Abstract
The stable fly, Stomoxys calcitrans, is an economically important pest of livestock. Prior studies demonstrated lymphocyte suppression by crude salivary gland extract (SGE) of the stable fly. A dominant 27 kDa protein identified in the SGE was reported to stimulate immunodominant antibody responses in exposed cattle. The purpose of this study was to determine if this protein, now identified as a homolog of insect proteins named antigen 5 (Ag5), was responsible for the lymphocyte suppression and if naïve calves can mount an immune response to it. Calves raised in the winter months were immunized with recombinant Ag5 (rAg5) expressed in Drosophila S2 cells or with “natural” Ag5 protein isolated by preparative gel electrophoresis of SGE. Control calves were immunized with adjuvant alone. Rising antibody concentrations to rAg5 were detected in two of three calves immunized with rAg5 and one of three calves immunized with natural Ag5. Recall lymphocyte responses to rAg5 were detected at 21 and 28 DPI in calves immunized with rAg5 but not in calves immunized with the natural Ag5 or those exposed to adjuvant alone. Mitogen-stimulated bovine lymphocyte responses were not suppressed by rAg5. Further investigation using immunoblotting revealed that rAg5 binds to the Fc and F(ab’)2 portions of bovine IgG, but not to an Fab fragment. These findings suggest that Ag5 of the stable fly salivary gland is not immunosuppressive, but has immunoglobulin binding properties and can invoke specific antibody and memory lymphocyte responses in immunized calves.
Keywords: bovine lymphocytes, lymphocyte suppression, antibody, stable fly, salivary gland protein
The stable fly, Stomoxys calcitrans (L.), is an economically important pest affecting the health of cattle in feedlots (Campbell et al. 1987) and dairies (Stork 1979). The latest estimate for economic loss to the U.S. livestock industry is $428 million/year (Kunz et al. 1991). Within the past decade several investigators have noted the stable fly has extended its pest distribution to range or pastured cattle (Campbell et al. 2001a, Campbell et al. 2001c). Studies indicate that the wasted hay/manure mixture at winter feeding sites of hay in round bales is the main source of stable flies in early spring and summer; in addition, this fly has also become quite an important nuisance in the urban landscape (Broce 1993, Hall et al. 1982). Behavioral responses, including bunching of the herd, foot stomping, and head throwing, lead to reduced feed consumption and weight gains (Wieman et al. 1992) and injured calves (Campbell et al. 2001a). Nebraska ranchers and veterinarians have reported weight gain losses of 40−50 pounds on yearling cattle and 25−30 pounds in calf weaning weights (Campbell et al. 2001a). The weight gain loss that occurred in steers/calves exposed to stable flies over a two-year grazing trial was not gained back even after the calves were placed in a feedlot and fed a finishing ration (Campbell et al. 2001b). The stable fly also causes considerable damage to the hide at the site of feeding, thus impacting the tanning industry (Torres et al. 1993).
There is experimental evidence that stable flies mechanically transmit significant pathogens to livestock (Knapp et al. 1992, Mellor et al. 1987, Potgieter et al. 1981) and potentially contribute to the spread of emerging foodborne pathogens (Hamilton et al. 2003). The feeding habits of the stable fly have the potential for mechanical transmission of pathogens, because the fly regurgitates at the feeding site on a second host after feeding is interrupted by the first host. This observation has broad public health implications, particularly since a recent study showed the infectivity of the human immunodeficiency virus is not reduced in regurgitates of the stable fly (Eigen et al. 2002). Stable flies effectively transmitted the retrovirus equine infectious anemia virus to horses (Hawkins et al. 1973). In Africa, Stomoxys calcitrans plays a significant role in interrupting the feeding habits of Glossina spp. (tsetse fly) such that the increase in biting rate of the tsetse fly potentates the risk of increased transmission of trypanosomiasis (Torr and Mangwiro 2000).
The best available tool for reducing stable fly populations in confined livestock operations is to reduce larval habitats by sanitation. However, control failures are common. The integration of other control practices, such as the use of residual pesticides and the release of pupal parasites aids in the control of these flies in confined livestock operations. There are no effective means of controlling stable flies attacking range cattle (Campbell and Raun 1971, Campbell and Wright 1976). Typical control strategies (wet sprays on the legs, dust bags, insecticide impregnated ear tags, oral larvicides, etc.) are either ineffective in lowering stable fly populations below economic levels, promote the development of insecticide resistant populations, or are becoming less desirable because of the increasing public concern about chemical residues in food. Therefore, new strategies and approaches for reducing stable fly populations near cattle are needed. In fact, recent immunization trials with a salivary protein of the horn fly demonstrated reduced blood meal sizes and delayed egg development in flies fed on immunized cattle (Cupp et al. 2004a).
In a previous study, we showed that stable fly salivary gland extract (SGE) inhibits mitogen driven proliferation of bovine lymphocytes and identified a dominant protein of 27 kDa in the stable fly salivary gland that appeared to be highly immunoreactive in cattle exposed to flies (Swist et al. 2002c). We have subsequently identified the 27 kDa protein as a homolog of “antigen 5” (Ag5) from other insect species (Wang, X., J. M. C. Ribeiro, M. J. Wilkerson, A. B. Broce, and M. R. Kanost, in preparation). We have determined the sequence of its cloned cDNA (Genbank accession number AY190321) and found that it is expressed specifically in adult salivary glands (Wang, X., A. B. Broce, and M. R. Kanost, in preparation). To further investigate these findings and to determine if this salivary gland protein would be a good immunogen in cattle, we conducted the present study. The objectives of this study were to i) determine specificity of bovine antibody response to recombinant Ag5, ii) to determine if a recombinant form of the protein suppresses bovine lymphocyte proliferation in culture, and iii) to determine if calves immunized with either recombinant or natural Ag5 preparations produce rising antibody and memory lymphocyte responses.
