Summary
This study examined prospectively the association of baseline plasma HDL-cholesterol levels with incidence of lung cancer in 14, 547 members of the Atherosclerosis Risk in Communities (ARIC) cohort. There were 259 cases of incident lung cancer identified during follow-up from 1987 through 2000. Results of this study indicated a relatively weak inverse association of HDL-cholesterol with lung cancer that was dependent on smoking status. The hazard ratio of lung cancer incidence in relation to low HDL-cholesterol, adjusted for race, gender, exercise, alcohol consumption, body mass index, triglycerides, age, and cigarette pack-years of smoking, was 1.45 (95% confidence interval 1.10, 1.92). This association was observed among former smokers (hazard ratio: 1.77, 95% confidence interval 1.05, 2.97), but not current smokers. The number of cases among never smokers in this study was too small (n=13) for meaningful interpretation of effect estimates. Excluding cases occurring within five years of baseline did not appreciably change the point estimates, suggesting lack of reverse causality. The modest association of low plasma HDL-cholesterol with greater incident lung cancer observed in this study is in agreement with existing case-control studies.
Keywords/Medical Subjects Headings (MeSH): cohort studies, HDL cholesterol, lipoproteins, lung neoplasms
Introduction
Lung cancer is the second most frequent non-cutaneous malignancy in men and women [1]. In 2005, an estimated 172,570 cases of lung cancer occurred in the United States [2]. This cancer has a high mortality rate, with a five year survival probability of approximately 10% [3]. Even though current trends in lung cancer incidence rates in the United States suggest an overall decline, there is a concerning trend of increase in incidence of lung cancer among never smokers and among women [4,5]. The vast majority of lung cancer cases can be attributed to smoking [6], however the increase in incidence rates of lung cancer among never smokers suggests a need for identification of other modifiable lung cancer risk factors.
Case-control studies of newly diagnosed lung cancer have shown that HDL-cholesterol levels are reduced in lung cancer cases relative to levels observed in the control groups[7–10]. This association was found to be independent of type and stage of lung cancer [7], but not all adjusted for smoking or other confounders. Furthermore, levels of plasma HDL-cholesterol may change as a consequence of lung cancer, and prospective studies are needed to better understand the temporal relationship between low plasma HDL-cholesterol levels and cancer incidence.
The purpose of the present study was to examine the association of baseline plasma HDL-cholesterol levels with incidence of lung cancer in the Atherosclerosis Risk in Communities (ARIC) study cohort.
Materials and Methods
This study was approved by the appropriate Institutional Review Boards and all participants provided written consent.
A detailed description of the ARIC study has been published elsewhere [11]. In brief, the ARIC cohort was selected as a probability sample of 15, 792 men and women, aged 45–64 years, from four US communities located in Forsyth County, North Carolina, city of Jackson, Mississippi, eight northwestern suburbs of Minneapolis, Minnesota and Washington County, Maryland. Physical examination and risk factor assessment was conducted during the baseline visit (1987–1989) and at three subsequent visits occurring every 3 years.
Blood was drawn from an antecubital vein into tubes containing EDTA at each clinical visit following a minimum of 8 hours of fasting. Plasma was separated by centrifugation at 4°C and aliquots were stored at −70°C until analysis.
HDL-cholesterol levels were measured using the method of Warnick et al [12]. There are no clearly defined cutpoints for HDL-cholesterol in relation to cancer outcomes. We chose to use the cardiovascular clinical cutpoints to define low HDL-cholesterol levels (<40 mg/dl for men, <50 mg/dl for women) as those have been extensively used in epidemiological research and would provide a well defined benchmark [13,14].
Additionally we performed an analysis with gender-specific HDL-cholesterol cutpoints for men: <34.7 mg/dl, ≥34.7 – <42.0 mg/dl, ≥42.0 – 51.0 mg/dl, and ≥51.0 mg/dl; for women: <45.3 mg/dl, ≥45.3–<54.9 mg/dl, ≥54.9–<67.4 mg/dl, and ≥67.4 mg/dl). Metabolic factors, other than HDL-cholesterol, were defined according to the National Cholesterol and Education Program (NCEP) Adult Treatment Panel III criteria [15]: elevated blood pressure (≥130/85 mm Hg), hypertriglyceridemia (≥150 mg/dl or 1.695 mmol/L), elevated fasting glucose (≥110 mg/dl) and large waist circumference (>88 cm in women and >102 cm in men). Triglyceride levels were measured by enzymatic methods, adapted for analysis using the Cobas Bioanalyzer (Roche) with the use of reagents supplied by Boehringer Mannheim Biochemicals [16]. Body mass index was calculated as the ratio of weight in kilograms to height in centimeters square which were measured at baseline. Waist circumference was measured at the umbilicus to the nearest centimeter with the subject standing. Presence of hypertension was based on use of antihypertensive medication within two weeks of baseline data collection or if systolic blood pressure measured at baseline was greater than 140 mm Hg or diastolic blood pressure was greater than 90 mm Hg. Diabetes status (yes/no) at baseline was based on either one of the following criteria: fasting plasma glucose levels greater than 126 mg/dL, non-fasting plasma glucose >200 mg/dL, use of anti-diabetic medication within two weeks of baseline data collection, or self-report of a diagnosis of diabetes. Exercise was based on self-reported level of sport activity during leisure time, at baseline. Alcohol consumption was based on baseline self-reported usual amount of ethanol intake per week.
