Abstract
The prefrontal cortex has been extensively implicated in autism to explain deficits in executive and other higher-order functions related to cognition, language, sociability and emotion. The possible changes at the level of the neuronal microcircuit are however not known. We studied microcircuit alterations in the prefrontal cortex in the valproic acid rat model of autism and found that the layer 5 pyramidal neurons are connected to significantly more neighbouring neurons than in controls. These excitatory connections are more plastic displaying enhanced long-term potentiation of the strength of synapses. The microcircuit alterations found in the prefrontal cortex are therefore similar to the alterations previously found in the somatosensory cortex. Hyper-connectivity and hyper-plasticity in the prefrontal cortex implies hyper-functionality of one of the highest order processing regions in the brain, and stands in contrast to the hypo-functionality that is normally proposed in this region to explain some of the autistic symptoms. We propose that a number of deficits in autism such as sociability, attention, multi-tasking and repetitive behaviours, should be re-interpreted in the light of a hyper-functional prefrontal cortex.
Keywords: autism, microcircuit, synaptic connectivity, synaptic plasticity, in vitro electrophysiology, hyper-connectivity, hyper-plasticity, hyper-functionality
Introduction
The prefrontal cortex is crucial for higher-order cognitive, language, social and normal emotion processing (Struss and Knight, 2002). For example, imaging studies suggest that the prefrontal cortex is a key substrate for social cognition, such as thinking about other's thoughts, feelings and intentions (Baron-Cohen et al., 1994; Castelli et al., 2002; Fletcher et al., 1995; Gallagher et al., 2000; Goel et al., 1995; Schultz et al., 2003). In addition, the medial prefrontal cortex (mPFC), by forming a system for regulating emotional processes through its dense reciprocal connections with medial temporal areas (Carmichael and Price, 1995), has been implicated in processing and integrating affect (Myers, 1972). For example, lesions of the mPFC result in abnormal social responsitivity and loss of social skills in non-human primates (Bachevalier and Mishkin, 1986), or in reduced fear extinction in rodents (Morgan et al., 1993).
Given that language, executive functions, social interaction and emotional behaviour are disrupted in autism, and given that these functions depend on integrative mechanisms within the prefrontal cortex, the most parsimonious explanation has been hypo-functionality of this brain region in autism spectrum disorders (Price, 2006). Numerous imaging studies on the prefrontal cortex in autism have been conducted, demonstrating abnormal activity. Misplaced or reduced medial prefrontal activation during social tasks has been consistently observed (Castelli et al., 2002; Happe et al., 1996; Pierce et al., 2004). A PET study demonstrated decreased medial prefrontal activation during cognitive tasks (Kennedy et al., 2006). fMRI studies also suggested reduced frontal activation in the autistic brain in a variety of tasks (Baron-Cohen et al., 1999; Ring et al., 1999). In addition, reduced long-distance functional connectivity between frontal and temporal higher cortices and between these cortices and other structures is commonly reported (Just et al., 2004; Koshino et al., 2005; Villalobos et al., 2005).
Based on these results, numerous cognitive theories of autism have been developed, such as the theory of mind deficit (Baron-Cohen et al., 1985), the weak central coherence (Abell et al., 1999), and the executive dysfunction (Hill, 2004). These theories are all centred on frontal lobe dysfunction and based on the observations demonstrating, with different approaches, hypo-functioning within the prefrontal cortex and diminished functional connectivity between the frontal cortex and other brain regions. The possible alterations in the microcircuitry of the mPFC has however never been conducted to better understand the origin of the whole brain abnormalities observed. Recent studies on the microcircuit effects in an animal model of autism suggested a potentially diametrically opposite interpretation of the data obtained and could challenge the conclusion that the prefrontal cortex is damaged and hypo-functioning if the alterations are also present in this region. The observed whole brain deficits may actually be secondary to a hyper-functioning of the prefrontal cortex, as observed in the somatosensory cortex and amygdala (Markram et al., 2008; Rinaldi et al., 2007, 2008).
