overwhelming immune response and supporting the humoral immune response42,43. Previous studies suggest a crucial immunosuppressive role for IL–10, as the specific cytokine induces the differentiation of a subset of regulatory CD4+ T cells (Tr1) that produce IL–10 and some IL–544,47. IL–5 is a cytokine primarily involved in the pathogenesis of atopic diseases and acts as a link between T cell activation and eosinophilic inflammation47. In our study, the number of IL–5 producing cells was significant decreased in OLP group (p<0.05).
Furthermore, anti–keratinocytes auto–cytotoxic T cell clones in OLP lesions suggest a role for autoimmunity and cell–mediated cytotoxity in OLP48.
Therefore, the decreased number of IL–10 producing cells in peripheral blood in OLP patients may indicate an inability of the immune system to inhibit the cytotoxic process following the antigen recognition in OLP lesions. Previous studies have shown defective peripheral immune suppressor function in OLP49 and have suggested that a defect in immunoregulation –specifically involving suppressor mechanisms– may facilitate cellular autoreactivity in OLP40.
In addition, the number of IL–6 producing cells was also found to be decreased in OLP patients, but non statististically important (p>0.05). IL–6 may be produced by various activated cells, including monocytes, macrophages, T cells and B lymphocytes IL–6 plays an important role in acute phase response, in B cell differentiation and stimulation, in T cell growth and differentiation49– 52. Previous studies have observed elevated IL–6 serum levels in OLP patients which reflect the chronic inflammatory nature of OLP. This could be due to local and systemic production of IL–6 by many cell types27,53. Moreover, Sun et al54 suggested that peripheral blood mononuclear cells and endothelial cells may be the systemic cellular sources of IL–6. Even though IL–6 plays an important role in acute phase reactions, endogenous IL–6 plays a crucial anti–inflammatory role in both local and systemic acute inflammatory responses by controlling the level of proinflammatory type 1 cytokine55. Thus, the decreased number of IL–6 producing cells in peripheral blood in OLP patients may indicate suppression of peripheral immune response in OLP patients that finally allows the OLP inflammatory process to proceed.
Even if the results in our study have shown decreased number of cells producing the type 2 cytokines IL–10, IL– 5 and IL–6 in peripheral blood of OLP patients, the number of IL–4 producing cells was found increased in OLP patients, although there was no statistical significance (p>0.05). IL–4 is the principal cytokine that promotes the development of type 2 cytokines secreting cells and stimulates B cell Ig heavy chain class switching to the IgE isotype56,57. Few studies in OLP sera have shown a slight increase of serum IL–4 levels or nearly the same levels as the control group11,58. Although IL–4 is not strictly an anti–inflammatory cytokine, in some cases it can regulate autoimmunity by antagonizing the development of type 1 responses59.
The lack of difference in the number of cytokine type 1 and type 2 producing cells (IFN–γ IL–12, TNFα and IL–10 IL–5, IL–4 and IL–6 respectively) suggests that the same deficiency of the immune system can be expressed with different clinical picture.
The decreased numbers of IFN–γ, IL–12, IL–2 and TNF–a producing cells in OLP indicates suppressed function and down regulation of type 1 immune response. Whether this alteration of the immune condition in OLP patients participates to the pathogenic pathway of the disease, remains to be determined. As for type 2 cytokines, the decreased number of the IL–10 producing cells in OLP could not be able to inhibit the activation and expansion of autoreactive lymphocytes and might allows cytotoxic reaction to proceed in OLP lesion.
