Abstract
Salient stimuli that modify behavior induce transcription of Activity regulated cytoskeleton-associated protein (Arc/Arg3.1) and transport Arc mRNA into dendrites, suggesting that local Arc translation mediates synaptic plasticity encoding such stimuli. Here we demonstrate that long-term synaptic depression (LTD) in hippocampal neurons induced by group 1 metabotropic glutamate receptors (mGluRs) relies on rapid translation of Arc. MGluR-LTD induction causes long-term increases in AMPA receptor endocytosis rate and dendritic synthesis of Arc, a component of AMPAR endocytosis machinery. Knockdown of Arc prevents mGluRs from triggering AMPAR endocytosis or LTD, and acute blockade of new Arc synthesis with antisense oligonucleotides blocks mGluR-LTD and AMPAR trafficking. In contrast, LTD induced by NMDA receptors does not persistently alter AMPAR endocytosis rate, induce Arc synthesis, or require Arc protein. These data demonstrate a role for local Arc synthesis specifically in mGluR-LTD and suggest that mGluR-LTD may be one consequence of Arc mRNA induction during experience.
Keywords: Arc/Arg3.1, group I mGluRs, long-term depression, AMPA receptor trafficking
Introduction
Learning and memory, as well as the underlying synaptic plasticity that mediates adaptive behaviors, require the synthesis of new genes and proteins (Davis and Squire, 1984; Kelleher et al., 2004). In particular, rapid synthesis of new proteins in dendrites or at individual synapses likely contributes to the longevity and synapse selectivity of plasticity (Steward and Schuman, 2001). Characterization of the mRNAs and protein targets whose synthesis is required for the cellular models of learning, long-term potentiation (LTP) and depression (LTD) will be instrumental in understanding how experience leads to plasticity of individual synapses. Activity-regulated cytoskeleton associated protein (Arc) (also termed Activity regulated gene of 3.1kb or Arg3.1) is an immediate early gene induced in response to sensory experience, learning, LTP, spatial exploration, and novelty (Gusev et al., 2005; Guzowski et al., 1999; Guzowski et al., 2006; Link et al., 1995; Lyford et al., 1995; Montag-Sallaz et al., 1999; Ons et al., 2004). These associations implicate a role for Arc in shaping plastic changes brought about by salient neuronal stimuli. Intriguingly, Arc mRNA is transported into dendrites following induction and specifically accumulates at active synapses, suggesting that local synthesis of Arc protein at synapses may mediate plasticity and encoding of Arc-inducing stimuli (Link et al., 1995; Lyford et al., 1995; Moga et al., 2004; Steward et al., 1998; Steward and Worley, 2001). In support of this idea, constitutive deletion of Arc or acute inhibition of Arc synthesis with antisense oligonucleotides inhibits memory consolidation (Guzowski et al., 2000; McIntyre et al., 2005; Plath et al., 2006).
The function of Arc protein remained elusive until recently when Chowdury et al., (2006) demonstrated that Arc interacts with components of the endocytosis machinery, dynamin and endophilin, to stimulate AMPA receptor endocytosis. Consequently, overexpression of Arc leads to decreases in postsynaptic AMPA receptor expression and excitatory synaptic transmission onto hippocampal CA1 neurons (Chowdhury et al., 2006; Rial Verde et al., 2006). Arc-induced decreases in synaptic function are thought to share mechanisms with acute forms of synaptic depression or LTD that is induced by low frequency synaptic stimulation (1 Hz) and NMDA receptor activation (Plath et al., 2006; Rial Verde et al., 2006). However Arc transcription and translation are induced by high frequency (> 100 Hz) synaptic stimulation that induces synapse strengthening or LTP, not low frequency, LTD-inducing, stimulation (Link et al., 1995; Steward et al., 1998). Therefore, it has been suggested that Arc may mimic LTD, but may not normally contribute to NMDAR-induced LTD (Rial Verde et al., 2006). Instead, Arc induced synaptic depression has been proposed to function as a homeostatic mechanism to reset total synaptic strength on a neuron during LTP or high levels of neuronal activity (Rial Verde et al., 2006; Shepherd et al., 2006). However, it remains unknown whether there are LTD paradigms that induce Arc or if Arc plays a physiological role in LTD.
Activation of group 1 metabotropic glutamate receptors (mGluRs) induces a form of LTD in CA1 neurons that is distinct from NMDAR-dependent LTD (Huber et al., 2000; Oliet et al., 1997). MGluR- and NMDAR-dependent LTD are both mediated by endocytosis and decreased surface expression of postsynaptic AMPARs, but they rely on distinct signaling cascades and do not occlude each other (Carroll et al., 1999; Colledge et al., 2003; Huber et al., 2001; Moult et al., 2006; Oliet et al., 1997; Snyder et al., 2001). The latter suggests that mGluR- and NMDAR-dependent LTD are expressed at different synapses and/or affect different populations of AMPARs. What determines which synapses or AMPARs are susceptible to mGluR- or NMDAR-LTD is unknown. MGluR-mediated effects are particularly interesting, because mGluR-LTD and the associated decreases in surface AMPARs rely on rapid dendritic protein synthesis, unlike NMDAR-LTD (Huber et al., 2000; Snyder et al., 2001). Consistent with the dependence on dendritic protein synthesis, mGluR-LTD is abnormal in a mouse model of Fragile X Syndrome mental retardation that is caused by a deficit in dendritic RNA binding protein Fragile X Mental Retardation Protein (FMRP) (Hou et al., 2006; Huber et al., 2002; Koekkoek et al., 2005; Nosyreva and Huber, 2006). From this work, it has been proposed that the mRNAs whose translation is required for mGluR-LTD (termed “LTD proteins”) are regulated by FMRP and code for proteins that regulate AMPAR trafficking. Interestingly, FMRP associates with Arc mRNA, although a direct interaction is controversial (Iacoangeli et al., 2008; Zalfa et al., 2007; Zalfa et al., 2003).
In an effort to identify LTD proteins, we evaluated how mGluR activation leads to long-term decreases in AMPAR surface expression. Here we report that brief activation of group 1 mGluRs results in a persistent increase (of at least 1 hour) in the endocytosis rate for GluR1 that requires new protein synthesis. This suggested that mGluRs stimulate synthesis of a rate-limiting component of the AMPAR endocytosis machinery. Due to its role in AMPAR endocytosis and dendritic mRNA expression, we focused our analysis on Arc. We present data that rapid Arc synthesis is required for mGluR-LTD and functions as an LTD protein.
Results
Brief group 1 mGluR activation induces a long-term, protein synthesis dependent increase in AMPAR endocytosis rate
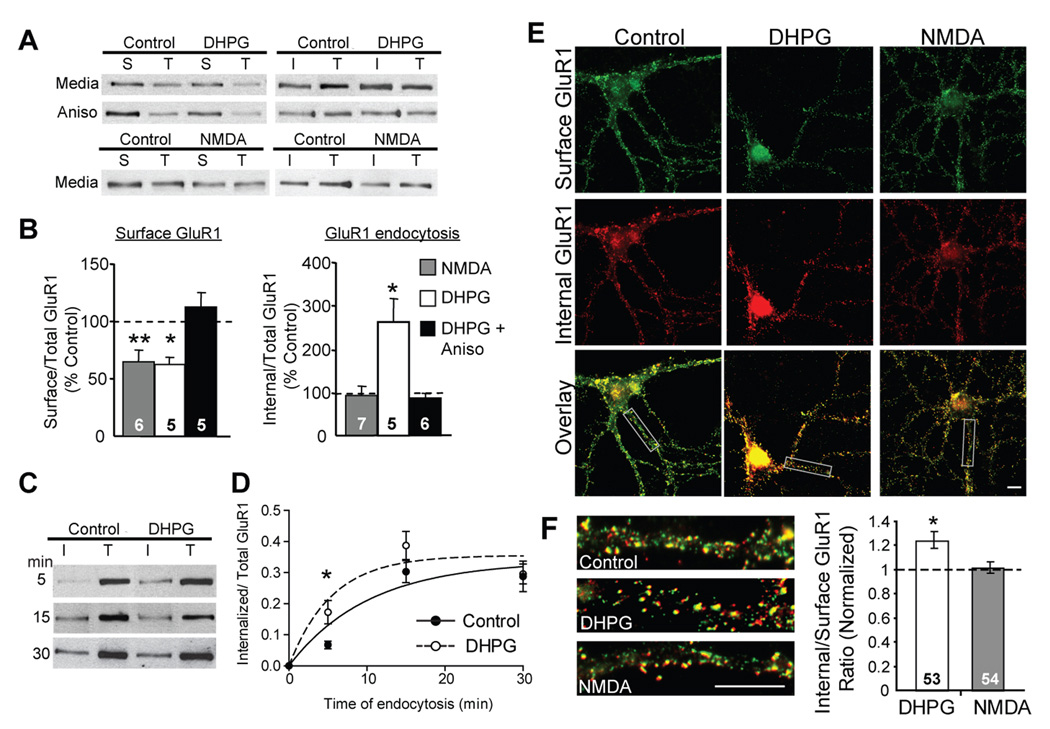
To determine how brief mGluR activation persistently alters AMPAR trafficking, we first measured surface AMPAR levels and the internalization rate of AMPA receptor subunit GluR1 using receptor biotinylation in high-density, dissociated, hippocampal neuron cultures. Application of the group I mGluR agonist DHPG (100 µM, 5 min) resulted in a long-term (1 hour) decrease in surface levels of the AMPAR subunits GluR1 (Fig. 1A, B) and GluR2/3 (Supp. Fig. 1C). To determine if mGluR-LTD correlates with persistent changes in GluR1 internalization rate, surface receptors were labeled with a cleavable form of biotin one hour after DHPG application and allowed to internalize at 37°C for 5 minutes. Biotin bound on remaining surface receptors was then cleaved, and internalized receptors, as well as total GluR1 levels, were measured. In these measurements, total GluR1 levels were not affected by DHPG (107 ± 7% of control; n = 17 cultures; p = 0.5) and therefore were used to normalize the internalized receptors within the same sample. According to this assay, the accumulation of intracellular GluR1 (during the 5 min endocytosis period), one hour after DHPG, increased to 263 ± 57% above control cultures (Fig. 1C, D; Supp. Table 1). These data indicate that even one hour after DHPG treatment, we observed an increase in endocytosis rate. In principle, this increased rate, accompanied by decreased surface GluR1, could be due to either an increase in the endocytosis rate constant (kendo) or a decrease in the exocytosis rate constant (kexo) (assuming a model of 1st order kinetics; see Supp. Fig. 2 legend). Two separate calculations, one made through measuring dynamic receptor internalization and the other made using steady state receptor levels, indicate the former. In a two pool receptor model where all surface receptors are available for endocytosis, the internalization rate constant can be calculated as the slope of a plot between the ratio of internalized to surface AMPAR versus time. With this approximation, the relative change in the endocytosis rate constant (kendo) for GluR1, an hour after DHPG washout, (described in Supp. Fig. 2) increased by a factor of 4.4 relative to control, untreated, sister cultures. Using steady state surface GluR1 levels, we also calculated the effects of DHPG on the ratio of the endocytosis to exocytosis rate constants (kendo/kexo), which reveals an increase of similar magnitude (Supp. Fig. 2). To obtain a time constant for endocytosis, GluR1 internalization was examined in control and DHPG treated cultures using longer periods for endocytosis of biotinylated receptors (15 and 30 min). A fit of this data using a single exponential function revealed a time constant of endocytosis (τ) of 10.2 ± 2 min for control cultures that is consistent with previous measurements (Ehlers, 2000; Lee et al., 2004; Lin et al., 2000). One hour after DHPG, the time constant for GluR1 endocytosis became more rapid (τ = 5.5 ± 1 min; Fig. 1C, D). With a first order kinetic model, τ = 1/(kendo + kexo). Therefore, if τ decreases after DHPG, then the sum of kendo and kexo must increase. Because the endocytosis rate goes up, this strongly points to an increase in kendo.