In the present study, we therefore explored the microcircuit of the mPFC in the valproic acid (VPA) rat model of autism using in vitro electrophysiological techniques. The VPA rat model of autism, established by Rodier et al. (1997), is now one of the most exhaustively established insult-based animal models of autism and is based on the observation that pregnant women treated with VPA in the 1960s, during a circumscribed time window of embryogenesis, had a much higher risk of giving birth to an autistic child than the normal population (Moore et al., 2000; Rasalam et al., 2005). Offspring of VPA-exposed pregnant rats show several anatomical and behavioural symptoms typical of autism, such as diminished number of cerebellar Purkinje neurons (Ingram et al., 2000), impaired social interaction, repetitive behaviours as well as other symptoms of autism (Markram et al., 2008; Schneider and Przewlocki, 2005) including enhanced fear memory processing (see Markram et al., 2008). At the microcircuit level, recent studies revealed alterations in the somatosensory cortex as well as in the lateral amygdala of the VPA rat model. In particular, increased probability of local connection among pyramidal cells (PCs) (Rinaldi et al., 2008) and enhanced plasticity (Rinaldi et al., 2007) have been demonstrated in the somatosensory cortex. Hyper-fear has also been identified as a key potential abnormality in autism, which is also associated with a hyper-reactive and hyper-plastic amygdala (Markram et al., 2008). Here we investigated whether the prefrontal cortex is similarly affected by hyper-functionality.
Materials and Methods
The valproic acid rat model of autism
Wistar Han rats (Charles River Laboratories, L'Arbresle, France) were mated, with pregnancy determined by the presence of a vaginal plug on embryonic day 0 (E0). The sodium salt of VPA (NaVPA, Sigma) was dissolved in 0.9% saline for a concentration of 150 mg/ml, pH 7.3. The dosing volume was 3.3 ml/kg; the dosage was adjusted according to the body weight of the pregnant rat on the day of injection. Treated rats received a single intraperitoneal injection of 500 mg/kg NaVPA on gestational day 11.5, control rats were untreated (Ingram et al., 2000). We verified that saline injected rats did not show any difference in behaviour compared to control rats (data not shown). Unchanged litter size, pup body weight and general health of the mothers and pups were indications of normal rearing conditions for treated rats. Rats were housed individually and were allowed to raise their own litters. All experimental procedures were carried out according to the Swiss Federation rules for animal experiments.
Acute slice preparation
Offspring were rapidly decapitated and coronal prefrontal slices (300 μm thick) were sectioned on a vibratome (HR2, Sigmann Elektronik) in iced artificial cerebral spinal fluid (ACSF). Slices were incubated for 30 min at 35°C and then at room temperature until transferred to the recording chamber (32°C). The ACSF contained (mM): 125 NaCl, 2.5 KCl, 25 glucose, 25 NaHCO3, 1.25 NaH2PO4, 2 CaCl2 and 1 MgCl2. Neurons in the mPFC were identified using IR-DIC microscopy with an upright microscope (Olympus BX51WI, fitted with a 60×/0.90 W objective, Olympus, Switzerland). Recorded PCs were determined unambiguously according to their typical soma size and shape and apical dendrite. They were selected up to 60 μm below the surface of the slice.
Electrophysiological recording
Simultaneous whole-cell recordings from clusters of up to four neurons (pipette resistance 4–10 MΩ) were made and signals were amplified using Axopatch 200B amplifiers (Axon Instruments). Neurons were submitted to different stimulation protocols. Voltages were recorded with pipettes containing (mM): 110 potassium gluconate, 10 KCl, 4 ATP-Mg, 10 phosphocreatine, 0.3 GTP, 10 HEPES and 0.5% biocytin (pH 7.3, 270–300 mOsm). Membrane potentials were not corrected for the junction potentials between pipette and bath solution (∼10 mV).
Intrinsic neuronal properties
During whole-cell current-clamp recordings, PCs were submitted to a set of stimulation protocols designed to capture their key active and passive electrical properties, as described in Le Be et al. (2007). In short, recordings were sampled at intervals of 10–400 μs using Igor Pro (Wavemetrics, Lake Oswego, OR, USA), digitised by an ITC-18 interface (Instrutech, Great Neck, NY, USA) and stored for off-line analysis. The number of cells studied was 21 for control, 31 for VPA-treated rats.
Connectivity
Direct synaptic connections were examined by eliciting short trains (eight pulses) of precisely timed action potentials (APs) at 30 Hz frequency followed by a recovery test response 500 ms later. The average synaptic response to the stimulation protocol allows the extraction of the basic parameters of the synaptic connection with a model of dynamic synaptic transmission (A, the absolute strength of the connection; Pr, equivalent to the probability of release; D, the time constant to recover from depression) (Tsodyks and Markram, 1997). These parameters were extracted by using the model for synaptic dynamics as previously described (Markram et al., 1998; Tsodyks et al., 1998). The number of connections studied was 29 for control, 32 for VPA-treated rats.
