Abstract
Background
Adenylyl cyclases (ACs) represent a diverse family of enzymes responsible for the generation of cAMP, a key intracellular second messenger. Ca2+/calmodulin-stimulated AC1 and AC8 isoforms, as well as the calcium-inhibited AC5 isoform, are abundantly expressed within limbic regions of the central nervous system. This study examines the contribution of these AC isoforms to emotional behavior.
Methods
Male and female AC1/8 double knockout mice (DKO) and AC5 knockout mice (AC5KO) were examined on a series of standard laboratory assays of emotionality. Mice were also assayed for hippocampal cell proliferation and for changes in BDNF signaling in the nucleus accumbens, amygdala, and hippocampus, three forebrain structures involved in the regulation of mood and affect.
Results
AC5KO mice showed striking anxiolytic and antidepressant phenotypes on standard behavioral assays. In contrast, AC1/8 DKO mice were hypoactive, exhibited diminished sucrose preference, and displayed alterations in neurotrophic signaling, generally consistent with a prodepressant phenotype. Neither line of mice displayed alterations in hippocampal cell proliferation.
Conclusions
These data illustrate the complex manner in which Ca2+/calmodulin-stimulated adenylyl cyclases contribute to emotional behavior. In addition, they support the possibility that a selective AC5 antagonist would be of therapeutic value against depression and anxiety disorders.
Keywords: AC1, AC8, AC5, nucleus accumbens, hippocampus, knockout mice, calcium-regulated adenylyl cyclases
INTRODUCTION
Adenylyl cyclases (ACs) catalyze the synthesis of cAMP (cyclic adenosine monophosphate), which in turn activates PKA (protein kinase A) signaling. Of the nine transmembrane isoforms (AC1–AC9) that have been identified in mammals (1, 2), Ca2+-regulated isoforms are particularly relevant to psychiatry, as they provide a crucial link between neuronal activity and intracellular cAMP (3). Ca2+-stimulated AC activity within limbic regions of the CNS is constituted by AC1 and AC8 (4), both of which are activated by Ca2+/calmodulin (5). Studies of mice that lack AC1 and/or AC8 have revealed an essential role for these enzymes in several neurobiological processes including long-term memory, sensitivity to drugs of abuse, and chronic pain (6–10). In contrast, physiological Ca2+ concentrations inhibit AC5 (11), a striatal-enriched isoform important for behavioral responses to morphine (12). AC5 knockout (AC5KO) mice show resistance against oxidative and genotoxic stresses; they also display an extended lifespan, reduced aging-induced cardiomyopathy, and improved bone quality (13), strongly implicating AC5 inhibition as an effective target against aging (14).
This study explores the relevance of Ca2+-regulated AC activity to mood and anxiety disorders by characterizing the performance of AC5KO and AC1/8 double knockout (DKO) mice and their controls on a series of standard behavioral assays. We complement these results with measures of hippocampal proliferation and neurotrophic signaling. Instead of examining the roles of AC1 and AC8 separately, we examine DKO mice since a simultaneous AC1/8 deletion is required to abolish Ca2+-stimulated AC activity within the CNS (8, 10), and several phenotypic changes related to loss of Ca2+-stimulated AC activity have only been observed in DKOs (6, 7, 10).
METHODS AND MATERIALS
Mice
Mice were: i) housed in groups of 3–4, ii) 8–12 weeks old, iii) fed ad libitum, and iv) housed at 23–25°C (lights ON between 0700–1900 hrs). AC5+/− breeder pairs were used to generate AC5KO mice and wildtype (WT) littermate controls (12, 15). AC1/8 DKO mice obtained through intercrosses between AC1+/−;AC8+/− doubly heterozygous breeders (16, 17) were employed to establish DKO breeding colonies in which all the offspring were DKO genotype. To control for genetic background, WT breeding colonies of the same lineage were simultaneously established. For immunoblotting and immunohistochemistry experiments, only males were employed.
Behavioral Testing
AC5KO and AC1/8DKO mice displayed no evidence of grossly abnormal behavior such as ataxia, seizures, stereotypy, etc., and displayed normal sensory and motor reflexes (see Supplementary Methods and Materials for detailed behavioral procedures). Testing was performed in the light phase using established procedures (18, 19). Locomotor activity was measured in a novel cage with photocells that measured total beam breaks during twelve 10 min intervals. Videotracking (Ethovision) was employed for elevated plus maze and open field testing (5 min), whereas dark-light testing (10 min) employed photocell fitted chambers. For social interaction testing, socially isolated mice (2–3 weeks isolation) were tested in a two-trial paradigm (20): in the first trial (2.5 min), mice were allowed to explore a square open field arena containing an empty wire-mesh cage. During the second trial (2.5 min), mice were reintroduced into this arena now containing an unfamiliar “social target” mouse (c57bl6, Jackson Labs) within the cage. Videotracking measured the time spent in an “interaction zone” – a 15 × 30 cm area surrounding the target enclosure during both “target absent” and “target present” conditions. Sucrose preference at increasing sucrose concentrations was measured through a two bottle choice paradigm (18).
BrdU Immunohistochemistry
Bromo-deoxyuridine (BrdU) labeling and cell counting was performed as described (21). 2 hrs following a single injection of BrdU (150 mg/kg), mice were perfused and fixed brains were sectioned (30 µm) and processed for BrdU with a rat monoclonal anti-BrdU antibody (1:400, Accurate).
Immunoblotting
Tissue punches of nucleus accumbens (NAc), amygdala, and hippocampus were obtained from 1 mm-thick fresh brain slices, frozen on dry ice, and subsequently lysed and sonicated in a lysis buffer (20 mM HEPES, 0.4 M NaCl, 20% glycerol, 5 mM MgCl2, 0.5 mM EDTA, 0.1 mM EGTA, 1% NP40, 50 mM DTT, 0.1% protease and phosphatase inhibitors [Sigma]), and then centrifuged at 14,000 rpm for 15 min. Supernatant protein concentrations were determined using the Bradford Assay (Biorad), following which 40 µg of protein were electrophoresed on 4–15% SDS gradient gels (Biorad). Proteins were transferred to PVDF membranes, washed thoroughly in 1x Tris-buffered saline with 0.1% Tween-20 (TBS-T), and subsequently blocked in a solution of 5% w/v nonfat dry milk in TBS-T for 1 hr at room temperature. Membranes were incubated in primary antibody: anti-BDNFN-20 (1:200; Santa Cruz), anti-actin (1:10,000; MP Biomedicals), anti-TrkB (1:5000; Upstate), anti-AKT, anti-phosphoAKTS473, anti-ERK1/2, anti-phosphoERK1/2T202 Y204, anti-PLCγ, and anti-phosphoPLCγY783 (1:500, Cell Signaling). After further washes, membranes were incubated with peroxidase-labeled goat anti-rabbit or horse anti-mouse IgG (1:40,000; Vector, Burlingame, CA). Bands were visualized with SuperSignal West Dura substrate (Pierce, Rockford, IL) and quantified with Scion Image (NIH).