Figure 1. Brief mGluR activation induces a persistent and protein synthesis dependent increase in GluR1 endocytosis rate.
A, Representative blots of surface (S), internalized (I) (100 µg pulldown) and total (T) GluR1 (10 µg protein) from high-density dissociated hippocampal neuron cultures one hour after DHPG or NMDA treatment in media or in the presence of anisomycin (Aniso). B, Brief DHPG treatment (100 µM; 5 min) results in persistent (60 min) decrease in surface GluR1 and increases in endocytosis rate for GluR1 measured with receptor biotinylation relative to control, or media treated, sister cultures. Preincubation of cultures in anisomycin (20 µM) blocks surface GluR1 decreases and GluR1 endocytosis rate increases. Although application of NMDA (20 µM; 3 min) results in a persistent (60 min) decrease in surface GluR1, it does not persistently alter GluR1 endocytosis rate. N = # cultures per condition is on each bar. Paired t-tests were performed on raw ratio values in treated vs. their respective control, media treated cultures (Supp. Table 1). C, Representative western blots of internalized (I) and total (T) GluR1 in high-density hippocampal cultures. One hour after treatment with media or DHPG, surface receptors were labeled with biotin and allowed to internalize at 37°C for 5,15 or 30 minutes, after which the remaining surface biotin was stripped off and internalized, biotinylated GluR1 was measured. D, Quantification of internalized/total GluR1 levels from data in C reveal that GluR1 endocytosis is not saturated at early time points (5 min) in either control or DHPG-treated cultures and therefore reflects endocytosis rate. N = 5 cultures per condition. A best fit single exponential association function (using the Marquardt and Levenberg method) was used to obtain a τ for endocytosis. E, Representative double-label images of surface (green) and internalized (red) staining of GluR1 in low-density hippocampal neurons. Live cultures were labeled with N-terminal GluR1 antibody one hour after treatment with media (Control), DHPG or NMDA. F, Left: Representative proximal dendrites from images of merged surface and intracellular GluR1 immunostaining. Right: Ratiometric analysis of internal to surface GluR1 puncta number reveal that one hour after treatment, DHPG, but not NMDA, persistently increases the internalization rate constant for GluR1. N = # cells per condition is on each bar. Scale bars = 10 µm. Data pooled from 4 cultures each. For all figures * p < 0.05; ** p < 0.01; ***p< 0.001*.
NMDA receptor dependent-LTD also results in long-term decreases in surface AMPARs (Carroll et al., 1999; Colledge et al., 2003). To determine if NMDAR-LTD is associated with persistent increases in GluR1 endocytosis rate, cultures were treated with NMDA (20 µM, 3 minutes), which elicited long-term decreases in surface GluR1 (Colledge et al., 2003), but did not persistently alter GluR1 endocytosis rate, in contrast to mGluR stimulation (Fig. 1A,B; Supp. Table 1). MGluR-induced long-term increases in GluR1 internalization were also detected using immunofluorescence and ratiometric measurements of internalized to surface GluR1 puncta within the same neuron (Lin et al., 2000) (Fig. 1E,F). One hour after DHPG treatment, the internal to surface ratio of GluR1 was elevated above control, untreated, neurons, whereas NMDA treated cultures showed no long-term change in GluR1 internalization rate.
Inhibition of mRNA translation prevents DHPG-induced LTD and the associated decreases in surface GluR1 (Huber et al., 2000; Snyder et al., 2001). Here, we observed that the translation inhibitor anisomycin (25 µM) blocked the late surface decreases in GluR1 expression as well as the persistent increase in GluR1 endocytosis rate after DHPG treatment (Fig. 1A,B; Supp. Table 1). In contrast, NMDA-induced decreases in surface GluR1 persisted in anisomycin (Supp. Fig. 3) (Davidkova and Carroll, 2007). These results suggest that prolonged increases in GluR1 endocytosis rate mediate mGluR-induced decreases in surface GluR1, and therefore LTD. They also suggest that mGluR-LTD requires new synthesis of a rate-limiting protein for GluR1 endocytosis.
MGluR activation induces rapid synthesis of Activity regulated cytoskeleton associated protein
Arc is a well known dendritic mRNA that has recently been shown to stimulate AMPAR endocytosis and is therefore a good candidate to mediate mGluR-LTD (Chowdhury et al., 2006; Lyford et al., 1995). If so, then Arc should be synthesized in response to mGluR activation. In support of this idea, we observed that DHPG treatment of cultures leads to rapid increases in dendritic Arc protein levels (within 10 min) as detected with immunocytochemistry (Fig. 2A,B). Anisomycin, but not the DNA transcription inhibitor actinomycin D, blocked mGluR-induced increases in Arc, suggesting that mGluRs stimulate the new synthesis of Arc protein from pre-existing mRNA. In contrast, treatment of dissociated hippocampal neurons with a chemical induction paradigm for NMDAR-LTD (20 µM NMDA; 3 min) did not elicit an increase in dendritic Arc immunofluorescence (Supp. Fig. 3). Increases in dendritic Arc levels could be due to local, dendritic synthesis of Arc or rapid transport of Arc from the cell body. To address this question, we locally perfused a distal region of dendrite with DHPG and then performed immunocytochemistry for Arc (Fig. 2B) (Sutton et al., 2007). To identify the perfused region, Alexa 488 hydrazide was included in the perfusion pipette. Arc levels were elevated in the DHPG perfused region in comparison to neighboring untreated dendrite (paired t-test; n = 8 dendrites from 4 cultures; p = 0.01). These increases in dendritic Arc were due to new protein synthesis, because they were blocked by the presence of anisomycin in the bath (Fig. 2B; DHPG vs DHPG + anisomycin; n = 8 dendrites from 4 cultures per condition; p= 0.04). This demonstrates that local, dendritic activation of mGluRs is sufficient to induce synthesis of dendritic Arc.
Figure 2. Activity-regulated cytoskeleton-associated protein (Arc) is rapidly synthesized in dendrites in response to mGluR stimulation.