Participants were classified as current, former and never smokers at baseline. Pack-years of cigarette smoking were determined as the average number of cigarettes smoked per day times the number of years of smoking divided by 20 (the number of cigarettes in a pack).
Incident lung cancer among the ARIC cohort members was ascertained from 1987 through 2000 on the basis of self-report by cohort participants of a cancer diagnosis (yes/no). The ARIC study asked participants to report all hospitalizations and hospital surveillance was carried out in each community. Cancer occurrence, primary site and diagnosis were identified by linkage to the following cancer registries: the Minnesota Cancer Surveillance System (completeness of records: 99.7 percent), the North Carolina Cancer Registry (completeness of records in Forsyth County only throughout the study follow-up time), the Washington County (Maryland) Cancer Registry (completeness of records: 90 percent), the (statewide) Maryland Cancer Registry (completeness of records: 90 percent) and the Mississippi Central Cancer Registry (from 1995; completeness of records: 90 percent in 1996, 96 percent in 2000). Because of a lack of a state cancer registry, lung cancer cases in Jackson, Mississippi (from 1987 until 1995) and in Minnesota (from 1987 to 1988), in addition to cases from other centers that were not identified through the registries, were identified based on review of discharge codes from retrieved medical records of persons who reported during the annual telephone follow-up call of having had a cancer-related hospitalization.
Statistical analysis
Participants in the 15, 792 member ARIC baseline cohort were excluded from the current study based on self-reported racial background other than white or black (n=48); failure to fast for ≥8 hours before venipuncture (n=587); missing baseline data on HDL- status (n=195), hypertension (n=128), pack years of smoking (n=279), cigarette smoking status (n=64); or missing information concerning lung cancer incidence (n=235). The final cohort for this analysis included 14,547 participants (92.1% of the baseline ARIC cohort).
The association between baseline HDL-cholesterol levels and lung cancer incidence was initially examined using the Nelson-Aalen estimate [17,18] of cumulative hazard, with incident lung cancer as the outcome of interest. The association between relative levels of HDL-cholesterol (quartiles) and lung cancer was also evaluated using age-adjusted incidence rate ratio estimates. The association between HDL-cholesterol and lung cancer incidence was estimated by Cox proportional hazard ratios adjusted for age, race, gender, body mass index, smoking status, cigarette pack-years of smoking, exercise, and alcohol consumption at baseline. Adherence to proportional hazard assumptions in the Cox regression analysis was determined for each covariate and for the overall regression model on the basis of smoothed plots of Schoenfeld residuals (log-negative log plots) [19] performed before and after stratifying on smoking status. Tests of the linear trend for the association between HDL-cholesterol and lung cancer incidence were performed using Cox regression models with HDL-cholesterol coded as a continuous variable or as an ordinal variable indicating HDL-cholesterol quartiles.
Interactions between HDL-cholesterol and selected covariates were evaluated using likelihood ratio tests comparing models with and without multiplicative interaction terms, and by comparing expected and observed incidence rates assuming multiplicative or additive effects. All hypothesis tests use α = 0.05 to determine statistical significance. All analyses were conducted using the STATA statistical software package, version 8.0.
Results
From 1987 through 2000, lung cancer was diagnosed in 259 of the 14,547 ARIC cohort members at risk (Table I). Participants who developed lung cancer were more likely than non-cases to have been male, and were also heavier smokers, older and leaner on average. They also were more likely to have low HDL-cholesterol levels and had a higher prevalence of coronary heart disease at baseline than the noncases. Cases and noncases were similar with respect to baseline prevalence of diabetes, average serum glucose, plasma triglyceride levels and use of lipid lowering medications.
TABLE I.
Selected baseline characteristics of the Atherosclerosis Risk in Communities (ARIC) study cohort. Lung cancer cases occurring between 1987–2000 (n=259).
| Characteristic | Total sample (n=14,547) | ||
|---|---|---|---|
| Cases (n=259) | Noncases (n=14,288) | p* | |
| Male (%) | 64.9 | 44.8 | <0.01 |
| Age, years (mean (SD)) | 57.2 (5.4) | 54.1 (5.8) | <0.01 |
| Smoking status: | |||
| current smokers (%) | 66.8 | 25.1 | <0.01 |
| former smokers (%) | 28.2 | 32.2 | 0.17 |
| never smokers (%) | 5.0 | 42.7 | <0.01 |
| Pack-years smoking (mean (SD)) | 42.5 (25.7) | 15.7 (21.4) | <0.01 |
| HDL-cholesterol (mg/dl (SD)) | 49.3 (17.7) | 51.6 (17.1) | 0.03 |
| Low plasma HDL-cholesterol (%)†† | 45.95 | 39.22 | 0.03 |
| High waist circumference (%)‡ | 39.8 | 49.9 | <0.01 |
| Diabetes (%) | 12.0 | 11.7 | 0.92 |
| High serum glucose (%)§ | 23.2 | 21.6 | 0.55 |
| Triglycerides (mmol/L (SD))# | 1.56 (1.0) | 1.49 (1.0) | 0.26 |
| High plasma triglycerides (%) | 30.1 | 27.5 | 0.35 |
| Cholesterol lowering drugs (%) | 3.5 | 3.0 | 0.63 |
| Prevalent CHD (%) | 7.3 | 5.0 | 0.08 |
p values were determined for the two-sided t-test
Low plasma HDL-cholesterol was defined as <50 mg/dl for women and <40 mg/dl for men
High waist circumference was defined as >88 cm for women and >102 cm for men
High serum glucose level was defined as ≥110 mg/dL
High plasma triglyceride level was defined as ≥150 mg/dl (1.695 mmol/L)
Stratification of the sample population according to smoking status indicated that among the former smokers, mean HDL-cholesterol was lower in lung cancer cases than noncases (Table II); however, mean HDL-cholesterol levels were comparable between cases and noncases in the other two smoking strata. The prevalence of low HDL-cholesterol was higher in cases than non-cases among both current and former smokers, but the difference was statistically significant for former smokers only.