References
- 1.Sugerman PB, Savage NW, Zhou X, Walsh LJ, Bigby M. Oral lichen planus. Clin Dermatol. 2000;18:533–539. doi: 10.1016/s0738-081x(00)00142-5. [DOI] [PubMed] [Google Scholar]
- 2.Axéll T, Rundqvist L. Oral lichen planus–a demographic study. Community Dent Oral Epidemiol. 1987;15:52–56. doi: 10.1111/j.1600-0528.1987.tb00480.x. [DOI] [PubMed] [Google Scholar]
- 3.Salonen L, Axéll T, Helldén L. Occurrence of oral mucosal lesions, the influence of tobacco habits and an estimate of treatment time in an adult Swedish population. J Oral Pathol Med. 1990;19:170–176. doi: 10.1111/j.1600-0714.1990.tb00819.x. [DOI] [PubMed] [Google Scholar]
- 4.McCreary CE, McCartan BE. Clinical management of lichen planus. Brit J Oral Maxillofacial Surg. 1999;5:338–343. doi: 10.1054/bjom.1999.0131. [DOI] [PubMed] [Google Scholar]
- 5.Thornhill MH. Immune mechanisms in oral lichen planus. Acta Odontol Scand. 2001;59:174–177. doi: 10.1080/000163501750266774. [DOI] [PubMed] [Google Scholar]
- 6.Sugerman PB, Savage NW, Walsh,, et al. The pathogenesis of oral lichen planus. Crit Rev Oral Biol Med. 2002;13:350–365. doi: 10.1177/154411130201300405. [DOI] [PubMed] [Google Scholar]
- 7.Edwards P, Kelsch R. Oral lichen planus. Clinical presentation and management. J Canad Dent Assoc. 2002;68:494–499. [PubMed] [Google Scholar]
- 8.Konttinen YT, Jungell P, Bergroth V, Hampf G, Kemppinen P, Malmstrom M. PHA stimulation of peripheral blood lymphocytes in oral lichen planus. Abnormality localized between interleukin–2 receptor ligand formation and gamma–interferon secretion. J Clin Lab Immunol. 1989;28:33–37. [PubMed] [Google Scholar]
- 9.Malmstrom M, Konttinen YT, Jungell P. Lymphocyte activation in oral lichen planus. Proc Finn Dent Soc. 1989;85:109–117. [PubMed] [Google Scholar]
- 10.Yamamoto T, Yoneda K, Ueta E, Osaki T. Cellular immunosuppression in oral lichen planus. J Oral Pathol Med. 1990;19:464–470. doi: 10.1111/j.1600-0714.1990.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Yoneda K, Ueta E, Hirota J, Osaki T. Serum cytokine levels in patients with oral mucous membrane disorders. J Oral Pathol Med. 1991;20:275–279. doi: 10.1111/j.1600-0714.1991.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 12.Paul WE. Pleiotropy and redundancy: T cell derived lymphokines in the immune response. Cell. 1989;57:521–524. doi: 10.1016/0092-8674(89)90121-9. [DOI] [PubMed] [Google Scholar]
- 13.Balkwill FR, Burke F. The cytokine network. Immunol Today. 1989;10:299–304. doi: 10.1016/0167-5699(89)90085-6. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann T, Sad S. The expanding universe of T cell subsets: TH1, TH2 and more. Immunology Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 15.Andreasen JO, Pindborg JJ. Cancerudvikling: Oral lichen planus: An literaturversigt. Nordisk Medicin. 1963;31:861–865. [PubMed] [Google Scholar]
- 16.Romagnani S. Induction of Th1 and T2 responses: a key role for the 'natural' immune response? Immunol Today. 1992;13:379–381. doi: 10.1016/0167-5699(92)90083-J. [DOI] [PubMed] [Google Scholar]
- 17.Paul WE, Seder RA. Lymphocytes responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 18.Gately ML, Warrier RR, Honasoge,, et al. Administration of recombinant IL–12 to normal mice enhances cytolytic lymphocytic activity and induces production of IFN gamma in vivo. Int Immunol. 1994;6:157–167. doi: 10.1093/intimm/6.1.157. [DOI] [PubMed] [Google Scholar]
- 19.Nastala CL, Edington HD, McKinney TG, et al. Recombinant IL–12 administration induces tumor regression in association with IFN gamma production. J Immunol. 1994;153:1697–1706. [PubMed] [Google Scholar]
- 20.Liblau R, Singer S, McDevitt H. Th1 and Th2 CD4+ T cells in the pathogenesis of organ–specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 21.Smith KA. Interleukin–2: inception, impact and implications. Science. 1988;240:1169–1176. doi: 10.1126/science.3131876. [DOI] [PubMed] [Google Scholar]