Statistical Analyses
Unpaired, two-tailed Student’s t tests were utilized for comparisons between WT and KO groups (BrDU counts, immunoblotting). For behavioral testing, two-way ANOVAs were used to examine significant main effects or interactions (genotype × sucrose concentration, genotype × target, or genotype × time interval). Significant post-hoc effects were revealed by Bonferroni tests; effects were considered significant at p< 0.05.
RESULTS
AC1/8 DKO and AC5KO Mice Display Contrasting Locomotor Habituation Phenotypes
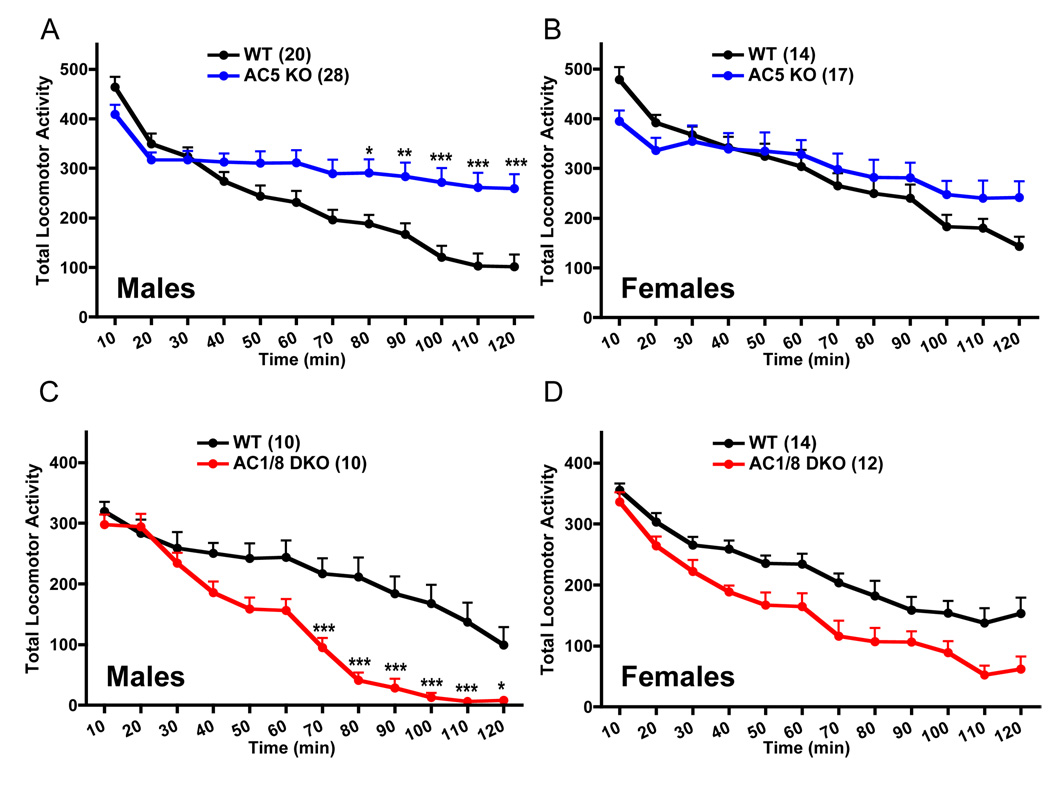
Male AC5KO mice were more hyperactive than their WT counterparts (Figure 1A), and this hyperactivity was limited to the last 50 min of testing. In contrast, AC1/8 DKO mice demonstrated a clear hypoactive phenotype (Figure 1C), with prominent differences observed in the last hour of testing. Female mice of either genotype did not demonstrate statistically significant changes in locomotor activity (ANOVA), although did display similar trends (Figure 1B, D). Thus, AC1/8 DKO and AC5KO mice do not differ from WT controls in the acute locomotor response to novelty, but rather do display opposite locomotor habituation phenotypes.
Figure 1.
Male AC5KO and AC1/8 DKO mice display opposite phenotypes on measures of locomotor habituation. A: Male AC5KO mice were more active (genotype × time intervalRM interaction, F11,506 = 18.18, p<0.0001), whereas B: female AC5KO and WT mice demonstrated similar locomotor habituation behavior (time intervalRM main effect, F11,319 = 49.94, p<0.0001). C: Male AC1/8DKO mice were less active (genotype × time intervalRM interaction, F11, 198 = 10.78, p<0.0001), whereas D: AC1/8DKO females were as active as WT mice (time intervalRM main effect, F11,264 = 65.78, p<0.0001). Data are presented as means+SEM, with * indicating significant post hoc differences (*:p<0.05, **:p<0.01, ***:p<0.001). (RM: repeated measure).