TABLE II.
Selected baseline characteristics of the ARIC study cohort: current smokers, former smokers, and never smokers. Lung cancer cases occurring from 1987 through 2000
| Characteristic | Current smokers (n=3,757) |
Former smokers (n=4,674) |
Never smokers (n=6,116) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Lung cancer | Lung cancer | Lung cancer | |||||||
| p* | p* | p* | |||||||
| Cases (n=173) |
Noncases (n=3,584) |
Cases (n=73) |
Noncases (n=4,601) |
Cases (n=13) |
Noncases (n=6,103) |
||||
| Male (%) | 63.0 | 47.1 | <0.01 | 76.7 | 62.1 | 0.01 | 23.1 | 30.4 | 0.57 |
| Age, years (mean (SD)) | 56.7 (5.5) | 53.5 (5.6) | <0.01 | 58.2 (5.1) | 54.8 (5.8) | <0.01 | 57.8 (5.2) | 54.1 (5.8) | 0.02 |
| Pack-years smoking (mean (SD)) | 46.3 (24.5) | 32.9 (21) | <0.01 | 41.3 (23.6) | 23.0 (21.7) | <0.01 | - | - | |
| HDL-cholesterol (mg/dl (SD)) | 50.2 (17.9) | 49.2 (17.2) | 0.46 | 45.8 (16.4) | 50.1 (16.6) | 0.03 | 56.8 (18.5) | 54.1 (16.9) | 0.57 |
| Low plasma HDL-cholesterol (%)† | 45.95 | 39.22 | 0.31 | 54.79 | 37.97 | <0.01 | 30.76 | 35.44 | 0.73 |
| High waist circumference (%)‡ | 34.7 | 43.6 | 0.02 | 49.3 | 47.9 | 0.81 | 53.9 | 55.0 | 0.94 |
| Diabetes (%) | 12.7 | 10.3 | 0.31 | 12.3 | 11.7 | 0.87 | 0 | 12.6 | |
| High serum glucose (%)§ | 21.4 | 19.6 | 0.57 | 30.1 | 24.1 | 0.23 | 7.69 | 21.0 | 0.24 |
| Triglycerides (mmol/L (SD))# | 1.44 (0.91) | 1.53 (1.0) | 0.37 | 1.91 (1.2) | 1.56 (1.1) | 0.01 | 1.15 (0.4) | 1.41 (1.0) | 0.34 |
| High plasma triglycerides (%)§ | 26.6 | 29.1 | 0.60 | 41.1 | 30.7 | 0.06 | 15.4 | 24.1 | 0.46 |
| Cholesterol lowering drugs (%) | 3.5 | 2.3 | 0.30 | 4.1 | 3.7 | 0.87 | 0 | 55.0 | |
| Prevalent CHD (%) | 7.5 | 5.4 | 0.24 | 8.2 | 7.6 | 0.85 | 0 | 2.7 | |
| Incident CHD preceding lung cancer (%) | 12.1 | - | 12.3 | - | 0 | 2.8 | |||
p values were determined for the two-sided t-test
†† Low plasma HDL-cholesterol was defined as <50 mg/dl for women and <40 mg/dl for men
High waist circumference was defined as >88 cm for women and >102 cm for men
High serum glucose level was defined as ≥110 mg/dL
High plasma triglyceride level was defined as ≥150 mg/dl (1.695 mmol/L)
Of other metabolic conditions, shown in Table II, notable is higher prevalence of high triglyceride levels among former smokers who developed lung cancer compared with former smokers who did not develop lung cancer. There was very little difference in the prevalence of high triglycerides in persons with lung cancer as compared to persons without lung cancer in the current smokers group. Among never smokers, high triglyceride levels were less prevalent in cases than in the noncases.
