Abstract
Alveolar soft– part sarcoma (ASPS) is a rare form of soft tissue sarcoma and is most often seen in adolescents and young adults. Surgical excision of the primary tumor and pulmonary metastases has resulted in prolonged survival in some patients while the benefit of adjuvant chemotherapy and/or radiotherapy has been disputed. An 11– year–old boy with ASPS which presented with a markedly vascular tumor in the left thigh, and multiple bilateral pulmonary metastases 8 months after diagnosis is described. The patient has remained disease–free for over 5 years since the initial diagnosis.
Keywords: alveolar soft – part sarcoma, children, pulmonary, metastases, long term survival
Alveolar soft–part sarcoma (ASPS) was first described as a separate entity in 19521. ASPS represents less than 1% of all sarcomas in children and adults. The disease primarily affects younger patients, with the median age at diagnosis significantly lower than that for the form of soft tissue sarcoma. The peak age incidence is between 15 and 35 years. The tumor is frequently localized in the lower extremities in adults and in the head and neck and particularly effects the tongue and orbit in children2–4,5. ASPS is a slow–growing, indolent tumor with metastases that may appear late in the lung, bone and brain4–6,7. Brain metastases have been described as a common feature metastatic ASPS, whereas they are reported to be relatively unusual with other high grade sarcomas8. ASPS tumor is a highly vascular soft – tissue tumor that is uniformly malignant and in the long run ASPS is usually a fatal disease. Death has occurred from disseminated sarcoma as late as 20 years from initial diagnosis6,8. The 5 year survival rate is reported to be to 45% – 88%, 38% at 10 years and 15% at 20 years8,10. Children have a better overall survival as compared with adults. Surgical excision with an attempt at obtaining tumor free margins is accepted as the treatment of choice for both primary tumors and metastatic tumors in the brain and lung.
As a consequence of the rarity of the disease, the majority of reports concerning ASPS are mostly individual of case reports and small collective series while there are a few large series reported in the literature today. As such, the literature base for clinical decision–making for patients with ASPS is quite limited– we present a young boy with ASPS and review the literature.
Case report
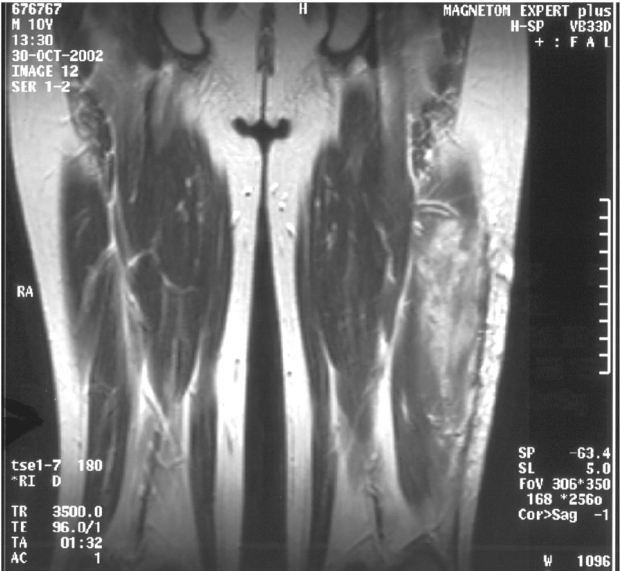
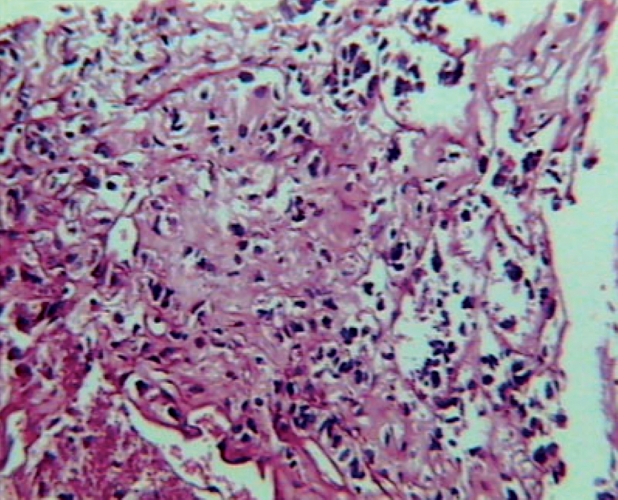
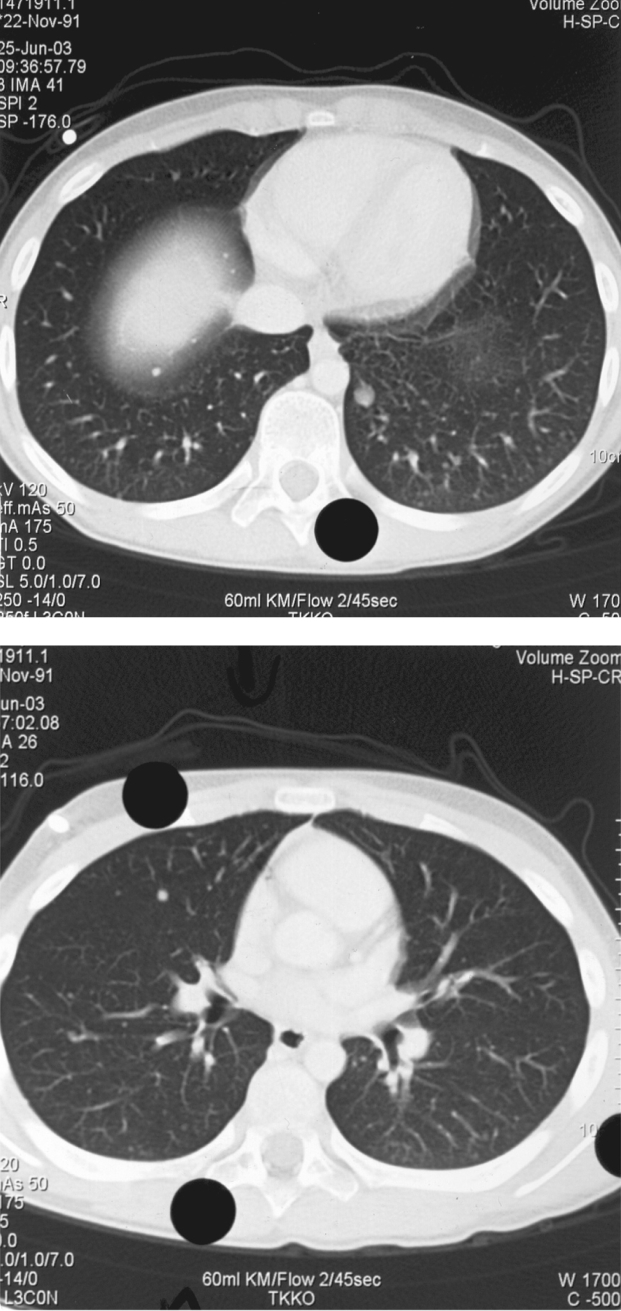