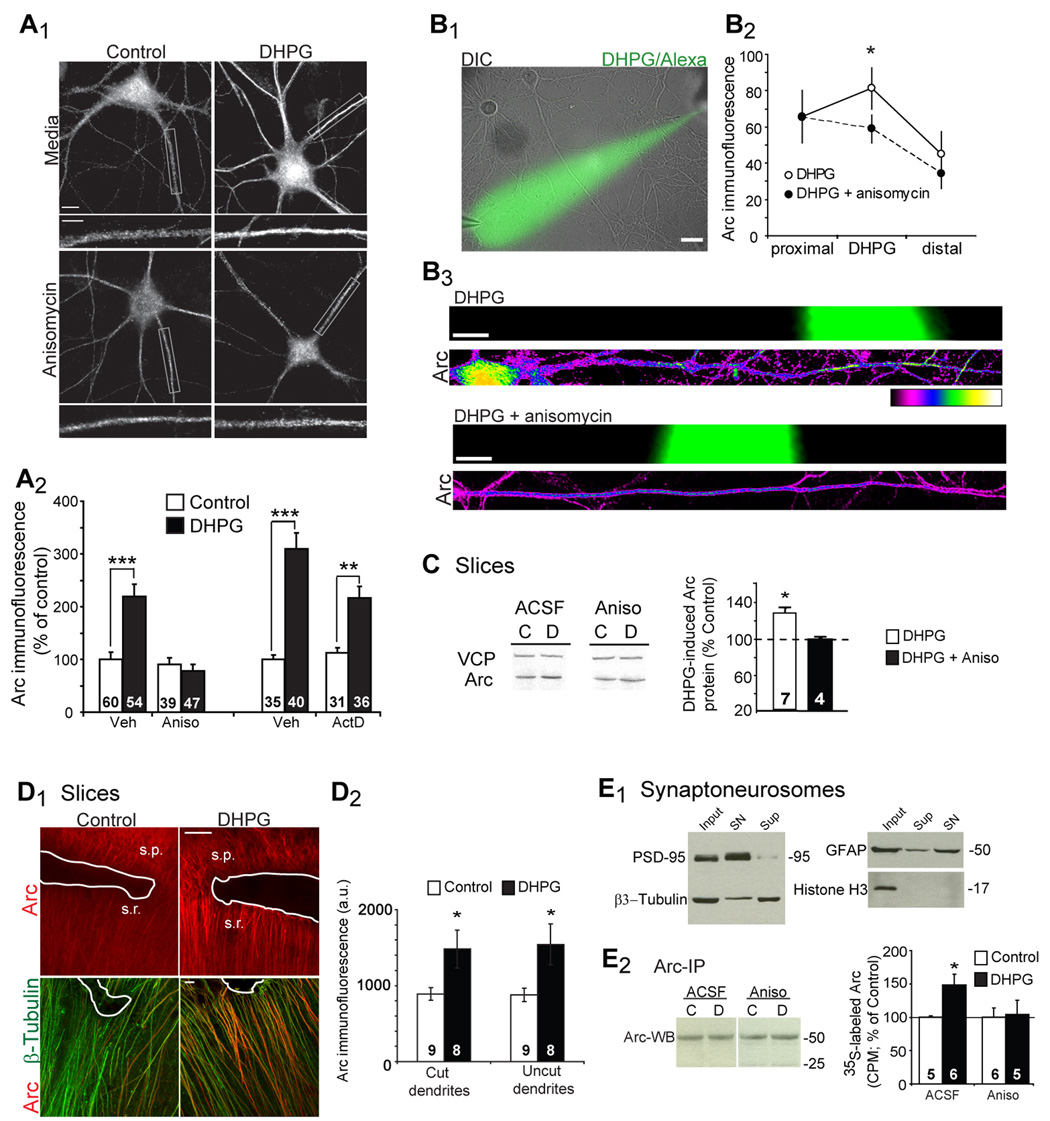
A, DHPG treatment of dissociated hippocampal neurons increases the total area of Arc immunofluorescence in dendrites 10 min after DHPG onset. Pretreatment with anisomycin, but not actinomycin D, blocks the DHPG-induced increases in Arc puncta. Scale bars = 10 and 5 µm. N = # cells each bar. Data from 4–5 cultures/ condition. B1, Local perfusion of DHPG and Alexa488 hydrazide to cultured hippocampal neurons. B2, Quantification of Arc immunofluorescence in the perfused dendritic region. n = 8 dendrites/4 cultures per condition. B3, Representative Arc immunofluorescence in dendritic regions perfused (as indicated by green) with either DHPG alone or DHPG + anisomycin. Scale bars = 10 µm. Two-way ANOVA was used to determine statistical significance. C, Representative western blots of Arc in acute hippocampal slices from mice treated with DHPG in the presence of ACSF or anisomycin (20 µM). Quantitative western blotting for Arc and VCP (valosin containing peptide; used as a loading control) of acute hippocampal slices frozen at 10 min after DHPG onset. Anisomycin blocks DHPG-stimulated rapid increases in Arc. N = # animals is on each bar. D1, Representative Arc immunofluorescence from CA1 regions where a cut (white line) severed dendrites from the pyramidal cell layer. s.p = Str. pyramidale, s.r.= Str. radiaum. β3-Tubulin immunoreactivity indicates the presence of dendrites. Scale bars = 100 and 10 µm. D2, Quantification of Arc immunofluorescence in dendrites that were severed (cut) from the soma or neighboring uncut dendrites. N = # of slices on each bar from 3 rats per condition. E1, Western blot of the synaptoneurosome preparation reveals enrichment of PSD-95 and a reduction in β3-tubulin, GFAP and Histone H3 in comparison to whole homogenate (input) or supernatant (Sup). E2, Western blot (WB) of Arc (using mAb) after Arc immunoprecipitation (using pAb) demonstrates that comparable amounts of Arc was IPed in different conditions. Quantification of 35S Met incorporation into Arc that was immunoprecipitated from synaptoneurosomes. N = # of animals on each bar.
To determine if mGluR stimulation increases total Arc protein levels, acute hippocampal slices were treated with DHPG (100 µM; 5 min), and Arc levels were quantified by western blot. Similar to cultured neurons, DHPG treatment of slices increased total Arc protein levels, and this increase was blocked with anisomycin (Fig. 2C). DHPG-induced LTD occurs in dendrites that have been severed from the CA1 cell body layer in acute slices and is therefore thought to be dependent on the local or dendritic synthesis of new proteins (Huber et al., 2000). To determine if DHPG increases Arc protein in isolated dendrites, we treated slices in which the CA1 pyramidal neuron soma had been mechanically severed from the dendrites. DHPG effectively increased Arc levels in isolated dendrites as detected by immunohistochemistry (Fig. 2D). To determine if mGluRs stimulated synaptic synthesis of Arc, 35S Methionine (Met) was added simultaneously with DHPG (100 µM) to hippocampal synaptoneurosomes (Fig. 2E1) and incubated for 15 min. Arc was subsequently immunoprecipitated from synaptoneurosomes. DHPG increased 35S Met incorporation into Arc which was blocked by preincubation of synaptoneurosomes in anisomycin (Fig. 2E2). Together, these results provide strong evidence for rapid and dendritic synthesis of Arc protein in response to mGluR-LTD induction.
Knockdown of Arc protein increases basal synapse function and surface AMPAR expression
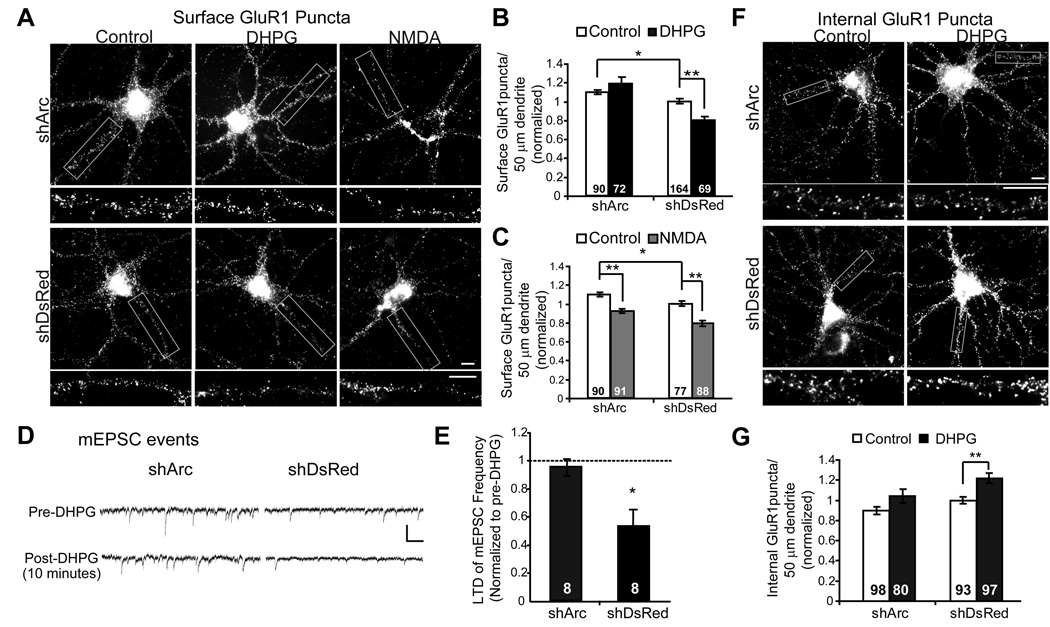
To determine if mGluR-mediated AMPAR trafficking and mGluR-LTD require Arc, we utilized a lentivirus expressing short-hairpin (sh) RNA to knockdown Arc mRNA and protein (shArc) (Rial Verde et al., 2006). Two weeks after infection of dissociated hippocampal neurons with shArc, total dendritic Arc-immunoreactivity was reduced by 83±2% (Fig. 3A,B) in comparison to control neurons infected with a lentivirus expressing a shRNA against DsRed (shDsRed). Consistent with previous work, knockdown of Arc lead to increased excitatory synaptic function as measured by mEPSC amplitude (Fig. 3C) (Rial Verde et al., 2006; Shepherd et al., 2006). There was also a trend for increased mEPSC frequency in neurons infected with shArc (Fig. 3C). Input resistance and capacitance were not different between shArc-, shDsRed- (Fig. 3B), or un-infected neurons (RM = 190 ± 20 MΩ, CM = 19 ± 2 pF, n=20 cells). The stability of these parameters, together with the observed increase in synaptic function, reduce concerns of off target effects of these shRNAs (Alvarez et al., 2006). Along with increased synaptic function, shArc infected neurons exhibited a slight increase in surface GluR1 levels in comparison to shDsRed infected cultures (Fig. 3D,E), consistent with a role for Arc in GluR1 endocytosis. These data indicate that endogenous levels of Arc in our cultures reduce excitatory synaptic function and surface AMPAR levels.
Figure 3. Knockdown of endogenous Arc increases synaptic transmission and surface GluR1 expression.