Materials and Methods
Flies
Stable flies were obtained from a colony maintained in the Department of Entomology (Kansas State University) and established from wild flies collected in Manhattan, KS in 1990. The larvae were reared in a vermiculite/wheat bran/fishmeal medium. Adults were fed daily on bovine blood stored in sodium citrate anticoagulant. Twenty-four hours prior to dissection, male and female flies (4 − 8 days after eclosion) were fed Gatorade™ to maintain nutrition without causing ovary development, and then anesthetized by lowering body temperature to 4°C. Salivary glands were dissected from the flies according to the method described elsewhere (Swist et al. 2002b).
Recombinant Ag5 Preparation
A cDNA for Ag5 obtained from a stable fly salivary gland cDNA library (accession number AY190321) was expressed from plasmid vector pMT/V5-His A (Invitrogen, Carlsbad, CA) in Drosophila Schneider 2 (S2) cells, and the secreted protein was purified by cation exchange chromatography (Wang, X., A. B. Broce, and M. R. Kanost, in preparation). Those fractions containing rAg5 were pooled, dialyzed against PBS at 4°C, concentrated using Centriplus YM-10 centrifugal filtration devices (Millipore) to 1 mg/mL, and stored at −80°C. The purity of rAg5 protein was confirmed by SDS-PAGE analysis. Supernatant harvested from untransfected S2 cells was used as the source of negative control protein (CP).
Preparation of Natural Ag5 for Immunization Studies
Salivary glands dissected from 1−7 day old adult male and female stable flies were used to prepare SGE (Swist et al., 2002). Twenty five microgram of SGE protein was separated by SDS-PAGE according to the method of Laemmli (Laemmli 1970) with a 10% separating gel and a 4% stacking gel. After Coomassie blue staining, the band with molecular weight of 27 kDa was excised. The gel slice containing the 27 kDa Ag5 protein was then homogenized and stored at −20°C. A similar gel slice was removed from a lane that did not contain protein to serve as the negative control.
Animals and Immunization Studies
Eight Holstein bull calves (3 to 4 months of age) were obtained from the Kansas State University-Dairy herd in the winter when stable flies were not present and after the calves had obtained colostrum. The calves were housed in an enclosed barn maintained under the guidelines of the Institutional Animal Care and Use Committee. Four additional adult steers housed at the KSU-Dairy herd were bled for serum samples and initial lymphocyte proliferation studies. Two calves were bled prior to ingestion of colostrum to obtain a source of antibody negative sera. Maternal antibody was confirmed to be absent in these serum samples by single radial immunodiffusion (VMRD, Pullman, WA) and serum electrophoresis (performed at the clinical pathology laboratory at Kansas State University, College of Veterinary Medicine). All calves were bled before immunization. Calves were immunized at 4 months of age, twice at one week intervals intra-muscularly (IM). Three calves (calf numbers 22, 23, and 73) were immunized with 100 μg of rAg5 in 200 μL of phosphate-buffered saline (PBS; 0.01 M, pH 7.2) emulsified with equal volumes of TiterMax® adjuvant (Sigma, St. Louis, MO). The other three calves (calf numbers 19, 20, and 21) were immunized with 100 μg of natural Ag5 from the preparative gel emulsified with equal volumes of TiterMax® adjuvant (Sigma, St. Louis, MO) as described previously (Yarnall et al. 1988). Two calves were immunized with adjuvant alone as negative controls. One control calf (No. 17) received adjuvant containing CP (prepared as described previously), whereas the other (No. 18) received adjuvant plus a piece of preparative gel that did not contain a protein band. All calves received two injections one week apart. Blood samples were collected at 0, 14, 21, 28, and 42 days post immunization (DPI) by jugular venipuncture. Whole blood was collected in tubes containing phosphate-buffered saline (PBS; 0.01 M, pH 7.2) and 20% acid-citrate dextrose (ACD, Fisher Scientific, Pittsburg, PA) from all calves for lymphocyte proliferation studies. Blood was also drawn directly into a serum clot tube using a vacutainer collection system. The samples were immediately transported to the laboratory in a cooler with ice packs. Processing of the samples was performed within 2 hours of blood collection. To collect serum samples for indirect ELISA studies, the samples in the clotting tubes were centrifuged at 2000 × g for 20 min at 4°C, and serum was stored at −20°C until analysis.
Isolation of Bovine Lymphocytes from Peripheral Blood
Lymphocytes harvested from venous blood of 4 adult steers housed at the KSU Dairy and exposed to stable fly biting was used to test whether rAg5 had immunosuppressive properties. Lymphocyte isolation were performed according to the protocol described elsewhere (Swist et al. 2002a) with some modification. Briefly, whole blood (30 − 60 mL) was placed into tubes containing PBS/ACD (20%). Lymphocytes were separated from other leukocytes by centrifugation on a density gradient (Ficoll-Paque 1.086, Sigma Co., St. Louis, MO). The mononuclear cell interface was collected and washed three times in PBS/ACD (20%). Ammonium chloride (0.8% NH4CL) was used to lyse red blood cells. After washing one more time, lymphocytes were counted using a Neubauer hemocytometer (AO Scientific, Buffalo, NY).