An 11 year–old boy was admitted to our department, on 22nd October 2002 with one and a half month history of gradually increasing left leg pain with a palpable mass on the external surface of the left thigh. The initial MRI showed a lesion of 9cmx3,5cmx3cm size on the middle of the external surface of the left quadriceps. The lesion was of hypointense signal intensity on T1–W images, showing homogeneous intense enhancement after intravenous (iv) contrast administration. The ragiologic features suggested that the lesion was probably benign and a tentative diagnosis of haemangioma was made (Figure 1A,1B). A radical resection of the tumor was performed, and at biopsy was confirmed the diagnosis of ASPS. The histopathlogy showed the characteristic features of ASPS. The most characteristic light microscopic feature was that of on organoid or nesting pattern. The nest tend to be uniform that were separated by delicate partitions of connective tissue containing sinusoidal vascular channels lined by flattened endothelium. The cytoplasm was abundant and the cells frequently contained rhomboid or rod shaped crystalline inclusions (Figure 2). The resected specimen had gross margins that were disease–free. The initial CT scan of the chest and the bone scan did not show evidence for metastases. Following informed consent, the patient was started a soft tissue sarcoma chemotherapy protocol of MMT–89 (SIOP). The therapy consisted of cyclic courses of vincristine (1.5 gr/m2) iv ifosfamide (3 gr/m2) with 3 gr/m2 of mesna [2–mercaptoethane sulfonate] iv (day 1, 2 and 3) and doxorubicin (30 mg/m2) iv (day 4) alternated every 3 weeks with vincristine iv (1.5 mg/m2) (day 1), Dactinomycin (1.5 mg/m2) iv (day 1) and ifosfamide iv ( 3 gr/m2 ), (day 1, 2 and 3) with 3 gr/m2 mesna iv. The patient received 6 cycles of chemotherapy and he presented median grade myelosuppression after every cycle, with anaemia (Hb: 8.5g/dl), neutropenia (granulocytes 500/µl) and thrombocytopenia platelets (20,000/µl). He was supported by GCSF, packed red cells and platelets. After chemotherapy he received local radiotherapy to the tumour bearing compartment of the left thigh, to a total dose of 50.4 Gy, including the resection scar, followed by a boost to the tumour area an additional 9 Gy. Even though the initial chest CT scan was negative, a second follow up CT scan of the chest (Figure 3A, 3B), 8 months from the initial diagnosis and two from the end of chemotherapy revealed bilateral pulmonary metastases (4 on the right and 2 on the left lung), but a brain CT scan and bone scan did not show evidence for metastases. The patient underwent sternotomy for exploration of the lungs and possible removal of as many metastases as possible. Histologically the lesions removed showed viable disease. The patient received 15Gy of bilateral pulmonary irradiation and was subsequently started on the less aggressive maintenance regimen of the German Soft Tissue Tumour Trial 2002 with: daily oral cyclophosphamide (2 x 25 mg/ m2) in combination with weekly vinblastine (3 mg/m2) iv on days 1, 8 and 15 to be repeated starting on day 28 for a year. The patient remains disease–free and clinically well with full activity over 5 years since the initial diagnosis. Now he is in a follow up every 6 months. The movement of the left arm, the heart and renal function are normal and our patient has an excellent quality of life.