Long-term potentiation
An extracellular electrode was placed 100–300 μm away from the whole-cell patched layer 5 PCs. An electrical stimulus that produced a postsynaptic response in the recorded neurons of 2–4 mV in amplitude was given before the pairing protocol. Responses to 30 such stimuli delivered at a rate of 0.1 Hz were recorded and averaged. The 3-s long pairing protocol consisted of a 30-Hz regular train stimulation to the extracellular electrode simultaneously with a depolarisation above threshold of the patched cell (giving rise to a mean firing rate above 20 Hz in the patched cell), applied twice with 30 s interval. After pairing, three further data-sets, each consisting of responses to 30 stimuli delivered at 0.1 Hz were collected with 5 min pauses separating these stimulus trains. The percent increase in the amplitude of response to the stimulation pulse after pairing compared to before pairing was measured. The number of cells studied was 15 for control, 9 for VPA-treated rats.
Statistical analysis
For comparison of frequencies (i.e. connection probabilities in our case), we used two-sided χ2 test. For comparison of means, we used the two-sample Student's t-tests (log-transformation was first applied to skewed distribution of the synaptic parameter A). Statistics reported in the text and figures represent the mean ± SEM.
Results
Hyper-connectivity of the pyramidal network
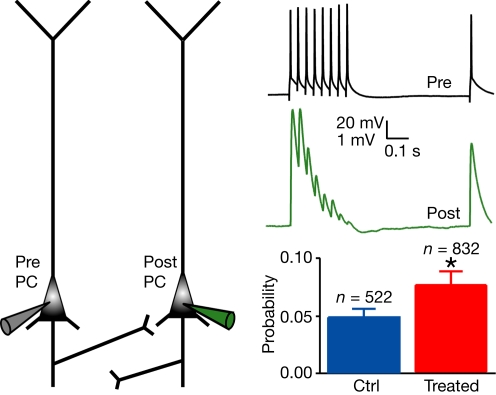
As in other neocortical regions, the pyramidal network receives distant input, generates the excitatory patterns and provides the executive output to multiple brain regions. We therefore recorded pairs of neighbouring layer 5 PCs in the mPFC of 2-week old control and VPA-treated offspring. We found that the probability of connection was significantly increased in the prefrontal cortex of VPA-treated rats as compared to controls (control: n = 832 pairs, 0.048 ± 0.007; treated: n = 522, 0.077 ± 0.012; P < 0.05, two-sided χ2 test; Figure 1). PCs of VPA-treated rats therefore connect more of their neighbouring PCs than in controls to produce a more distributed excitation.
Figure 1.
Increased connectivity between layer 5 pyramidal neurons. Experimental scheme of a pair of recorded PC, and example of the voltage traces recorded in the presynaptic cell and in the connected postsynaptic cell; probability of connection for control and treated rats. Data show mean ± SEM (*P < 0.05).
Weaker pyramidal synaptic connections
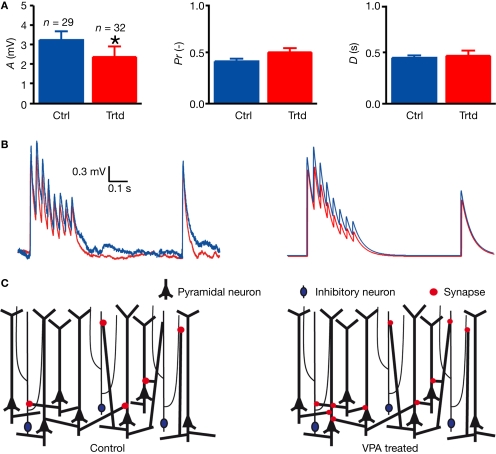
In order to understand whether the electrical signal sent between two PCs presents the same dynamic parameters in VPA-treated as in control rats, we then studied the connection dynamics. Using a model of dynamic synaptic transmission established by Tsodyks and Markram (1997) (see Section “Materials and Methods”), we fitted the traces (Figure 2B) and extracted three parameters characterising the connection dynamic: the absolute synaptic efficacy A is a measure of the strength of the connection, the utilisation Pr is a measure of the probability of release of the connection and the time constant for depression D is a measure of the short-term depression dynamic of the connection. VPA-treated rats exhibited significantly smaller absolute synaptic efficacy, A (control: n = 29, 3.30 ± 0.44 mV; treated: n = 32, 2.37 ± 0.52 mV; P < 0.05, two-sample Student's t-test; Figure 2A), indicating that the connections are weaker in the prefrontal cortex of VPA-treated rats. The probability of release (Pr) as well as the time constant to recover from depression (D) did not differ between control and VPA-treated rats (Figure 2A). This means that although connections among layer 5 PCs are more numerous, they are weaker in VPA-treated rats (Figure 2C).