To evaluate effect modification by smoking on the association between low HDL-cholesterol and lung cancer, we estimated incidence rates and crude Cox proportional hazard ratios for the independent and joint effects of low HDL-cholesterol and smoking. Ever smokers were defined as current and former smokers. Never smokers with high levels of HDL-cholesterol were defined as the common reference group (Table IIIA). Joint effect estimates for ever smokers with low HDL-cholesterol levels were consistent with additive and multiplicative risks, however the small number of cases (n=13) among never smokers contributed to a very imprecise analysis (note width of confidence intervals). We performed an analogous analysis of the independent and joint effects of low levels of HDL-cholesterol among current and former smokers only, with former smokers with high levels of HDL-cholesterol defined as the common reference group (Table IIIB). Our results were consistent with a subadditive effect of current cigarette smoking and HDL-cholesterol on lung cancer incidence, such that low HDL-cholesterol was associated with lung cancer incidence only among former smokers and did not appear to contribute any additional hazard to the incidence of lung cancer among current smokers. Likelihood ratio tests comparing Cox regression models with and without a multiplicative HDL-cholesterol-smoking interaction term showed a statistically significant departure from expectations for multiplicative risks (observed adjusted joint hazard ratio for low HDL cholesterol and current smoking 3.89 vs. expected hazard ratio of 7.78, likelihood ratio χ2=6.00, p=0.01). The interaction remained statistically significant after adjusting for confounding.
TABLE III.
Analysis of potential effect modification of cigarette smoking on the estimate of the association of HDL-cholesterol with lung cancer in the ARIC study cohort
| Smoking status | HDL-cholesterol* | Crude incidence rate × 106 (95% CI) | HR (95% CI) | ||
|---|---|---|---|---|---|
| Crude | Adjusted** | ||||
| Never | High | 0.48 (0.25, 0.92) | 1.00 (reference) | 1.00 (reference) | |
| Never | Low | 0.40 (0.15, 1.07) | 0.84 (0.26, 2.71) | 1.00 (0.31, 3.25) | |
| Ever | High | 5.90 (4.97, 7.01) | 12.32 (6.27, 24.21) | 5.60 (2.79, 11.23) | |
| Ever | Low | 7.28 (6.06, 8.73) | 15.15 (7.69, 29.86) | 7.88 (3.90, 15.92) | |
| A. | |||||
| Smoking status | HDL-cholesterol* | Crude incidence rate × 106 (95% CI) | HR (95% CI) | ||
|---|---|---|---|---|---|
| Crude | Adjusted** | ||||
| Former | High | 2.46 (1.75, 3.46) | 1.00 (reference) | 1.00 (reference) | |
| Former | Low | 4.95 (3.63, 6.75) | 2.01 (1.27, 3.19) | 1.99 (1.24, 3.18) | |
| Current | High | 11.18 (9.17, 13.6) | 4.56 (3.08, 6.77) | 3.91 (2.60, 5.88) | |
| Current | Low | 9.70 (7.78, 12.2) | 3.97 (2.67, 5.94) | 3.89 (2.56, 5.92) | |
| B. | |||||
Cut-points for HDL-cholesterol were 40 mg/dl for men, 50 mg/dl for women
Adjustment for race, gender, body mass index, age, exercise, alcohol, cigarette years of smoking HR: Cox proportional hazards ratio
Given the observed modification of the hazard ratio for HDL-cholesterol and lung cancer incidence by current cigarette smoking, subsequent analyses were stratified by smoking status among current and former smokers only.
Likelihood ratio tests indicated no statistically significant (α=0.05) modification of the HDL-cholesterol and lung cancer hazard ratio by cigarette pack-years of smoking (p=0.73 for former smokers; p=0.63 for current smokers) or gender (p=0.42 for former smokers; p=0.93 for current smokers).
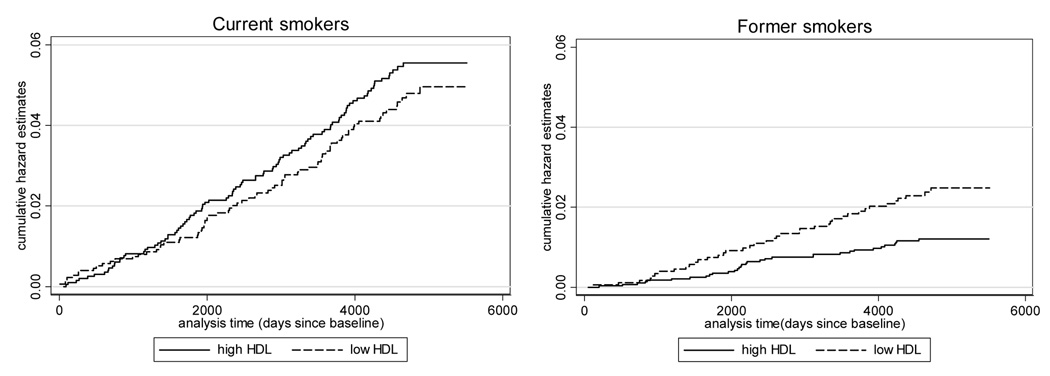
Crude Nelson-Aalen cumulative hazard estimates indicated that cumulative hazards for lung cancer were lower among current smokers with low HDL-cholesterol (logrank test χ2= 0.72, p= 0.40), while among former smokers hazards were higher in association with low HDL-cholesterol (logrank test χ2= 4.90, p=0.03) (Figure 1).
Figure 1.
Crude Nelson-Aalen cumulative hazard function of lung cancer incidence for the current and former smokers in the ARIC study cohort.