AC5KO Mice Display a Prominent Anxiolytic Phenotype
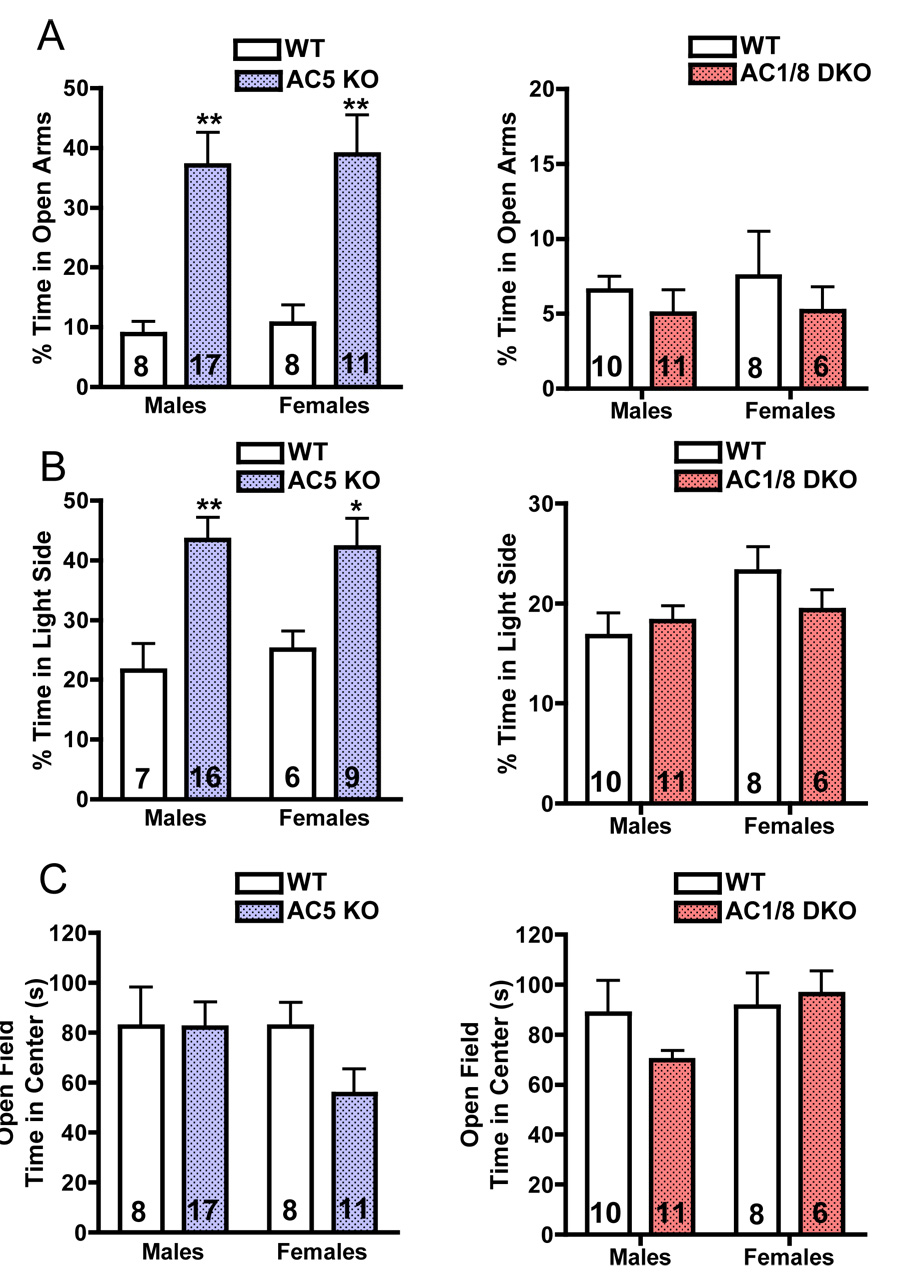
We next examined AC5KO and AC1/8 DKO mice on tests of exploratory behavior (Figure 2, Supplementary Figures S1 and S2). In the elevated plus maze, AC5KO mice spent a significantly greater amount of time in the open arms of the maze (Figure 2A), whereas AC1/8 DKO mice were no different from their WT counterparts. Similar results were obtained on the light-dark test, where AC5KO mice displayed greater exploration of the light side (Figure 2B). However, in the open field test, both AC5KO and AC1/8 DKO mice performed comparably to WTs (Figure 2C), suggesting that the striking anxiolytic effects of AC5 disruption are limited to certain types of anxiety-provoking situations.
Figure 2.
AC5KO mice display a prominent anxiolytic phenotype. A: On the elevated plus maze test, AC5KO mice spent significantly greater time on the open arms of the plus maze (genotype main effect, F1,40 = 23.33, p<0.0001), while AC1/8 DKO mice were no different from their WT controls (genotype, F1,31 = 0.04, p>0.5). B: Similar results were obtained in the dark-light test, where AC5KO mice explored the light compartment significantly more than their WT controls (genotype main effect, F1,34 = 16.99, p<0.01), while AC1/8 DKO mice performed comparably to WT mice (genotype, F1,30 = 0.30, p>0.5). C: The open field tests did not reveal anxiety phenotypes in either AC5KO (genotype, F1,40 = 1.28, p>0.2) or AC1/8 DKO (genotype, F1,31 = 0.41, p>0.5). Data are presented as means + SEM (sample size denoted within bars), with * indicating significant posthoc differences (*: p<0.05, **:p<0.01).