Figure 2.
Decreased connection strength between layer 5 pyramidal neurons. (A) Mean values of the parameters characterising the connection dynamic: the absolute synaptic efficacy A, the probability of release Pr and the time constant to recover from depression D. Data show mean ± SEM (*P < 0.05). (B) Examples of the voltage trace recorded in a connected postsynaptic cell (left), and model fitting for the mean values of A, Pr and D (right) for control (blue) and treated (red) rats. (C) Scheme representing the increasing connectivity (number of red dots) and decreased connection strength (size of red dots) in treated rats as compared to control.
Hypo-excitability of pyramidal neurons
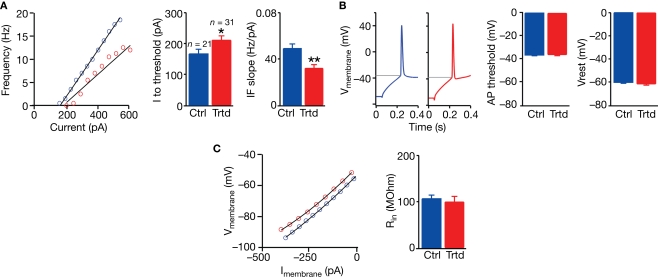
Increased connection probability gives the possibility to each PC to communicate with a higher number of neighbouring PCs. However, a change in the intrinsic properties of this type of cell could also modify the signal transmission. We therefore examined the key passive and active electrical properties of layer 5 PCs of the mPFC in control and VPA-treated offspring. By submitting the current-clamped neurons to a set of stimulation protocols designed to capture their electrical properties, we found that PCs exhibited depressed electrical excitability (Figure 3). The amount of current required to reach AP threshold was significantly higher in slices from treated rats (control: n = 21, 166 ± 15 pA; treated: n = 31, 211 ± 14 pA; P < 0.05, two-sample Student's t-test; Figure 3A), and the slope for the current–discharge (mean discharge during the whole stimulation) relationship was significantly lower (control: n = 21, 0.049 ± 0.004 Hz/pA; treated: n = 31, 0.032 ± 0.003 Hz/pA; P < 0.001, two-sample Student's t-test; Figure 3A). The AP threshold, the resting membrane potential (Figure 3B), the input resistance (Figure 3C), as well as other intrinsic properties (for example the AP width, the AP amplitude, the time to hyperpolarisation, the average delay to first spike; data not shown), were unchanged between the two populations. These results indicate that more current must be injected into PCs of VPA-treated rats to reach to the same output signal, i.e. to give rise to the same amount of APs.
Figure 3.
Decreased excitability of layer 5 pyramidal neurons. (A) Example of the current–frequency relationship for a control (blue) and a treated (red) layer 5 PC; mean values for the current needed to reach action potential threshold (I to threshold) and for the slope of the current–frequency relationship (IF slope). (B) Example of a response in a patched neuron from a control and a treated cell to a depolarising step current; mean values of the AP threshold and membrane resting potential (Vrest). (C) Example of the current–voltage relationship from a control and a treated cell; mean values of the input resistance (Rin). Data show mean ± SEM (*P < 0.05, **P < 0.01).
Hyper-plasticity of pyramidal synaptic connections
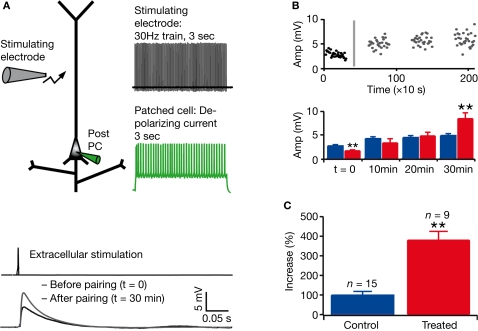
We next examined whether the observed hyper-connectivity and weaker connections were associated with alterations in synaptic plasticity. We studied synaptic plasticity in VPA-treated offspring following a classical Hebbian pairing protocol (see Section “Materials and Methods,” Figure 4A). Using extracellular stimulation and whole-cell patch-clamp recording in acute slice preparation, we found a significantly larger increase in the amplitude of the synaptic response up to 30 min after pairing in VPA-treated rats as compared to control (percentage increase in control rats: n = 15, 99 ± 19%; in treated rats: n = 9, 381 ± 44%; P < 0.001, two-sample Student's t-test; Figure 4B,C). Enhanced long-term potentiation (LTP) introduces a surprising perspective to the potential functional consequences of the hyper-connected prefrontal cortex of VPA-treated offspring.