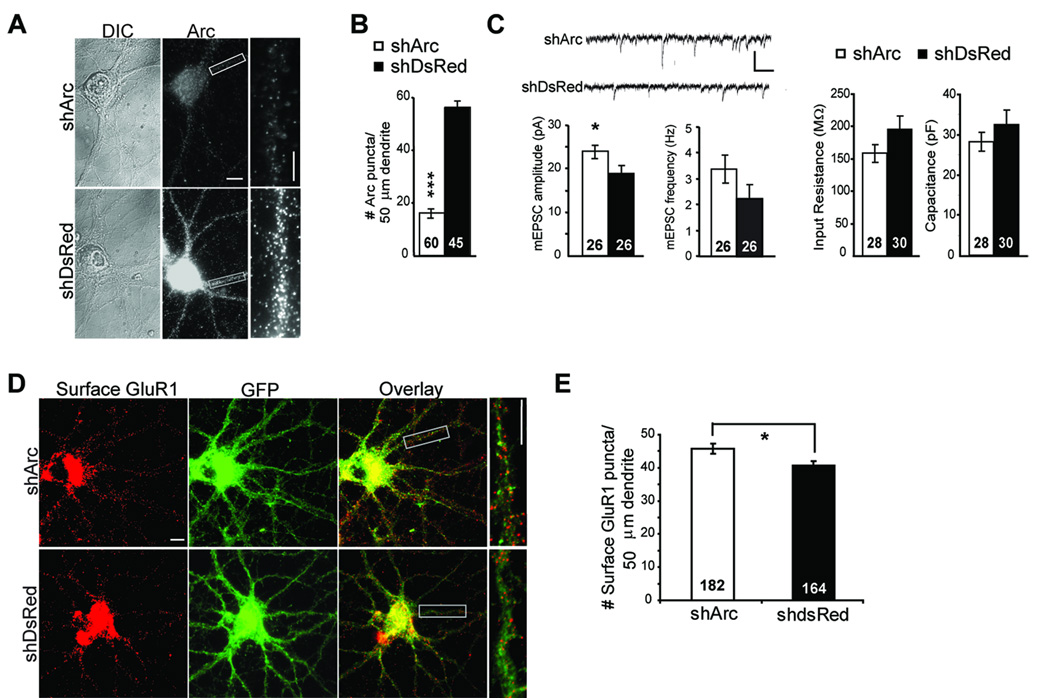
A, Lentivirus expressing a shRNA against Arc (shArc) mRNA in comparison to sister cultures infected with a control lentivirus expressing shRNA against DsRed (shDsRed). Dissociated rat hippocampal neurons were infected at 7 DIV with shArc or shDsRed that co-expresses GFP. 10–12 days postinfection, neurons were fixed, and Arc protein was visualized using immunofluorescence. Scale bar = 10 and 5 µm B, Quantified group data reveal an ~80% knockdown in the total Arc immunofluorescence in dendrites of shArc infected neurons. N = # cells/condition from 2 cultures. C, Representative mEPSCs from shArc and shDsRed infected neurons. Scale bars = 30 pA/100 msec. Neurons expressing shArc exhibited increased mEPSC amplitude, but no difference in mEPSC frequency, input resistance or capacitance in comparison to neurons infected with shDsRed. N= number of cells/condition from 4 cultures. D, E, Representative surface GluR1 on dendrites of neurons infected with either shDsRed or shArc. Scale bars = 10 µm. F, Group data reveal that knockdown of Arc increases basal surface GluR1 in control cultures. N = # cells/condition from 8 cultures.
Arc knockdown abolishes mGluR-induced changes in surface AMPAR expression and LTD
We next utilized shArc-infected neurons to examine whether mGluR-mediated AMPAR trafficking requires Arc protein. Depleting Arc levels in neurons abolished DHPG-induced decreases in GluR1 surface expression observed 15 min after DHPG treatment. In contrast, NMDA-induced decreases in surface GluR1 remained intact (Fig. 4A,B). Because knockdown of endogenous Arc blocked surface GluR1 changes relatively quickly after DHPG (within 15 min), Arc may be necessary for mGluRs to trigger endocytosis of GluR1. In support of this idea, DHPG did not induce significant increases in GluR1 endocytosis in shArc-expressing neurons as measured using live antibody labeling of surface GluR1, in contrast to sister cultures expressing shDsRed (Fig. 4F,G).
Figure 4. Endogenous Arc is required for mGluR stimulated AMPAR endocytosis and LTD.
A, Representative images of surface GluR1 immunofluorescence in low-density dissociated hippocampal cultures infected with lentivirus expressing shArc or shDsRed treated with media (control), DHPG, or NMDA and fixed 15 min after treatment onset. B, Group data reveal that knockdown of Arc blocks DHPG- induced decreases in surface GluR1. Data pooled from 5 cultures. C, Arc knockdown does not affect NMDA-induced decreases in surface GluR1. N = # cells on each from 3 cultures/condition. D, Representative mEPSCs recorded from neurons infected with shArc or shDsRed before DHPG treatment and 10 min after DHPG washout. Recordings performed in the presence of 1 µM TTX and 100 µM picrotoxin. E, mEPSC frequency 15 minutes after onset of DHPG treatment in shArc and shDsRed infected neurons plotted as a percentage of pre-treatment mEPSC frequency. Group data reveal that Arc knockdown blocks DHPG-induced decreases in mEPSC frequency. N = # cells on each bar from 4 cultures/condition. F, Representative images of internalized GluR1 immunofluorescence in low-density dissociated hippocampal cultures infected with lentivirus expressing shArc or shDsRed. Neurons were live labeled with N-terminal GluR1 antibody immediately after treatment with media (control) or DHPG (100 µM; 5 min) and fixed 15 min later. G, Group data reveal that knockdown of Arc reduces DHPG- induced endocytosis of surface GluR1. Data pooled from 5 cultures. In all images scale bars = 10 µm.
DHPG treatment of dissociated hippocampal cultures results in a long-term decrease in mEPSC frequency (Moult et al., 2006; Snyder et al., 2001). To determine if Arc was required for mGluR-LTD of synaptic function in culture, we performed whole cell patch clamp recordings from neurons infected with lentivirus expressing either shArc or shDsRed. In control cultures expressing shDsRed, DHPG application induced a decrease in mEPSC frequency 10 minutes after drug washout (p=0.02). In contrast, DHPG was ineffective in decreasing mEPSC frequency in shArc- expressing neurons (p = 0.78; Fig. 4D,E). Furthermore, the magnitude of DHPG-induced LTD of mEPSC frequency was different between shArc and shDsRed infected neurons (p = 0.02). These findings demonstrate that endogenous Arc is required for mGluR-induced decreases in surface AMPARs and synaptic function.
Rapid translation of Arc is necessary for mGluR-induced decreases in surface GluR1 and LTD
Although knockdown of endogenous Arc blocks mGluR-induced synaptic changes, these experiments do not address whether new synthesis of Arc in response to mGluRs is required for plasticity. To acutely block new synthesis of Arc, we employed an antisense oligonucleotide against Arc mRNA to specifically block its translation (described in Exp. Procedures). We acutely introduced the Arc antisense oligo, or a control mismatch oligo (with 5 mismatched nucleotides) into cultured neurons using lipid mediated transfer. Thirty minutes after oligo delivery, neurons were treated with DHPG and processed for Arc immunofluorescence. DHPG induced a rapid increase (within 10 min) in dendritic Arc immunoreactivity in the presence of Arc mismatch oligo. Pretreatment with Arc antisense oligo effectively blocked DHPG-induced increases in Arc without altering basal Arc levels (Fig. 5A). The Arc antisense oligos do not generally impair mGluR stimulated protein synthesis, because they did not affect DHPG-induced increases in dendritic MAP1b (Supp. Fig. 4).
Figure 5. Acute blockade of Arc translation blocks the long-term effects of mGluR activation on GluR1 trafficking.