Indirect ELISA
The solid phase was prepared by coating the wells of ELISA plates with rAg5 at 1 μg/mL (100 μL per well) in PBS (0.01 M, pH 7.2) at 4°C overnight. After removal of unbound rAg5 by washing the plate with PBS containing 0.05 % of Tween-20 (PBS-T, v/v) three times, calf sera at 0, 14, 21, 28, and 42 dpi (1/1000 dilution in PBS-T) were added to the wells (100 μL per well) and incubated for 45 min at room temperature. The wells were then washed three times with PBS-T. The bovine immunoglobulin that bound to the stable fly protein was detected by adding HRP-goat anti-bovine Fab (Jackson ImmunoResearch, West Grove, PA) at 1:2000 dilution in PBS-T to each well and the plate was incubated for 45 min at room temperature. After washing the wells with PBS-T, the substrate TMB (100 μL/well; Sigma, St. Louis, MO) was used to develop the colorimetric reaction. Finally, stop solution (H2SO4, 0.5 M) was added to each well at 50 μL per well. The optical density (OD) at 450 nm of each well was measured on an automatic ELISA plate reader (Universal Microplate Reader, EL800, Bio-Tek Instrument, Inc., Winooski, VT). The assay was performed in duplicate.
Lymphocyte Proliferation Assay
Bovine lymphocytes collected from the calves immunized with either rAg5, natural Ag5, or adjuvant alone (control calves) were stimulated in vitro with a T-cell specific mitogen concanavalin A (Con A; Sigma Co., St. Louis, MO) in the presence of various concentrations of rAg5 or natural Ag5. Lymphocytes were plated in flat bottom, 96-well plates (Corning Inc., Corning, NY) at a concentration of 1 × 106 cells/mL in RPMI-1640 medium (Gibco/BRL, Rockville, MD) containing 10% heat-inactivated fetal bovine serum (HyClone Lab, Logan, UT), L-glutamine (0.3 mg/mL), penicillin (10,000 units/mL), streptomycin (10 mg/mL) and gentamicin (12.5 mg/mL). Assays were performed in triplicate. Wells were stimulated with Con A (1 μg/mL) with or without addition of rAg5 or natural Ag5 at 1 and 5 μg/mL. Lymphocyte cultures we incubated for 72 hours at 37°C with 5% CO2. Unstimulated lymphocytes were used as the background control for the nonspecific proliferation by medium alone. To measure cell proliferation, 0.2 μCi of [3H]thymidine was added to cells 18 hours prior to harvest. The cells were harvested using an automated multi-well cell harvester (Inotech Biosystems Intl, Inc., Rockville, Md). The amounts of [3H] incorporated into DNA of proliferating lymphocytes were expressed as the radioactive counts per minute (cpm) determined by liquid scintillation counting (Beckman Instruments Inc., Fullerton, CA). Proliferation was expressed as a stimulation index (SI), a ratio of the cpm for mitogen stimulated cells divided by the cpm of nonstimulated cells. To determine the suppressive effects of the stable fly protein preparations, a range of 1 − 10.0 μg/mL of either rAg5, CP, or SGE was added with Con A to the lymphocyte cultures. The percent inhibition (% I) of mitogen driven lymphocyte proliferation imparted by the presence of recombinant or native protein was determined by the following formula: SI (Con A) – SI (Con A + protein)/ SI (Con A).
Immunoblotting
Four micrograms of protein per lane of protein (rAg5 or SGE) were treated with the reducing sample buffer and separated by SDS-PAGE according the method of Laemmli (1970) with a 10% separating gel and a 4% stacking gel. The proteins were electrophoretically transferred from gels to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA) using Trans-Blot® SD Semi-Dry transfer Cell system (Bio-Rad Laboratories, Hercules, CA). The immunoblots were developed under the following conditions at room temperature. The PVDF membranes were blocked with 1% (w/v) bovine serum albumin (BSA; 99%; γ-globulin free; Sigma-Aldrich Co., St. Louis, MO) in PBS-T at room temperature for 1 h and cut into strips. After washing 3 times with PBS-T, two strips from each membrane were incubated for 1 h with serum (diluted 1:100 in PBS-T) from two different steers that had been exposed to stable fly bites. Two additional strips from each membrane were incubated for 1 h with serum (diluted 1:100 in PBS-T) from two newborn calves collected prior to ingestion of colostrum. One strip from each membrane was not incubated with a primary antibody. After washing three times in PBS-T, all five strips were incubated with a horse radish peroxidase (HRP) conjugated sheep-anti-bovine IgG (H + L) diluted 1:1000 in PBS-T. Three additional strips from each membrane were incubated with HRP-bovine IgG Fab, HRP-bovine IgG Fc, and HRP-bovine IgG F(ab’)2 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) diluted 1:1000 in PBS-T for another hour. For detection, all membranes were washed twice for 5 minutes each with PBS (pH 7.2). The 3,3′-diaminobenzidine tetra hydrochloride (DAB) liquid substrate system (Sigma, St. Louis, MO) was added to develop the color reaction.
Statistical Analysis
Differences in the lymphocyte proliferation among the treatment groups were determined by one-way analysis of variance (ANOVA) and Holm-Sidak method for pairwise comparison procedures SIGMASTAT (Jandel Software, San Rafael, CA). A one way repeated measures analysis of variance and multiple comparisons versus the control animal was performed to determine differences among the lymphocyte recall responses (performed in triplicate) between individual calves. Differences in antibody concentration between the calf treatment groups and days post immunization were determined using a two-way analysis of variance with repeated measures model and multiple comparisons with computations from PROC GLIMMIX of the SAS system (Version 9.1.3; SAS Institute Inc., Cary, NC, USA). Statistical significance was established at P values < 0.05.
Results
SDS-PAGE analysis
The rAg5 protein was detected as a 27 kDa single band by SDS-PAGE followed by Coomassie Blue staining (Fig. 1A). The SDS-PAGE analysis of the SGE stained with silver stain revealed a major band running at 27 KDa as shown in Fig. 1B.
Fig. 1.