Figure 4.
Enhanced long-term potentiation in layer 5 pyramidal neurons. (A) Experimental scheme and pairing protocol; example of mean response in a patched cell, before and after pairing. (B) Example of response amplitude as a function of time (the grey line represents the timing of the pairing protocol); mean absolute values for control (blue) and treated (red) rats before pairing (t = 0) and at three different times after pairing. (C) Mean of percentage increase in the amplitude of the response to extracellular stimulation 30 min after pairing. Data show mean ± SEM (**P < 0.01).
Comparison of effects with somatosensory cortex
The neuronal and circuit alterations found in the prefrontal cortex were very similar to those previously found in the somatosensory cortex (Table 1). In both cases, the intrinsic properties displayed the same profile of changes, with increased current needed to discharge the neurons and a shallower current-frequency response. The connection rate increases were approximately the same and the synaptic strength decreases were similar. In the somatosensory study, this decrease could be accounted for by the deployment of fewer synapses in each connection. Both the hypo-excitability and the weakened connection strength have been argued to be compensatory effects to the hyper-connectivity since the microcircuitry was found to be ultimately hyper-reactive to stimulation, which cannot be caused by hypo-excitability of the pyramidal neurons and weaker connections between individual neurons (Rinaldi et al., 2008).
Table 1.
Comparison of different properties between the medial prefrontal cortex and the somatosensory cortex in control and VPA-treated rats. All results were obtained with the same protocols as described in the Section “Materials and Methods”. Values significantly increased in treated rats as compared to control are in red, values significantly decreased are in blue. All results are for layer 5 PCs, apart from the result for the long-term potentiation in the somatosensory cortex that are for layer 2/3 PCs.
| Medial prefrontal cortex | Somatosensory cortex | |||
|---|---|---|---|---|
| INTRINSIC PROPERTIES | ||||
| Control (n = 21) | Treated (n = 31) | Control (n = 51) | Treated (n = 31) | |
| I to threshold (pA) | 166 ± 15 | 211 ± 14 | 400 ± 16 | 444 ± 16 |
| IF slope (Hz/pA) | 0.049 ± 0.004 | 0.032 ± 0.003 | 0.029 ± 0.001 | 0.025 ± 0.001 |
| Vrest (mV) | −60.4 ± 0.9 | −61.9 ± 0.9 | −60.2 ± 0.3 | −59.6 ± 0.4 |
| AP threshold (mV) | −36.7 ± 0.7 | −36.5 ± 1.2 | −33.0 ± 0.6 | −32.8 ± 0.7 |
| Rin (MΩ) | 106 ± 8 | 100 ± 10 | 69.9 ± 3.0 | 63.0 ± 3.4 |
| CONNECTION PROBABILITY | ||||
| Control (n = 832) | Treated (n = 522) | Control (n = 458) | Treated (n = 420) | |
| Probability (−) | 0.048 ± 0.007 | 0.077 ± 0.012 | 0.10 ± 0.01 | 0.16 ± 0.02 |
| CONNECTION DYNAMIC | ||||
| Control (n = 29) | Treated (n = 32) | Control (n = 50) | Treated (n = 46) | |
| A (mV) | 3.3 ± 0.4 | 2.4 ± 0.5 | 4.2 ± 0.4 | 3.3 ± 0.3 |
| Pr (−) | 0.44 ± 0.04 | 0.52 ± 0.04 | 0.52 ± 0.02 | 0.55 ± 0.02 |
| D (s) | 0.47 ± 0.03 | 0.49 ± 0.05 | 0.47 ± 0.02 | 0.49 ± 0.02 |
| LONG-TERM POTENTIATION | ||||
| Control (n = 15) | Treated (n = 9) | Control (n = 12) | Treated (n = 14) | |
| Increase (%) | 99 ± 19 | 381 ± 44 | 69 ± 22 | 207 ± 59 |
Discussion
In summary, we demonstrate that connections among layer 5 PCs of the mPFC, although weaker, are significantly more numerous in VPA-treated rats as compared to control. We also observe that these cells are less excitable. Furthermore, the synaptic connections between layer 5 PCs show significantly enhanced LTP following Hebbian pairing.