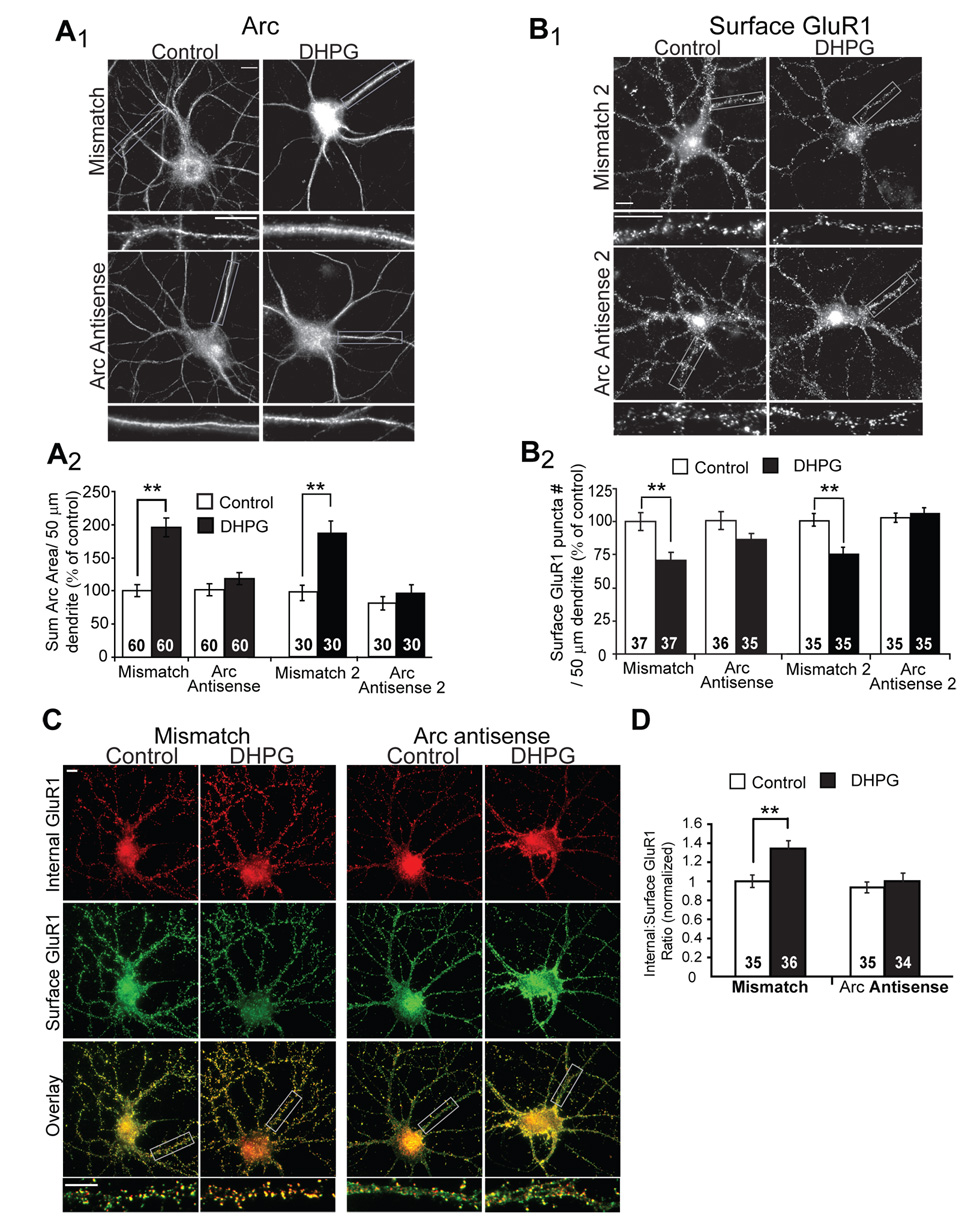
A1, Representative images of Arc immunofluorescence in dissociated hippocampal neurons. Antisense oligonucleotides directed against Arc mRNA, or mismatch oligonucleotides were introduced into 19–21 DIV neurons via a lipid-based delivery system. Neurons were treated with media (control) or DHPG and fixed 10 min after onset of treatment. A2, Quantification of the area of the dendritic Arc fluorescence reveal that either one of two unique Arc antisense oligonucleotides (Arc antisense and Arc antisense 2) block DHPG-induced increases in Arc protein without affecting basal Arc levels (in control mismatch oligo treated cultures). Data from 2 cultures per condition. B1, Representative images of surface GluR1 in neurons treated with antisense or mismatch oligonucleotides 30 min prior to media (control) or DHPG treatment. One hour after DHPG, neurons were fixed and processed for surface GluR1 immunofluorescence. B2, Quantified group data reveal that DHPG fails to induce long-term decreases in surface GluR1 in neurons pretreated with either Arc antisense oligonucleotides, in contrast to neurons treated with mismatch oligonucleotides. Data from 2–3 cultures/condition. C, Representative double-label images of surface (green) and internalized (red) GluR1 in dissociated hippocampal neurons treated with Arc antisense oligo or mismatch oligo. One hour after media (control) or DHPG treatment (100 µM, 5 min), live neurons were labeled with GluR1 antibody. D, Ratiometric quantification of internal to surface GluR1 in the same dendrites reveal that Arc antisense oligonucleotide blocks DHPG-induced, long-term increases in GluR1 endocytosis rate. Data from 2 cultures per condition. In all images scale bars = 10 µm.
Using the antisense oligo, we tested the effects of blocking rapid Arc synthesis on mGluR-induced GluR1 trafficking. Delivery of Arc antisense oligo into neurons did not affect basal levels of surface GluR1, but reduced the decrease of surface GluR1 observed one hour after DHPG treatment (Fig. 5B). The ability to acutely block mGluR stimulated increases in dendritic Arc and decreases in surface GluR1 was replicated with another Arc antisense oligo that targeted a different region of the Arc mRNA (Arc antisense 2; Fig. 5A,B) (Messaoudi et al., 2007). We hypothesize that mGluR-stimulated Arc translation mediates persistent increases in GluR1 endocytosis rate. To test this idea, we examined the effects of the Arc antisense oligos (or mismatch control oligos) on mGluR-stimulated, long-term increases in GluR1 endocytosis rate using the ratiometric immunofluorescence method. One hour after DHPG treatment, GluR1 endocytosis rate was increased in cultures pretreated with mismatch control oligos. In contrast, cultures pretreated with Arc antisense oligos failed to demonstrate persistent increases in GluR1 endocytosis rate after DHPG (Fig. 5C,D). These data support our hypothesis that mGluR-induced synthesis of Arc leads to long-term increases in AMPAR endocytosis rate.
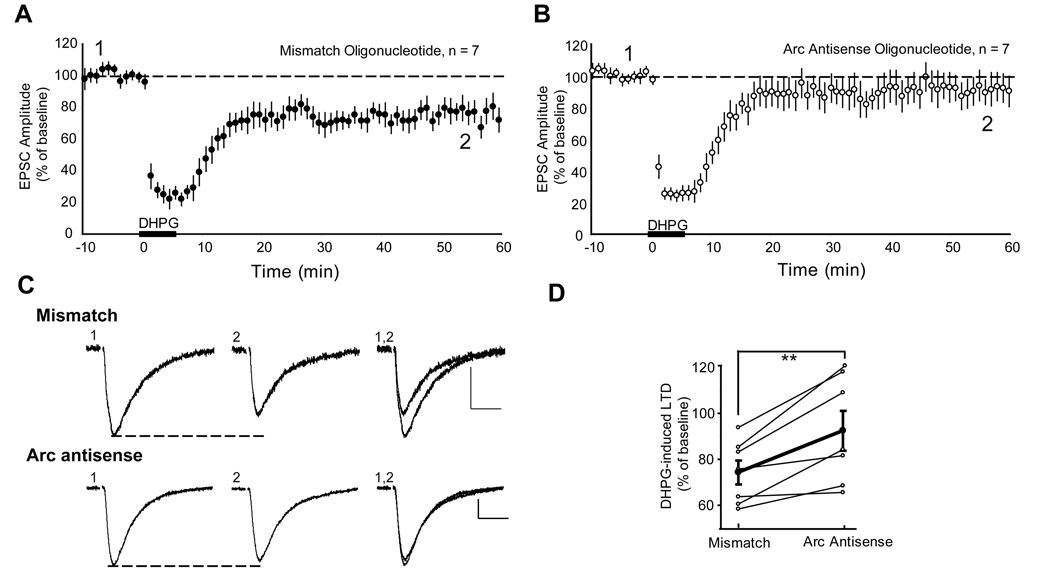
To determine if rapid Arc synthesis is required for DHPG-induced LTD of evoked EPSCs, we introduced Arc antisense oligos (or mismatch oligo; 250 µM) into CA1 pyramidal neurons of acute rat hippocampal slices via a whole cell recording pipette. At least 30 min after breaking into the cell, LTD induction was attempted with bath DHPG application (100 µM; 5 min). LTD was observed in cells infused with control mismatch oligos (74 ±5% of baseline 40–50 min post-DHPG; n = 7; p < 0.001; Fig. 6A). In contrast, DHPG failed to induce LTD in neurons infused with Arc antisense oligo (92 ± 8%; p = 0.43; n = 7; Fig. 6B). All LTD experiments were performed either within the same slice using two simultaneous patch clamp recordings of neighboring CA1 neurons (one filled with each oligo) or on the same day from a sister slice. The level of LTD in cells filled with Arc antisense oligos was reduced in comparison to cells filled with control mismatch oligo (paired t-test with same day control; p=0.007; Fig. 6D).
Figure 6. Acute blockade of Arc translation blocks mGluR induced LTD in CA1 neurons of acute hippocampal slices.
A,B Average normalized EPSC amplitudes of CA1 neurons from acute hippocampal slices recorded with pipettes containing 250 µM mismatch oligonucleotide (A) or Arc antisense oligonucleotide (B). DHPG (100 µM, 5 min) applied to the bath resulted in LTD of EPSC amplitudes in cells filled with mismatch oligonucleotide. In contrast, intracellular introduction of Arc antisense oligonucleotide via patch pipette blocks DHPG-induced LTD. N = # of neurons. C, Representative EPSCs from cells filled with Arc antisense oligonucleotide or mismatch oligonucleotide taken during pre-DHPG baseline (1) or 50 min after LTD induction (2; indicated in A and B). Scale bars = 50 pA/ 10 msec. D, LTD of EPSC amplitude (plotted as a percent of pre-DHPG baseline) at 60 min in Arc antisense oligonucleotide infused neurons that were paired with same day or same slice control neurons infused with mismatch oligonucleotide. Average of 7 experiments is plotted in bold.