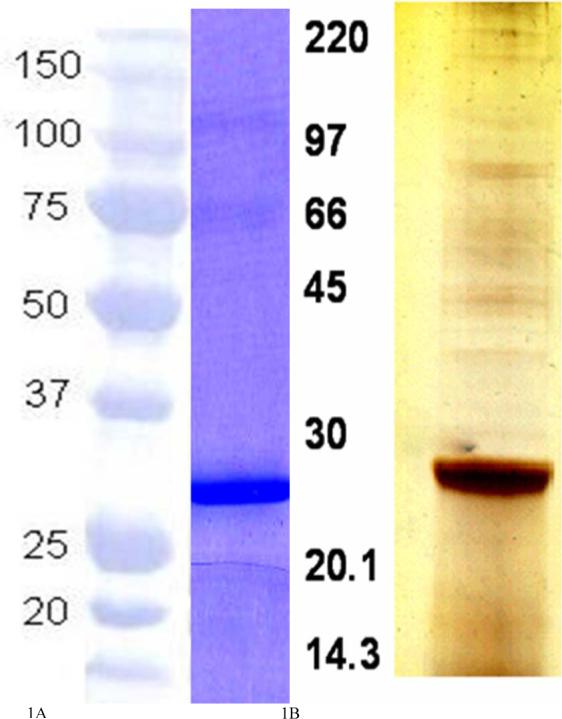
SDS-PAGE analysis of rAg5 stained with Coomassie blue (A) and SGE stained with silver stain (B) depicting the dominant 27 KD protein (native Ag5) of the extract.
Effect of rAg5 on Mitogen Induced Lymphocyte Proliferation
Lymphocytes from four steers were stimulated in culture with the T-cell mitogen, Con A. The lymphocytes from all steers proliferated in response to the mitogen (Fig. 2), with incorporation of [3H]thymidine significantly higher than unstimulated cells (P < 0.01). SGE suppressed Con A stimulated lymphocyte proliferation in all steers, ranging from 55% (steer 2) to 90% (steer 4) inhibition. However, rAg5 at 3.0 μg/mL did not suppress Con A stimulated lymphocyte proliferation; the mean cpm for Con A + rAg5 (25,165) was no different than Con A stimulated proliferation when control protein was added (27,125 ± 2218). (Fig. 2). Moreover, the addition of lower and higher protein concentrations of SGE or AG5 ranging between 0.1 to 10 μg did not significantly change the results (data not shown).
Fig. 2.
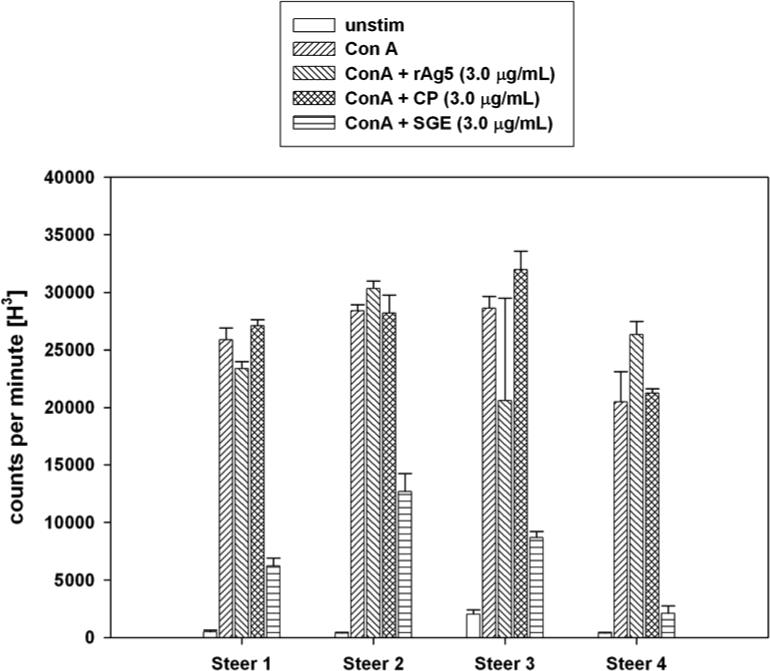
Lymphocyte proliferation assay determined by incorporation of tritiated thymidine defined as counts per minute of radioactivity. Lymphocytes from four steers were treated with five separate conditions: no stimulation (no mitogen), stimulated with Con A alone, Con A plus 3.0 μg/mL of rAg5, control protein (CP) or crude SGE. Error bars represent one standard error of the mean of three replicate experiments.
Antibody Responses to Ag5 in immunized Calves
Antibody concentration measured by ELISA for control calves (C18 and C17) exposed to adjuvant alone did not significantly change over time. Two of three calves immunized with rAg5 produced antibody responses to rAg5, detected beginning at 21 DPI peaking at 28 DPI for one calf (C22) and 42 DPI for the other calf (C23). There was a significant increase in the overall group mean antibody concentrations at 28 and 42 DPI compared to 0 DPI for calves immunized with rAg5 protein (P < 0.01), consistent with a rising antibody response following vaccination. One calf immunized with Ag5 protein isolated by preparative gel electrophoresis of SGE produced a slight increase in antibody concentration peaking at 21 DPI (C19), whereas the other two calves (C73 and C20) did not respond to Ag5. There were no significant differences at 21 DPI compared to 0 DPI for the Ag5 group (Fig. 3).
Fig. 3.