Immobility, Social Interaction, and Sucrose Preference
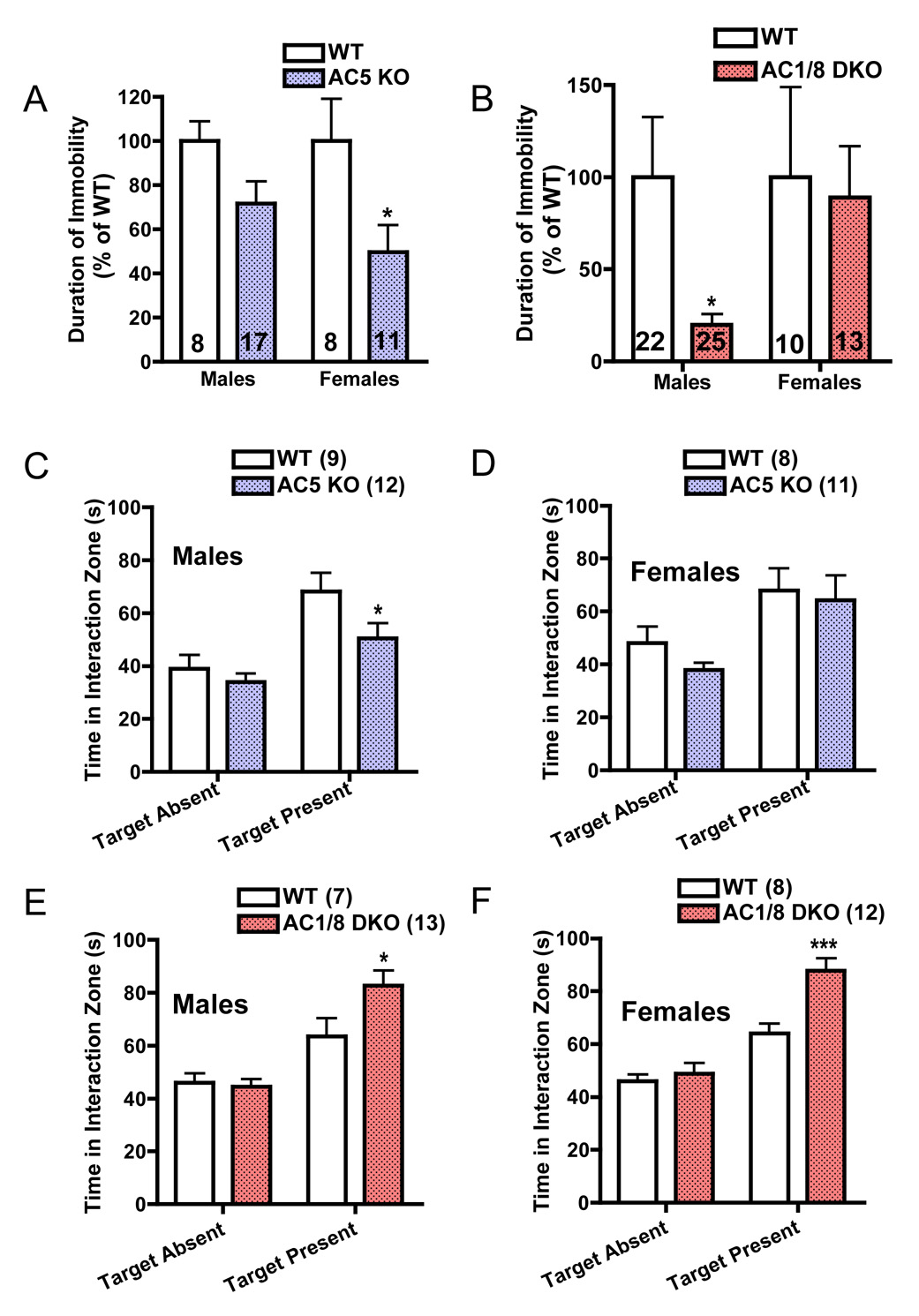
The FST gauges the duration of immobility during an inescapable situation, a measure sensitive to antidepressant treatments and genetic manipulations (19, 22). AC5KO mice displayed a reduction in immobility (antidepressant-like behavior), with particularly striking effects in females (Figure 3A). Interestingly, male AC1/8DKO mice also displayed reduced immobility (Figure 3B), while female DKO mice performed identically to WT. We also examined these mice on a modified social interaction test (Supplementary Figure S3A–C). During the presence of a social cue, AC5KO mice were significantly less interactive than WT mice (Figure 3C), an effect that was not observed in females (Figure 3D). AC1/8 DKO displayed the opposite phenotype: they interacted considerably more than their WT counterparts (Figure 3E, F), suggesting that disrupting these two types of Ca2+-regulated AC activities may confer opposite effects on social behavior.
Figure 3.
A,B: Durations of immobility during the last 4 min period of forced swim testing (FST, 6 min test) measured by an experienced observer blind to condition. AC5KO mice spent significantly less time immobile than their wild-type counterparts (genotype main effect, F1,39 = 9.71, p<0.01), with a prominent phenotype emerging in females. AC1/8DKO mice also displayed an decreased immobility on the FST (genotype main effect, F1,71 = 3.993, p<0.05), an effect mainly observed in males. C, D: In a two-trial social interaction test (see Methods and Materials), male AC5KO mice were significantly less interactive than WT mice (genotype × target interaction, F1,38 = 4.68, p<0.05). This effect was not observed in females (genotype × target interaction, F1,38 =0.91, p>0.3). E, F: AC1/8DKO mice were significantly more interactive than WT mice, and this effect was observed both in males (genotype × target interaction, F1,36 = 2.92, p<0.05) and females (genotype × target interaction, F1,36 =9.78, p<0.01). Data are presented as means + SEM (sample size denoted within bars), with * indicating significant post-hoc differences (*:p<0.05, ***:p<0.001).
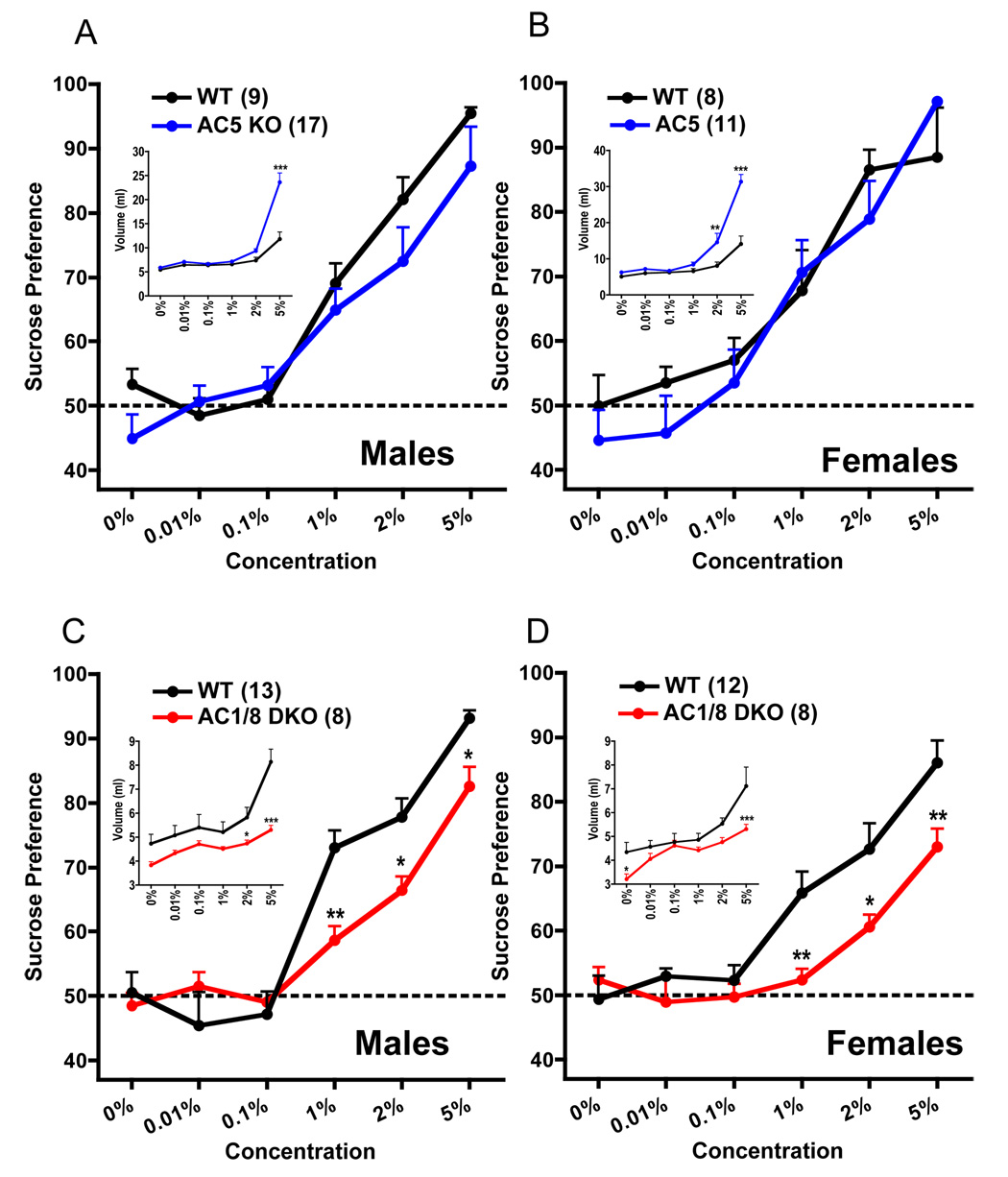