Cutpoints for HDL-cholesterol: 40 mg/dl for men and 50 mg/dl for women
In an analysis of age-standardized relative rate of lung cancer incidence as a function of HDL-cholesterol quartiles (Table IV) an inverse association between lung cancer incidence and HDL-cholesterol was observed for former smokers (p for trend = 0.02). No obvious gradient of association was seen for current smokers.
TABLE IV.
Association of plasma HDL-cholesterol quartiles with incidence of lung cancer in the ARIC study cohort.
| Total sample | Current smokers | Former smokers | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HDL-chol. quartiles | Lung cancer cases | Person-years | Incidence rate (×106) (95% CI) | Relative rate (95%CI) | Lung cancer cases | Person-years | Incidence rate (×106) (95% CI) | Relative rate (95%CI) | Lung cancer cases | Person-years | Incidence rate (×106) (95% CI) | Relative rate (95%CI) | ||||
| 1.17 | 9.46 | 0.76 | 4.97 | 1.97 | ||||||||||||
| 1 | 75 | 16,116,858 | 4.65 (3.71, 5.84) |
(0.84, 1.62) | 49 | 5,177,047 | (7.15, 12.5) | (0.51, 1.13) | 23 | 4,624,827 | (3.30, 7.48) | (1.01, 3.82) | ||||
| 2 | 64 | 16,211,473 | 3.95 (3.09, 5.04) |
0.98 (0.70, 1.39) |
41 | 4,035,332 | 10.16 (7.48, 13.8) |
0.78 (0.52, 1.19) |
20 | 5,378,433 | 3.72 (2.4, 5.76) |
1.44 (0.73, 2.84) |
||||
| 3 | 53 | 17,876,052 | 2.96 (2.27, 3.88) |
0.75 (0.52, 1.07) |
35 | 3851,054 | 9.09 (6.53, 12.7) |
0.68 (0.44, 1.05) |
16 | 5,939,775 | 2.69 (1.65, 4.40) |
1.08 (0.53, 2.12) |
||||
| 4 | 67 | 16,530,050 | 4.05 (3.19, 5.15 |
1.00 | 48 | 3,432,223 | 13.99 (10.5, 18.6) |
1.00 | 14 | 5,558,144 | 2.52 (1.49, 4.25) |
1.00 | ||||
| p-for-trend | p=0.12 | p=0.35 | p=0.02 | |||||||||||||
Relative incidence rates were age adjusted. P-for-trend denotes trend across quartile values of HDL-cholesterol.
HDL-cholesterol quartiles were defined for men as <34.7 mg/dl, ≥34.7 - <42.0 mg/dl, ≥42.0 – 51.0 mg/dl, and ≥51.0 mg/dl, and for women as <45.3 mg/dl, ≥45.3-<54.9 mg/dl, ≥54.9-<67.4 mg/dl, and ≥67.4 mg/dl.
A Cox regression analysis of the association of HDL-cholesterol, as a dichotomous variable, and incidence of lung cancer (Table V) was consistent with the above observations. A modest association between low HDL-cholesterol and lung cancer incidence was observed for the total sample population and among former smokers. In those two groups the magnitude of the association increased after adjustment for covariates (race, age, gender, body mass index, triglycerides, exercise, alcohol consumption, and cigarette pack-years of smoking). No association was seen in current smokers. We noted a modest association among the never smokers; however the number of cases in this group was small, rendering the measurement of effect imprecise and difficult to interpret. The results of the analysis of association of HDL-cholesterol as a continuous variable and incidence of lung cancer (Table V) revealed a pattern similar to that observed with the dichotomous variable.
TABLE V.
Cox proportional hazard ratios of the association of low HDL and the incidence of lung cancer in the ARIC study cohort.
| Cox proportional hazard ratios | ||||
|---|---|---|---|---|
| Risk factor | Total sample (95% CI) | Current smokers (95% CI) | Former smokers (95% CI) | Never smokers (95% CI) |
| Low vs. high HDL-cholesterol: | ||||
| Crude | 1.35 (1.05, 1.72) |
0.89 (0.66, 1.20) |
2.01 (1.27, 3.19) |
0.83 (0.25, 2.69) |
| Adjusted* | 1.45 (1.10, 1.92) |
1.04 (0.74, 1.47) |
1.77 (1.05, 2.97) |
1.56 (041, 5.86) |
| HDL-cholesterol as a continuous variable: | ||||
| Crude | 0.86 (0.75, 0.99) |
1.05 (0.91,1.22) |
0.72 (0.55, 0.96) |
1.16 (0.69, 1.94) |
| β=−0.16 (−0.29, −0.03) |
β=0.05 (−0.10, 0.20) |
β=−0.32 (−0.60, −0.04) |
β=0.15 (−0.37, 0.66) |
|
| p=0.02 | p=0.50 | p=0.02 | p=0.58 | |
| Adjusted* | 0.92 (0.78, 1.08) |
1.07 (0.90, 1.28) |
0.87 (0.62, 1.22) |
0.78 (0.39, 1.56) |
| β=−0.09 (−0.25, 0.07) |
β=0.07 (−0.11, 0.25) |
β=−0.14 (−0.48, 0.20) |
β=−0.25 (−0.94, 0.45) |
|
| p=0.29 | p=0.45 | p=0.42 | p=0.49 | |
Adjustment for age, race, body mass index, cigarette pack-years of smoking, gender, triglycerides, exercise and alcohol consumption
Adjustment for smoking status was performed only for the total sample. No adjustment for smoking, or pack years of smoking was performed for never smokers.