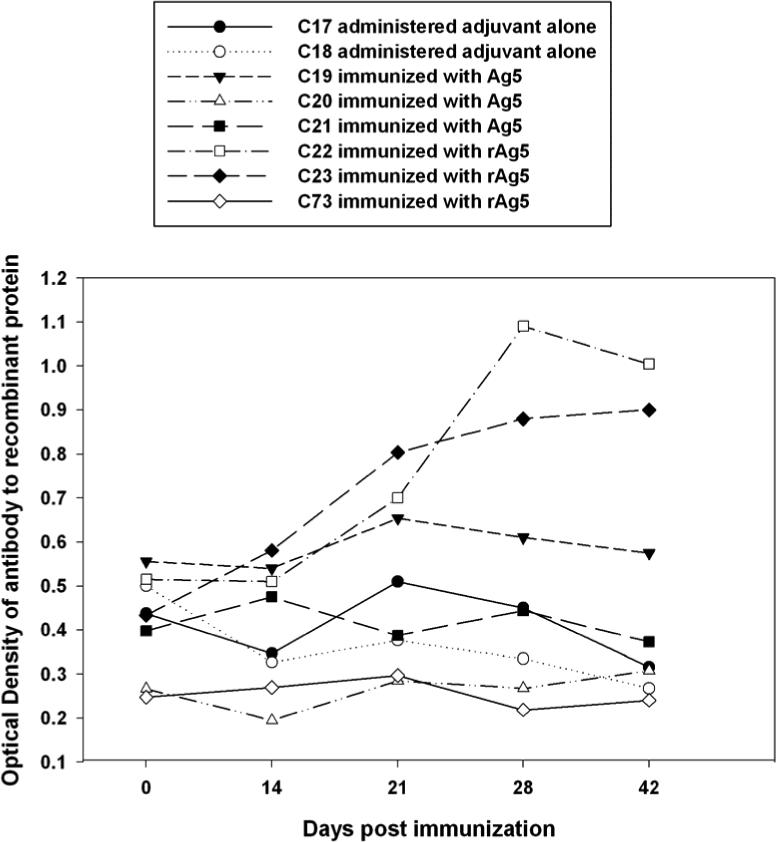
Detection of rising antibody concentration against rAg5 in immunized calves following 21, 28, and 42 days post immunization. Serum samples were diluted 1:1000 and reacted with rAg5 (1 μg/mL) on the solid phase of ELISA plates. Each point represents the average value of the OD450 obtained from duplicate ELISA results.
Recall Lymphocyte Responses to Ag5
All calves immunized with rAg5 had recall lymphocyte responses to rAg5 at 21 DPI or 28 DPI. The lymphocyte response of calf 22 was significantly higher than the control calf at 28 DPI (P< 0.05). Lymphocyte responses to rAg5 of calf 23 were significantly higher than the control calf at both 21 and 28 DPI (P< 0.05), whereas calf 73 had significantly higher lymphocyte responses than the control calf only at 21 DPI (P< 0.05). Lymphocytes from calves immunized with natural Ag5 did not respond to rAg5 protein in vitro and were not significantly different than the control calf (Fig. 4).
Fig. 4.
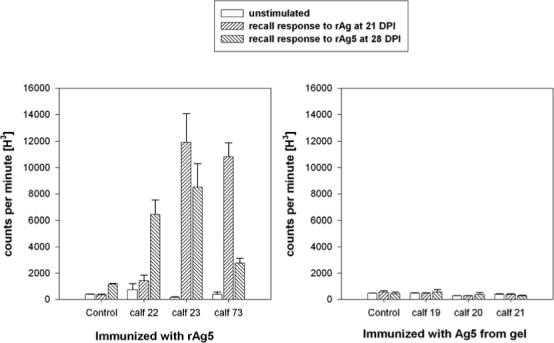
Lymphocyte proliferation assay determined by incorporation of tritiated thymidine defined as counts per minute of radioactivity. Lymphocytes collected from eight calves at 21 and 28 DPI were not stimulated (medium alone) or stimulated with rAg5 (3.0 μg/mL). Three calves (Nos. 22, 23, 73) were immunized with rAg5, and control calf 18 received adjuvant with control protein (A), whereas three calves (Nos. 19, 20, 21) were immunized with natural Ag5 isolated from a preparative acrylamide gel, and control calf 19 received adjuvant plus preparative gel lacking protein. Error bars represent one standard error of the mean (n = 3).
Immunoglobulin Binding properties of rAg5
Serum from cattle exposed to stable fly bites reacted with a prominent band at 27 kDa in the SGE (Fig. 5A, lanes 1 and 2). However, we noted that serum from newborn calves never exposed to stable flies and known to be deficient in antibodies (determined by serum protein electrophoresis and radial immunodiffusion assays to be below detectable limits, < 0.2 g/dL of IgG) also reacted with the 27 kDa protein (Fig. 5A, lanes 3 and 4). This reaction, albeit less intense, was found to be due to the binding of the secondary antibody to the 27 kDa protein (Fig. 5B, lane 5). Furthermore, fragments of bovine IgG including Fc and F(ab’)2 reacted with the 27 kDa Ag5 protein in SGE (Fig. 5A, lanes 7 and 8), but the Fab fragment did not (Fig. 5A, lane 6).
Fig. 5.
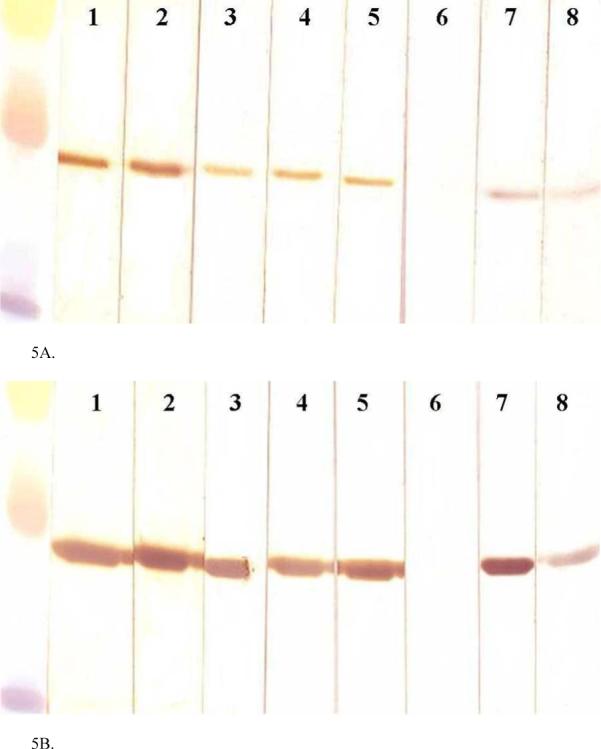
Western blot immunoassay demonstrates immunoglobulin-binding properties of the natural Ag5 from SGE (A) and rAg5 (B). Lanes 1 and 2 were incubated with serum from two different steers that had been exposed to stable fly bites; lanes 3 and 4 were incubated with serum from two newborn calves collected prior to ingestion of colostrum. Lanes 1−5 were incubated with HRP-sheep-anti-bovine IgG (H + L). Lanes 6−8 were incubated with HRP-bovine IgG Fab, HRP-bovine IgG Fc, or HRP-bovine IgG F(ab’)2, respectively. Molecular weight markers (Rainbow markers; Amersham, Piscataway, NJ) included 35, 30, and 20 kDa.