Many chronic stresses produce a stable reduction in the preference for a sweet solution, interpreted as a form of anhedonia (18, 23); this reduction is reversed by chronic antidepressant treatments (24). When compared with WT mice, AC5KO mice displayed normal sucrose preference (Figure 4A, B) as well as comparable levels of fluid intake (Figure 4A, B insets), except at higher concentrations where KO mice drank significantly higher volumes. This pattern cannot be explained by body weight, which is normal in AC5KO mice at the ages tested. In contrast, AC1/8 DKO mice displayed a prominent reduction in sucrose preference (Figure 4C, D). This cannot be explained by alterations in gustation, which is normal in these mice (6). Total fluid intake was reduced in AC1/8 DKO mice (Figure 4C, D insets), possibly partly to the significant reductions in body weight observed in DKO mice (Supplementary Figure S3D).
Figure 4.
Sucrose preference data for male and female AC5KO and AC1/8DKO mice depicted as a function of sucrose concentration in bottle “A” (x axis, [w/v]), with inset graphs displaying fluid volume intake. A: Male AC5KO and WT mice showed comparable sucrose preference (genotype × concentration interaction, F5,144 = 0.74, p>0.5), and displayed increased fluid intake at higher sucrose concentrations (F5,144 = 13.20, p<0.0001). B: Female AC5KO mice also displayed sucrose preference scores similar to WT (genotype × concentration interaction, F5,102 = 0.85, p>0.5), as well as increased fluid intake at higher sucrose concentrations (F5,102 = 12.85, p<0.0001). C: Male AC1/8DKO mice exhibited significantly lowered sucrose preference (genotype × concentration interaction, F5,114 = 4.59, p<0.001) together with overall decreases in fluid intake (genotype main effect, F1,113 = 53.18, p<0.0001). D: Female AC1/8DKO mice demonstrated significantly lowered sucrose preference (genotype × concentration interaction, F5,108 = 2.98, p<0.05), with reduced overall fluid intake (genotype main effect, F1,107 = 22.85, p<0.0001). Data are presented as means ± SEM, with * indicating significant post-hoc differences (*:p<0.05, **:p<0.01, ***:p<0.001).
Hippocampal Cell Proliferation is Not Altered in AC KO Mice
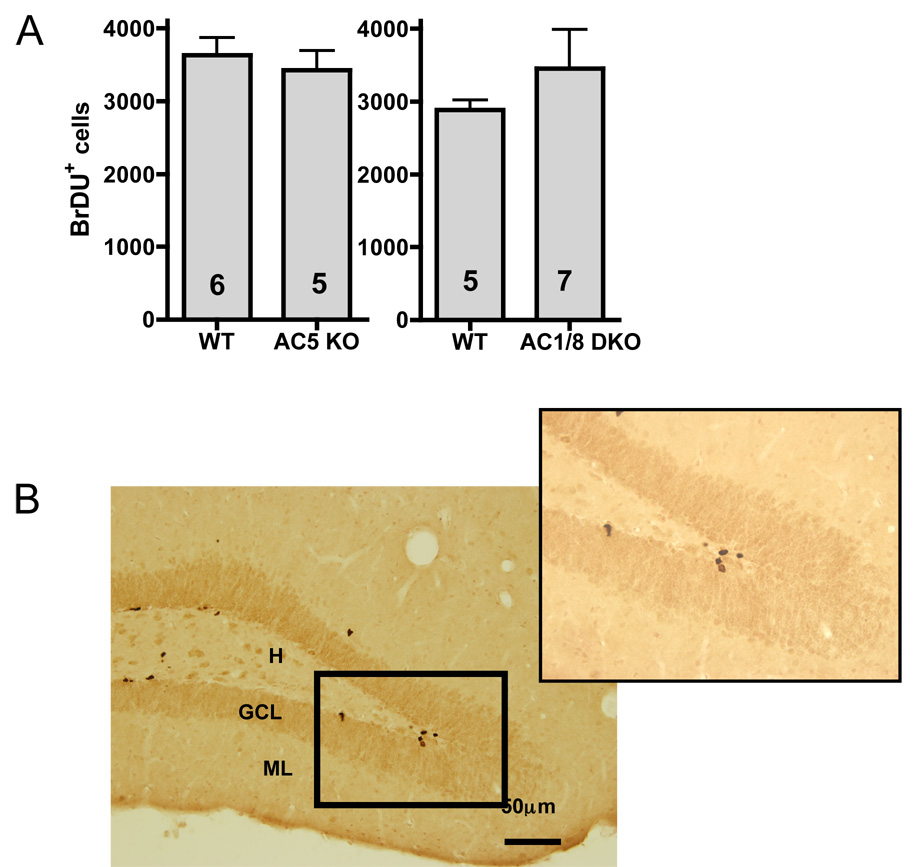
Decreased hippocampal cell proliferation has been postulated as a key mechanism for stress-related pathology, with antidepressants causing the opposite effect (25). AC5KO and AC1/8 DKO mice were assayed for changes in levels of proliferation using BrDU as an S-phase marker. BrDU+ cells were enriched in the subgranular zone of the granule cell layer (Figure 5B). There were no significant differences in the number of BrDU+ cells in either AC5KO or AC1/8 DKO mice compared to their respective controls (Figure 5A).