- 22.Gullberg M, Smith K. Regulation of T cell autocrine growth. T4+ cells become refractory to interleukin 2. J Exp Med. 1986;163:270–284. doi: 10.1084/jem.163.2.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cousens LP, Orange JS, Biron CA. Endogenous IL–2 contributes to T cell expansion and IFN-gamma production dyring lymphocytic choriomeningitis virus infection. J Immunol. 1995;155:5690–5699. [PubMed] [Google Scholar]
- 24.Kasahara T, Hooks JJ, Dungherty SF, Oppenheim JJ. Interleukin–2 mediated immune interferon (IFN–γ) production by human T cells and T cell subsets. J Immunol. 1983;130:1784–1789. [PubMed] [Google Scholar]
- 25.Vassali P. The pathophysiology of tumor necrosis factor. Ann Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 26.Porter SR, Kirby A, Olsen I, Barrett W. Immunological aspects of dermal and oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:358–366. doi: 10.1016/s1079-2104(97)90244-4. [DOI] [PubMed] [Google Scholar]
- 27.Karagouni E, Dotsika E, Sklavounou A. Alteration in peripheral blood mononuclear cell function and serum cytokines in oral lichen planus. J Oral Pathol Med. 1994;23:28–35. doi: 10.1111/j.1600-0714.1994.tb00250.x. [DOI] [PubMed] [Google Scholar]
- 28.Walsh LJ, Savage W, Ishii T, Seymour GJ. Immunopathogenesis of oral lichen planus. J Oral Pathol Med. 1990;19:389–396. doi: 10.1111/j.1600-0714.1990.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 29.Sugerman PB, Satterwhite K, Bigby M. Auto–cytoxic T cell clones in lichen planus. Br J Dermatol. 2000;142:449–456. doi: 10.1046/j.1365-2133.2000.03355.x. [DOI] [PubMed] [Google Scholar]
- 30.Khan A, Farah CS, Savage NW, Walsh LJ, Harbrow DJ, Sugerman PB. Th1 cytokines in oral lichen planus. J Oral Pathol. 2003;32:77–83. doi: 10.1034/j.1600-0714.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 31.Ueta E, Umazume M, Yamamoto T, Osaki T. Leukocyte dysfunction in oral mucous membrane diseases. J Oral Pathol. 1993;22:120–125. doi: 10.1111/j.1600-0714.1993.tb01042.x. [DOI] [PubMed] [Google Scholar]
- 32.Simark M, Jontell M, Bergenholtz G, Heyden M, Dahlgren UI. Distribution of interferon–gamma mRNA–positive cells in oral lichen planus lesions. J Oral Pathol Med. 1998;27:483–488. doi: 10.1111/j.1600-0714.1998.tb01917.x. [DOI] [PubMed] [Google Scholar]
- 33.Simark–Mattsson C, Bergenholtz G, Jontell M, Eklund C, Seymour GJ, Sugerman PB. Distribution of interleukin–2, –4, –10, tumour necrosis factor–alpha and transforming growth factor-beta mRNAs in oral lichen planus. Arch Oral Biol. 1999;44:499–507. doi: 10.1016/s0003-9969(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto T, Osaki T. Characteristics cytokines generated by keratinocytes and mononuclear infiltrates in oral lichen planus. J Invest Dermatol. 1995;104:784–788. doi: 10.1111/1523-1747.ep12606990. [DOI] [PubMed] [Google Scholar]
- 35.Rabinovitch A. Immunoregulatory and cytokine imbalances in the pathogenesis of IDDM. Diabetes. 1994;43:613–621. doi: 10.2337/diab.43.5.613. [DOI] [PubMed] [Google Scholar]
- 36.La Cava A, Sarvetnick N. The role of cytokines in autoimmunity. Curr Dir Autoimmunity. 1999;1:56–71. doi: 10.1159/000060495. [DOI] [PubMed] [Google Scholar]
- 37.Cope AP. Regulation of autoimmunity by proinflammatory cytokines. Curr Opin Immunol. 1998;10:669–676. doi: 10.1016/s0952-7915(98)80087-3. [DOI] [PubMed] [Google Scholar]
- 38.Hill N, Sarvetnick N. Cytokines. promoters and dampeners of autoimmunity. Curr Opin Immunol. 2002;14:791–797. doi: 10.1016/s0952-7915(02)00403-x. [DOI] [PubMed] [Google Scholar]
- 39.Sugerman PB, Voltz MJ, Savage NW, Basford KE, Seymour GJ. Phenotypic and functional analysis of peripheral blood lymphocytes in oral lichen planus. J Oral Pathol Med. 1992;21:445–450. doi: 10.1111/j.1600-0714.1992.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 40.Sugerman PB, Rollason PA, Savage NW, Seymour GJ. Suppressor cell function in oral lichen planus. J Dent Res. 1992;71:1916–1919. doi: 10.1177/00220345920710121201. [DOI] [PubMed] [Google Scholar]