The recombinant Ag5 reacted with all sources of antibody tested in a similar manner (Fig. 5B). The secondary sheep anti-bovine IgG antibody reacted with the recombinant protein with or without the addition of steer serum samples or newborn calf serum samples (Fig. 5B, lanes 1 − 5). The Fc fragment of purified HRP-labeled bovine IgG (Fig. 5B, lane 7), but not the HRP-labeled Fab fragment of purified bovine IgG (Fig. 5B, lane 6) reacted with the recombinant protein. In addition, the F(ab’)2 fragment of HRP-labeled purified bovine IgG reacted weakly to rAg5 (Fig. 5B, lane 8).
Discussion
Our findings demonstrated that rAg5 does not suppress mitogen-driven lymphocyte proliferation in culture, indicating either the recombinant form of the protein lacks immunosuppressive properties or that other salivary gland substances may be responsible for the immunosuppressive activity present in SGE of the stable fly. The immunization trial demonstrated that naïve calves raised in the winter months (when stable flies were not present) can produce rising antibody responses to rAg5 and in one calf to the natural Ag5 from SGE. This finding indicates that specific antibody responses can be produced to Ag5 in the bovine even though the protein binds bovine immunoglobulin nonspecifically via the Fc or F(ab’)2 portions. However, the ELISA that was used in this study incorporated anti-bovine Fab, which was shown by western blot to not react to Ag5. Although Ag5 binds to antibodies nonspecifically, the presence of rising antibody concentrations over time after immunization indicates induction of specific antibody responses to the protein instead of nonspecific binding. In contrast, calves immunized with adjuvant alone produced no change in the signal for the indirect ELISA throughout the 42 day investigation period. Similarly, in other systems, specific antibody responses to Fc receptor-like binding proteins of bacteria have been reported in immunized cattle (Yarnall and Corbeil 1989).
Furthermore, calves immunized with rAg5 develop memory lymphocyte responses when lymphocytes were restimulated in culture with rAg5. Since only the calves immunized with rAg5 developed recall lymphocyte responses, memory epitopes may have been altered in the natural Ag5 preparations isolated by gel electrophoresis.
The immunization trial demonstrated that rAg5 evokes bovine immune responses (antibody and lymphocyte) and is potentially a good immunogen to pursue further challenge studies to determine if prior immunization of calves decreases the feeding and reproductive efficiency of stable flies. Although vaccination of the host against salivary proteins, such as maxadilan of the sand fly, has proven to be protective against infection by Leishmania major, an intracellular parasite carried by the sand fly (Morris et al. 2001, Wikel and arcon-Chaidez 2001, Valenzuela et al. 2001), effects on the feeding and reproductive capabilities of flies exposed to immunized hosts are just beginning to be studied. Recently, an immunization trial using recombinant horn fly thrombostasin proteins demonstrated reduced blood meal sizes and delayed egg development in flies fed on immunized cattle (Cupp et al. 2004b). Vaccine trials against stable flies on rabbit immunized with gut and other tissue derived antigens showed increased mortality and reduced egg viability in flies fed on blood of immunized rabbits (Schlein and Lewis 1976, Webster et al. 1992).
A unique observation in our study is the demonstration that S. calcitrans Ag5 from natural and recombinant sources binds to sheep, goat, and bovine antibodies, apparently by interaction with the Fc portion of IgG rather than through a typical antigen-antibody binding interaction. This result is consistent with additional experiments, using antibodies from other animal sources, which also demonstrated non-specific binding of Ag5 to antibodies and to the isolated Fc fragment of mouse IgG (Wang, X., A. B. Broce, and M. R. Kanost, in preparation). This finding may explain our previous dichotomous results in which there was a strong apparent antibody reaction to this protein in the serum of cattle exposed to stable flies even though the crude extract was suppressive to lymphocyte responses in vitro assays. We have now found that Ag5 binds nonspecifically to IgG, and that it binds to the Fc portion but not to the Fab fragment. The F(ab’)2 fragment of HRP-labeled purified bovine IgG bound weakly to both forms of Ag5. The reason for this binding of F(ab’)2 fragment to Ag5 is not known, however, it may be due to the presence of the hinge region in this antibody fragment, as pepsin cleaves on the C-terminal side of the disulfide bonds linking the two heavy chains and including the hinge region. Alternatively, it is possible that F(ab’)2 portion of the antibody was contaminated with some Fc fragments. It appears that Ag5 from stable fly salivary gland has immunoglobulin binding properties (IgB), which to our knowledge has not been described in salivary gland secretions of biting flies.