Figure 5.
A: Neither AC5KO nor AC1/8 DKO mice showed abnormal rates of hippocampal proliferation, as measured by the total number of BrDU immunopositive cells found in the dentate gyrus (DG) subgranular zone (AC5: t9 = 0.57, p>0.5, AC1/8: t10 = 0.87, p>0.3, sample size denoted within bars). B: Representative photomicrograph of a coronal section through the hippocampus showing darkly stained BrDU labeled cell clusters in the apex of the DG (20X). Inset: 40X magnified view. Abbreviations: H (Hilus), GCL (Granule Cell Layer), ML (Molecular Layer).
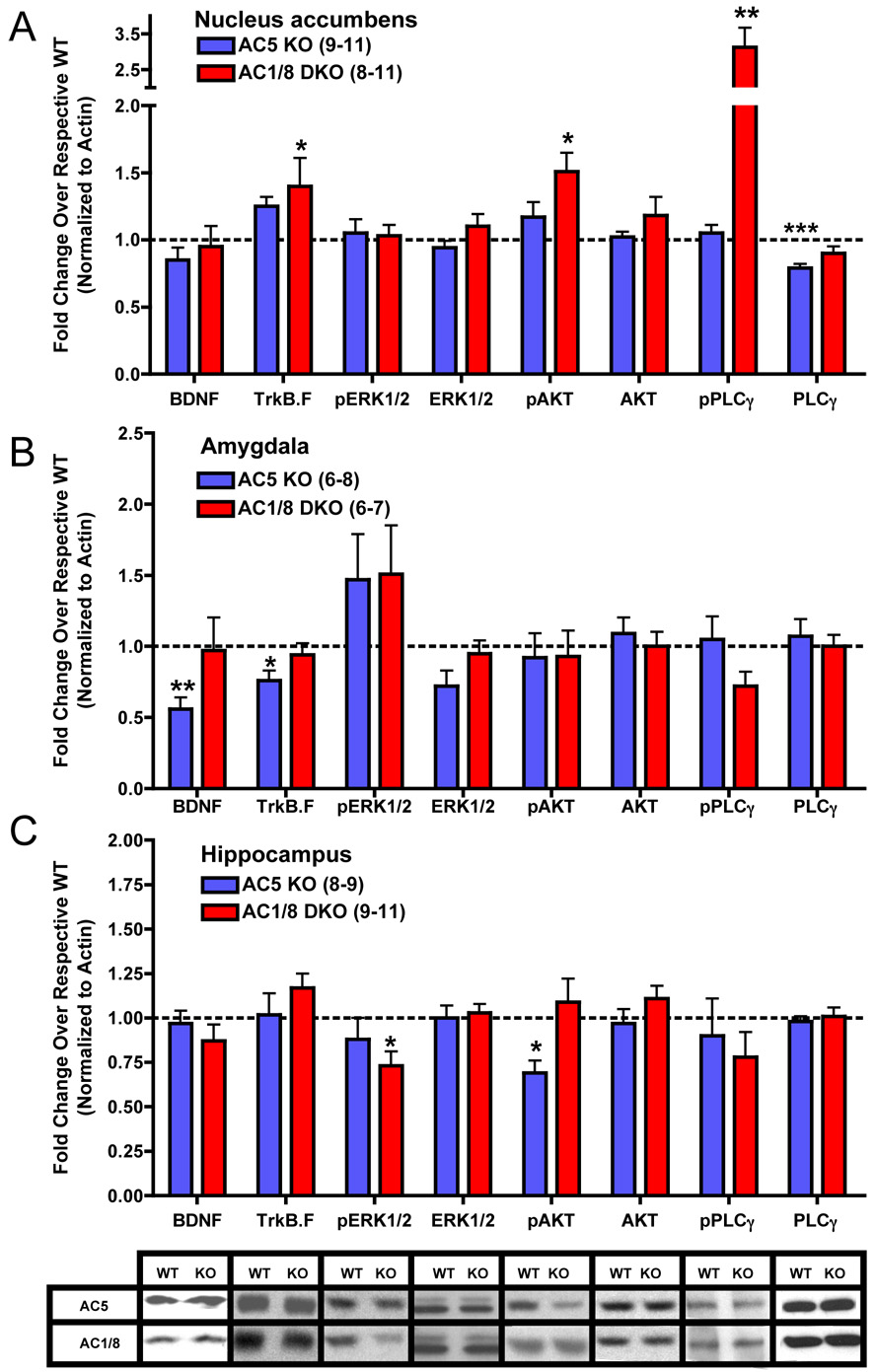
Altered BDNF Signaling in AC KO Mice
Abnormalities in BDNF (brain-derived neurotrophic factor) signaling are thought to represent a candidate pathophysiological mechanism for depression (26); postmortem studies of depressed individuals reveal decreased BDNF protein levels in hippocampus (27) and increased BDNF levels in NAc (18), a forebrain structure involved in reward-related processes. Through immunoblotting, we probed samples of hippocampus, NAc, and the amygdala, which plays a prominent role in anxiety-related processes (28), from AC1/8 DKO and AC5KO mice for abnormalities in BDNF and downstream signaling. In NAc, AC1/8 DKO mice displayed strong inductions of neurotrophic signaling (Figure 6A), including increased levels of the full-length BDNF receptor, TrkB.F (tropomyosin-related kinase B), phospho-AKT, and phospho-PLCγ (phosholipase Cγ). In contrast, NAc from AC5KO mice only displayed a reduction in levels of total PLCγ. Amygdala samples from AC5KO mice displayed significant reductions in both BDNF and TrkB.F levels (Figure 6B). In hippocampus (Figure 6C), AC1/8 DKO mice displayed significantly lower levels of phospho-ERK1/2 (extracellular signal-related kinase 1,2), whereas AC5KO mice showed reduced phospho-AKT levels.