Figure1: 1A,1B.MRI of the left thigh. (The ragiologic features suggested that the lesion was probably benign).
Figure2: Photomicrograph of patient's primary tumor of the left thigh. The neoplasm shown the characteristic features of ASPS.
Figure3: 3A, 3B Chest CT scan showing pulmonary metastases.
Discussion
ASPS is one of the rarest soft tissue sarcomas. The mean age at diagnosis is about 22 years in females and 27 years in males, but the tumor can occur in children as young as 2 year old. It tends to grow slowly and insidiously often with a long clinical history and a large mass at presentation. The most common site of origin is the lower limb, followed by the trunk and the upper limbs. Almost all cases appear to arise in skeletal muscles. Numerous case reports describe ASPS, in diverse anatomic locations as the mediastinum, stomach, larynx and there is the first documentation of primary ASPS of the liver9. Despite the slow growth rate of the primary tumor, metastases are common. They are detected in about 20–25% of patients at diagnosis. Metastases are found most often in the lungs, followed in frequency by bone and brain 10. ASPS is typically very vascular a feature, reflected on imaging studies, which may show large intra–and extra tumoral blood vessels11. MR features of our patient lesions simulated those of haemangioma but the histology showed ASPS.
While surgical resection of ASPS primary and metastatic tumors remains the treatment of choice, the decision making of the optimal an adjuvant chemotherapy remains a challenge12. Adjuvant radiation therapy has been given to some children but the results are difficult to evaluate. Our patient – a 11–year old boy with an ASPS of the left thigh – has received intense chemotherapy as per MMT – 89 (SIOP) protocol (6 cycles) followed by radiotherapy (50,4 Gy). Nonetheless, bilateral pulmonary metastases appeared on the chest CT scan 8 months from the initial diagnosis and two months from the end of his adjuvant chemotherapy revealed bilateral pulmonary metastases which showed chemotherapy resistant disease. He subsequently received a less aggressive maintenance regimen, aiming at slowing the disease progression by inducing as much antiangiogenesis as possible.
Chemotherapy has been used in several reported adolescent patients with ASPS, but only one of 8 has had a long term survival longer that 46 months. Regimens containing doxorubicin, cyclophosphamide, cisplatin, vincristine, dacarbazine and other agents have not been shown to be effective as preoperative and /– or post operative treatment of ASPS12 . However, Baum et al reported a case in which 92 malignant pulmonary ASPS metastases where surgically excised followed by adjuvant combination chemotherapy (vincristine, actinomycin D, cyclophosphamide and doxorubicin) resulting in disease free survival of at least 5 years13. Portera et al presented a comprehensive review of the clinical presentation, treatment, outcome and patterns of treatment failure in a consecutive series of patients with localized or metastatic ASPS. The authors presented 74 patients with stage II or III (35%) and IV (65%) disease. The 5–year actuarial local recurrence free, distant recurrence free, disease free and overall survival rate among the 22 patients with localized ASPS were 88%, 84%, 71% and 87% respectively. At a medium follow up of 9 years, 2 of 20 patients of localized disease had developed local recurrences and 3 had developed metastatic disease (all to the lung) only. The median survival of patients with metastatic disease was 40 months with a 5–year survival rate of 20%14.