We examined the pyramidal network in the mPFC in this animal model of autism, but it is important to bear in mind that the layer 5 pyramidal network in this region is the most complex in the neocortex displaying at least three sub-networks involving different types of PCs interconnected with multiple types of synapses (Wang et al., 2006). Our study focussed on what has been classified as the simple PCs, which have simpler axonal and dendritic arbors (more similar to other neocortical regions), display spike train accommodation, and are interconnected with depressing synapses and at lower connection rates (see Wang et al., 2006). Further studies would be required to examine the alterations in other sub-networks of the prefrontal cortex. Of particular interest would be to determine how the complex PC sub-network is affected since these PCs are more tightly coupled with facilitating synapses that could be a key component in supporting persistent activity and working memory (see Wang et al., 2006). A recent modelling study explored how such a network of PCs with facilitating synapses could support working memory (Barak and Tsodyks, 2007).
The microcircuit alterations described in this study are similar to the findings in the somatosensory cortex, another cortical region studied in the VPA-treated rats (Rinaldi et al., 2007, 2008) (see Table 1). Increased connectivity, decreased connection strength, decreased cell excitability and enhanced plasticity are therefore not unique to one cortical region. These changes have been shown to underlie hyper-reactivity of the neocortical microcircuitry (Rinaldi et al., 2008) and have been proposed to lead to hyper-sensitive neocortical columns that could easily become autonomous once activated and difficult to control (Markram et al., 2007). Autonomous columns in the prefrontal cortex may excessively lock attention onto specific tasks, which could provide an alternative explanation for the deficits in multi-tasking, specialised interests, behavioural inflexibility or repetitive tendencies. The prefrontal cortex provides top down executive control over multiple brain regions (for review, see Struss and Knight, 2002), which probably demands projection of the most complex orchestration of activity patterns in the brain. Thus, not only would complex patterns needed for global orchestration be difficult to generate in the prefrontal cortex, but hyper-sensitive and autonomous columns in the rest of the neocortex could make the task of executive control over neocortical activity, even more challenging. Hyper-functionality may therefore provide an opposing explanation for the inability of the prefrontal cortex to activate remote neocortical regions during complex tasks (Just et al., 2004; Koshino et al., 2005; Villalobos et al., 2005). This may also explain why imaging studies reveal deficits for complex tasks, but unaffected or even enhanced activity for simpler tasks (Baron-Cohen et al., 1999; Kennedy et al., 2006; Pierce et al., 2004; Ring et al., 1999; Sotres-Bayon et al., 2004). The hyper-plasticity in this animal model was linked to a massive over-expression of NMDA receptors subunits in the somatosensory cortex (Rinaldi et al., 2007). Such enhanced plasticity might boost memory and learning capabilities, but combined with easily triggered autonomous neocortical columns, may lock memory talents into highly specialised domains, as proposed in the Intense World Syndrome (Markram et al., 2007).
Hyper-functional microcircuitry is not restricted to the neocortex since the amygdala, a sub-cortical component of the limbic system, is also hyper-reactive and hyper-plastic (Markram et al., 2008). The latter study linked hyper-reactivity and hyper-plasticity in this region to hyper-fear, over generalisation and resistance to extinction. The amygdala provides emotionally charged signals to the prefrontal cortex and the prefrontal cortex in turn controls the amygdala's dissemination of emotional content to the neocortex and plays a role in extinguishing fears (Sotres-Bayon et al., 2004). The prefrontal cortex may face a similar difficulty as proposed for controlling general neocortical activity, since this region would be severely challenged to orchestrate and apply inhibitory signals to the amygdala to control emotional determination of behaviour, generalisation and extinction of fears.
These results together indicate that the observed microcircuit alterations are not unique to specific brain regions. Regionally unspecified alterations are not surprising as the VPA insult affects gene transcription (as a potent teratogen) at the time of neural tube closure and the effects may unfold with development and be clinically evident when the functions served by these brain regions are demanded. As the insult happens so early in development, it is also evident that VPA does not act on the microcircuit properties directly, and that the alterations described here are indirect consequences of the primary neonatal insult. A better understanding of the actions of VPA during embryogenesis will be required to determine the specific molecular pattern or syndrome activated to drive the unfolding of an autistic-like disorder.