Figure 6.
Effects on BDNF signaling within the NAc, amygdala and hippocampus. A–C: Optical density measurements for each sample were first normalized to the respective β-actin band intensity; group averages were then normalized to WT. Representative bands are shown for hippocampal samples. Data are presented as means ± SEM, with * indicating significant differences in comparison to WT controls (*:p<0.05, **:p<0.01, ***:p<0.001). Abbreviations: BDNF (brain-derived neurotrophic factor), TrkB.F (full-length tropomyosin-related kinase B), ERK1/2 (extra-cellular signal regulated kinase 1/2), AKT (thymoma viral proto-oncogene), PLCγ (phospholipase Cγ), pERK1/2, pAKT, and p PLCγ (phosphorylated forms of these proteins).
DISCUSSION
Achieving a detailed understanding of the biology of individual AC isoforms in vivo has been challenging: in addition to complex and redundant tissue distribution patterns, catalytic domains of AC isoforms are highly homologous, which has precluded the generation of isoform-specific antibodies. ACs have also been neglected as pharmacological targets: current small molecule modulators of AC function such as forskolin act on virtually all ACs (29). However, important insights have come from examining phenotypes of specific AC KO mice, an approach which may provide insight into any therapeutic potential of isoform-specific small-molecule modulators. Given the contribution of calcium-regulated ACs to memory and addiction-related processes, we examined AC5KO and AC1/8 DKO mice in paradigms of depression and anxiety, two common and complex psychiatric syndromes.
Our data highlight a novel role for AC5 in the regulation of affective behavior. While male AC5KO mice displayed hyperactivity, this effect was only observed during the “habituation phase”, and therefore does not confound the results of other assays, which can be sensitive to changes in novelty-induced hyperactivity. AC5KO mice displayed a strong anxiolytic phenotype in two of three standard assays, as well as an antidepressant phenotype in the FST. Together, these features resemble other mouse models of “manic-like” behavior such as Clock mutant mice (30, 31), which display hyperactivity as well as hyperhedonic, anxiolytic, and antidepressant phenotypes. Indeed, the mood stabilizer lithium has been shown to inhibit AC5 in vitro (32), as well as AC7, another isoform that produces an antidepressant phenotype when genetically eliminated in mice (33). Interestingly, levels of BDNF and TrkB were reduced within the amygdala, an important neural substrate for anxiety, while transgenic overexpression of BDNF in this region causes potent anxiogenic effects (34). We also observed decreased levels of phospho-AKT in hippocampus, and AKT is known to phosphorylate and inhibit GSK3β (glycogen synthase kinase 3β). Transgenic mice overexpressing a constitutively active form of GSK3β in broad forebrain regions also display a manic phenotype (35). Further studies will be important to discriminate the “antidepressant” versus “pro-manic” effects of AC5 disruption, and to more causatively link our molecular changes with the observed behavior phenotypes.
AC1/8 DKO mice displayed a more complex phenotype, and since the generation of an appropriately large number of DKO mice required non-littermate comparisons, we acknowledge that these behavioral changes could be a result of different maternal effects. While male AC1/8 DKO mice displayed reduced immobility in the FST, both males and female DKO mice displayed lower body weights and strong deficits in sucrose preference, abnormalities often observed in mouse models of depression (18, 24). Depressed patients display reductions in platelet adenylyl cyclase activity (36), which is predominantly constituted by AC3 (37), an isoform structurally and functionally similar to AC1 and AC8 (11). Immunoblotting of NAc revealed strong activation of BDNF signaling in the DKOs, which is also observed in mice after chronic social defeat (18). Additionally, we observed a significant reduction in ERK phosphorylation in the hippocampus. Such an effect has also been observed after prolonged corticosterone exposure (38), and systemic pharmacological inhibition of ERK1/2 produces prodepressant effects (39). DKO mice did not display abnormalities in hippocampal cell proliferation, an important negative finding in light of the critical role of AC1 and AC8, and subgranular zone proliferation, in memory (10). Our social interaction tests revealed that DKO mice socially interacted more than WTs. Social interaction is a complex behavior, mediated by several components including anxiety, social dominance, and motivation (40). In contrast to AC1/8 DKO mice, male AC5KO mice displayed a social deficit, and these opposite findings correlate with levels of PLCγ signaling within the NAc. Further studies are clearly required to dissect the molecular basis for these social phenotypes.
In summary, constitutive knockout of AC1 and AC8 produces a deficit in sucrose reward, weight loss, and decreased locomotor activity. Together with previous studies that demonstrate altered drug reward and robust memory deficits in AC1/8 DKO mice (see Introduction), these findings suggest that inhibitors of Ca2+/calmodulin-stimulated ACs would have largely adverse behavioral consequences. In contrast, knockout of AC5 causes strong antidepressant and anxiolytic phenotypes. Indeed, novel pharmacotherapies aimed at selectively antagonizing AC5 function would promote longevity and protect against oxidative stress (13). Our findings suggest that such agents would also promote resilience against depression and anxiety disorders.
Supplementary Material
Acknowledgments
The authors would like to thank Megumi Adachi and Cathy Steffen for excellent technical assistance. This work was supported by grants from the National Institute on Drug Abuse (AJE), the Canadian Institutes of Health Research (DCL) and National Institute of Mental Health (EJN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure
All the authors declare that they have no competing financial interests or conflicts of interest.