The long term outcome of patients with stage IV (metastatic) ASPS also merits comment. In the 50–year experience of 192 patients reported from the Memorial Sloan–Kettering Cancer Center 22 patients with metastatic ASPS had long term follow up date available and at least 2 of those 22 patients survived beyond 5 years15. In our case, the patient is alive with full activity >5 years from the initial diagnosis. Because of the indolent nature of ASPS and the prolonged interval between primary presentation and the appearance of metastases the disease–free survival of > 5 years in our patient is encouraging.
References
- 1.Christopherson VΜ, Foote FM, Stewart FW. Alveolar soft part sarcoma: structurally characteristic tumors of uncertain histogenesis. Cancer. 1952;5:100–111. doi: 10.1002/1097-0142(195201)5:1<100::aid-cncr2820050112>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Nickerson HJ, Silberman T, Jacobsen FS, et al. Alveolar soft – part sarcoma responsive to intensive chemotherapy. J Pediatr Hematol Oncol. 2004;26:233–235. doi: 10.1097/00043426-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Kim HS, Lee HK, Weon YC, Kim HS. Alveolar soft –part sarcoma of the head and neck: clinical and imaging features in five cases. Am J Neuroradiol. 2005;26:1331–1335. [PMC free article] [PubMed] [Google Scholar]
- 4.Aliken AH, Stone JA. Alveolar soft – part sarcoma of the tongue. Amer J Neuroradiol. 2003;24:1156–1158. [PMC free article] [PubMed] [Google Scholar]
- 5.Marchac A, Picard A, Landman–Parker J, et al. A pediatric case of Alveolar Soft Part Sarcoma. Rev Stomatol Chir Maxillofac. 2007;108:547–550. doi: 10.1016/j.stomax.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Kodama Κ, Doi O, Higashiyama Μ, et al. Surgery for multiple lung metastasis from alveolar soft–part sarcorna. Surg Today. 1997;27:806–811. doi: 10.1007/BF02385270. [DOI] [PubMed] [Google Scholar]
- 7.Κuriyama Κ, Todo S, Hibi S, et al. Alveolar soft part sarcoma with lung metastases. Response to interferon alpha-2a? Med Pediatr Oncol. 2001;37:482–483. doi: 10.1002/mpo.1237. [DOI] [PubMed] [Google Scholar]
- 8.Auerbach HE, Brooks J. Alveolar soft – part sarcoma. A clinicopathologic and immunohistochemical study. Cancer. 1987;60:66–73. doi: 10.1002/1097-0142(19870701)60:1<66::aid-cncr2820600112>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Shaddix KK, Fachre GP, Nields WW, et al. Primary alveolar soft – part sarcoma of the liver: anomalous presentation of a rare disease. Am Surg. 2008;74:43–46. [PubMed] [Google Scholar]
- 10.Zamani F, Jabhar M, Alimohamad S, et al. Primary alveolar soft part sarcoma of chest wall: A case report and review. Meds Gen Med. 2006;8:2. [PMC free article] [PubMed] [Google Scholar]
- 11.Suh IS, Cho J, Lee SH, et al. Alveolar soft part sarcoma: MR and angiographic findings. Skeletal Radiol. 2000;29:680–689. doi: 10.1007/s002560000285. [DOI] [PubMed] [Google Scholar]
- 12.Miser JS, Kinsel1a TJ, Τriche TJ, et al. Ifosfamide with mesna uroprotection and etoposide: an effective regimen in the treatment of recuπent sarcomas and other tumors of children and young adults. J Clin Oncol. 1987;5:1191–1198. doi: 10.1200/JCO.1987.5.8.1191. [DOI] [PubMed] [Google Scholar]
- 13.Baum E, Fickenscher I, Nachman JB, et al. Pulmonary resection and chemotherapy for metastatic alveolar sot – part sarcoma. Cancer. 1981;47:1946–1948. doi: 10.1002/1097-0142(19810415)47:8<1946::aid-cncr2820470805>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 14.Portera CA, Jr, Ηο V, Pate! SR, et al. Alveolar soft part sarcoma: clinical course and patterns of metastasis in 70 patients treated at a single institution. Cancer. 2001;91:585–591. doi: 10.1002/1097-0142(20010201)91:3<585::aid-cncr1038>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman PH, Brennan MF, Kimmel M, Erandson RA, Garin–Chesa P, Flehinger BY. Alveolar soft–part sarcoma. A clinicopathologic study of half a century. Cancer. 1989;63:1–13. doi: 10.1002/1097-0142(19890101)63:1<1::aid-cncr2820630102>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]