Other IgB proteins have been discovered in SGE of unfed ticks including Rhipicephalus appendiculatus, Amblyomma variegatum and Ixodes hexagonus (Wang and Nuttall 1995a) . IgB proteins in tick saliva are hypothesized to serve as a means for the tick to evade the host immune system. Studies involving the ixodid tick Rhipicephalus appendiculatus revealed that host IgG found in the tick was excreted via salivation at feeding, and the immunoglobulin binding proteins in tick hemolymph and salivary glands are thought to be responsible for such excretion. The discovery of an immunoglobulin excretion system in ticks indicates that they have a highly developed mechanism to protect themselves from their host's antibody attack (Wang and Nuttall 1994, Wang and Nuttall 1999). Salivary glands of R. appendiculatus male ticks contain a number of IgB proteins. It has been postulated that males secrete a cocktail of IgB proteins into the feeding lesion where they help protect adjacent females from the host's anti-tick immune response (Wang and Nuttall 1995b).
Collectively, these findings show promise that the very abundant Ag5 protein in salivary glands of the stable fly may be a good candidate immunogen to pursue future challenge studies to determine if bovine immune responses generated toward this unique protein could decrease feeding and reproductive efficiency of the stable fly.
Acknowledgements
We thank Tammy Koopman and Kent Hampton, and Sandi Youngeberg for their technical support and John Reese for use of a microscope for salivary gland dissections. This work was supported by Kansas State University Agricultural Experimental Station Grant 418343 and Dean's Fund supported by the College of Veterinary Medicine, Kansas State University and by NIH grant GM41247.
Reference List
- Broce AB. North Central Regional Res. University of Nebraska; Lincoln: 1993. Dispersal of house flies and stable flies. Proceedings Symposium on rural flies in the Urban Environment. [Google Scholar]
- Campbell JB, Berry IL, Boxler DJ, Davis RL, Clanton DC, Deutscher GH. Effects of stable flies (Diptera: Muscidae) on weight gain and feed efficiency of feedlot cattle. J Econ. Entomol. 1987;80:117–119. doi: 10.1093/jee/80.1.117. [DOI] [PubMed] [Google Scholar]
- Campbell JB, Bozza M, Aksoy S, Davis R. The stable fly - an emerging pest of range cattle. Livestock Insect Workers’ Conference; Cody, Wyoming. 2001a. [Google Scholar]
- Campbell JB, Raun ES. Aerial ULV and LV applications of insecticides for control of the stable fly and the horn fly. J. Econ. Entomol. 1971;64:1170–1173. doi: 10.1093/jee/64.5.1170. [DOI] [PubMed] [Google Scholar]
- Campbell JB, Skoda SR, Berkebile DR, Boxler DJ, Thomas GD, Adams DC, Davis R. Effects of stable flies (Diptera: Muscidae) on weight gains of grazing yearling cattle. J. Econ. Entomol. 2001c;94:780–783. doi: 10.1603/0022-0493-94.3.780. [DOI] [PubMed] [Google Scholar]
- Campbell JB, Skoda SR, Berkebile DR, Boxler DJ, Thomas GD, Adams DC, Davis R. Effects of stable flies (Diptera: Muscidae) on weight gains of grazing yearling cattle. J. Econ. Entomol. 2001b;94:780–783. doi: 10.1603/0022-0493-94.3.780. [DOI] [PubMed] [Google Scholar]
- Campbell JB, Wright JE. Field evaluations of insect growth regulators, insecticides, and a bacterial agent for stable fly control in feedlot breeding areas. J. Econ. Entomol. 1976;69:566–568. doi: 10.1093/jee/69.5.566. [DOI] [PubMed] [Google Scholar]
- Cupp MS, Cupp EW, Navarre C, Wisnewski N, Brandt KS, Silver GM, Zhang D, Panangala V. Evaluation of a recombinant salivary gland protein (thrombostasin) as a vaccine candidate to disrupt blood-feeding by horn flies. Vaccine. 2004a;22:2285–2297. doi: 10.1016/j.vaccine.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Cupp MS, Cupp EW, Navarre C, Wisnewski N, Brandt KS, Silver GM, Zhang D, Panangala V. Evaluation of a recombinant salivary gland protein (thrombostasin) as a vaccine candidate to disrupt blood-feeding by horn flies. Vaccine. 2004b;22:2285–2297. doi: 10.1016/j.vaccine.2003.11.024. [DOI] [PubMed] [Google Scholar]
- Eigen M, Kloft WJ, Brandner G. Transferability of HIV by arthropods supports the hypothesis about transmission of the virus from apes to man. Naturwissenschaften. 2002;89:185–186. doi: 10.1007/s00114-002-0319-x. [DOI] [PubMed] [Google Scholar]
- Hall RD, Thomas GD, Morgan CE. Stable fly, Stomoxys calcitrans (L.), breeding in large round hay bales: Initial associations. J. Kansas Entomol. Soc. 1982;55:617–620. [Google Scholar]
- Hamilton JV, Lehane MJ, Braig HR. Isolation of Enterobacter sakazakii from midgut of Stomoxys calcitrans. Emerg. Infect. Dis. 2003;9:1355–1356. doi: 10.3201/eid0910.030218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins JA, Adams WV, Cook L, Wilson BH, Roth EE. Role of horse fly (Tabanus fuscicostatus Hine) and stable fly (Stomoxys calcitrans L.) in transmission of equine infectious anemia to ponies in Louisiana. Am. J. Vet. Res. 1973;34:1583–1586. [PubMed] [Google Scholar]
- Knapp FW, Charron AE, Burg G. Proc. Symp. on The Stable Fly: A pest of Humans and Domestic Animals. Vol. 64. University of Nebraska Research Publication; 1992. Disease transmission by the stable fly: A Review. pp. 25–38. [Google Scholar]