REFERENCES
- 1.Bernatchez R, Belkacemi L, Rassart E, Daoud G, Simoneau L, Lafond J. Differential expression of membrane and soluble adenylyl cyclase isoforms in cytotrophoblast cells and syncytiotrophoblasts of human placenta. Placenta. 2003;24:648–657. doi: 10.1016/s0143-4004(03)00060-2. [DOI] [PubMed] [Google Scholar]
- 2.Hanoune J, Pouille Y, Tzavara E, Shen T, Lipskaya L, Miyamoto N, et al. Adenylyl cyclases: structure, regulation and function in an enzyme superfamily. Molecular and cellular endocrinology. 1997;128:179–194. doi: 10.1016/s0303-7207(97)04013-6. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Storm DR. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Molecular pharmacology. 2003;63:463–468. doi: 10.1124/mol.63.3.463. [DOI] [PubMed] [Google Scholar]
- 4.Conti AC, Maas JW, Jr, Muglia LM, Dave BA, Vogt SK, Tran TT, et al. Distinct regional and subcellular localization of adenylyl cyclases type 1 and 8 in mouse brain. Neuroscience. 2007;146:713–729. doi: 10.1016/j.neuroscience.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S, Lee ML, Bruchas MR, Chan GC, Storm DR, Chavkin C. Calmodulin-stimulated adenylyl cyclase gene deletion affects morphine responses. Molecular pharmacology. 2006;70:1742–1749. doi: 10.1124/mol.106.025783. [DOI] [PubMed] [Google Scholar]
- 6.Maas JW, Jr, Vogt SK, Chan GC, Pineda VV, Storm DR, Muglia LJ. Calcium-stimulated adenylyl cyclases are critical modulators of neuronal ethanol sensitivity. J Neurosci. 2005;25:4118–4126. doi: 10.1523/JNEUROSCI.4273-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vadakkan KI, Wang H, Ko SW, Zastepa E, Petrovic MJ, Sluka KA, et al. Genetic reduction of chronic muscle pain in mice lacking calcium/calmodulin-stimulated adenylyl cyclases. Molecular pain. 2006;2:7. doi: 10.1186/1744-8069-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei F, Qiu CS, Kim SJ, Muglia L, Maas JW, Pineda VV, et al. Genetic elimination of behavioral sensitization in mice lacking calmodulin-stimulated adenylyl cyclases. Neuron. 2002;36:713–726. doi: 10.1016/s0896-6273(02)01019-x. [DOI] [PubMed] [Google Scholar]
- 9.Wei F, Vadakkan KI, Toyoda H, Wu LJ, Zhao MG, Xu H, et al. Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice. J Neurosci. 2006;26:851–861. doi: 10.1523/JNEUROSCI.3292-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, et al. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- 11.Willoughby D, Cooper DM. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiological reviews. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 12.Kim KS, Lee KW, Lee KW, Im JY, Yoo JY, Kim SW, et al. Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3908–3913. doi: 10.1073/pnas.0508812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 14.Chester JA, Watts VJ. Adenylyl cyclase 5: a new clue in the search for the "fountain of youth"? Sci STKE. 2007;2007:pe64. doi: 10.1126/stke.4132007pe64. [DOI] [PubMed] [Google Scholar]
- 15.Lee KW, Hong JH, Choi IY, Che Y, Lee JK, Yang SD, et al. Impaired D2 dopamine receptor function in mice lacking type 5 adenylyl cyclase. J Neurosci. 2002;22:7931–7940. doi: 10.1523/JNEUROSCI.22-18-07931.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong AH, Gottesman II, Petronis A. Phenotypic differences in genetically identical organisms: the epigenetic perspective. Hum Mol Genet. 2005;14(Spec No 1):R11–R18. doi: 10.1093/hmg/ddi116. [DOI] [PubMed] [Google Scholar]
- 17.Zachariou V, Liu R, Laplant Q, Xiao G, Renthal W, Chan GC, et al. Distinct Roles of Adenylyl Cyclases 1 and 8 in Opiate Dependence: Behavioral, Electrophysiological, and Molecular Studies. Biological psychiatry. 2008 doi: 10.1016/j.biopsych.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular Adaptations Underlying Susceptibility and Resistance to Social Defeat in Brain Reward Regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, et al. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biological psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biological psychiatry. 2006;59:468–476. doi: 10.1016/j.biopsych.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 21.Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17:175–180. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- 22.Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology. 2001;155:315–322. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- 23.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 24.Strekalova T, Gorenkova N, Schunk E, Dolgov O, Bartsch D. Selective effects of citalopram in a mouse model of stress-induced anhedonia with a control for chronic stress. Behav Pharmacol. 2006;17:271–287. doi: 10.1097/00008877-200605000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nature neuroscience. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 26.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nature neuroscience. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 27.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain research. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nature neuroscience. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annual review of pharmacology and toxicology. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- 30.McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, et al. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mann L, Heldman E, Shaltiel G, Belmaker RH, Agam G. Lithium preferentially inhibits adenylyl cyclase V and VII isoforms. Int J Neuropsychopharmacol. 2008:1–7. doi: 10.1017/S1461145707008395. [DOI] [PubMed] [Google Scholar]
- 33.Hines LM, Hoffman PL, Bhave S, Saba L, Kaiser A, Snell L, et al. A sex-specific role of type VII adenylyl cyclase in depression. J Neurosci. 2006;26:12609–12619. doi: 10.1523/JNEUROSCI.1040-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, et al. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hines LM, Tabakoff B. Platelet adenylyl cyclase activity: a biological marker for major depression and recent drug use. Biological psychiatry. 2005;58:955–962. doi: 10.1016/j.biopsych.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 37.Katsel PL, Tagliente TM, Schwarz TE, Craddock-Royal BD, Patel ND, Maayani S. Molecular and biochemical evidence for the presence of type III adenylyl cyclase in human platelets. Platelets. 2003;14:21–33. doi: 10.1080/0953710021000062905. [DOI] [PubMed] [Google Scholar]
- 38.Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, et al. Regionally Specific Regulation of ERK MAP Kinase in a Model of Antidepressant-Sensitive Chronic Depression. Biological psychiatry. 2007 doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biological psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 40.Sousa N, Almeida OF, Wotjak CT. A hitchhiker's guide to behavioral analysis in laboratory rodents. Genes, brain, and behavior. 2006;5 Suppl 2:5–24. doi: 10.1111/j.1601-183X.2006.00228.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.