Cut-points for the dichotomous HDL-cholesterol variable were 40 mg/dl for men, 50 mg/dl for women.
In analysis of the hazard ratios of the continuous HDL-cholesterol variable, the Cox proportional hazard ratios indicate change in hazard rate per one standard deviation increase in HDL-cholesterol levels (SD=17.07), the value of the beta coefficient defines a logarithm of the relative risk of lung cancer incidence per one standard deviation increase in HDL-cholesterol levels; p designates p for trend.
We explored the impact of possible variations in the measured HDL-cholesterol levels by restricting our analysis to the sample remaining in the cohort after the second visit and classifying low HDL-cholesterol based on the average of measurements from visit 1 and visit 2. Adjusted Cox proportional hazard ratios decreased for the total sample (hazard ratio 1.23; 95% confidence interval 0.93;1.64) and for former smokers (hazard ratio 1.38; 95% confidence interval 0.77, 2.47), but the difference between former and current smokers persisted (hazard ratio for current smokers: 1.10; 95% confidence interval: 0.74, 1.64)).
Subclinical cancer may itself decrease plasma levels of HDL-cholesterol, suggesting that observed reduced HDL-cholesterol levels may be a result and not a cause of lung cancer. We therefore eliminated from the analyses those persons for whom the diagnosis of lung cancer was made within 5 years after baseline data collection (n=74). Estimates of the hazard ratios in that subset did not differ substantially from the estimates for the entire sample, although the confidence intervals were, as expected, wider after the exclusion.
Discussion
We found evidence of a weak inverse association of HDL-cholesterol and lung cancer incidence in the ARIC study cohort. We observed that low HDL-cholesterol levels were associated with a higher incidence of lung cancer in the total sample and among the former smokers in this study; however no consistent association was seen between low HDL-cholesterol and lung cancer incidence for the current smokers. The association observed among the former smokers was probably not an effect of depletion of plasma HDL-cholesterol by existing preclinical cancer [20] since exclusion of lung cancer cases within 5 years or less from baseline did not alter the relative lung cancer incidence rates.
Biological mechanisms that might link low plasma levels of HDL-cholesterol with cancer are not well understood [10,21–23]. The function of HDL-cholesterol in reverse cholesterol transport [24] is important in development of atherosclerosis; however it is not obvious how this function of HDL-cholesterol could influence carcinogenesis. The pleiotropic properties of the HDL-cholesterol particle [25], including its anti-oxidative function, modulation of cytokine production, blockage of apoptosis and stimulation of cell proliferation and migration, are more likely to play a role in the development of cancer. HDL-cholesterol levels are decreased during chronic inflammation. Reduction in the above mentioned functions of HDL-cholesterol, resulting from decreased HDL-cholesterol levels, may contribute to a milieu conducive to cancer development. Case-control studies have shown that low plasma HDL-cholesterol is associated with newly diagnosed cancer at various sites [10,21–23,26,27]. That association has also been seen in case-control studies of lung cancer [7–9], however those studies were based on relatively small numbers of cases and compared mean levels of HDL-cholesterol in cases and controls without always adjusting for age or smoking status. The most comprehensive of those studies [10], examined association of lipoproteins (LDL-cholesterol, HDL-cholesterol, triglycerides) and multiple cancer types. Their findings suggest that the association with low HDL-cholesterol is shared among different malignancies, suggesting a common pathway.
A major strength of the present study is its prospective design, which adds support to the existing case-control studies of the association of low HDL-cholesterol and lung cancer incidence. The ARIC cohort is a representative sample of populations of four selected communities and as such represents an ethnically heterogeneous study population. This heterogeneity and large size of the cohort are further strong advantages of our study.
The major methodological issue that we had to confront in this analysis was the effect of cigarette use on the association of HDL-cholesterol and incidence of lung cancer. Smoking is a well documented and highly significant independent risk factor for lung cancer [28]. Furthermore, smoking has been shown to reduce plasma HDL-cholesterol levels, possibly through induction of an inflammatory response and an associated increase in lipases that hydrolyze HDL-cholesterol and enhance clearance of HDL-cholesterol molecules from the bloodstream [29]. From our data, it is not possible to discern whether the lower mean HDL-cholesterol levels observed among the participants destined to develop lung cancer were due to smoking or other factors. We tried to address the confounding effect of cigarette smoking by stratifying the analysis by smoking status. This approach was limited by the small number of cases of lung cancer among never smokers and could not eliminate all of the confounding effects due to smoking. Residual confounding by smoking may persist among the current and former smokers despite adjusting for cigarette pack-years, age at onset of smoking and years of smoking cessation (former smokers). This residual confounding could result from self-reporting of smoking status, change in smoking status during the follow-up time, or differences in smoking intensity.