Overexpression of Arc protein decreases basal levels of surface AMPARs and occludes mGluR-dependent decreases in AMPAR surface expression
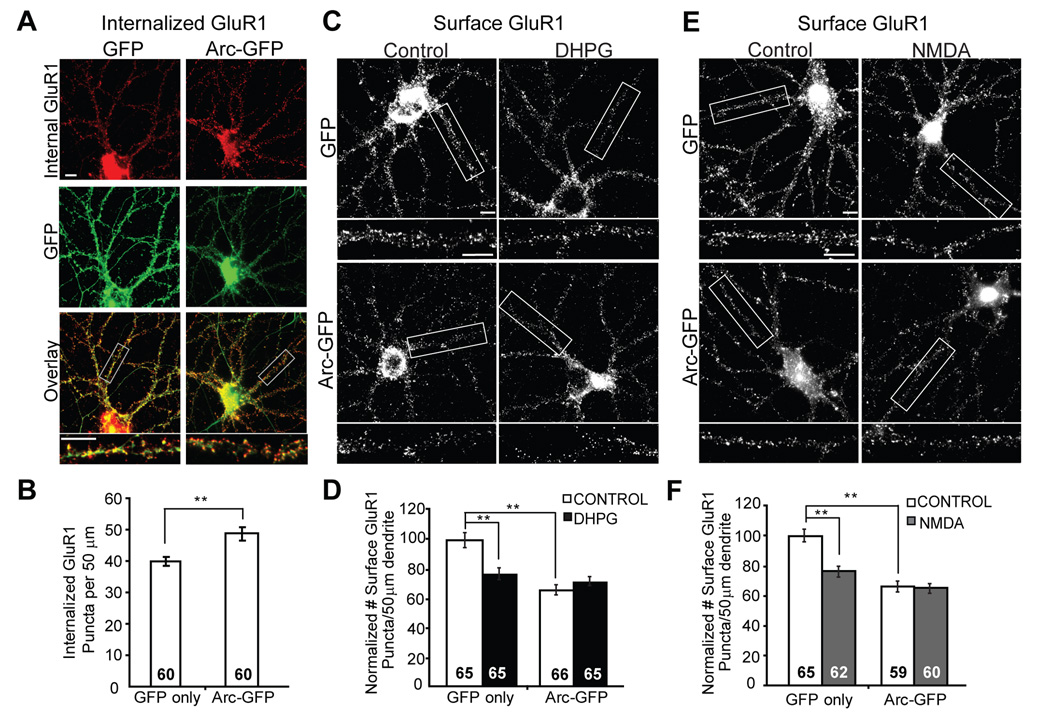
Our data indicate that Arc and rapid synthesis of Arc are necessary for mGluR-LTD and decreases in surface AMPARs. Expression of Arc alone may be sufficient to stimulate endocytosis and decrease surface expression of GluR1 and thereby prevent or occlude subsequent mGluR-induced changes in AMPAR trafficking. To test this, we overexpressed an N-terminally GFP-tagged Arc in neurons via lentiviral mediated expression (Chowdhury et al., 2006). As previously observed, Arc overexpression increased GluR1 internalization and decreased surface GluR1 to 64 ± 5% of that observed in neurons infected with lentivirus expressing GFP only (Fig. 7A–D) (Chowdhury et al., 2006; Rial Verde et al., 2006). Overexpression of Arc protein also occluded the effect of mGluR stimulation on AMPAR surface levels, suggesting that Arc expression alone is sufficient to mimic mGluR-LTD. Interestingly, Arc overexpression also occluded NMDA stimulated decreases in surface GluR1 (Fig. 7E,F). Together with the results from Arc knockdown experiments (Fig. 4), our data suggest that endogenous Arc is primarily utilized for mGluR-mediated AMPAR surface expression changes, and Arc overexpression impacts both mGluR and NMDAR-mediated AMPAR trafficking mechanisms.
Figure 7. Overexpression of Arc in hippocampal neurons increases GluR1 endocytosis and occludes mGluR induced decreases in surface GluR1.
A, Representative immunofluorescence of internalized GluR1 in hippocampal neurons infected with lentivirus containing GFP or Arc-GFP (green). B, Quantification of internalized GluR1 puncta reveal that neurons with Arc-GFP expression have increased GluR1 endocytosis rate as compared to neurons expressing GFP only. Data from 3 cultures. C, Representative images of surface GluR1 staining in neurons infected with lentivirus containing GFP or Arc-GFP. Neurons were treated with media (control) or DHPG (100 µM, 5 min) and fixed one hour after onset of treatment. D, Quantification of surface GluR1 puncta number in GFP or Arc-GFP infected neurons treated with media (control) or DHPG reveal that Arc-GFP overexpression decreases basal surface GluR1 levels and blocks DHPG-induced decreases in surface GluR1. Data from 3 cultures. E, Representative images of surface GluR1 staining in neurons infected with lentivirus containing GFP or Arc-GFP. Neurons were treated with media (control) or NMDA and fixed one hour after onset of treatment. F, Quantification of surface GluR1 puncta number in GFP or Arc-GFP infected neurons treated with media (control) or NMDA reveal that Arc-GFP overexpression decreases basal surface GluR1 levels and blocks NMDA-induced decreases in surface GluR1. Data from 3 cultures. In all images scale bars = 10 µm.
Discussion
Arc is a fascinating gene, since its induction coincides with recent salient experience and activity in neurons. Upon induction, Arc mRNA is transported to dendrites, but little is known about the role of local Arc synthesis in synaptic plasticity. Furthermore, mGluR-dependent LTD depends on the rapid synthesis of new dendritic proteins, but the proteins that mediate LTD were unknown. Here we provide evidence that induction of LTD with brief mGluR activation in cultured neurons is associated with persistent increases in GluR1 endocytosis rate that relies on new Arc synthesis. MGluR-LTD inducing stimulation causes rapid synthesis of Arc protein in isolated dendrites and synaptoneurosomes, implicating local or synaptic synthesis of Arc (Fig. 2). Knockdown of endogenous Arc results in increased surface GluR1 and mEPSC amplitudes, but prevented mGluR-induced changes in AMPAR trafficking and mEPSCs, demonstrating a role for Arc in both constitutive and mGluR-triggered AMPAR endocytosis (Fig. 2, Fig. 3). Arc overexpression is sufficient to mimic and occlude mGluR-induced decreases in surface GluR1, providing evidence for a common, shared mechanism between Arc-induced depression and mGluR-LTD. Together, these data support a model by which mGluRs trigger AMPAR endocytosis through existing Arc protein, as well as stimulate local synthesis of Arc that results in a long-term increase in AMPAR endocytosis rate. The increased endocytosis rate then maintains decreased surface GluR1 expression and LTD.
MGluR-LTD is associated with persistent increases in GluR1 endocytosis rate
Using two methods to label surface GluR1, receptor biotinylation and live antibody feeding, we observed greater intracellular accumulation of labeled GluR1 one hour after induction of mGluR-, but not NMDAR-dependent LTD. Because GluR1 accumulation was measured for a brief period of time (5 min in biotinylation experiments; before saturation; Fig. 1A, B), we assert that this is primarily a reflection of endocytosis rate. This is based in part on previous work in cultured neurons that the time constant for GluR1 recycling is about 10 min (Ehlers, 2000). Therefore, recycling is not likely to significantly decrease the intracellular GluR1 accumulation measurements. The increases in GluR1 endocytosis rate observed using biotinylation were greater than observed with ratiometric immunofluorescence (Fig. 1B, 1F), which is likely due to recycling of GluR1 back to the surface during the longer endocytosis periods required for the immunofluorescence experiments. Additionally, antibody-labeling of GluR1 slows internalization in comparison to biotin labeling (Lin et al., 2000). To maintain a steady state level of AMPARs during LTD in the face of a persistently elevated endocytosis rate, the exocytosis rate must also increase. Exocytosis rate increases could result from either an increase in the internal pool of AMPARs or the exocytosis rate constant (kexo). However, because we did not directly measure these variables we cannot address the source of any exocytosis rate changes. In addition, we observe decreases in both surface GluR1 and GluR2/3 (Supp. Fig. 1), suggesting that similar persistent alterations in GluR2 endocytosis rates may also occur after DHPG.
Endogenous Arc is required for mGluR- but not NMDAR-dependent LTD
Our findings support recent studies that Arc is associated with synaptic weakening through stimulation of AMPAR endocytosis (Chowdhury et al., 2006; Plath et al., 2006; Rial Verde et al., 2006; Shepherd et al., 2006). However, we find that Arc is specifically required for an mGluR-, as opposed to, NMDAR-dependent LTD. Although NMDA treatment elicits a prolonged decrease in surface AMPAR levels, induction of NMDAR-LTD does not require protein synthesis, Arc protein, nor is it associated with persistent increases in AMPAR internalization rates (Fig. 1, Fig 4; Supp. Fig. 3). Furthermore, stimuli that typically induce NMDAR-LTD, such as low frequency stimulation (1Hz; LFS) do not induce Arc transcription or translation (Steward and Worley, 2001). Similarly, we observed that chemical induction of NMDAR-LTD did not increase Arc levels (Supp. Fig. 3). Previous work observed that LFS-induced LTD is absent in the Arc knockout (KO) mouse (Plath et al., 2006). However, it was unclear from this study whether an mGluR- or NMDAR-dependent LTD was deficient in the Arc KO. Alternatively, in our study, the 20% of endogenous Arc remaining after Arc knockdown may be sufficient to support NMDAR-LTD (Fig. 3, Fig. 4). NMDAR and mGluR-LTD in area CA1 utilize distinct mechanisms and affect different synapses or populations of AMPARs (Huber et al., 2000; Huber et al., 2001; Oliet et al., 1997). Our data suggest that the presence of Arc at synapses or with AMPARs makes them selectively susceptible to mGluR induced endocytosis and LTD.
In contrast to the effects of Arc knockdown with shArc, overexpression of GFP-tagged Arc inhibited both NMDA and mGluR induced decreases in surface GluR1 (Fig. 4 and Fig. 6). Because Arc-GFP alone reduced surface GluR1 levels by 40%, we conclude that both LTD mechanisms were saturated, which prevented further surface GluR1 decreases in response to agonist. This is consistent with the previous finding that Arc overexpression in organotypic hippocampal slice cultures occludes an LFS-induced LTD that is presumably NMDAR dependent (Rial Verde et al., 2006). Therefore, extremes of Arc expression such as a complete knockout or overexpression may impact both mGluR and NMDAR-LTD mechanisms, whereas mGluR-LTD is more sensitive to Arc protein levels and blockade of new Arc synthesis.
MGluRs induce rapid and local translation of Arc
Upon induction, Arc mRNA is rapidly transported into dendrites, but it is unknown if Arc is locally translated in response to synaptic activity (Steward et al., 1998; Steward and Worley, 2001). Here we provide strong evidence for dendritic and synaptic synthesis of Arc in response to mGluR activation (Fig. 2): 1) Bath application of DHPG induced a rapid (10 min) increase in dendritic Arc levels that relied on protein synthesis from preexisting mRNA. 2) Local dendritic activation of mGluRs was sufficient to induce an anisomycin sensitive increase in dendritic Arc. 3) MGluR activation of synaptoneurosomes increased 35S Met incorporation into Arc. 4) DHPG treatment of slices in which the dendrites were severed from their cell bodies was capable of increasing Arc levels. The latter result indicates that the increase in dendritic Arc does not stem from soma mediated synthesis and transport of Arc into dendrites. Although our results suggest that Arc mRNA is present in dendrites of CA1 neurons in acutely prepared slices, this needs to be confirmed. Together these results provide strong evidence that mGluRs locally synthesize Arc and utilize new Arc to maintain enhanced GluR1 endocytosis rates and LTD. Future experiments are required to establish the role of dendritic transport and the 3’UTR of the Arc mRNA in mGluR-LTD. To study mGluR-induced AMPAR trafficking and Arc synthesis during LTD in cultured neurons, we utilized DHPG. However, we have previously demonstrated on a number of levels that DHPG-induced LTD shares common mechanisms with a synaptically induced form of mGluR-LTD (Huber et al., 2002; Huber et al., 2000; Huber et al., 2001; Volk et al., 2007) and would predict that synaptically induced mGluR-LTD would stimulate and require dendritic synthesis of Arc.