Further studies are required to determine whether the alterations found in animal models are also reflected in the human condition. Genetically based animal models of diseases are traditionally more commonly accepted as valid models, but in the case of autism, the heredity component is complex and specific models are debatable and none have been well established. The VPA animal model is today the most exhaustively validated animal model of autism, mapping remarkably well with many of the macro and micro-anatomical, biochemical and behavioural alterations found in the human disorder (for review see Markram et al., 2007). The model is an insult-based model, as a number of insults are associated with a high incidence of autism, but this does not discount the strong heredity component, which confers a polygenetic predisposition for autism. A systematic study to combine the insult-based and genetic models to determine whether such an insult can more readily trigger autism in genetically predisposed animals is yet to be performed.
In summary, we report that a pyramidal network in the mPFC displays similar alterations as previously found in the somatosensory cortex and propose that these alterations could lead to hyper-functional neocortical columns in this region. We suggest that past data and conclusions supporting diminished prefrontal function should be revisited in the light of the potential consequences of hyper-functionality in the prefrontal cortex.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Thomas Berger and Kamila Markram for their help. This work was supported by the National Alliance for Autism Research (NAAR) and by a grant from the European Commission for the EUSynapse project.
References
- Abell F. (1999). The neuroanatomy of autism: a voxel-based whole brain analysis of structural scans. Neuroreport 10, 1647–1651 10.1097/00001756-199906030-00005 [DOI] [PubMed] [Google Scholar]
- Bachevalier J. (1986). Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav. Brain Res. 20, 249–261 10.1016/0166-4328(86)90225-1 [DOI] [PubMed] [Google Scholar]
- Barak O. (2007). Persistent activity in neural networks with dynamic synapses. PLoS Comput. Biol. 3, e35. 10.1371/journal.pcbi.0030035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. (1985). Does the autistic child have a “theory of mind”? Cognition 21, 37–46 10.1016/0010-0277(85)90022-8 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. (1994). Recognition of mental state terms. Clinical findings in children with autism and a functional neuroimaging study of normal adults. Br. J. Psychiatry 165, 640–649 10.1192/bjp.165.5.640 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. (1999). Social intelligence in the normal and autistic brain: an fMRI study. Eur. J. Neurosci. 11, 1891–1898 10.1046/j.1460-9568.1999.00621.x [DOI] [PubMed] [Google Scholar]
- Carmichael S. T. (1995). Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol. 363, 642–664 10.1002/cne.903630409 [DOI] [PubMed] [Google Scholar]
- Castelli F. (2002). Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain 125, 1839–1849 10.1093/brain/awf189 [DOI] [PubMed] [Google Scholar]
- Fletcher P. C. (1995). Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition 57, 109–128 10.1016/0010-0277(95)00692-R [DOI] [PubMed] [Google Scholar]
- Gallagher H. L. (2000). Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia 38, 11–21 10.1016/S0028-3932(99)00053-6 [DOI] [PubMed] [Google Scholar]
- Goel V. (1995). Modeling other minds. Neuroreport 6, 1741–1746 10.1097/00001756-199509000-00009 [DOI] [PubMed] [Google Scholar]
- Happe F. (1996). ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport 8, 197–201 [DOI] [PubMed] [Google Scholar]
- Hill E. L. (2004). Executive dysfunction in autism. Trends Cogn. Sci. 8, 26–32 10.1016/j.tics.2003.11.003 [DOI] [PubMed] [Google Scholar]
- Ingram J. L. (2000). Prenatal exposure of rats to valproic acid reproduces the cerebellar anomalies associated with autism. Neurotoxicol. Teratol. 22, 319–324 10.1016/S0892-0362(99)00083-5 [DOI] [PubMed] [Google Scholar]
- Just M. A. (2004). Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain 127, 1811–1821 10.1093/brain/awh199 [DOI] [PubMed] [Google Scholar]