We did not observe a clear association between HDL-cholesterol and lung cancer among current smokers, in contrast with a positive association between low HDL cholesterol among former smokers. One possible explanation for this finding is that there is in fact no association and the weak association we did find in former smokers is random or is the result of residual confounding. Another explanation is that smoking is such a strong risk factor for lung cancer that the effect of a relatively weak risk factor, such as HDL-cholesterol, is unobservable among current smokers. Furthermore, our data indicate that the reduction in HDL-cholesterol levels seen among current smokers relative to never smokers, resulting presumably from cigarette smoking, is independent of lung cancer status. Given this smoking-related reduction in HDL-cholesterol levels the hazard ratio for the association of HDL-cholesterol and lung cancer, observed in the group of current smokers, may not be representative of the actual risk.
Plasma levels of HDL-cholesterol are closely related to levels of triglycerides. In hypertriglyceridemic states, which can occur as a consequence to an inflammatory process [30], there is an increase in plasma triglyceride levels and a net increased movement of triglycerides into HDL-cholesterol molecules. The thus triglyceride-enriched HDL-cholesterol is cleared more readily from the blood stream, resulting in decreased plasma HDL-cholesterol levels [31]. Smoking cessation does not appear to affect levels of triglycerides [32]. In our study we observed a higher prevalence of elevated triglyceride levels among former smokers who developed lung cancer relative to the former smokers who did not develop lung cancer, but did not observe such differences among current smokers for whom levels of triglycerides were not significantly associated with lung cancer status. It is possible, that the low mean HDL-cholesterol levels observed among cases in the sample of former smokers were reflective of the increased triglyceride levels.
Conclusion
Our data underscore the difficulty in examining the role of factors other than smoking in the development of lung cancer. The modest inverse association between plasma HDL-cholesterol and incidence of lung cancer, which we have noted in former smokers, but not in current smokers, should be confirmed by further studies performed in never smokers as well as studies in which cancers other than lung cancer are the outcome of interest. Likewise, the potential clinical and public health significance of this association should be explored further.
Acknowledgements
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022. The authors thank the staff and participants of the ARIC study for their important contributions.
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- HDL-cholesterol
high density lipoprotein cholesterol
- HR
hazard ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
No disclosures
References
- 1.A.C.S.S. 2006 [Google Scholar]
- 2.Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 3.Bofetta P, Trichopoulos D. Cancer of the Lung, Larynx, and PLeura. In: Adami HO, et al., editors. Textbook of Cancer Epidemiology. Oxford: Oxford University Press, Inc; 2002. pp. 248–281. [Google Scholar]
- 4.Patel JD. Lung cancer in women. J Clin Oncol. 2005;23:3212–3218. doi: 10.1200/JCO.2005.11.486. [DOI] [PubMed] [Google Scholar]
- 5.Wingo PA, Ries LA, Giovino GA, Miller DS, Rosenberg HM, Shopland DR, Thun MJ, Edwards BK. Annual report to the nation on the status of cancer, 1973–1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91:675–690. doi: 10.1093/jnci/91.8.675. [DOI] [PubMed] [Google Scholar]
- 6.Shields PG. Molecular epidemiology of smoking and lung cancer. Oncogene. 2002;21:6870–6876. doi: 10.1038/sj.onc.1205832. [DOI] [PubMed] [Google Scholar]
- 7.Siemianowicz K, Gminski J, Stajszczyk M, Wojakowski W, Goss M, Machalski M, Telega A, Brulinski K, Magiera-Molendowska H. Serum HDL cholesterol concentration in patients with squamous cell and small cell lung cancer. Int J Mol Med. 2000;6:307–311. doi: 10.3892/ijmm.6.3.307. [DOI] [PubMed] [Google Scholar]
- 8.Umeki S. Decreases in serum cholesterol levels in advanced lung cancer. Respiration. 1993;60:178–181. doi: 10.1159/000196195. [DOI] [PubMed] [Google Scholar]
- 9.Dessi S, Batetta B, Pulisci D, Spano O, Cherchi R, Lanfranco G, Tessitore L, Costelli P, Baccino FM, Anchisi C, et al. Altered pattern of lipid metabolism in patients with lung cancer. Oncology. 1992;49:436–441. doi: 10.1159/000227088. [DOI] [PubMed] [Google Scholar]
- 10.Fiorenza AM, Branchi A, Sommariva D. Serum lipoprotein profile in patients with cancer. A comparison with non-cancer subjects. Int J Clin Lab Res. 2000;30:141–145. doi: 10.1007/s005990070013. [DOI] [PubMed] [Google Scholar]