Endogenous Arc is required for mGluRs to trigger AMPAR endocytosis
Knockdown of endogenous Arc caused a decrease in surface GluR1, mEPSC amplitudes as well as a trend towards decreased mEPSC frequency in unstimulated or control cultures, indicating a role for Arc in constitutive AMPAR cycling. This supports previous findings with siRNA mediated knockdown or knockout of Arc (Rial Verde et al., 2006; Shepherd et al., 2006). Surprisingly, we observed that endogenous Arc is required for mGluRs to trigger endocytosis and decreases in surface GluR1 (Fig. 4). Arc interacts with components of the endocytosis machinery endophilin 3 and dynamin 2, but not AMPARs (Chowdhury et al., 2006). Therefore, mGluRs may stimulate association of existing Arc with endocytosis machinery, the synaptic membrane or interacting proteins of GluR1 to induce endocytosis. In addition, blockade of mGluR-induced Arc synthesis using antisense oligonucleotides inhibited the long-term effects of mGluRs on GluR1 surface expression, endocytosis rate and evoked EPSCs (Fig. 5). Therefore, in order to maintain plasticity, mGluRs synthesize more of an existing and limited component of the AMPAR endocytosis machinery, as opposed to a novel protein.
MGluR-dependent LTD exists in a number of brain regions, where it has a common requirement for new protein synthesis, despite differences in LTD expression mechanism (Mameli et al., 2007; Snyder et al., 2001). For example, expression of mGluR-LTD onto dopaminergic neurons in the ventral tegmental area is mediated by insertion of low conductance, GluR2-containing AMPARs that is supported by new synthesis of GluR2 (Mameli et al., 2007). Furthermore, although we have demonstrated a requirement of Arc synthesis in mGluR-LTD in CA1, other candidate LTD proteins, such as MAP1b, regulate AMPAR endocytosis and are synthesized in response to mGluRs (Davidkova and Carroll, 2007; Hou et al., 2006). Therefore, mGluRs may stimulate synthesis of a number of, perhaps functionally related, proteins that contribute to LTD.
Rapid translation of Arc is required for the persistence of plasticity
Acute blockade of Arc synthesis using antisense oligos was first demonstrated to inhibit the late-phase of LTP in vivo, as well as hippocampal memory consolidation (Guzowski et al., 2000). More recently, it has been shown using this same method that LTP in the dentate gyrus requires a sustained synthesis of Arc for at least 2 hours after induction (Messaoudi et al., 2007), and Arc KO mice have a deficit in late-phase LTP (Plath et al., 2006). Together with our work, this indicates that rapid translation of Arc is important to maintain both synapse strengthening and weakening. Different functional domains of Arc may have distinct roles in LTP or LTD. During LTP, Arc is thought to function in the regulation of cofilin dephosphorylation and actin polymerization (Messaoudi et al., 2007). If Arc is required for stabilization of structural synaptic plasticity, then multiple forms of plasticity could conceivably utilize Arc. MGluR activation induces structural changes in dendritic spines that require new protein synthesis, but it is unknown whether these changes contribute to LTD or require Arc (Vanderklish and Edelman, 2002).
Implications of Arc involvement in mGluR-LTD
The discovery of a protein whose synthesis is required for mGluR-LTD, such as Arc, may have important implications for understanding the neurobiological mechanisms of Fragile X Syndrome (FXS). In the mouse model of Fragile X Syndrome, Fmr1 KO mice, mGluR-LTD is enhanced and no longer requires new protein synthesis (Hou et al., 2006; Huber et al., 2002; Koekkoek et al., 2005; Nosyreva and Huber, 2006). MGluR-LTD is associated with a reduction in surface AMPARs in Fmr1 KO mice, which, unlike their wild-type counterparts, these decreases in surface AMPARs persist in anisomycin (Nosyreva and Huber, 2006). FMRP associates with Arc mRNA, and Arc mRNA translation may be dysregulated in Fmr1 KO mice (Iacoangeli et al., 2008; Zalfa et al., 2007; Zalfa et al., 2003). This work, together with our findings that Arc synthesis is necessary for mGluR-LTD at wild-type synapses, suggest that altered regulation of Arc at Fmr1 KO synapses may account for the abnormal LTD.
Arc mRNA is induced and transported to dendrites within one hour after neuronal activation (Link et al., 1995; Steward et al., 1998; Steward and Worley, 2001). Once Arc mRNA is in dendrites, it accumulates at active synapses within 15 minutes (Steward and Worley, 2001). Our findings would predict that synaptic mGluR activation is more likely to induce LTD in a time window following salient, Arc-inducing events. LTD in the hippocampus, in general, is facilitated during exposure to novelty and is hypothesized to encode novel objects in a spatial context (Kemp and Manahan-Vaughan, 2004, 2007; Manahan-Vaughan and Braunewell, 1999). Novelty is a robust Arc induction stimulus and may underlie the facilitation of LTD and the encoding of novel objects (Guzowski et al., 2006; Kelly and Deadwyler, 2002; Ons et al., 2004). MGluR-LTD, in particular, is enhanced after exposure to stress or cocaine, stimuli known to induce Arc mRNA (Chaouloff et al., 2007; Fosnaugh et al., 1995; Mameli et al., 2007; Mikkelsen and Larsen, 2006; Ons et al., 2004). Therefore, the local translation of Arc by mGluRs and facilitation of LTD may function to depress synapses whose activation occurs within a time window after behaviorally relevant stimuli.
Experimental Procedures
Dissociated Hippocampal Culture
CA1/CA3 regions of hippocampi were isolated from P0–P2 hooded Long Evans rats and cultures prepared as described previously (Lin et al., 2000). Briefly, dissected hippocampi were trypsinized for 10 min, and dissociated by trituration. After centrifugation, neurons were plated in Neurobasal A medium supplemented with B27, 0.5 µ M glutamine, and 1% fetal bovine serum at high density (800 neurons/mm2; for biotinylation experiments) onto 6 well plates or low density (250 neurons/mm2; for immunofluorescence) on glass coverslips coated overnight with 50 µg/ml poly-D-lysine and 25 µg/ml laminin. Cultures were fed at 1 day in vitro (DIV) and every 3 days afterwards by replacing half the media with serum-free media containing 1µM Cytosine arabinoside. Experiments were performed at 19–22 DIV.
Drug/oligo treatments
Receptor Biotinylation and measurement of GluR1 endocytosis rate
High density hippocampal cultures were treated with media, DHPG or NMDA. After 10 min., neurons were cooled to 4°C to halt endocytosis and were incubated in 1 mg/mL EZ-link-NHS-biotin (Pierce) for 20 min. After washing with Tris-buffered saline (TBS), neurons were lysed in Radio-Immuno Precipitation Assay (RIPA) Buffer containing: 50 mM Tris-HCl, pH 7.4, 1% Triton X100, 0.1 % SDS, 0.5% Na-deoxycholate, 150 mM NaCl, 2 mM EDTA, 50 mM NaH2PO4, 50 mM NaF, 10 mM Na4P2O7, 1mM Na3VO4, and protease inhibitor cocktail III (Calbiochem, La Jolla, CA), and centrifuged at 14,000 g for 15 min at 4°C. Ten µg of0 0protein were removed for total (T) protein measurements and 100 µg of protein were mixed with 100 µl of Immobilized NeutrAvidin beads (Pierce) by rotating for 3 h at 4°C. Beads were washed with 10 volumes of RIPA buffer. Biotinylated proteins were eluted by boiling for 20 min in sample buffer. Eluted proteins were separated by SDS-PAGE and immunoblotted with 1° antibodies against GluR1 (1:200, Chemicon), anti-GluR2/3 N-terminal antibody (1:1000, Chemicon) and HRP-conjugated 2° antibody. Immunoreactive bands were visualized by enhanced chemiluminescence (ECL) and captured on Biomax autoradiography film (Kodak). Digital images, created by densitometric scans of autoradiographs on a ScanJet 4300C (Hewlett Packard) were quantified using ImageJ software. Surface GluR levels were normalized to GluR values from total protein samples.
To measure internalized receptors, cultures were treated 1 hour after stimulation with 2 minutes of cleavable EZ-link-NHS-SS-biotin (Pierce) at 37°C, and subsequently incubated for 5, 15 or 30 minutes at 37°C to allow for endocytosis. Remaining surface biotin was stripped off with 50 mM cold glutathione twice for 20 min. After quenching with cold 50 mM iodoacetamide, neurons were lysed in RIPA buffer and processed as described above.