- 41.Hagiwara E, Abbasi F, Mor G, Ishigatsubo Y, Klinman D. Phenotype and frequency of cells secreting IL–2, IL–4, IL–6, IL–10, IFN and TNF–A in human peripheral blood. Cytokine. 1995;7:815–822. doi: 10.1006/cyto.1995.0098. [DOI] [PubMed] [Google Scholar]
- 42.Beissert S, Hossoi J, Kuhn R, Rajewsky K, Muller W, Granstein RD. Impaired immunosuppressive response to ultraviolet radiation in interleukin–10–deficient mice. J Invest Dermatol. 1996;107:553–557. doi: 10.1111/1523-1747.ep12582809. [DOI] [PubMed] [Google Scholar]
- 43.Spits H, de Waal MR. Functional characterization of human IL–10. Int Arch Allergy Immunol. 1992;99:8–15. doi: 10.1159/000236329. [DOI] [PubMed] [Google Scholar]
- 44.Cottrez F, Hurst SD, Coffman RL, Groux H. T regulatory cells inhibit a Th2 specific response in vivo in process citation. J Immunol. 2000;165:4848–4853. doi: 10.4049/jimmunol.165.9.4848. [DOI] [PubMed] [Google Scholar]
- 45.Groux H, Cottrez F. The complex role of interleukin–10 in autoimmunity. Journal of Autoimmunity. 2003;4:281–285. doi: 10.1016/s0896-8411(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 46.Groux H, Bigler M, O'Garra A, Rouleau M, Antonenko S, de Vries J. A CD4+ T cell subset inhibits antigen– specific T cells responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 47.Schwenger G, Mordvinov V, Fournier R, Czabotar P, Peroni S, Sanderson C. IL–5. In: Oppenheim J, Feldmann M, editors. Cytokine Reference. 1st ed. San Diego: Academic Press; 2001. pp. 862–862. [Google Scholar]
- 48.Sugerman PB, Satterwhite K, Bigby M. Auto–cytoxic T cell clones in lichen planus. Br J Dermatol. 2000;142:449–456. doi: 10.1046/j.1365-2133.2000.03355.x. [DOI] [PubMed] [Google Scholar]
- 49.Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon b2/B–cell stimulatory factor type 2 shares identity with monocyte–derived hepatocyte–stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci USA. 1987;84:7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Helfgott DC, May LT, Sthoeger Z, Tamm I, Sehgal PB. Bacterial lipopolysaccharide (endotoxin) enhances expression and secretion of beta 2 interferon by human fibroblasts. J Exp Med. 1987;166:1300–1309. doi: 10.1084/jem.166.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka Y, Saito K, Shirakawa, et al. Production of B cell –stimulatory factors by B cell in patients with systemic lupus erythematosus. J Immunol. 1988;141:3043–3049. [PubMed] [Google Scholar]
- 52.Van Snick J, Vink A, Cayphas S, Uyttenhove C. Inteleukin–HP1, a T cell–derived hybridoma growth factor that supports the in vitro growth of murine plasmacytomas. J Exp Med. 1987;165:641–649. doi: 10.1084/jem.165.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu G, Martin M, Darveau R, Truelove E, Epstein J. Oral and serum IL–6 levels in oral lichen planus patients. Oral Surg Oral Med Oral Pathol Oral Radiol Oral Endod. 2004;98:673–678. doi: 10.1016/j.tripleo.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 54.Sun A, Chia JS, Chang YF, Chiang CP. Serum inteleukin–6 level is a useful marker in evaluating therapeutic effects of levemisole and Chinese medicinal herbs on patients with oral lichen planus. J Oral Pathol Med. 2002;31:196–203. doi: 10.1034/j.1600-0714.2002.310402.x. [DOI] [PubMed] [Google Scholar]
- 55.Xing Z, Gaultie J, Cox G, et al. IL–6 is an anti–inflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abbas A, Lichtman A, Pober J. Cellular and molecular immunology. 4th ed. Philadelphia: Saunders; 2000. 259 pp. [Google Scholar]
- 57.Mossman TR, Coffman RL. TH1 and TH2 cells:different patterns of lymphokine secretion lead to different functional properties. Ann Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto T, Yoneda K, Ueta E, Osaki T. Serum cytokines, interleukin–2 receptor and soluble intercellular adhesion molecule–1 in oral disorders. Oral Surg Oral Med Oral Pathol. 1994;78:727–737. doi: 10.1016/0030-4220(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 59.Hill N, Sarvetnick N. Cytokines: promoters and dampeners of autoimmunity. Cur Opinion Immunol. 2002;14:791–797. doi: 10.1016/s0952-7915(02)00403-x. [DOI] [PubMed] [Google Scholar]