- 11.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 12.Warnick GR, Mayfield C, Benderson J, Chen JS, Albers JJ. HDL cholesterol quantitation by phosphotungstate-Mg2+ and by dextran sulfate-Mn2+-polyethylene glycol precipitation, both with enzymatic cholesterol assay compared with lipid research method. Am J Clin Pathol. 1982;78:718–723. doi: 10.1093/ajcp/78.5.718. [DOI] [PubMed] [Google Scholar]
- 13.Brown SA, Hutchinson R, Morrisett J, Boerwinkle E, Davis CE, Gotto AM, Jr, Patsch W The Atherosclerosis Risk in Communities (ARIC) Study. Plasma lipid, lipoprotein cholesterol, and apoprotein distributions in selected US communities. Arterioscler Thromb. 1993;13:1139–1158. doi: 10.1161/01.atv.13.8.1139. [DOI] [PubMed] [Google Scholar]
- 14.McNeill AM, Katz R, Girman CJ, Rosamond WD, Wagenknecht LE, Barzilay JI, Tracy RP, Savage PJ, Jackson SA. Metabolic Syndrome and Cardiovascular Disease in Older People: The Cardiovascular Health Study. Journal of the American Geriatrics Society. 2006;54:1317–1324. doi: 10.1111/j.1532-5415.2006.00862.x. [DOI] [PubMed] [Google Scholar]
- 15.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 16.Shahar E, Chambless LE, Rosamond WD, Boland LL, Ballantyne CM, McGovern PG, Sharrett AR. Plasma Lipid Profile and Incident Ischemic Stroke: The Atherosclerosis Risk in Communities (ARIC) Study. Stroke. 2003;34:623–631. doi: 10.1161/01.STR.0000057812.51734.FF. [DOI] [PubMed] [Google Scholar]
- 17.Nelson W. Theory and applications of hazard plotting for censored failure data. Technometrics. 1972;14:945–966. [Google Scholar]
- 18.Aalen OO. Non parametric inference for a family of counting processes. Annals of Statistics. 1978;6:701–726. [Google Scholar]
- 19.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14:1707–1723. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 20.Sherwin RW, Wentworth DN, Cutler JA, Hulley SB, Kuller LH, J, S Serum cholesterol levels and cancer mortality in 361,662 men screened for the Multiple Risk Factor Intervention Trial. JAMA. 1987;257:943–948. [PubMed] [Google Scholar]
- 21.Boyd NF, McGuire V. Evidence of association between plasma high-density lipoprotein cholesterol and risk factors for breast cancer. J Natl Cancer Inst. 1990;82:460–468. doi: 10.1093/jnci/82.6.460. [DOI] [PubMed] [Google Scholar]
- 22.Moorman PG, Hulka BS, Hiatt RA, Krieger N, Newman B, Vogelman JH, Orentreich N. Association between high-density lipoprotein cholesterol and breast cancer varies by menopausal status. Cancer Epidemiol Biomarkers Prev. 1998;7:483–488. [PubMed] [Google Scholar]
- 23.Furberg AS, Veierod MB, Wilsgaard T, Bernstein L, Thune I. Serum high-density lipoprotein cholesterol, metabolic profile, and breast cancer risk. J Natl Cancer Inst. 2004;96:1152–1160. doi: 10.1093/jnci/djh216. [DOI] [PubMed] [Google Scholar]
- 24.Tall AR. An overview of reverse cholesterol transport. Eur Heart J. 1998;19 Suppl:A31–A35. [PubMed] [Google Scholar]
- 25.von Eckardstein A, Hersberger M, Rohrer L. Current understanding of the metabolism and biological actions of HDL. Curr Opin Clin Nutr Metab Care. 2005;8:147–152. doi: 10.1097/00075197-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Hoyer AP, Engholm G. Serum lipids and breast cancer risk: a cohort study of 5,207 Danish women. Cancer Causes and Control. 1992;3:403–408. doi: 10.1007/BF00051352. [DOI] [PubMed] [Google Scholar]
- 27.Gerber M, Cavallo F, Marubini E, Richardson S, Barbieri A, Capitelli E, Costa A, Crastes de Paulet A, Crastes de Paulet P, Decarli A, et al. Liposoluble vitamins and lipid parameters in breast cancer. A joint study in northern Italy and southern France. Int J Cancer. 1988;42:489–494. doi: 10.1002/ijc.2910420403. [DOI] [PubMed] [Google Scholar]
- 28.Peto R, Darby S, Deo H, Silcocks P, Whitley E, R D. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. Brithish Medical Journal. 2000;321:323–329. doi: 10.1136/bmj.321.7257.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tietge UJ, Maugeais C, Lund-Katz S, Grass D, deBeer FC, Rader DJ. Human secretory phospholipase A2 mediates decreased plasma levels of HDL cholesterol and apoA-I in response to inflammation in human apoA-I transgenic mice. Arterioscler Thromb Vasc Biol. 2002;22:1213–1218. doi: 10.1161/01.atv.0000023228.90866.29. [DOI] [PubMed] [Google Scholar]
- 30.Esteve E, Ricart W, Fernandez-Real JM. Dyslipidemia and inflammation: an evolutionary conserved mechanism. Clin Nutr. 2005;24:16–31. doi: 10.1016/j.clnu.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Lamarche B, Rashid S, Lewis GF. HDL metabolism in hypertriglyceridemic states: an overview. Clin Chim Acta. 1999;286:145–161. doi: 10.1016/s0009-8981(99)00098-4. [DOI] [PubMed] [Google Scholar]
- 32.Maeda K, Noguchi Y, Fukui T. The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: a meta-analysis. Prev Med. 2003;37:283–290. doi: 10.1016/s0091-7435(03)00110-5. [DOI] [PubMed] [Google Scholar]