Western blotting
Immunofluorescence
Surface changes of AMPAR subunit GluR1 were observed in low density cultures treated with media, DHPG or NMDA. After treatment, neurons recovered in conditioned media with TTX for 10 or 60 minutes before fixation with 4% paraformaldehyde (PFA) for 15 min. at 37°C. After blocking with 5% goat serum in PBS at room temperature (RT), neurons were labeled with N-terminal GluR1 antibody (1:50; Calbiochem) for 1 hour and stained with AlexaFluor488-conjugated 2° antibody (Molecular Probes) for 1 hour. Ratiometric measurements of internalized and surface GluR1 were performed as described (Lin et al., 2000). One hour after treatment with DHPG or NMDA, live neurons were incubated with the N-terminal GluR1 antibody (1:10) for 15 min. at 37°C. Neurons were fixed under nonpermeabilizing conditions (4% PFA; 37°C; 15 min) and incubated for 1 hour with AlexaFluor488 2° antibody. After permeabilization with 0.1% TritonX-100 for 10 min., neurons were labeled for 1 hour with AlexaFluor555 2° antibody. To measure internalized GluR1 only, live neurons were labeled with GluR1 antibody immediately after media or DHPG treatment for 15 min (37°C), fixed and remaining surface GluR1 was blocked with unlabeled donkey anti-rabbit IgG (0.2 mg/ml) (Jackson Immunoresearch) as described (Ehlers, 2000). After permeabilization, neurons are labeled for 1 hour with AlexaFluor555 2° antibody. Fluorescence images were acquired on a Nikon TE2000 microscope with a cooled CCD camera (CoolSnap HQ; Roper Scientific) and quantified with Metamorph software (Molecular Devices). Healthy neurons are first identified by their smooth soma and multiple processes under DIC microscopy. GluR1 immunoreactive puncta are defined as discrete points along a dendrite (within 50 µm from the soma) with fluorescence intensity at least twice the background staining of a region adjacent to the dendrite. For each neuron GluR1 puncta are analyzed in 3 dendrites. GluR1 puncta number from each dendrite was averaged and represents the value for that cell and this value equals an n of 1. 5–30 cells were analyzed per condition in each culture. N values are indicated on the bar graphs of group data in each figure. Statistical comparisons were performed with the n = to number of cells. Each experiment was performed on 2–8 separate cultures (indicated in Fig. legends) with three different coverslips per condition.
For Arc staining, neurons were fixed and permeabilized with cold methanol (−20°C) for 10 minutes prior to addition of 1° antibodies to Arc (Santa Cruz; C7 mouse monoclonal; 1:100 or Synaptic Systems; rabbit polyclonal; 1:800). The total area of dendritic fluorescence (at least twice the background) was quantified and normalized to the control (untreated) condition for each experiment.
Electrophysiology
Statistics
Independent or paired t-tests were used for most statistical analysis, as indicated. For multiple comparisons a Bonferroni correction (Figs. 2A, Fig. 4B,C, and G, Fig. 5A, B and D, Fig. 7D,F) or 2 way ANOVA (Fig. 2B) was performed. In all figures group data is presented as average ± SEM and * p < 0.05; **p < 0.01, ***p< 0.001.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the grants from the National Institutes of Health NS045711, HD052731 (KMH), 1F31NS050992 (BEP), and the UTSW Medical Scientist Training Program T32 GM08014 (MWW) and FRAXA Research Foundations (EDN, JAR). We would like to thank K. Loerwald for technical assistance and J. Gibson, E. Kavalali, J. Albanesi and I. Bezprozvanny for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alvarez VA, Ridenour DA, Sabatini BL. Retraction of synapses and dendritic spines induced by off-target effects of RNA interference. J Neurosci. 2006;26:7820–7825. doi: 10.1523/JNEUROSCI.1957-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RC, Lissin DV, von Zastrow M, Nicoll RA, Malenka RC. Rapid redistribution of glutamate receptors contributes to long-term depression in hippocampal cultures. Nat Neurosci. 1999;2:454–460. doi: 10.1038/8123. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Hemar A, Manzoni O. Acute stress facilitates hippocampal CA1 metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2007;27:7130–7135. doi: 10.1523/JNEUROSCI.1150-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidkova G, Carroll RC. Characterization of the role of microtubule-associated protein 1B in metabotropic glutamate receptor-mediated endocytosis of AMPA receptors in hippocampus. J Neurosci. 2007;27:13273–13278. doi: 10.1523/JNEUROSCI.3334-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Fosnaugh JS, Bhat RV, Yamagata K, Worley PF, Baraban JM. Activation of arc, a putative "effector" immediate early gene, by cocaine in rat brain. J Neurochem. 1995;64:2377–2380. doi: 10.1046/j.1471-4159.1995.64052377.x. [DOI] [PubMed] [Google Scholar]
- Gusev PA, Cui C, Alkon DL, Gubin AN. Topography of Arc/Arg3.1 mRNA expression in the dorsal and ventral hippocampus induced by recent and remote spatial memory recall: dissociation of CA3 and CA1 activation. J Neurosci. 2005;25:9384–9397. doi: 10.1523/JNEUROSCI.0832-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Miyashita T, Chawla MK, Sanderson J, Maes LI, Houston FP, Lipa P, McNaughton BL, Worley PF, Barnes CA. Recent behavioral history modifies coupling between cell activity and Arc gene transcription in hippocampal CA1 neurons. Proc Natl Acad Sci U S A. 2006;103:1077–1082. doi: 10.1073/pnas.0505519103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile-X mental retardation. Proc. Natl. Acad. Sci. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent LTD. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Huber KM, Roder JC, Bear MF. Chemical induction of mGluR5- and protein synthesis--dependent long-term depression in hippocampal area CA1. J Neurophysiol. 2001;86:321–325. doi: 10.1152/jn.2001.86.1.321. [DOI] [PubMed] [Google Scholar]
- Iacoangeli A, Rozhdestvensky TS, Dolzhanskaya N, Tournier B, Schütt J, Brosius J, Denman RB, Khandjian EW, Kindler S, Tiedge H. On BC1 RNA and the fragile X mental retardation protein. Proc Natl Acad Sci U S A. 2008;105:734–739. doi: 10.1073/pnas.0710991105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Deadwyler SA. Acquisition of a novel behavior induces higher levels of Arc mRNA than does overtrained performance. Neuroscience. 2002;110:617–626. doi: 10.1016/s0306-4522(01)00605-4. [DOI] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression and long-term potentiation encode different aspects of novelty acquisition. Proc Natl Acad Sci U S A. 2004;101:8192–8197. doi: 10.1073/pnas.0402650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Manahan-Vaughan D. Hippocampal long-term depression: master or minion in declarative memory processes? Trends Neurosci. 2007;30:111–118. doi: 10.1016/j.tins.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Koekkoek SK, Yamaguchi K, Milojkovic BA, Dortland BR, Ruigrok TJ, Maex R, De Graaf W, Smit AE, VanderWerf F, Bakker CE, et al. Deletion of FMR1 in Purkinje cells enhances parallel fiber LTD, enlarges spines, and attenuates cerebellar eyelid conditioning in Fragile X syndrome. Neuron. 2005;47:339–352. doi: 10.1016/j.neuron.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Lee SH, Simonetta A, Sheng M. Subunit rules governing the sorting of internalized AMPA receptors in hippocampal neurons. Neuron. 2004;43:221–236. doi: 10.1016/j.neuron.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Lin JW, Ju W, Foster K, Lee SH, Ahmadian G, Wyszynski M, Wang YT, Sheng M. Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nat Neurosci. 2000;3:1282–1290. doi: 10.1038/81814. [DOI] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Mameli M, Balland B, Lujan R, Luscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci U S A. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc Natl Acad Sci U S A. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soule J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen JD, Larsen MH. Effects of stress and adrenalectomy on activity-regulated cytoskeleton protein (Arc) gene expression. Neurosci Lett. 2006;403:239–243. doi: 10.1016/j.neulet.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Moga DE, Calhoun ME, Chowdhury A, Worley P, Morrison JH, Shapiro ML. Activity-regulated cytoskeletal-associated protein is localized to recently activated excitatory synapses. Neuroscience. 2004;125:7–11. doi: 10.1016/j.neuroscience.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Montag-Sallaz M, Welzl H, Kuhl D, Montag D, Schachner M. Novelty-induced increased expression of immediate-early genes c-fos and arg 3.1 in the mouse brain. J Neurobiol. 1999;38:234–246. [PubMed] [Google Scholar]
- Moult PR, Gladding CM, Sanderson TM, Fitzjohn SM, Bashir ZI, Molnar E, Collingridge GL. Tyrosine phosphatases regulate AMPA receptor trafficking during metabotropic glutamate receptor-mediated long-term depression. J Neurosci. 2006;26:2544–2554. doi: 10.1523/JNEUROSCI.4322-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosyreva ED, Huber KM. Metabotropic receptor-dependent long-term depression persists in the absence of protein synthesis in the mouse model of fragile x syndrome. J Neurophysiol. 2006;95:3291–3295. doi: 10.1152/jn.01316.2005. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Malenka RC, Nicoll RA. Two distinct forms of long-term depression coexist in CA1 hippocampal pyramidal cells. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]
- Ons S, Marti O, Armario A. Stress-induced activation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: relationship to c-fos mRNA. J Neurochem. 2004;89:1111–1118. doi: 10.1111/j.1471-4159.2004.02396.x. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder EM, Philpot BD, Huber KM, Dong X, Fallon JR, Bear MF. Internalization of ionotropic glutamate receptors in response to mGluR activation. Nat Neurosci. 2001;4:1079–1085. doi: 10.1038/nn746. [DOI] [PubMed] [Google Scholar]
- Steward O, Schuman EM. Protein synthesis at synaptic sites on dendrites. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Vanderklish PW, Edelman GM. Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons. Proc Natl Acad Sci U S A. 2002;99:1639–1644. doi: 10.1073/pnas.032681099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk LJ, Pfeiffer BE, Gibson JR, Huber KM. Multiple Gq-coupled receptors converge on a common protein synthesis-dependent long-term depression that is affected in fragile X syndrome mental retardation. J Neurosci. 2007;27:11624–11634. doi: 10.1523/JNEUROSCI.2266-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S, di Penta A, Tabolacci E, Chiurazzi P, Neri G, Grant SG, Bagni C. A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci. 2007;10:578–587. doi: 10.1038/nn1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F, Giorgi M, Primerano B, Moro A, Di Penta A, Reis S, Oostra B, Bagni C. The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses. Cell. 2003;112:317–327. doi: 10.1016/s0092-8674(03)00079-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.