- Kunz S, Murrell K, Lambert G, James LF, Terrill CE. Estimated losses of livestock to pests. CRC; Boca Raton, FA: 1991. pp. 69–98. [Google Scholar]
- Laemmli UK. Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4. Nature. 1970;227:680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mellor PS, Kitching RP, Wilkinson PJ. Mechanical transmission of capripox virus and African swine fever virus by Stomoxys calcitrans. Res. Vet. Sci. 1987;43:109–112. [PubMed] [Google Scholar]
- Morris RV, Shoemaker CB, David JR, Lanzaro GC, Titus RG. Sandfly maxadilan exacerbates infection with Leishmania major and vaccinating against it protects against Leishmania major infection. J. Immunol. 2001;167:5226–5230. doi: 10.4049/jimmunol.167.9.5226. [DOI] [PubMed] [Google Scholar]
- Potgieter FT, Sutherland B, Biggs HC. Attempts to transmit Anaplasma marginale with Hippobosca rufipes and Stomoxys calcitrans. Onderstepoort J. Vet. Res. 1981;48:119–122. [PubMed] [Google Scholar]
- Schlein Y, Lewis CT. Lesions in haematophagous flies after feeding on rabbits immunized with fly tissues. Physiol. Entomol. 1976;1:55–59. [Google Scholar]
- Stork MG. The epidemiological and economic importance of fly infestation of meat and milk producing animals in Europe. Vet Rec. 1979;105:341–343. doi: 10.1136/vr.105.15.341. [DOI] [PubMed] [Google Scholar]
- Swist SL, Wilkerson MJ, Wyatt CR, Broce AB, Kanost MR. Modulation of bovine lymphocyte response by salivary gland extracts of the stable fly, Stomoxys calcitrans (Diptera: Muscidae). J. Med. Entomol. 2002a;39:900–907. doi: 10.1603/0022-2585-39.6.900. [DOI] [PubMed] [Google Scholar]
- Swist SL, Wilkerson MJ, Wyatt CR, Broce AB, Kanost MR. Modulation of bovine lymphocyte response by salivary gland extracts of the stable fly, Stomoxys calcitrans (Diptera: Muscidae). J. Med. Entomol. 2002b;39:900–907. doi: 10.1603/0022-2585-39.6.900. [DOI] [PubMed] [Google Scholar]
- Swist SL, Wilkerson MJ, Wyatt CR, Broce AB, Kanost MR. Modulation of bovine lymphocyte response by salivary gland extracts of the stable fly, Stomoxys calcitrans (Diptera: Muscidae). J. Med. Entomol. 2002c;39:900–907. doi: 10.1603/0022-2585-39.6.900. [DOI] [PubMed] [Google Scholar]
- Torr SJ, Mangwiro TN. Interactions between cattle and biting flies: effects on the feeding rate of tsetse. Med. Vet. Entomol. 2000;14:400–409. doi: 10.1046/j.1365-2915.2000.00257.x. [DOI] [PubMed] [Google Scholar]
- Torres PR, Cicchino AC, Abrahamovich AH, Nunes JL. Damage to the skin and leather caused by the horn fly (Diptera: Muscidae) in Argentina. IULTCS Congress; Porto Alegre, Brazil: 1993. pp. 544–545. [Google Scholar]
- Valenzuela JG, Belkaid Y, Garfield MK, Mendez S, Kamhawi S, Rowton ED, Sacks DL, Ribeiro JM. Toward a defined anti-Leishmania vaccine targeting vector antigens: characterization of a protective salivary protein. J Exp. Med. 2001;194:331–342. doi: 10.1084/jem.194.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Nuttall PA. Excretion of host immunoglobulin in tick saliva and detection of IgG-binding proteins in tick haemolymph and salivary glands. Parasitology. 1994;109(Pt 4):525–530. doi: 10.1017/s0031182000080781. [DOI] [PubMed] [Google Scholar]
- Wang H, Nuttall PA. Immunoglobulin-G binding proteins in the ixodid ticks, Rhipicephalus appendiculatus, Amblyomma variegatum and Ixodes hexagonus. Parasitology. 1995a;111(Pt 2):161–165. doi: 10.1017/s0031182000064908. [DOI] [PubMed] [Google Scholar]
- Wang H, Nuttall PA. Immunoglobulin-binding proteins in ticks: new target for vaccine development against a blood-feeding parasite. Cell Mol. Life Sci. 1999;56:286–295. doi: 10.1007/s000180050430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Nuttall PA. Immunoglobulin G binding proteins in male Rhipicephalus appendiculatus ticks. Parasite Immunol. 1995b;17:517–524. doi: 10.1111/j.1365-3024.1995.tb00882.x. [DOI] [PubMed] [Google Scholar]
- Webster KA, Rankin M, Goddard N, Tarry DW, Coles GC. Immunological and feeding studies on antigens derived from the biting fly, Stomoxys calcitrans. Vet. Parasitol. 1992;44:143–150. doi: 10.1016/0304-4017(92)90152-y. [DOI] [PubMed] [Google Scholar]
- Wieman GA, Campbell JB, Deshazer JA, Berry IL. Effects of stable flies (Diptera: Muscidae) and heat stress on weight gain and feed efficiency of feeder cattle. J Econ. Entomol. 1992;85:1835–1842. doi: 10.1093/jee/85.5.1835. [DOI] [PubMed] [Google Scholar]
- Wikel SK, arcon-Chaidez FJ. Progress toward molecular characterization of ectoparasite modulation of host immunity. Vet Parasitol. 2001;101:275–287. doi: 10.1016/s0304-4017(01)00556-8. [DOI] [PubMed] [Google Scholar]
- Yarnall M, Corbeil LB. Antibody response to Haemophilus somnus Fc receptor. J. Clin. Microbiol. 1989;27:111–117. doi: 10.1128/jcm.27.1.111-117.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnall M, Widders PR, Corbeil LB. Isolation and characterization of Fc receptors from Haemophilus somnus. Scand. J. Immunol. 1988;28:129–137. doi: 10.1111/j.1365-3083.1988.tb02424.x. [DOI] [PubMed] [Google Scholar]