- Kennedy D. P. (2006). Failing to deactivate: resting functional abnormalities in autism. Proc. Natl. Acad. Sci. USA 103, 8275–8280 10.1073/pnas.0600674103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshino H. (2005). Functional connectivity in an fMRI working memory task in high-functioning autism. Neuroimage 24, 810–821 10.1016/j.neuroimage.2004.09.028 [DOI] [PubMed] [Google Scholar]
- Le Be J. V. (2007). Morphological, electrophysiological, and synaptic properties of corticocallosal pyramidal cells in the neonatal rat neocortex. Cereb. Cortex 17, 2204–2213 10.1093/cercor/bhl127 [DOI] [PubMed] [Google Scholar]
- Markram H. (2007). The Intense World Syndrome – an alternative hypothesis for autism. Front. Neurosci. 1, 77–96 10.3389/neuro.01.1.1.006.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H. (1998). Differential signaling via the same axon of neocortical pyramidal neurons. Proc. Natl. Acad. Sci. USA 95, 5323–5328 10.1073/pnas.95.9.5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram K. (2008). Abnormal fear conditioning and amygdala processing in an animal model of autism. Neuropsychopharmacology 33, 901–912 10.1038/sj.npp.1301453 [DOI] [PubMed] [Google Scholar]
- Moore S. J. (2000). A clinical study of 57 children with fetal anticonvulsant syndromes. J. Med. Genet. 37, 489–497 10.1136/jmg.37.7.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M. A. (1993). Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci. Lett. 163, 109–113 10.1016/0304-3940(93)90241-C [DOI] [PubMed] [Google Scholar]
- Myers R. E. (1972). Role of prefrontal and anterior temporal cortex in social behavior and affect in monkeys. Acta Neurobiol. Exp. 32, 567–579 [PubMed] [Google Scholar]
- Pierce K. (2004). The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain 127, 2703–2716 10.1093/brain/awh289 [DOI] [PubMed] [Google Scholar]
- Price J. L. (2006). Prefrontal cortex. In Understanding Autism: From Basic Neuroscience to Treatment, Moldin S. O., eds (Boca Raton, CRC Press; ), pp. 205–226 [Google Scholar]
- Rasalam A. D. (2005). Characteristics of fetal anticonvulsant syndrome associated autistic disorder. Dev. Med. Child Neurol. 47, 551–555 10.1017/S0012162205001076 [DOI] [PubMed] [Google Scholar]
- Rinaldi T. (2007). Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid. Proc. Natl. Acad. Sci. USA 104, 13501–13506 10.1073/pnas.0704391104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi T. (2008). Hyperconnectivity of local neocortical microcircuitry induced by prenatal exposure to valproic acid. Cereb. Cortex 18, 763–770 10.1093/cercor/bhm117 [DOI] [PubMed] [Google Scholar]
- Ring H. A. (1999). Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of embedded figures task performance. Brain 122(Pt 7), 1305–1315 10.1093/brain/122.7.1305 [DOI] [PubMed] [Google Scholar]
- Rodier P. M. (1997). Linking etiologies in humans and animal models: studies of autism. Reprod. Toxicol. 11, 417–422 10.1016/S0890-6238(97)80001-U [DOI] [PubMed] [Google Scholar]
- Schneider T. (2005). Behavioral alterations in rats prenatally exposed to valproic acid: animal model of autism. Neuropsychopharmacology 30, 80–89 10.1038/sj.npp.1300518 [DOI] [PubMed] [Google Scholar]
- Schultz R. T. (2003). The role of the fusiform face area in social cognition: implications for the pathobiology of autism. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 358, 415–427 10.1098/rstb.2002.1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F. (2004). Emotional perseveration: an update on prefrontal–amygdala interactions in fear extinction. Learn. Mem. 11, 525–535 10.1101/lm.79504 [DOI] [PubMed] [Google Scholar]
- Struss D. (2002). Principles of Frontal Lobe Function. Oxford, Oxford University Press. [Google Scholar]
- Tsodyks M. (1998). Neural networks with dynamic synapses. Neural Comput. 10, 821–835 10.1162/089976698300017502 [DOI] [PubMed] [Google Scholar]
- Tsodyks M. V. (1997). The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc. Natl. Acad. Sci. USA 94, 719–723 10.1073/pnas.94.2.719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos M. E. (2005). Reduced functional connectivity between V1 and inferior frontal cortex associated with visuomotor performance in autism. Neuroimage 25, 916–925 10.1016/j.neuroimage.2004.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. (2006). Heterogeneity in the pyramidal network of the medial prefrontal cortex. Nat. Neurosci. 9, 534–542 10.1038/nn1670 [DOI] [PubMed] [Google Scholar]